Abstract
Objectives
Involuntary weight loss affects 20% of community dwelling older adults. The underlying mechanism for this disorder is unknown. Objective is to determine if failure of older persons to regain weight is associated with elevated pro-inflammatory cytokine and leptin levels.
Design
Prospective diet intervention study.
Setting
University of Washington Medical Center from 2001–2005.
Participants
Twenty-one younger (18–35 years old) and nineteen older (≥ 70 years old) men and women.
Intervention
Each subject was placed for two weeks on a weight-maintaining diet, followed in sequence by 2 weeks of 30% caloric restriction, then 4 weeks of ad libitum food intake.
Measurements
Plasma leptin levels, fasting serum pro-inflammatory cytokine levels, and peripheral blood mononuclear cell cytokine levels were measured.
Results
Leptin levels in the two cohorts decreased after caloric restriction and increased after ad-libitum food consumption resumed. Plasma TNF α levels were higher in older subjects compared to younger adults. However, there was no association between changes in TNF α levels and changes in AUC leptin.
Conclusion
Leptin levels in healthy older individuals responded appropriately in a compensatory manner to changes in body weight. These data do not support a cytokine dependent elevation in leptin levels as being responsible for the failure of older adults to regain weight.
Keywords: Weight loss, leptin, pro-inflammatory cytokines, aging
Introduction
Involuntary weight loss is a common problem associated with adverse outcomes such as poor wound healing and infections among community dwelling older adults (1, 2). Weight loss is a component of the frailty syndrome (3), and previous studies have shown increased mortality rates in older persons with involuntary weight loss (1, 2). Body weight regulation is a complex interaction between appetite-driven food intake and both resting and non-resting energy expenditure. Previous studies have demonstrated abnormalities of body weight regulation in healthy older adults, but not younger persons, after a brief period of reduced food intake (4, 5). We have previously studied the effect of aging on ghrelin response to acute weight loss and shown that ghrelin levels respond in a compensatory manner to changes in body weight (6). However, the influence of aging on leptin response to acute weight loss is unknown. Understanding the physiology of body weight regulation in healthy older individuals is necessary to distinguish between normal aging and pathological change in weight regulation due to underlying diseases.
Leptin, a product of adipocytes (7–8), serves as an endocrine marker of fat mass. It is thought to reduce food intake and to increase energy expenditure and fat oxidation by acting on receptors both in the hypothalamus and periphery (9). Plasma leptin concentrations have been extensively investigated in medical conditions associated with cachexia (10–13). In all of these studies, except for one study of hemodialysis patients (11), leptin levels were measured only after patients had lost significant amounts of weight. Leptin levels were found to be lower after weight loss, as expected, due to the decrease in fat mass (14). It would be more informative to compare leptin levels before and after weight loss to assess the appropriateness of the decline in leptin levels. The importance of measuring integrated 24-hour plasma leptin levels (AUC leptin) in the regulation of body composition has been appreciated (15), although most studies fail to report this data.
Levels of pro-inflammatory cytokines, specifically interleukin-6 (IL-6) and tumor necrosis factor α (TNF α), increase with age (16–18). Their levels are elevated in conditions characterized by anorexia, cachexia, and, in the elderly, weight loss and disability (19–23). Interleukin-1 (IL-1) and TNF α stimulate leptin synthesis in adipose tissue (24–25). Thus, it is plausible that increased levels of pro-inflammatory cytokines contribute to involuntary weight loss in older persons through both leptin-dependent and leptin-independent mechanisms.
We measured serum levels and mitogen-stimulated, peripheral blood mononuclear cell (PBMC) production of proinflammatory cytokines before and after weight loss in older subjects and young controls. Although PBMC stimulation experiments reflect the production and release of cytokines in vivo better than measurement of serum levels of cytokines (26–27), serum cytokine levels were also obtained to assess production by adipocytes and other non-lymphoid cells (28). AUC leptin was measured as a possible mediator of any cytokine-induced increase in satiety. We hypothesized that older but not younger individuals would fail to regain weight after acute weight loss due to high pro-inflammatory cytokine levels leading to increased AUC leptin.
