Abstract
Objective:
To test the hypothesis that different neurocognitive networks underlie verbal fluency deficits in frontotemporal lobar degeneration (FTLD).
Methods:
Letter (“FAS”) and semantic (“animal”) fluency tests were administered to patients with a behavioral/dysexecutive disorder (bvFTLD; n = 71), semantic dementia (SemD; n = 21), and progressive nonfluent aphasia (PNFA; n = 26). Tests measuring working memory, naming/lexical retrieval, and semantic knowledge were also obtained. MRI voxel-based morphometry (VBM) studies were obtained on a subset of these patients (bvFTLD, n = 51; PNFA, n = 11; SemD, n = 10).
Results:
Patients with SemD were disproportionately impaired on the semantic fluency measure. Reduced output on this test was correlated with impaired performance on naming/lexical retrieval tests. VBM analyses related reduced letter and semantic fluency to anterior and inferior left temporal lobe atrophy. Patients with bvFTLD were equally impaired on both fluency tests. Poor performance on both fluency tests was correlated with low scores on working memory and naming/lexical retrieval measures. In this group, MRI-VBM analyses related letter fluency to bilateral frontal atrophy and semantic fluency to left frontal/temporal atrophy. Patients with PNFA were also equally impaired on fluency tests. Reduced semantic fluency output was correlated with reduced performance on naming/lexical retrieval tests. MRI-VBM analyses related semantic fluency to the right frontal lobe and letter fluency to left temporal atrophy.
Conclusions:
Distinct neurocognitive networks underlie impaired performance on letter and semantic fluency tests in frontotemporal lobar degeneration subgroups.
GLOSSARY
- AD
= Alzheimer disease;
- ANOVA
= analysis of variance;
- bvFTLD
= behavioral/dysexecutive subgroup;
- FTLD
= frontotemporal lobar degeneration;
- MMSE
= Mini-Mental State Examination;
- MNI
= Montreal Neurological Institute;
- MR
= magnetic resonance;
- PNFA
= progressive nonfluent aphasia;
- SemD
= semantic dementia;
- TE
= echo time;
- TR
= repetition time;
- VBM
= voxel-based morphometry.
Frontotemporal lobar degeneration (FTLD) is a progressive neurodegenerative illness associated with frontal and temporal lobe atrophy.1 Clinical subgroups of patients with FTLD include a decline in behavior, comportment, and executive functioning (bvFTLD)2,3; semantic dementia (SemD)4 associated with fluent progressive aphasia, impaired word comprehension, and poor object knowledge; and progressive nonfluent aphasia (PNFA)5 associated with effortful speech and impaired grammatic comprehension. Imaging studies have shown that these patients have different distributions of cortical atrophy.6
Recent research has demonstrated that these FTLD subgroups can present with impaired executive control as seen by reduced performance on tests of letter (“FAS”) and semantic (“animals”7,8) fluency. Because autopsy-confirmed patients with FTLD produce differing patterns of impairment on letter as compared with semantic fluency tests, these tests may help differentiate between Alzheimer disease (AD) and FTLD and between FTLD subtypes.9–12 Functional neuroimaging studies of healthy adults associate these tasks with partially distinct activation patterns.13,14
In the current research, FTLD patients diagnosed with bvFTLD, SemD, and PNFA were compared on letter (FAS) and semantic verbal fluency tests. In addition, measures of working memory, lexical retrieval, and semantic knowledge were evaluated to help interpret the basis for difficulty on these tests. MRI voxel-based morphometry (VBM) studies of cortical atrophy were also obtained in a subset of patients to help establish the neuroanatomic correlates of impaired fluency performance. We hypothesized that letter and semantic fluency measures depend on partially overlapping large-scale neural networks that support search and retrieval mechanisms in the mental lexicon and that partially distributed patterns of impairment would be evident in each FTLD subgroup.
METHODS
Patients.
We examined 118 patients evaluated from the Department of Neurology, University of Pennsylvania. The initial clinical diagnosis of FTLD was based on published criteria,1,15 and determined by a structured clinical interview, a neurologic examination conducted by the attending neurologist (M.G., H.B.C., A.C.), a detailed mental status assessment using the Philadelphia Brief Assessment of Cognitive Functions (PBAC),16 the results of serum studies looking for reversible causes for dementia, structural imaging studies such as MRI or CT, clinical studies of CSF (when available), and clinical functional neuroimaging studies such as SPECT or PET (these studies were not available to the consensus committee). Exclusion criteria included conditions such as stroke/white matter disease, hydrocephalus (as determined by imaging studies), primary psychiatric disorders (e.g., depression or psychosis), or a systemic illness that can interfere with cognitive functioning. Some of these patients were taking a fixed dosage of an acetylcholinesterase inhibitor, a low dosage of a nonsedating antidepressant (e.g., serotonin-specific reuptake inhibitors), or an atypical neuroleptic agent (e.g., quetiapine), as indicated clinically. No patient demonstrated any sedation. Informed consent was obtained following procedures approved by the University of Pennsylvania Institutional Review Board.
