INTRODUCTION
The introduction of methicillin in 1959 was a ground-breaking achievement in the war against penicillin-resistant Staphylococcus aureus. Methicillin was developed to overcome the primary mode of resistance found with resistant strains of S. aureus, inactivation of penicillin by beta-lactamase. In 1961, this monumental achievement was overshadowed by the discovery of several strains of S. aureus in the United Kingdom, which had developed resistance to methicillin (methicillin-resistant S. aureus [MRSA]). MRSA was subsequently isolated throughout the world and, in addition to causing hospital-acquired infections, has now spread to the community. With this resistance mechanism, MRSA has proved to be resistant to all subsequent beta-lactam molecules developed over the past several decades. Ceftobiprole, a new-generation cephalosporin, is the first beta-lactam agent to demonstrate potent in vitro and in vivo activity against MRSA.
MRSA is a major cause of hospital and community-acquired infections worldwide and a major cause of morbidity and mortality. Klein et al. determined that from 1999 to 2005, the estimated number of hospitalizations involving S. aureus infections increased by 62% (from 294,570 to 477,927), with MRSA-related infections more than doubling during this same period (from 127,036 to 278,203).1 In 2005, approximately 94,360 patients in the U.S. developed a serious MRSA infection, with 18,650 deaths (20%) related to the hospital stay.2 Of these severe MRSA infections, 85% were associated with health care exposure and one-third occurred during hospitalization.
Methicillin resistance is conferred by a penicillin-binding protein (PBP) that is encoded by the mecA gene found in the staphylococcal cassette chromosome mec (SCCmec).3–5 These mobile genetic elements may carry additional genetic material that encode resistance to other classes of antimicrobials. Penicillin resistance in Streptococcus pneumoniae is mediated through a similar adaptive mechanism by the bacteria. Alterations of PBP 2 to PBP 2x by S. pneumoniae may lead to a decrease in activity of penicillin, necessitating higher doses to achieve adequate activity, or may prevent the binding altogether (penicillin-resistant S. pneumoniae [PRSP]).
Ceftobiprole (BAL 9141) is the first of a new generation of extended-spectrum cephalosporins with activity against clinically important gram-positive bacteria, including MRSA, PRSP, and Enterococcus faecalis.6–10 If approved, ceftobiprole would become the only cephalosporin with established activity against E. faecalis and MRSA. The drug has shown activity against clinically important gram-negative pathogens, including Citrobacter spp., Escherichia coli, Enterobacter spp., Klebsiella spp., Serratia marcescens, and Pseudomonas aeruginosa.
The limited number of approved drugs with activity against multidrug-resistant bacteria such as MRSA and P. aeruginosa has increased the demand for new agents with a novel mechanism of action or an ability to overcome bacterial resistance. Ceftobiprole is a broad-spectrum cephalosporin with additional properties that circumvent many of the mechanisms of resistance to beta-lactams. Ceftobiprole has been evaluated in phase 3 trials for treating complicated skin and soft-tissue infections (cSSSIs) caused by gram-positive and gram-negative bacteria. Recent studies examining the use of ceftobiprole for the treatment of community-associated and hospital-associated pneumonia have also been completed but have been published only in abstract form.
PHARMACOLOGY AND MECHANISM OF ACTION
Ceftobiprole medocaril is a water-soluble prodrug developed to facilitate the intravenous (IV) administration of the active parent drug, ceftobiprole.5,11 As a result of its limited oral bioavailability, it will likely be available in an IV formulation only. After IV administration, ceftobiprole medocaril is converted to the active drug, ceftobiprole, by type A plasma esterases. This process is rapid (less than one minute) and complete, with minimal influence from other medications or disease states.
Ceftobiprole is a beta-lactam anti-microbial agent that shows potent bactericidal activity by binding to PBP, inhibiting transpeptidation and formation of the bacterial cell wall, leading to cell lysis and death. The drug can bind to several different PBPs found in both gram-negative and gram-positive bacteria.12,13 Ceftobiprole rapidly binds and forms a stable inhibitory acyl-enzyme complex with PBP 2′ (PBP 2a) and PBP 2x, which provide activity against beta-lactam–resistant staphylococci and streptococci, respectively. The stability of the acyl-enzyme complex, in combination with the long side chain that sits deep in the PBP 2′-binding pocket, enhances the stability of the bond and inhibition of the enzyme.
MICROBIOLOGY
Ceftobiprole has demonstrated activity against clinically important gram-positive bacteria, including penicillin-resistant S. pneumoniae (PRSP), methicillin-resistant S. aureus (MRSA), and E. faecalis with MIC90 values of 0.25, 2, and 2 mcg/mL, respectively (Table 1).6–10 Ceftobiprole has also demonstrated potent in vitro activity against several clinical isolates of community-associated methicillin-resistant S. aureus (CA-MRSA), vancomycin-intermediate S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA), with a minimum inhibitory concentration (MIC) of 2 mcg/mL.14,15 The clinical utility of ceftobiprole for infections caused by VISA and VRSA, however, has not been determined. In vitro resistance to ceftobiprole has been found in specific strains of S. aureus, with high-volume broth cultures containing subinhibitory concentrations of ceftobiprole.16
Table 1.