Methods
Subjects
Advertisements were placed in both local and University of Washington campus newspapers to recruit healthy younger (18–35 years old) and older (≥ 70 years old) men and women from the community between years 2001 and 2005. Specific inclusion and exclusion criteria of the subjects have been previously published (6). Briefly, all subjects had a stable body mass index (BMI) between 22 and 30 kg/m2 for at least one year. They could not exercise more than 30 minutes three times per week or express interest in losing weight. Potential participants were excluded if they had diseases known to be associated with weight loss or anorexia (6). The study protocol was approved by the Human Subjects Division of the University of Washington. Each subject provided a written informed consent prior to enrollment into the study. The General Clinical Research Center (GCRC) of the University of Washington was the main site to conduct the study procedure.
Dietary Protocol
A detailed description of the dietary protocol has been previously published (6). Briefly, each subject was placed on two weeks of a weight-maintaining diet provided by the Nutrition Research Kitchen of the General Clinical Research Center (GCRC). Subjects were admitted to the GCRC (study visit “CRC1”) for evaluation on day 14 of the diet. This evaluation consisted of 24 hours of hourly blood sampling for leptin and pro-inflammatory cytokine levels. At 0800h on the morning following admission, body composition data was obtained by a dual x-ray absorptiometry scan (Lunar Prodigy scanner, Madison WI). In addition, resting energy expenditure (REE) was determined using a metabolic cart and ventilated hood system (SensorMedics, Deltatrac MBM-100, Yorba Linda, CA and Parvo Medics Metabolic Cart, Sandy UT). Following this GCRC admission, subjects were placed on a caloric-restriction diet (30% decreases in calorie intake from their baseline total calorie intake per day) for two weeks. On Day 14 of the caloric-restriction diet, they were re-admitted to the GCRC (study visit “CRC2”) to undergo the same study procedures as the previous visit. Afterwards subjects were placed on 4 weeks of an ad-libitum diet, and they could eat as much or as little food provided by the GCRC as they wished. Their energy intake and body weights were monitored biweekly. At the end of the ad-libitum diet, each subject was re-admitted to the GCRC to undergo the study protocol one final time (study visit “CRC3”).
Peripheral blood mononuclear cell (PBMC) culture
PBMC were isolated from 20 ml of heparinized blood drawn from subjects after an overnight fast. Blood samples diluted with an equal volume of RPMI 1640 medium were centrifuged over Ficoll-Paque (Pharmacia NJ), and the interphase cells were washed twice in RPMI 1640 supplemented with 2 mmol/L L-glutamine, 50 U/ml penicillin, 50 mg/ml streptomycin and 10% fetal calf serum (Life Technologies, Grand Island NY). Cells were plated at 1.25×106 per well in flat-bottom tissue culture-treated 48-well cell culture clusters (Costar, Cambridge MA).
PBMCs were stimulated with Phytohemagglutinin (PHA) at final concentrations of 10 µg/mL (Difco, Detroit MI) and with Lipopolysaccarides (LPS) extracted from Escherichia coli 055:B5, at 1000 pg/mL final concentration (Sigma Chemical Co, St Louis MO). PBMC were cultured for 24 hours at 37°C in a water-saturated atmosphere with 5% CO2. Culture supernatants from three replicate wells were pooled and frozen at −70°C for determinations of IL-1β, IL-6 and TNF α levels.
Assays
Leptin
Plasma leptin levels were measured using an active human leptin enzyme-linked immunosorbent assay (ELISA) kit. (Diagnostic Systems laboratories, Inc. Webster, TX) The lower and upper limits of detection were 0.0 and 50.0 ng/ml respectively. Samples with initial leptin values ≥ 50 ng/ml were re-assayed after dilution. The intra-assay coefficient of variation (CV) was 6.2% and the inter-assay CV was 5.3%. All samples from a single individual were run in duplicate in the same assay. AUC leptin was determined by using the trapezoidal rule for calculating the area under the curve.
Pro-inflammatory cytokines
Pro-inflammatory cytokine (TNFRp55, TNFRp75, TNF α, IL-1β and IL-6) analyses were performed using an ELISA [29]. The intra-assay CV for TNF α, TNFRp55, TNFRp75, IL-1β and IL-6 were 5.4%, 1.2%, 5.1%, 2.0% and 1.8% respectively. The inter-assay CV for TNF α, TNFRp55, TNFRp75, IL-1β and IL-6 were 5.1%, 7.0%, 10.9%, 2.9% and 10.3% respectively.