At least 2 trained reviewers of a consensus committee assigned patients to an FTLD subgroup based on an independent of available in formation. The subgroups were classified based on published criteria modified to improve reliability.17,18 When there was disagreement, cases were discussed by the entire committee to arrive at a consensus diagnosis. Seventy-one patients had a bvFTLD profile presenting with social/behavioral difficulties and impaired executive functioning. Twenty-one patients with SemD were included characterized by fluent/circumlocutory spontaneous speech that was often empty in content with a prominent naming deficit and difficulty understanding single words and objects. Twenty-six patients with PNFA were studied who presented with effortful speech, dysarthria, phonemic substitutions, or impaired grammatic comprehension but relatively good single word comprehension.
Patients with bvFTLD were younger than patients with SemD (p < 0.044) and PNFA (p < 0.017) (table 1). The PNFA group was less educated compared with the bvFTLD group (p < 0.013). However, the subgroup of patients with available imaging studies did not differ on these characteristics. There were no differences for illness duration or performance on the Mini-Mental State Examination.19
Table 1 Demographic information, neuropsychological test data (means and standard deviations), and correlations between fluency and neuropsychological test data
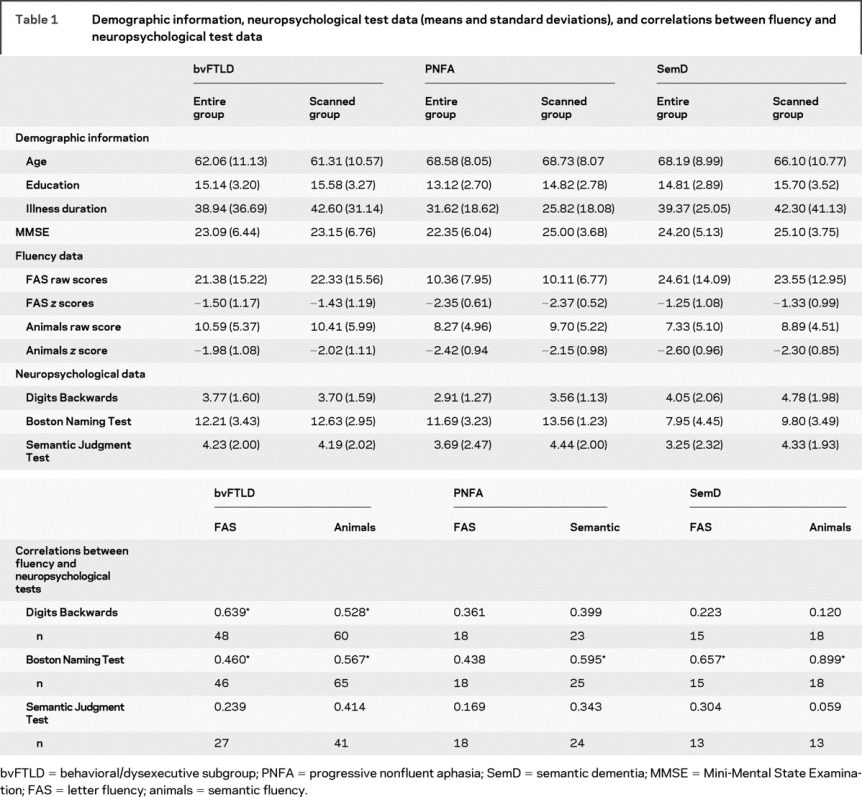
Fluency and neuropsychological tests.
Letter fluency.
Patients were given 60 seconds each to generate words beginning with a specified letter (FAS), excluding proper nouns, numbers, and multiple forms of the same word.20 The dependent variable was the number of correct responses summed across the 3 letters.
Semantic fluency.
Patients were given 60 seconds to produce as many names of animals as possible.21 The dependent variable was the total number of responses, excluding perseverations and extracategory intrusion responses.
Working memory.
Working memory was assessed with the Digits Backwards test condition from the Wechsler Adult Intelligence Scale–Revised.22 Prior research has shown a relationship between Digits Backwards performance and impairments on other working memory tests.23 The longest backward span was the dependent variable.
Lexical retrieval.
This was assessed with the 15-item version of the Boston Naming Test.24 The stimuli were equally divided among high-, middle-, and low-frequency items. The dependent variable was the total number of correct responses.
Semantic category membership task.
Semantic knowledge was assessed by asking patients to judge the semantic category membership of 48 individually presented stimuli in response to a simple probe (“Is it an X?”).25,26 One target category was tools and the other target category was vegetables. Stimuli were presented in a manner blocked by category and material. Patients were given 10 seconds to judge each stimulus. The dependent variable was the number of correct responses (range 0–48).