In Vitro Activity of Ceftobiprole against Clinically Significant Pathogens
| Bacterial Isolate | MIC50 | MIC90 | Range |
|---|---|---|---|
| Gram-positive pathogens | |||
| • Methicillin-susceptible Staphylococcus aureus (MSSA) | 0.25 | 0.5 | 0.25–2 |
| • Methicillin-resistant S. aureus (MRSA) | 1 | 2 | 0.12–2 |
| • Methicillin-susceptible coagulase-negative Staphylococcus spp. | 0.12 | 0.25 | ≤0.015–1 |
| • Methicillin-resistant coagulase-negative Staphylococcus spp. | 1 | 2 | ≤0.015–4 |
| • Penicillin-susceptible Streptococcus pneumoniae (PSSP) | ≤0.06 | ≤0.06 | ≤0.015–0.03 |
| • Penicillin-intermediate S. pneumoniae (PISP) | ≤0.06 | 0.12 | ≤0.015–0.5 |
| • Penicillin-resistant S. pneumoniae (PRSP) | 0.25 | 0.25 | ≤0.015–1 |
| • Beta-hemolytic Streptococcus spp. | ≤0.06 | ≤0.06 | ≤0.015–0.06 |
| • Enterococcus faecalis | 0.5 | 2 | 0.12– >8 |
| • Enterococcus faecium | >8 | >8 | 0.25– >8 |
| Gram-negative pathogens | |||
| • Citrobacter freundii | ≤0.06 | 2 | ≤ 0.015– >8 |
| • Enterobacter spp. | ≤0.06 | >8 | ≤0.015– >8 |
| • Enterobacter cloacae | ≤0.06 | 0.12 | ≤ 0.03– >8 |
| • Escherichia coli | ≤0.06 | 0.12 | ≤ 0.015–2 |
| • Escherichia coli (ESBL-positive) | >8 | >8 | 0.03– >8 |
| • Klebsiella pneumoniae | ≤0.06 | >8 | ≤ 0.015– >8 |
| • Klebsiella pneumoniae (ESBL-positive) | >8 | >8 | ≤ 0.015– >8 |
| • Proteus mirabilis | ≤0.06 | 0.12 | ≤ 0.015–0.03 |
| • Indole-positive Proteus spp.* | ≤0.06 | >8 | ≤ 0.015– >8 |
| • Serratia marcescens | ≤0.06 | 1 | 0.03– >8 |
| • Haemophilus influenzae | ≤0.06 | ≤0.06 | 0.12–0.25 |
| • Moraxella catarrhalis | ≤0.06 | 0.12 | ≤ 0.015–1 |
| • Acinetobacter spp. | >8 | >8 | ≤ 0.015– >8 |
| • Pseudomonas aeruginosa | 2 | >8 | 0.12– >8 |
| • Stenotrophomonas maltophilia | >8 | >8 | >8 |
Banerjee et al. determined that one possible mechanism of ceftobiprole resistance was associated with multiple mecA mutations in several isolates expressing the mecA gene and another potential role for chromosomal genes mediating resistance in particular strains lacking mecA.
Ceftobiprole is active against clinically important gram-negative pathogens, including Citrobacter spp., Escherichia coli, Enterobacter spp., Klebsiella spp., Serratia marcescens, and Pseudomonas aeruginosa.6–10 Although ceftobiprole has demonstrated activity against isolates expressing AmpC beta-lactamases, it has not consistently shown activity against isolates expressing extended spectrum beta-lactamases (ESBLs). Investigators have not been able to demonstrate reliable in vitro activity of this agent against isolates of Enterococcus faecium, Stenotrophomonas maltophilia, Burkholderia cepacia, Acinetobacter spp., or clinically significant anaerobic bacteria like Bacteroides spp. (MIC90 values above 8 mcg/mL). Breakpoints for susceptibility of ceftobiprole have not been established.
PHARMACOKINETICS AND PHARMACODYNAMICS
The pharmacokinetic properties of ceftobiprole have been evaluated in healthy volunteers, in patients with varying degrees of renal dysfunction, and in patients enrolled in clinical trials for the treatment of cSSSIs.11,17–20 The volume of distribution at steady state (Vss) is approximately 18 to 20 liters. Like other beta-lactams, this drug is comparable to the extracellular fluid compartment in adults.