Statistical analysis
The effect of the three different diets on leptin and proinflammatory cytokine levels within younger and older subjects was examined with paired t-tests. Due to the skewed data for serum and PBMC pro-inflammatory cytokine levels, all values were log transformed prior to analysis. Partial correlation analyses were performed to evaluate the association between changes in AUC leptin and pro-inflammatory cytokine levels. The Bonferroni correction was applied to adjust for multiple comparisons made for different cytokines (30). The criterion for statistical significance was P ≤0.05, and two-sided tests were used for all analyses. The SPSS version 14 (SPSS Inc., Chicago, IL) was used to analyze data.
Results
Body composition, REE, and calorie consumption
The detailed results of body composition, REE and calorie consumption have been previously reported (6). Briefly, both younger (mean age = 25 ± 5 years old) and older (mean age = 75 ± 4 years old) subjects lost weight after the caloric restriction diet and both groups failed to regain their weight back to their baseline after the 4-weeks of ad-libitum diet (6). There were no significant differences in the magnitude of changes in weight, REE, fat mass or lean mass after caloric restriction or after ad-libitum diet when younger and older subjects were compared with one another (6).
AUC leptin and fasting morning leptin levels
Neither unadjusted AUC leptin levels nor AUC leptin levels adjusted for fat mass at baseline (CRC 1) were significantly different between the two age groups (unadjusted AUC leptin for younger and older subjects were 556 ± 139 and 726 ± 151 ng/ml respectively; P = 0.41. Adjusted AUC leptin for younger and older subjects were 23.8 ± 4.7 and 25.6 ± 4.7 ng/ml respectively; P = 0.79). Younger and older women had significantly higher AUC leptin per fat mass at baseline compared to younger and older men (Adjusted AUC leptin for younger women and men were 36.3 ± 6.4 vs. 10.1 ± 3.7 ng/ml respectively; P = 0.003. Adjusted AUC leptin for older women and men were 35.4 ± 5.4 vs. 12.2 ± 5.6 ng/ml respectively; P = 0.009).
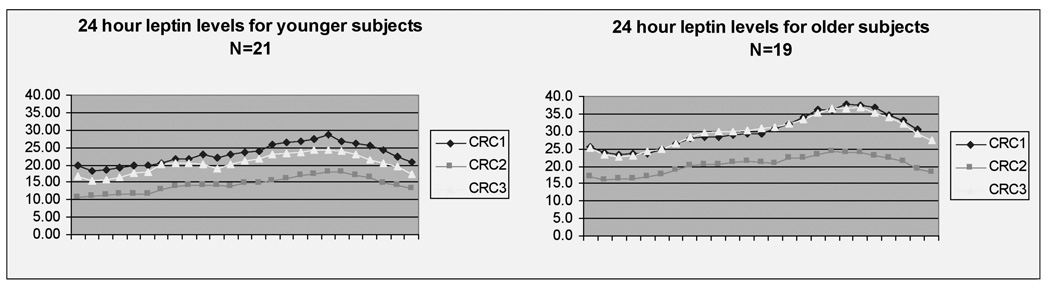
In both age groups, AUC leptin levels decreased appropriately after caloric restriction (CRC2) and returned to baseline after the ad-libitum diet (CRC3) (Figure 1). There was a trend towards a greater decrease in AUC leptin levels induced by caloric restriction among younger versus older subjects (−38.7% ±3.9 vs. −28.9% ±3.0 respectively; P = 0.06). The increase in AUC leptin values after the ad-libitum diet was instituted did not differ between the two cohorts (30.9% ± 6.5 for younger and 36.5% ± 6.7 for older, P = 0.55). There was a significant correlation between the overnight fasting leptin levels and AUC leptin values for both younger and older subjects (r =0.978, P = 0.001, and r =0.982, P = 0.001, respectively).
Figure 1. 24 hour leptin levels.
All values are mean. CRC1 = Clinical Research Center visit after weight maintenance phase, CRC2 = Clinical Research Center visit after 30 % caloric restriction phase, CRC3 = Clinical Research Center visit after ad-libitum phase.