Performance on each test was converted to z scores based on the performance of a normal control group (n = 25) matched for age and education to minimize task-related nonspecific differences in difficulty.
Imaging methods.
Structural MRI scans were available for a subset of 72 patients (bvFTLD = 51, SemD = 10, PNFA = 11) to establish cortical atrophy using a modulated version of VBM. Images were acquired on 2 scanners. Images acquired with a SIEMENS Treo 3-T MRI scanner (n = 43) started with a rapid sagittal T1-weighted image to determine patient position. Next, high-resolution T1-weighted 3-dimensional spoiled gradient echo images were acquired with repetition time (TR) = 1,620 msec, echo time (TE) = 3 msec, slice thickness = 1.0 mm, flip angle = 15°, matrix size = 192 × 256, and in-plane resolution = 0.9 × 0.9 mm. Images from a GE Horizon Echospeed 1.5-T MRI scanner (n = 17) were high-resolution T1-weighted 3-dimensional spoiled gradient echo images acquired with TR = 35 msec, TE = 6 msec, slice thickness = 1.3 mm, flip angle = 30°, matrix size = 128 × 256, and a rectangular field of view giving an in-plane resolution of 0.9 × 0.9 mm. All images used a novel symmetric diffeomorphism procedure to normalize these high-resolution (1-mm3) T1-weighted magnetic resonance (MR) images for shape and intensity27 using a local template. The template consisted of 16 healthy seniors and 16 patients. This sample size was deemed more than large enough to yield convergence in the template while minimizing and individual bias. Half of the template images within both groups were acquired from the 1.5-T scanner and half were acquired from the 3-T scanner. We used images from both scanners to reduce any scanner bias in the template and therefore provide a controlled method for comparing individual MR images from different scanners in the experimental groups under study. We used high-dimensional normalization and template-based cortical segmentation to quantify gray matter changes. A spatially dense mapping, or correspondence, between the template and a population of experimental images was first computed. Each brain image was modeled as a dense continuum, sampled at individual voxels, that was accompanied by a transformation model that preserved neighborhood relationships among voxels even under very large deformations while maintaining global gray matter volume. This mapping process was fully unbiased because the mapping uses a bidirectional algorithm that builds unbiased maps from the set of experimental images into a template and, simultaneously, from the template into the experimental images. The symmetry achieved in this optimization significantly improves normalization compared with unidirectional template mapping.28 These types of high-dimensional, unbiased maps also benefited normalization accuracy because of the reduced variance in the probable location of a structure after deformation.27,29 The reduced variance in the estimated location of the neuroanatomy also reduced the amount of smoothing required in the final statistical treatments of these data. The statistical superiority of symmetric diffeomorphisms, relative to available parametric and elastic methods, has been established experimentally in the propagation of neuroanatomic labels across a population of elderly and neurodegenerative brains,27 and in localizing activation in small brain structures such as the hippocampus.30 After normalization, the resulting whole brain images were then skull-stripped31 and segmented using the Automated Segmentation Tool (FAST),32 which labeled the brain volumes into gray matter, white matter, CSF, and “other,” with inhomogeneity correction. The resulting gray matter images for each subject were then multiplied by their corresponding jacobian registrations to template space. This process yielded a normalized gray matter volume for each subject which contains spatially varying estimates incorporating both the deformation values and information about the subject’s global gray matter intensity.27 The multiplied volumetric images were then subsampled to 2 × 2-mm voxel sizes and then warped into Montreal Neurological Institute (MNI) space using the jacobians of the MNI space-warped template. Last, images were smoothed with a 4-mm full-width at half-maximum gaussian filter and contrasted with a cohort of 42 age-matched controls using an independent samples t test, as described elsewhere.9 The analysis included all voxels containing any gray matter in the volume, thus resulting in a true whole brain analysis. Explicit masking was used to limit comparisons to include only voxels that contained gray matter values greater than zero, and global calculation was omitted. We set a statistical height threshold of p < 0.005 uncorrected and only accepted clusters with an extent of 100 adjacent voxels because a cluster of this size would also demonstrate a consistent effect in a particular neuroanatomical distribution while minimizing false positives.33 To further reduce likelihood of false positives, we only report significant gray matter atrophy for clusters that survived a statistical threshold of p < 0.05 corrected for multiple comparisons. We then used the multiple regression module of Statistical Parametric Mapping version 2005 (SPM5) to relate both semantic and letter fluency tests to cortical volume within areas of significant gray matter atrophy for each subgroup, with a voxel height threshold of p < 0.05 corrected for multiple comparisons and a cluster extent of 20 adjacent voxels.33
RESULTS
Output on fluency tests.