After a two-hour infusion of 500 mg, the peak plasma concentration (Cmax) and the area-under-the-curve (AUC) concentration in healthy volunteers were 29.2 mcg/mL and 90 mcg • hour/mL, respectively. Accumulation of ceftobiprole was not apparent after five days of administration of 500 mg every eight hours infused over two hours, Cmax 33 mcg/mL and an AUC of 102 mcg • hour mL. Protein binding is limited (16%) and is independent of the drug concentration; therefore, alterations in protein binding are unlikely to affect its overall activity.11
Ceftobiprole is neither an inhibitor of nor a substrate for the cytochrome P450 (CYP 450) system.11 Studies with cyclosporine have also demonstrated that ceftobiprole is neither an inhibitor of nor a substrate of the p-glycoprotein (PGP) transporter system. Based on combination studies with probenecid, ceftobiprole is eliminated by the kidneys as unchanged drug via glomerular filtration, not through active tubular secretion.11
The half-life of ceftobiprole is approximately three hours, with more than 80% of the active drug recovered in the urine within 12 hours after the administration.
Although slight variations in the drug’s pharmacokinetic properties are apparent based on a patient’s sex, no dosing adjustments are necessary.11,21 Pharmacokinetic properties, in terms of race and optimal dosing for pediatric patients, have not been published.
The pharmacokinetic parameters of ceftobiprole in patients with normal renal function and in those with mild, moderate, and severe renal impairment have been determined.11,19,20 Twenty male subjects with varying degrees of renal function were studied to determine the optimal dosing schedule for those patients with renal impairment for use in future clinical trials. Roos et al. determined that systemic exposure, as measured by the AUC concentration, was elevated in the patients with renal impairment.
Renal impairment was defined as a creatinine clearance (CrCl) below 80 mL/minute; normal clearance was defined as a CrCl above 80 mL/minute. Compared with subjects with normal renal function, patients with mild renal impairment (CrCl, 50 to 80 mL/minute) experienced an increase of 29% in the AUC concentration; patients with moderate renal impairment (CrCl, 30 to 50 mL/minute), an AUC increase of 250%; and patients with severe renal impairment (CrCl, below 30 mL/minute), an AUC increase of 330%.
The half-life of ceftobiprole increased with decreasing renal function. The longest half-life occurred in patients with severe renal impairment (11 hours). As a result, dosage adjustment is necessary in patients with renal insufficiency.
The in vitro and in vivo pharmacodynamics of ceftobiprole have been studied extensively and are similar to those of other beta-lactam antimicrobial drugs.20,22 The pharmacodynamic parameter most correlated with clinical efficacy of ceftobiprole is the percentage of time in which the free serum concentration (%fT) is above the MIC. Like other cephalosporins, the optimal %fT above the MIC required for ceftobiprole to achieve a bacteriostatic effect is 30% of the dosing interval for staphylococci and 40% of the dosing interval for gram-negative bacilli, respectively. For maximal bactericidal activity, the %fT above the MIC should be at least 50% (staphylococci) and 60% (gram-negative bacilli) of the dosing interval.
Using pharmacokinetic data from 150 subjects, Lodise et al. performed a Monte Carlo simulation to determine the probability of target attainment (PTA) of ceftobiprole against gram-positive and gram-negative pathogens.20 Several different dosing strategies, in vitro MIC data, and the four goals for target attainment, as just described, were used.
When the investigators used 500 mg every 12 hours over one hour, they found no significant difference in the PTA for gram-positive isolates with MIC values of 1 mcg/mL or below, using a target for the PTA of 50% for bactericidal activity. Using the dosing scheme of 500 mg every eight hours and three different lengths of infusions (30 minutes, one hour, two hours), they determined that the two-hour infusion improved the likelihood of target attainment for gram-positive and gram-negative organisms with an MIC of 2 mcg/mL or greater.
For isolates with MIC values of 1 mcg/mL or below, the infusion time had little effect on the PTA for a %fT above the MIC target of 40%, 50%, or 60%. The investigators did notice a decreased PTA for the two-hour dosing scheme against AmpC-producing gram-negative isolates and P. aeruginosa (MIC50 = 4), compared with isolates that were non–AmpC-producing gram-negative isolates, PTA (of 60%fT > MIC) 87.8%, 62%, and 94.1%, respectively.
CLINICAL EFFICACY
Skin and Skin-Structure Infections23–26
Two randomized, multicenter, double-blind, phase 3 trials (STRAUSS 1 and 2) evaluated the clinical efficacy of ceftobiprole for hospitalized patients with complicated skin and skin structure infections (cSSSIs).
The STRAUSS 1 Study24
STRAUSS 1 compared ceftobiprole with vancomycin for the treatment of cSSSIs caused by documented or suspected gram-positive pathogens. Patients were considered eligible for enrollment if they were older than 18 years of age and had a cSSSI caused by gram-positive pathogens based on predefined criteria. Patients were classified according to infection type, and the investigators limited the percentage of patients with cellulitis to less than 20%. Patients with diabetic foot infections, infections from animal or human bites, or osteomyelitis were excluded.
Patients were randomly assigned, in a 1:1 ratio, according to the type of infection (abscess, wound infections, or cellulitis) to receive either IV ceftobiprole 500 mg every 12 hours as a 60-minute infusion or IV vancomycin 1,000 mg every 12 hours as a 60-minute infusion for 7 to 14 days.