Serum pro-inflammatory cytokine levels
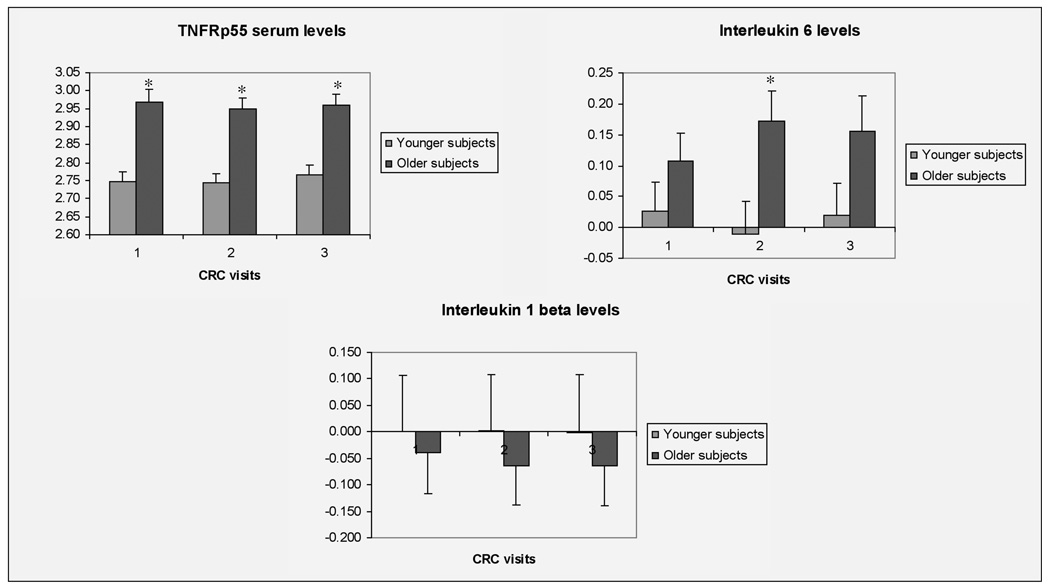
Older subjects had higher TNFRp55 levels than younger subjects at CRC 1, 2 and 3 (Figure 2). IL-6 levels were elevated in healthy older individuals compared to younger subjects only after caloric restriction (CRC 2) (Figure 2). There were no statistically significant differences in TNFRp55, TNFRp75, IL 1β or IL6 before or after the caloric restriction period for either age group (data not shown).
Figure 2. Log transformed serum cytokine levels.
All values are mean ± SEM. * P ≤ 0.05 values for differences between younger and older subjects, two-tailed independent t-test. CRC1 = Clinical Research Center visit after weight maintenance phase, CRC2 = Clinical Research Center visit after 30 % caloric restriction phase, CRC3 = Clinical Research Center visit after ad-libitum phase.
PBMC pro-inflammatory cytokine levels
The pro-inflammatory cytokine levels produced by unstimulated PBMC or PBMC stimulated with LPS or PHA at CRC 1, 2 or 3 did not differ significantly by age group (data not shown). There were no statistically significant differences in PBMC production of TNF α, IL-1β or IL6 before or after caloric restriction for either age group (data not shown).
AUC leptin and pro-inflammatory cytokine levels
After correcting for multiple comparisons, the change in AUC leptin with caloric restriction (CRC 1 to CRC 2) did not correlate significantly with changes in pro-inflammatory cytokine production by unstimulated PBMC or PBMC stimulated with LPS or PHA for both age groups (Table 1). Similarly, there were no significant correlations between changes in AUC leptin after ad-libitum diet phases (CRC 2–3) and change in pro-inflammatory cytokine levels in either cohort (Table 1).
Table 1.