Fluency raw scores and z scores, and neuropsychological test results are displayed in table 1. A repeated-measures analysis of variance (ANOVA) of z scores covaried for age and education yielded a significant task-by-group interaction [F(2,76) = 4.86, p < 0.01]. Separate 1-way ANOVAs using z scores covarying for age and education yielded significant group effects for letter fluency [F(2,78) = 4.22, p < 0.018] and semantic fluency tests [F(2,112) = 3.44, p < 0.035]. Post hoc analysis for letter fluency using Tukey tests indicated that patients with PNFA produce fewer words compared with patients with bvFTLD (p < 0.011) and SemD (p < 0.011). For the semantic fluency test, patients with PNFA produced fewer words than patients with SemD (p < 0.048). Within-group analyses revealed that patients with SemD produced fewer responses on semantic than letter fluency tests (p < 0.002). No within-group difference was noted for patients with PNFA and bvFTLD.
Different patterns emerged when fluency performance was correlated with neuropsychological measures (table 2). Patients with SemD showed correlations between letter and semantic fluency output only with performance on the Boston Naming Test (p < 0.001). For patients with PNFA, only performance on the semantic fluency test was related to performance on the Boston Naming Test (p < 0.002). For patients with bvFTLD, letter and semantic fluency tests were related to performance on the Digits Backwards and Boston Naming Tests (p < 0.001).
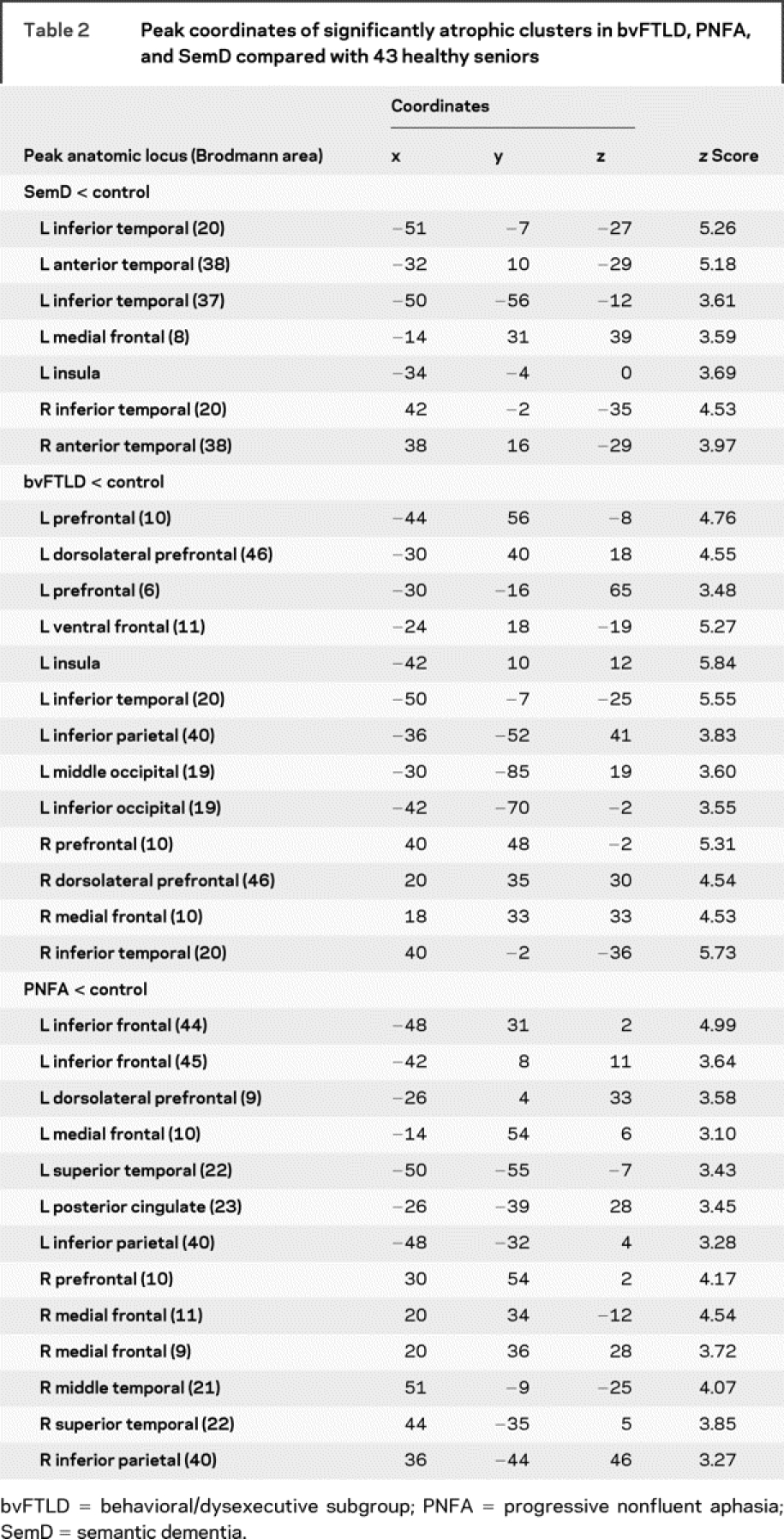
Table 2 Peak coordinates of significantly atrophic clusters in bvFTLD, PNFA, and SemD compared with 43 healthy seniors
Fluency output related to cortical atrophy.