The primary efficacy endpoint of this non-inferiority trial was a clinical cure rate at the test-of-cure (TOC) visit (7 to 14 days after the end of therapy). This outcome was assessed in the clinically evaluable and intent-to-treat (ITT) populations (Figures 1 and 2). The ITT population included all randomized patients. The clinically evaluable patients had a protocol-defined cSSSI, completed the TOC visit (6 to 14 days after therapy), and received 80% or more of the study drug course. Based on an expected clinical cure rate of 80%, ceftobiprole was considered non-inferior if the lower limit of the 95% con fidence interval (CI) for the difference in clinical cure rate was −10% or more.
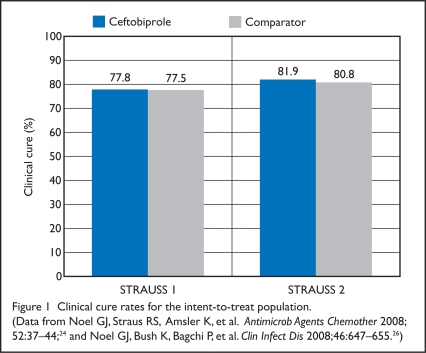
Figure 1.
Clinical cure rates for the intent-to-treat population. (Data from Noel GJ, Straus RS, Amsler K, et al. Antimicrob Agents Chemother 2008; 52:37–44;24 and Noel GJ, Bush K, Bagchi P, et al. Clin Infect Dis 2008;46:647–655.26)
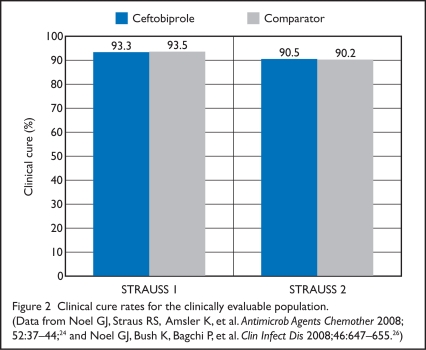
Figure 2.
Clinical cure rates for the clinically evaluable population. (Data from Noel GJ, Straus RS, Amsler K, et al. Antimicrob Agents Chemother 2008; 52:37–44;24 and Noel GJ, Bush K, Bagchi P, et al. Clin Infect Dis 2008;46:647–655.26)
The ITT analysis included a total of 784 patients, with 42% patients from the U.S.; 397 patients were randomly as signed to receive ceftobiprole, and 387 patients received vancomycin. The clinically evaluable population consisted of 559 patients with a clinical outcome evaluated at the TOC visit (282 in the ceftobiprole group, 277 in the vancomycin group).
In the ITT population, there were no significant differences in demographics, baseline characteristics, type of infection, or duration of treatment between the two treatment groups. The proportion of men in the clinically evaluable group was significantly lower in the ceftobiprole group (55%) than in the vancomycin group (61%) (P = 0.025). Clinical cure rates in the ceftobiprole and vancomycin groups were similar in the ITT group (77.8% vs. 77.5%; CI, −5.5 to 6.1) and in the clinically evaluable group (93.3 vs. 93.5%; CI, −4.4 to 3.9).
The STRAUSS 2 Study26
In STRAUSS 2, investigators compared ceftobiprole monotherapy with vancomycin and ceftazidime (Fortaz, GlaxoSmithKline) in combination for the treatment of cSSSIs. Patients were considered eligible if they were older than 18 years of age and had a cSSSI, including patients with diabetic foot infections. Patients with foreign-body infections, critical limb ischemia, septic arthritis, or osteomyelitis were excluded. The patients were stratified by infection type, and the investigators limited the percentage of patients with cellulitis to less than 20%.
Patients were randomly assigned, in a 2:1 ratio, to receive ceftobiprole plus placebo or vancomycin plus ceftazidime. Unlike STRAUSS 1, in which patients received ceftobiprole 500 mg every 12 hours, patients in STRAUSS 2 received ceftobiprole 500 mg every eight hours as a 120-minute infusion and placebo every 12 hours as a 60-minute infusion, or vancomycin 1,000 mg every 12 hours as a 60-minute infusion and ceftazidime 1,000 mg every eight hours as a 120-minute infusion for 7 to 14 days. Metronidazole (e.g., Flagyl, Pfizer) could be added empirically in either group for 48 hours, pending the results of culture, at the discretion of the clinician if suspected or documented anaerobic pathogens were present.
The trial was of a non-inferiority design. The primary efficacy endpoint was the clinical cure rate at the TOC visit (7 to 14 days after the end of therapy). As with STRAUSS 1, the primary outcome was the rate of clinical cure, as measured in both the clinically evaluable and ITT populations. Based on an expected non-evaluable rate of 30%, 816 patients were needed to reject the null hypothesis of inferiority for ceftobiprole of 10%, using a power of 80% and a two-sided alpha of 0.05. Ceftobiprole was considered non-inferior to the combination of vancomycin and ceftazidime if the lower limit of the 95% CI for the difference in clinical cure rate was −10% or above. A secondary outcome in the group of microbiologically evaluable patients was analyzed to determine the microbiologic eradication rate at the TOC visit.