Association between change in Log 10 cytokine levels and change in AUC leptin levels after adjusting for change in fat mass
| Δ AUC leptin in younger | Δ AUC leptin in older | Δ AUC leptin in younger | Δ AUC leptin in older | |||||
|---|---|---|---|---|---|---|---|---|
| CRC 1–2 | CRC 1–2 | CRC 2–3 | CRC 2–3 | |||||
| r | P | r | P | r | P | r | P | |
| ΔSeram TNFRp 55 (pg/ml) | 0.21 | 0.89 | −0.03 | 0.91 | −0.22 | 0.35 | −0.22 | 0.39 |
| TNFRp 75 (pg/ml) | 0.03 | 0.91 | −0.14 | 0.56 | −0.43 | 0.05 | 0.27 | 0.30 |
| IL-1β (pg/ml) | −0.39 | 0.09 | 0.14 | 0.53 | −0.33 | 0.14 | −0.18 | 0.52 |
| IL-6 (pg/ml) | −0.02 | 0.95 | −0.35 | 0.11 | −0.06 | 0.81 | −0.47 | 0.08 |
| Δ PBMC unstimulated | −0.05 | 0.83 | 0.19 | 0.36 | −0.27 | 0.24 | 0.35 | 0.15 |
| TNF α (pg/ml) | ||||||||
| IL-1β (pg/ml) | −0.21 | 0.37 | 0.21 | 0.33 | −0.25 | 0.29 | 0.33 | 0.19 |
| IL-6 (pg/ml) | −0.36 | 0.12 | 0.13 | 0.54 | −0.24 | 0.31 | 0.30 | 0.22 |
| Δ PBMC stimulated with LPS | −0.003 | 0.99 | 0.13 | 0.56 | −0.10 | 0.66 | 0.17 | 0.51 |
| TNF α (pg/ml) | ||||||||
| IL-1 β (pg/ml) | −0.01 | 0.69 | −0.16 | 0.44 | 0.11 | 0.64 | 0.04 | 0.87 |
| IL-6 (pg/ml) | −0.14 | 0.56 | 0.12 | 0.59 | −0.04 | 0.88 | 0.20 | 0.43 |
| Δ PBMC stimulated with PHA | ||||||||
| TNF α (pg/ml) | 0.28 | 0.22 | −0.28 | 0.26 | 0.15 | 0.53 | −0.09 | 0.73 |
| IL-1 β (pg/ml) | 0.05 | 0.86 | −0.44 | 0.05 | 0.04 | 0.86 | −0.07 | 0.78 |
| IL-6 (pg/ml) | 0.04 | 0.87 | −0.05 | 0.83 | 0.21 | 0.39 | 0.03 | 0.92 |
Partial correlation analysis. * P < 0.05 values for significant association between change in cytokine levels and change in AUC leptin. ΔAUC leptin = Change in 24 hour leptin levels. Δ PBMC = Change in peripheral blood mononuclear cells. CRC 1–2 = Change between Clinical Research Center visit before and after 30% caloric restriction phase, CRC 2–3 = Change between Clinical Research Center visit after 30% caloric restriction and after ad-libitum phase. LPS = lipopolysaccarides, PHA = phytohemagglutinin, TNFRp55= TNF receptor 55, TNFRp75=TNF receptor 75, IL-1β = Interleukin 1 beta, and IL-6 = Interleukin 6.
Discussion
This study demonstrates that caloric restriction induced similar decreases in AUC leptin levels in healthy older and younger adults, and the two groups did not differ in the degree to which their leptin levels recovered following 4 weeks of ad libitum refeeding. The fasting leptin levels and AUC leptin values were highly correlated in both subject groups. Therefore, in future studies, fasting blood samples may be used as a surrogate for diurnal blood sampling. Surprisingly, only one of the pro-inflammatory cytokines, TNF α, was higher in older subjects compared to younger adults during all three diet phases. Previous studies have shown that IL-6 and TNF α increase with age [16–18], however, the older subjects in this study probably had better overall health than those enrolled in previous studies. Although serum TNF α levels were higher in older subjects, there was no significant correlation between change in TNF α and change in AUC leptin. Our data do not support the hypothesis that elevation of serum proinflammatory cytokine levels prevents a decline in leptin levels with weight loss, thereby impairing body weight recovery in older individuals.