Fluency output was related to cortical atrophy in the patients who received high-resolution structural MRI scans. Significant cortical atrophy in each clinical subgroup is illustrated in figure 1, and regression analyses are summarized in each group in figure 2. The loci of these areas are provided in tables 2 and 3.
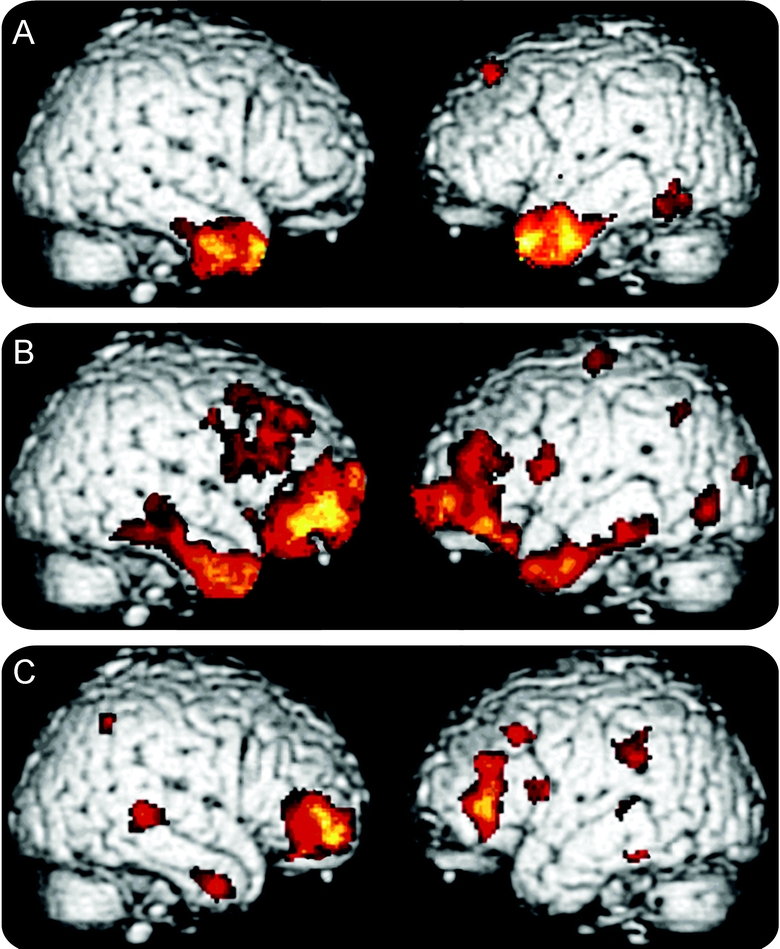
Figure 1 Cortical atrophy in subgroups of patients with frontotemporal lobar degeneration
(A) Semantic dementia; (B) behavioral/dysexecutive disorder; (C) progressive nonfluent aphasia.
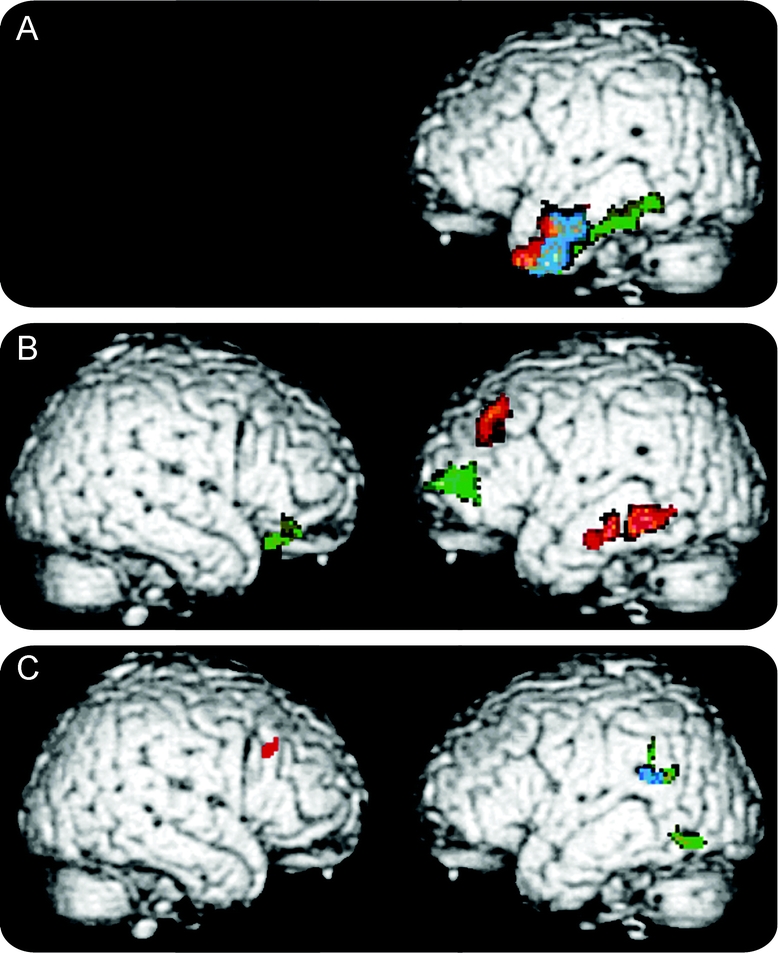
Figure 2 Reduced category naming fluency related to cortical volume in frontotemporal lobar degeneration
(A) Semantic dementia; (B) behavioral/dysexecutive disorder; (C) progressive nonfluent aphasia. Red = semantic fluency; green = FAS fluency; blue = both semantic and FAS fluency.