A total of 828 patients were initially randomized and were included in the ITT analysis, with 33% of patients from the U.S.; 547 (66%) received ceftobiprole, and 281 (33%) were assigned to the comparator group. There were no significant differences between groups in terms of demographics, baseline characteristics, type of infection, or duration of treatment.
Infections of the fascial plane or muscle (36%) and the number of patients undergoing surgical debridement (39%) as part of initial therapy were similar in both groups. Ninety-two percent of the ceftobiprole patients and 90% of the vancomycin/ceftazidime patients completed the trial. The clinically evaluable population consisted of 729 patients. Clinical outcomes were evaluated at the TOC visit for 485 (89%) patients receiving ceftobiprole group and 244 (87%) receiving vancomycin/ceftazidime.
For the primary outcome of clinical cure rates in the ITT and CE populations, ceftobiprole was considered non-inferior to vancomycin/ceftazidime at the TOC visit (7 to 14 days after therapy). Clinical cure rates in the ceftobiprole and vancomycin/ceftazidime groups were similar in the ITT group (81.9% vs. 80.8%; CI, −4.5% to 6.7%) and in the clinically evaluable group (90.5% vs. 90.2%; CI −4.2% to 4.9%). There were no significant differences in clinical cure rate or microbiologic eradication based on the type of infection. Diabetic foot infection was the most common diagnosis in 31% of patients in the ceftobiprole group and in 32% of those receiving vancomycin/ceftazidime. Response rates were 86.2% for the ceftobiprole patients and 81.8% for the other group (CI, −5.4% to 15.7%).
For the secondary outcome, the microbiologically evaluable population consisted of 590 patients in the clinically evaluable group with microbiologic data available—391 patients (71%) received ceftobiprole, and 199 (71%) received vancomycin/ceftazidime. Clinical cure and microbiological eradication rates at the TOC visit were similar in the microbiologically evaluable population for both gram-positive and gram-negative infections. In most patients (53%), infections had been caused by gram-positive pathogens, and more than 64% of pathogens in the microbiologically evaluable population were S. aureus. Of those, only 32.8% of the isolates were methicillin-resistant.
Of the 12 patients in the ceftobiprole group with P. aeruginosa isolated as the only pathogen, three patients (25%) failed to respond to therapy; all of the isolates from these three patients demonstrated an MIC of 8 mcg/mL or more. All nine vancomycin/ceftazidime patients with P. aeruginosa infection achieved clinical cure at the TOC visit.
Pneumonia Trials27–30
Phase 3 trials demonstrating the clinical efficacy of ceftobiprole for the treatment of community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP) have been completed. Preliminary data from one randomized, double-blind, multicenter study demonstrated the non-inferiority of ceftobiprole when compared with ceftriaxone (Rocephin, Roche) with or without the addition of linezolid, for hospitalized patients with CAP.27,28
Investigators enrolled 666 patients into the study (328 patients in the ceftobiprole arm and 338 patients in the comparator arm). Patients were stratified before randomization according to Pneumonia Outcomes Research Team (PORT) Severity Index scores and the need for linezolid in patients with proven or suspected MRSA or ceftriaxone-resistant S. pneumoniae. Non-inferiority was defined in both the clinically evaluable and ITT populations as a difference of 10% in the cure rate between treatment groups.
The primary outcome clinical cure rate at the TOC visit (7 to 14 days after therapy) for the clinically evaluable population was 86.7% for ceftobiprole and 87.6% for the comparator drug. Clinical cure rates in the ITT population were 77.4% with ceftobiprole and 80.2% with the comparator agent.
Microbiologic eradication rates in both groups were also similar: 88% with ceftobiprole and 92% with the comparator drug. Patients with S. pneumoniae infection had comparable cure rates in each arm: 90% with ceftobiprole and 89% with the comparator drug; however, the authors did not report rates of penicillin-resistant S. pneumoniae (PRSP).
In patients with baseline PORT scores above 90, clinical cure rates were 90.2% for the clinically evaluable ceftobiprole patients and 84.5% for the comparator arm (95% CI, −6.7 to 18.1). Details of the study, including inclusion and exclusion criteria, dose, length of therapy, and differences between the groups, have not yet been published.
The preliminary results of a phase 3 trial in patients with hospital-acquired pneumonia showed the non-inferiority of ceftobiprole versus ceftazidime/linezolid for the primary outcome;29,30 primary outcome clinical cure rates at the TOC visit (7 to 14 days after therapy) for the clinically evaluable population were 69% and 72%, respectively.