Two limitations of this study deserve comment. First, since neither age group regained their weight completely after 4 weeks of the ad libitum diet (6), the AUC leptin levels measured during CRC3 may not have accurately reflected a state of energy balance. Nevertheless, our data provide absolutely no indication for a difference in diurnal leptin profiles between younger and older subjects at any point during the study. The second potential limitation is that this study was powered to detect a significant difference in calorie intake between younger and older adults during recovery from induced weight loss rather than differences in pro-inflammatory cytokine levels. However, the significant differences we observed between groups for 2 out of the 3 cytokines, or cytokine receptors measured in serum, is reassuring that our methodology was sufficiently sensitive to detect major diet and age related changes. The absence of diet or age effects on cytokine production by stimulated PBMC in vitro further supports a lack of cytokine modulation of leptin levels during recovery from weight loss.
In conclusion, leptin levels in healthy older individuals responded appropriately to acute weight loss, and serum IL-1β and IL-6 levels were not significantly higher in older subjects compared to younger controls. There was no association between changes in pro-inflammatory cytokine levels and changes in AUC leptin levels for either younger or older adults. Our results establish that the homeostatic system acting to stabilize body composition is intact in older individuals who are free of medical illness. It is uncertain how older individuals with co-morbid illnesses will respond to acute weight loss; this is an important area for future investigation.
Acknowledgment
The authors would like to acknowledge the laboratory assistance provided by Pam Yang of the University of Washington; Del Landicho and Teresa Whisler of Clinical Nutrition Research Unit Core Laboratory of the University of Washington; and Richard Lawler and George McDonald, MD of Cytokine laboratory of the Fred Hutchinson Cancer Research Center.
References
- 1.Wallace JI, Schwartz RS, LaCroix AZ, Uhlmann RF, Pearlman RA. Involntary weight loss in older outpatients: Incidence and clinical significance. J Am Geriatr Soc. 1995;43:329–337. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 2.Payette H, Coulombe C, Boutier V, Gray-Donald K. Weight loss and mortality among free-living frail elders: Prospective study. J Gerontol. 1999;54A:M440–M445. doi: 10.1093/gerona/54.9.m440. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol Med Science. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Robert SB, Fuss P, Heyman MB, et al. Control of food intake in older men. JAMA. 1994;272:1601–1606. doi: 10.1001/jama.1994.03520200057036. [DOI] [PubMed] [Google Scholar]
- 5.Moriguti JC, Das SK, Saltzman E, et al. Effects of a 6-week hypocaloric diet on changes in body composition, hunger, and subsequent weight regain in healthy young and older adults. J Gerontol. 2000;55A:BS80–BS87. doi: 10.1093/gerona/55.12.b580. [DOI] [PubMed] [Google Scholar]
- 6.Yukawa M, Cummings DE, Matthys CC, et al. Effect of aging on the response of ghrelin to acute weight loss. J Am Geriatr Soc. 2006;54:648–653. doi: 10.1111/j.1532-5415.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffeir M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 8.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:542–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 9.Grunfeld C, Pang M, Shigenaga JK, et al. Serum leptin levels in the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1996;81:4342–4346. doi: 10.1210/jcem.81.12.8954039. [DOI] [PubMed] [Google Scholar]
- 10.Lin SY, Wang YY, Sheu WH. Increased serum leptin concentrations correlate with soluble tumuor necrosis factor receptor levels in patients with cirrhosis. Clin Endo. 2002;57:805–811. doi: 10.1046/j.1365-2265.2002.01672.x. [DOI] [PubMed] [Google Scholar]
- 11.Odamaki M, Furuya R, Yoneyama T, et al. Association of the serum leptin concentration with weight loss in chronic hemodialysis patients. Am J Kidney Dis. 1999;33:361–368. doi: 10.1016/s0272-6386(99)70313-6. [DOI] [PubMed] [Google Scholar]