Table 3 Atrophic areas significantly correlated with semantic and letter fluency in each of the 3 FTLD subgroups
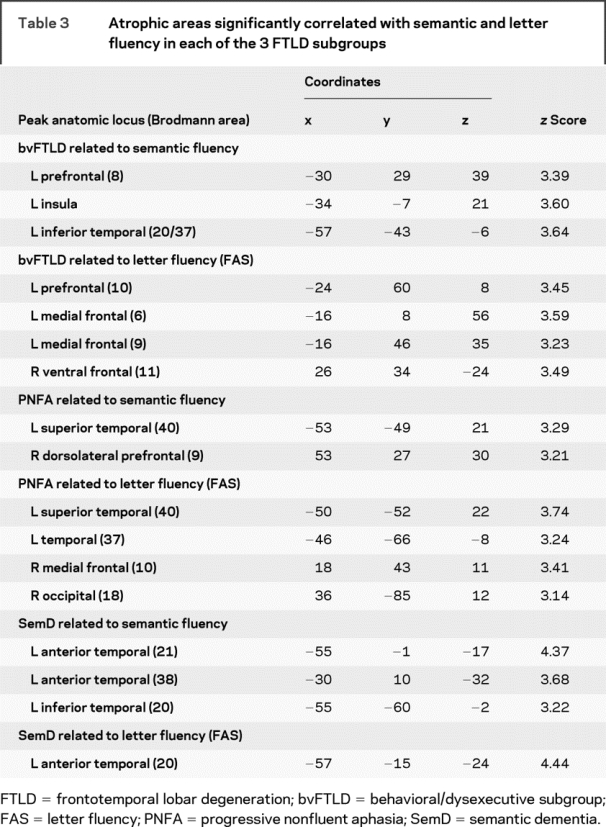
Patients with SemD had significant bilateral anterior temporal atrophy. Regression analyses related both letter and semantic fluency to atrophy in anterior and inferior left temporal regions. Patients with bvFTLD showed atrophy in a frontal and temporal distribution bilaterally that was more prominent in the right hemisphere than the left hemisphere. Letter fluency was related to frontal atrophy bilaterally, whereas semantic fluency was related to left frontal and temporal atrophy. Patients with PNFA showed significant frontal atrophy bilaterally that was more extensive on the left than the right. Semantic fluency was related to right frontal and left temporal atrophy, and letter fluency was related to left temporal atrophy.
DISCUSSION
Performance on letter and semantic fluency tests is heavily reliant on the frontal lobes to sustain the required search of the mental lexicon and maintain output in working memory to avoid repetitions.34,35 However, letter and semantic fluency tests differ in that the mental search required for letter fluency is guided by a grapheme cue, whereas the mental search required for semantic fluency (i.e., animals) is guided by the semantic attributes of the category. The artificial nature of a letter target places greater demands on executive resources needed to formulate and sustain a mental search. The semantic value inherent in a mental search of a semantic category lessens the need for executive resources but places an additional burden on semantic representations mediated by the temporal lobe.4 Functional imaging studies have generally upheld this distinction with letter and semantic fluency test performance associated with frontal and temporal brain regions.13,14
We found cognitive and neuroanatomic dissociations between FTLD subgroups consistent with these distinctions between fluency tasks. Patients with SemD were more impaired on semantic than letter fluency.7,10,12,16 Nevertheless, output on both fluency tests was highly correlated with confrontation naming. Thus, for patients with SemD, fluency performance may be determined, in part, by impaired lexical/semantic access more than mental search limitations. This resembles the pattern of fluency impairment in patients with AD.35 The low correlation found between output on the semantic fluency test and the semantic categorization test may have been because the measure of semantic categorization we used relies minimally on retrieval. Rather, the retrieval of a lexical semantic representation is reflected in the confrontation naming measure. While letter-guided fluency is more dependent on executive resources, the retrieved words nevertheless have a semantic representation that plays a relatively minor role on this task. The imaging data are consistent with the neuropsychological data in that reduced output on both fluency tests was related to anterior/inferior left temporal lobe atrophy, an area implicated in lexical semantic representations.36
Patients with bvFTLD presented with equal impairment on letter and semantic fluency tests. Output on both fluency tests was highly correlated with the Digits Backwards test, a measure of working memory.23 This emphasizes the role of executive limitations in the fluency deficits of patients with bvFTLD. Working memory clearly contributes to performance of these tasks, such as maintaining the mental search and keeping track of previously mentioned items. Patients with bvFTLD also showed positive correlations between both fluency tests and performance on the BNT. Although not aphasic, patients with bvFTLD do have naming difficulty.7 This may be a reflection of the bilateral cortical atrophy seen in these patients.9 VBM analyses related poor letter fluency performance to bilateral frontal atrophy, and related impaired semantic fluency performance to left frontal and temporal atrophy. Presumably, involvement of the frontal lobes implicates this area in components of the mental search. Involvement of the temporal lobe for semantic fluency reflects the semantic component of a mental search guided by a semantic target.