In the subgroup of patients with ventilator-associated pneumonia (25% of patients), non-inferiority could not be established, because clinical cure rates were lower with ceftobiprole than with the comparator drug. Details of the study, including inclusion and exclusion criteria, dose, duration of therapy, and differences between the groups, have not been published. A subgroup analysis will probably be performed to determine potential differences in the patients with ventilator-associated pneumonia that might have led to the decreased response.
ADVERSE DRUG REACTIONS
Ceftobiprole is as tolerable and safe as other agents used for the treatment of cSSSIs, including vancomycin and ceftazidime.24,26 The pooled analysis from STRAUSS 1 and 2 showed similar adverse events reported for both ceftobiprole and the comparator. At least one adverse event was reported in more than 50% of patients in both studies, and most events were considered mild or moderate in intensity.
Adverse events leading to discontinuation of the study drugs were similar in both trials. The most common adverse reactions in clinical trials included gastrointestinal effects (nausea, vomiting, diarrhea), dysgeusia (sense of distorted taste), headache, and infusion-site reactions (Table 2). These events occurred with similar frequency in both treatment groups.
Table 2.
Pooled Incidence of Treatment-Related Adverse Events For Complex Skin and Skin Structure Infections
| Adverse Event |
Ceftobiprole (n= 932) |
Comparator Drug* (n= 661) |
|---|---|---|
| No. (%) | No. (%) | |
| Nausea | 113 (12) | 49 (7) |
| Vomiting | 61 (7) | 27 (4) |
| Diarrhea | 62 (7) | 32 (5) |
| Constipation | 33 (4) | 25 (4) |
| Dysgeusia† | 30 (3) | 2 (1) |
| Headache | 68 (7) | 39 (6) |
| Dizziness† | 14 (4) | 8 (2) |
| Insomnia‡ | 26 (5) | 13 (5) |
| Infusion-site reaction† | 48 (9) | 26 (9) |
| Hypersensitivity§ | 49 (5) | 62 (9) |
| Overall adverse drug events | ||
| • One or more adverse drug events | 507 (54) | 352 (53) |
| • One or more serious adverse drug events | 63 (7) | 47 (7) |
| • Discontinued therapy because of adverse drug events | 39 (4) | 32 (5) |
Comparator regimen: vancomycin (STRAUSS 1); vancomycin plus ceftazidime (STRAUSS 2).
Data reported for STRAUSS 1 only.
Data reported for STRAUSS 2 only.
Data for STRAUSS 1 is a combination of rash and pruritus. The overall incidence of hypersensitivity was not reported in this trial.
DRUG INTERACTIONS
The potential for clinically significant drug interactions with ceftobiprole is considered low because of its favorable pharmacokinetic profile. To date, no published studies have indicated a clinically significant drug interaction that would call for dose adjustments or discontinuation of therapy. Like all antimicrobial agents, ceftobiprole has the potential to decrease the effectiveness of oral contraceptives.
Coadministration of warfarin (Coumadin, Bristol-Myers Squibb) with ceftobiprole sometimes causes an increased prothrombin time and an International Normalized Ratio (INR). Compatibility data for the coadministration of other medications with ceftobiprole, however, have not been published.
DOSAGE AND ADMINISTRATION
Based on the pharmacokinetic, pharmacodynamic, and clinical data published, ceftobiprole dosing is likely to be based on the indication and the intended bacterial coverage. For cSSSIs caused by culture-proven or presumed gram-positive infections, the dose of ceftobiprole is expected to be 500 mg every 12 hours infused over one hour.23,24 For cSSSIs (including diabetic foot infections) caused by culture-proven or presumed gram-negative or mixed infections or for patients with CAP or HAP, the predicted dosing for ceftobiprole is expected to be 500 mg every eight hours infused over two hours.26–30
Ceftobiprole is eliminated primarily via the kidneys; thus, the dosage would probably need to be adjusted in patients with renal insufficiency. Preliminary data suggest that for patients with mild renal impairment (CrCl of 50 to 80 mL/minute), no dosage adjustment is needed.11,19,20
In patients with moderate renal impairment (CrCl of 30 to 50 mL/minute), the predicted dosing of ceftobiprole would probably be 500 mg every 12 hours.
In patients with severe impairment (CrCl of below 30 mL/minute), the predicted dosing of ceftobiprole would be 250 mg every 12 hours.
Of note, patients were excluded from STRAUSS 1 and STRAUSS 2 if they had severe renal dysfunction or oliguria (a urine output below 20 mL/hour that was unresponsive to fluid challenge).24,26
Pharmacokinetic data for ceftobiprole in patients receiving hemodialysis, peritoneal dialysis, or continuous renal replacement therapy have not been published; however, it is unlikely that a dosage adjustment would be necessary for patients with hepatic dysfunction.
The physical compatibility of ceftobiprole with commonly administered IV medications has been reported.31 Chan et al. diluted ceftobiprole to a test concentration of 2 mg/mL using three separate solutions: 0.9% sodium chloride injection, 5% dextrose in water, and lactated Ringer’s solution. The researchers then mixed the solutions in equal amounts with 70 medications using simulated Y-site administration. Of the 70 drugs tested in combination with ceftobiprole, 32 (45.7%) were considered incompatible for Y-site administration regardless of the diluent used or the order of admin istration. For seven of the medications (10%), compatibility was dependent on the type of solution used to dilute ceftobiprole.