- 12.Papathanassoglou EDE, Moynihan JA, Ackerman MH, Mantzoros CS. Serum leptin levels are higher but are not independently associated with severity or mortality in the multiple organ dysfunction/systemic inflammatory response syndrome: a matched case control and a longitudinal study. Clin Endo. 2001;54:225–233. doi: 10.1046/j.1365-2265.2001.01209.x. [DOI] [PubMed] [Google Scholar]
- 13.Takabatake N, Nakamura H, Abe S, et al. Circulating leptin in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1215–1219. doi: 10.1164/ajrccm.159.4.9806134. [DOI] [PubMed] [Google Scholar]
- 14.Weigle DS, Ganter SL, Kuijper JL, Leonetti DL, Boyko EJ, Fujimoto WY. Effect of regional fat distribution and Prader-Willi Syndrome on plasma leptin levels. J Clin Endocrinol Metab. 1997;82:566–570. doi: 10.1210/jcem.82.2.3761. [DOI] [PubMed] [Google Scholar]
- 15.Havel PJ, Townsend R, Chaump L, Teff K. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes. 1999;48:334–341. doi: 10.2337/diabetes.48.2.334. [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R, Harris TB, Abad LW, Wilson PWF, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: Effect of age and inflammation. J Gerontol. 1998;53A:M20–M26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- 17.Mysliwska J, Bryl E, Foerster J, Mysliwski A. Increase of interleukin 6 and decrease of interleukin 2 production during the aging process are influenced by the health status. Mech Aging Dev. 1998;100:313–328. doi: 10.1016/s0047-6374(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 18.Ruscin JM, Page RL, Yeager BF, Wallace JI. Tumor necrosis factor-alpha and involuntary weight loss in elderly, community-dwelling adults. Pharmacotherapy. 2005;25:313–319. doi: 10.1592/phco.25.3.313.61607. [DOI] [PubMed] [Google Scholar]
- 19.Filippatos GS, Tsilias K, Venetsanou K, Karambinos E, Manolatos D, Kranidis A, et al. Leptin serum levels in cachectic heart failure patients relationship with tumor necrosis factor-alpha system. Int J Cardiol. 2000;76:117–122. doi: 10.1016/s0167-5273(00)00397-1. [DOI] [PubMed] [Google Scholar]
- 20.Dobbs RJ, Charlett A, Perkiss AG, Dobbs SM, Weller C, Peterson DW. Association of circulating TNF-α and IL-6 with aging and Parkinsonism. Acta Neurol Scand. 1999;100:34–41. doi: 10.1111/j.1600-0404.1999.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 21.Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer. 2003;97:1211–1216. doi: 10.1002/cncr.11178. [DOI] [PubMed] [Google Scholar]
- 22.Yeh SS, Hafnew A, Chang CK, Levine DM, Parker TS, Schuster MW. Risk factors relating blood markers of inflammation and nutritional status to survival in cachectic geriatric patients in a randomized clinical trial. J Am Geriatr Soc. 2004;52:1708–1712. doi: 10.1111/j.1532-5415.2004.52465.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen HJ, Pieper CF, Harris T, Murali K, Rao K, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol. 1997;52A:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 24.Janik JE, Curti BD, Considine RV, et al. Interleukin 1α increases serum leptin concentrations in humans. J Clin Endocrinol Metab. 1997;82:3084–3086. doi: 10.1210/jcem.82.9.4214. [DOI] [PubMed] [Google Scholar]
- 25.Zumback MS, Boehme MWJ, Wahl P, Stremmel W, Ziegler R, Nawroth PP. Tumor necrosis factor increases serum leptin levels in humans. J Clin Endocrinol Metab. 1997;82:4080–4082. doi: 10.1210/jcem.82.12.4408. [DOI] [PubMed] [Google Scholar]
- 26.Hansen MB, Svenson M, Diamant M, Bendtzen K. High affinity IgG autoantibodies to IL-6 in sera of normal individuals are competitive inhibitors of IL-6 in vitro. Cytokine. 1993;5:72–80. doi: 10.1016/1043-4666(93)90026-2. [DOI] [PubMed] [Google Scholar]
- 27.Dandona F, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-α in sera of obese patients: Fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–2910. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 28.House RV. Cytokine bioassays: an overview. Dev Biol Stand. 1999;97:13–19. [PubMed] [Google Scholar]
- 29.Williams MA, Mahomed K, Farrand A, et al. Plasma tumor necrosis factor-alpha soluble receptor p55 (sTNFp55) concentrations in eclamptic, preeclamptic and normotensive pregnant Zimbabwean women. J Reprod Immunol. 1998;40:159–173. doi: 10.1016/s0165-0378(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 30.Moore DS, McCabe GP. Introduction to the practice of Statistics. 4th Ed. New York, NY: WH Freeman Co; 2003. pp. 773–775. [Google Scholar]