Patients with PNFA were also equally impaired on both fluency tests,12 and a positive correlation between semantic fluency performance and the Boston Naming Test was obtained, suggesting that impaired lexical access may play more of a role in performance of semantic fluency in these patients than their working memory limitation. Fluency performance was related to frontal and temporal atrophy in PNFA. In contrast to SemD and bvFTLD, the anatomic extent of these related areas was surprisingly modest. Corticobasal degeneration is often associated with PNFA,37 including this series. There is extensive white matter disease in this condition.38 This seems to be reflected in the extensive subcortical changes seen on diffusion tensor imaging studies of PNFA.39 The disproportionate interruption of white matter projections may fragment the large-scale neural network on which category naming fluency tasks depend. This may explain the disproportionate fluency impairment relative to the apparently more modest reduction in cortical volume that is associated with their difficulty on this task. While the relationship between semantic fluency and left posterior superior temporal lobe atrophy in PNFA differs from the anatomic distribution of left temporal disease seen in SemD, it is important to note multiple studies relating this posterior superior temporal region to word meaning.39,40 Diffusion tensor imaging studies show that projections to the posterior superior temporal region are compromised in PNFA, and performance on measures involving lexical retrieval such as semantic fluency and visual confrontation naming are significantly related to reduced fractional anisotropy in the left posterior superior temporal region in these patients.37
Several caveats should be kept in mind when interpreting this work, i.e., VBM analyses were obtained for only a portion of patients and correlation analyses may have been altered if different neuropsychological tests were used. Moreover, we do not have pathologic confirmation of dementia etiology. With these caveats in mind, we find differential patterns of impairment on tests of letter and semantic fluency that are corroborated by neuropsychological test performance. This is associated with specific areas of frontal and temporal atrophy implicating the disruption of a large-scale neural network involving executive search and lexical semantic retrieval components of category naming fluency. Selective patterns of impairment on fluency measures in FTLD seem to reflect the particular way in which this large-scale network is compromised in each subgroup.
DISCLOSURE
Dr. Coslett serves on the editorial board of Brain and Language, Journal of the International Neuropsychological Society, and Journal of Experimental Psychology: General, Cognitive Neuropsychology; and is supported by US Public Health Service (NS08130 and MH076227). Dr. Grossman serves on a scientific advisory board for Allon Pharmaceutical; serves as editor of Cognitive and Behavioral Neurology and as an editorial board member for Neurology; and receives research support from the US Public Health Service (AG17586, AG15116, AG10124, and NS44266). The remaining authors report no disclosures.
Address correspondence and reprint requests to Dr. David J. Libon, Drexel University College of Medicine, New College Building, Mail Stop 423, 245 North 15th St., Philadelphia, PA 19102 dlibon@drexelmed.edu
Disclosure: Author disclosures are provided at the end of the article.
Received December 23, 2008. Accepted in final form May 15, 2009.