Some of the clinically important medications found to be incompatible with ceftobiprole include aminoglycosides, amiodarone (Cordarone), calcium gluconate, diltiazem (Cardizem), dopamine, dobutamine, fluoroquinolones, human regular insulin, hydromorphone, labetalol (Normodyne, Trandate), magnesium sulfate, midazolam (Versed), morphine sulfate, and potassium phosphate. The timing of administration and availability of IV lines are expected to be a concern for patients receiving ceftobiprole with incompatible medications.
CONCLUSION
Ceftobiprole is an advanced-generation cephalosporin with a broad spectrum of activity against gram-negative pathogens, including P. aeruginosa and Enterobacteriaceae (ESBL-negative) with the added advantage of enhanced gram-positive activity against MRSA, PRSP, and ampicillin-susceptible E. faecalis. Activity against other clinically important pathogens (such as ESBL-producing Enterobacteriaceae, Acinetobacter spp., S. maltophilia, and Bacteroides spp.) remains limited; most of the isolates tested have an MIC above 8 mcg/mL.
Ceftobiprole’s pharmacokinetic and pharmacodynamic profile is similar to that of other cephalosporins, including cefepime (Maxipime, Elan) and ceftazidime (Fortaz). Like other beta-lactams, the pharmacodynamic parameter most correlated with efficacy is the percentage of time in which the free serum concentration is above the MIC. The activity of ceftobiprole against clinically important gram-negative pathogens is comparable to that of ceftazidime.
Ceftobiprole has proven efficacy in two phase 3 clinical trials for the treatment of cSSSIs, including diabetic foot infections, caused by gram-positive or gram-negative bacteria. Additional studies of ceftobiprole for the treatment of CAP and hospital-acquired pneumonia (HAP) have also been completed but are available only in abstract form. Investigators noted in the trial for HAP that non-inferiority could not be established in the subgroup of patients with ventilator-assisted pneumonia (VAP), because clinical cure rates were significantly lower in the ceftobiprole group of patients than in the comparator group. It is not clear why ceftobiprole underperformed in this group of patients; the use of ceftobiprole for the treatment of VAP warrants further research.
With antimicrobial resistance on the rise and the pipeline of active agents against gram-negative pathogens relatively nonexistent, hospitals and clinicians are constantly being challenged to develop new strategies to treat complicated infections while preserving antimicrobials for the future. Although ceftobiprole provides us with another option in our antimicrobial armamentarium, further research of its role in clinical practice is still required. The judicious use of this agent will be imperative, in view of the lack of newer antimicrobial agents (with activity against multidrug-resistant, gram-negative pathogens) that are expected to be available in the near future.
Footnotes
Disclosure: Dr. Whitney has received an honorarium from Ortho-McNeil and has been a one-time advisory board consultant for ceftobiprole and doripenem.
REFERENCES
- 1.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13(12):1840–1846. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Utsui Y, Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985;3:397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livermore DM. Can beta-lactams be re-engineered to beat MRSA? Clin Microbiol Infect. 2006;12(Suppl 2):11–6. doi: 10.1111/j.1469-0691.2006.01403.x. [DOI] [PubMed] [Google Scholar]
- 5.Adis R&D profile Ceftobiprole medocaril. Drugs RD. 2006;7(5):305–311. doi: 10.2165/00126839-200607050-00003. [DOI] [PubMed] [Google Scholar]
- 6.Walkty A, DeCorby M, Nichol K, et al. In vitro activity of ceftobiprole against clinical isolates of Pseudomonas aeruginosa obtained from Canadian intensive care unit (ICU) patients as part of the CAN–ICU Study. J Antimicrob Chemother. 2008;62(1):206–208. doi: 10.1093/jac/dkn140. [DOI] [PubMed] [Google Scholar]
- 7.Jones ME. In vitro profile of a new beta-lactam, ceftobiprole, with activity against methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2007;13(Suppl 2):17–24. doi: 10.1111/j.1469-0691.2007.01722.x. [DOI] [PubMed] [Google Scholar]
- 8.Fritsche TR, Sader HS, Jones RN. Anti-microbial activity of ceftobiprole, a novel anti–methicillin-resistant Staphylococcus aureus cephalosporin, tested against contemporary pathogens: Results from the SENTRY Antimicrobial Surveillance Program (2005–2006) Diagn Microbiol Infect Dis. 2008;61(1):86–95. doi: 10.1016/j.diagmicrobio.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Jones RN, Deshpande LM, Mutnick AH, et al. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J Antimicrob Chemother. 2002;50:915–932. doi: 10.1093/jac/dkf249. [DOI] [PubMed] [Google Scholar]
- 10.Pillar CM, Aranza MK, Shah D, et al. In vitro activity profile of ceftobiprole, an anti-MRSA cephalosporin, against recent gram-positive and gram-negative isolates of European origin. J Antimicrob Chemother. 2008;61(3):595–602. doi: 10.1093/jac/dkm492. [DOI] [PubMed] [Google Scholar]
- 11.Murthy B, Schmitt-Hoffman A. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin Pharmacokinet. 2008;47:21–33. doi: 10.2165/00003088-200847010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Hebeisen P, Heinze-Krauss I, Angehrn P, et al. In vitro and in vivo properties of RO 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob Agents Chemother. 2001;45:825–836. doi: 10.1128/AAC.45.3.825-836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovering A, Daniel F, Page MG, et al. Mechanism of action of ceftobiprole: Structural bases for anti-MRSA activity (poster 1586). Presented at the 16th European Congress of Clinical Microbiology and Infectious Diseases; Nice, France. April 1–4, 2006. [Google Scholar]
- 14.Bogdanovich T, Ednie LM, Shapiro S, et al. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 2005;49:4210–4219. doi: 10.1128/AAC.49.10.4210-4219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard SN, Cheung CM, Rybak MJ. Activities of ceftobiprole, linezolid, vancomycin, and daptomycin against community-associated and hospital-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52:2974–2976. doi: 10.1128/AAC.00257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee R, Gretes M, Basuino L, et al. In vitro selection and characterization of ceftobiprole-resistant methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52:2089–2096. doi: 10.1128/AAC.01403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt-Hoffmann A, Roos B, Schleimer M, et al. Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob Agents Chemother. 2004;48:2570–2575. doi: 10.1128/AAC.48.7.2570-2575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt-Hoffmann A, Nyman L, Roos B, et al. Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob Agents Chemother. 2004;48:2576–2580. doi: 10.1128/AAC.48.7.2576-2580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos B, Schmidt-Hoffman A, Schleimer M. Safety and pharmacokinetics of BAL5788 in healthy subjects with normal or impaired renal function (Abstract A-23). Presented at the 43rd Annual Inter-science Conference on Antimicrobial Agents and Chemotherapy; Chicago. September 14–17, 2003. [Google Scholar]
- 20.Lodise TP, Pypstra R, Kahn JB, et al. Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects. Antimicrob Agents Chemother. 2007;51:2378–2387. doi: 10.1128/AAC.01181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitt-Hoffmann AH. Influence of gender on the pharmacokinetics of BAL9141 after intravenous infusion of Pro-drug BAL5788 (Abstract 902). Presented at the 14th European Congress of Clinical Microbiology and Infectious Diseases; Prague. May 1–4, 2004. [Google Scholar]
- 22.Andes DR, Craig WA. In vivo pharmacodynamics of RO 63-9141 against multiple bacterial pathogens (Abstract F-1079). Presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; Toronto. 2000. [Google Scholar]
- 23.Noel GJ. Clinical profile of ceftobiprole, a novel beta-lactam antibiotic. Clin Microbiol Infect. 2007;13(Suppl 2):25–29. doi: 10.1111/j.1469-0691.2007.01725.x. [DOI] [PubMed] [Google Scholar]
- 24.Noel GJ, Straus RS, Amsler K, et al. Treatment of complicated skin and skin structure infections caused by gram-positive bacteria with ceftobiprole: Results of a double-blind, randomized trial. Antimicrob Agents Chemother. 2008;52:37–44. doi: 10.1128/AAC.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 26.Noel GJ, Bush K, Bagchi P, et al. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin structure infections. Clin Infect Dis. 2008;46:647–655. doi: 10.1086/526527. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson SC, Strauss RS, Michiels B, et al. Efficacy of ceftobiprole for the treatment of severely ill patients hospitalized with community-acquired pneumonia (poster C-17). Presented at the American Thoracic Society International Conference; Toronto. May 16–21, 2008. [Google Scholar]
- 28.Basilea announces positive top-line data from phase III study of ceftobiprole in community-acquired pneumonia requiring hospitalization. Basel: Basilea Pharmaceutica, Ltd.; September 14, 2007. Available at: www.basilea.com
- 29.Nicholson SC, Strauss RS, Michiels B, et al. Efficacy of ceftobiprole compared to ceftriaxone +/− linezolid for the treatment of subjects hospitalized with community-acquired pneumonia (poster C-16). Presented at American Thoracic Society International Conference; Toronto. May 16–21, 2008. [Google Scholar]
- 30.Basilea announces positive top-line data from phase III study of ceftobiprole in hospital-acquired pneumonia. Basel: Basilea Pharmaceutica, Ltd.; October 9, 2007. Available at: www.basilea.com
- 31.Chan P, Bishop A, Kupiec TC, et al. Compatibility of ceftobiprole medocaril with selected drugs during simulated Y-site administration. Am J Health Syst Pharm. 2008;65(16):1545–1551. doi: 10.2146/ajhp080032. [DOI] [PubMed] [Google Scholar]