REFERENCES
- 1.McKhann G, Trojanowski JQ, Grossman M, Miller BL, Dickson D, Albert M. Clinical and pathological diagnosis of frontotemporal dementia: report of a work group on frontotemporal dementia and Pick’s disease. Arch Neurol 2001;58:1803–1809. [DOI] [PubMed] [Google Scholar]
- 2.Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 2004;62:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioral profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 2001;70:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 2007;6:1004–1014. [DOI] [PubMed] [Google Scholar]
- 5.Peelle JE, Troiani V, Gee J, et al. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics 2008;21:418–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossman M, McMillan C, Moore P, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia, and corticobasal degeneration. Brain 2004;127:628–649. [DOI] [PubMed] [Google Scholar]
- 7.Libon DJ, Xie S, Moore P, et al. Patterns of neuropsychological impairment associated with frontotemporal dementia: a factor analytic study. Neurology 2007;68:368–375. [DOI] [PubMed] [Google Scholar]
- 8.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003;16:211–218. [DOI] [PubMed] [Google Scholar]
- 9.Grossman M, Libon DJ, Forman MS, et al. Distinct antemortem profiles in pathologically defined patients with frontotemporal dementia. Arch Neurol 2007;64:1601–1609. [DOI] [PubMed] [Google Scholar]
- 10.Hodges JR, Patterson K, Ward R, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: a comparative neuropsychological study. Neuropsychology 1999;13:31–40. [DOI] [PubMed] [Google Scholar]
- 11.Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology 2007;21:20–30. [DOI] [PubMed] [Google Scholar]
- 12.Rogers TT, Ivanoiu A, Patterson K, Hodges JR. Semantic memory in Alzheimer’s disease and the frontotemporal dementias: a longitudinal study of 236 patients. Neuropsychology 2006;20:319–335. [DOI] [PubMed] [Google Scholar]
- 13.Gourovitch ML, Kirkby BS, Goldberg TE, et al. A comparison of RCBF patterns during letter and semantic fluency. Neuropsychology 2000;14:353–360. [DOI] [PubMed] [Google Scholar]
- 14.Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating “tiger” as an animal name or a word beginning with t: differences in brain activation. Proc Biol Sci 1996;263:989–995. [DOI] [PubMed] [Google Scholar]
- 15.Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 1994;57:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libon DJ, Massimo L, Moore P, et al. The Philadelphia Brief Assessment of Cognition (PBAC): a brief neuropsychological protocol that distinguishes frontotemporal dementia from Alzheimer’s disease. Dement Geriatr Cogn Disord 2007;24:441–447. [DOI] [PubMed] [Google Scholar]
- 17.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 18.Grossman M, Ash S. Primary progressive aphasia: a review. Neurocase 2004;10:3–18. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the mental state of patients for a clinician. J Psychiatr Res 1974;12:189–198. [DOI] [PubMed] [Google Scholar]
- 20.Spreen O, Struss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1990. [Google Scholar]
- 21.Carew TG, Lamar M, Cloud BS, Grossman M, Libon DJ. Impairment in category fluency in ischemic vascular dementia. Neuropsychology 1997;11:400–412. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale–Revised. San Antonio: The Psychology Corporation; 1987. [Google Scholar]
- 23.Lamar M, Price CC, Libon DJ, et al. Alterations in working memory as a function of leukoaraiosis in dementia. Neuropsychologia 2007;45:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 25.Grossman M, D’Esposito M, Hughes E, et al. Language comprehension difficulty in Alzheimer’s disease, vascular dementia, and fronto-temporal degeneration. Neurology 1996;47:183–189. [DOI] [PubMed] [Google Scholar]
- 26.Grossman M, Payer F, Onishi K, et al. Constraints on the cerebral basis for semantic processing from neuroimaging studies of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1997;63:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape and intensity averaging. Neuroimage 2004;23:S139–S150. [DOI] [PubMed] [Google Scholar]
- 28.Beg MF, Khan A. Symmetric data attachment terms for large deformation image registration. IEEE Trans Med Imaging 2007;26:1179–1189. [DOI] [PubMed] [Google Scholar]
- 29.Avants B, Anderson C, Grossman M, Gee JC. Symmetric normalization for patient-specific tracking of longitudinal change in frontotemporal dementia. Med Image Analysis (in press).
- 30.Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc Natl Acad Sci USA 2005;102:9685–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Brady M, Smith S. Hidden Markov random field model for segmentation of brain MR images. SPIE Proc 2000;3979:1126–1138. [Google Scholar]
- 33.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Res Med 1995;33:636–647. [DOI] [PubMed] [Google Scholar]
- 34.Stuss D, Alexander MP, Hamper L, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. J Intl Neuropsychological Soc 1998;4:265–278. [PubMed] [Google Scholar]
- 35.Mickanin J, Grossman M, Onishi K, Auriacombe S, Clark C. Verbal and non-verbal fluency in patients with probable Alzheimer’s disease. Neuropsychology 1994;8:385–394. [Google Scholar]
- 36.Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology 2000;54:2277–2284. [DOI] [PubMed] [Google Scholar]
- 37.Murray R, Neumann M, Forman MS, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology 2007;68:1274–1283. [DOI] [PubMed] [Google Scholar]
- 38.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal lobe dementia: clinicopathological correlations. Ann Neurol 2006;952:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenig P, Grossman M. Process and content in semantic memory. In: Hart J, Kraut MA, eds. Neural Basis of Semantic Memory. Cambridge: Cambridge University Press; 2007:247–264. [Google Scholar]
- 40.DeLeon J, Gottesman RF, Kleinman JT, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain 2007;130:1408–1422. [DOI] [PubMed] [Google Scholar]


