Abstract
Deep brain stimulation (DBS), a surgical therapy for advanced Parkinson's disease (PD), is known to change neuronal activity patterns in the pallidothalamic circuit. Whether these effects translate to motor cortex and, if so, how they might modulate the functional responses of individual neurons in primary motor cortex remains uncertain. A 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkey was implanted with a DBS lead spanning internal and external segments of globus pallidus. During therapeutic stimulation (135 Hz) for rigidity and bradykinesia, neurons in primary motor cortex (M1) exhibited an inhibitory phase-locking (2-5 ms) to the stimulus, an overall decrease in mean discharge rate, and an increase in response specificity to passive limb movement. Sub-therapeutic DBS (30 Hz) still produced entrainment to the stimulation, but the mean discharge rate and specificity to movement were not changed. Lower stimulation intensities (at 135 Hz), which no longer improved motor symptoms, had little effect on M1 activity. These findings suggest that DBS improves parkinsonian motor symptoms by inducing global changes in firing pattern and rate along the pallido-thalamocortical sensorimotor circuit.
Keywords: DBS, M1, globus pallidus, MPTP, specificity
Introduction
While the theoretical rate model (DeLong, 1990) has been useful to describe how loss of dopamine signaling affects activity within the basal ganglia and relates to the manifestation of parkinsonian motor symptoms, recent studies have not supported the model's downstream predictions of a persistent inhibition in motor thalamus (Pessiglione, et al., 2005) and decreased motor cortical output (Doudet, et al., 1990, Goldberg, et al., 2002). Changes in thalamocortical activity following administration of the neurotoxin MPTP have instead been characterized by increased synchrony and decreased specificity to passive (Goldberg, et al., 2002, Pessiglione, et al., 2005) and voluntary movement (Doudet, et al., 1990, Watts and Mandir, 1992), suggestive of a distorted capacity to process spatiotemporal information (Leblois, et al., 2006, Mink, 1996). Abnormal firing patterns and loss of spatial selectivity have also been reported in the external globus pallidus (GPe) (Filion, et al., 1988) and internal globus pallidus (GPi) (Boraud, et al., 2000) in parkinsonian non-human primates. Interestingly, disrupting the propagation of pathological firing patterns in the globus pallidus and thalamus, through therapies like deep brain stimulation (DBS), correlates with improvement in motor symptoms (Hashimoto, et al., 2003, Montgomery, 2006, Xu, et al., 2008). In this study, we investigated the hypothesis that pallidal DBS, with parameters that relieve bradykinesia and rigidity, modulates neuronal activity in primary motor cortex.
A rhesus macaque was treated with two left intracarotid injections of MPTP to induce a moderate hemi-parkinsonian state (0.4-0.6 mg/kg per treatment) (Johnson, et al., 2008). A scaled version of a human DBS lead was implanted through a cephalic chamber such that the electrode contacts spanned both GPe and GPi (Fig. S1, also see (Elder, et al., 2005)). Additional methodological details are provided as supplementary material. The placement of the stimulating electrodes in the globus pallidus provided an opportunity to correlate M1 activity with improvement in motor symptoms without the added complication of antidromic activation of M1, which can occur during subthalamic nucleus stimulation (Dejean, et al., 2008, Li, et al., 2007). Activity in primary motor cortex (n=92 cells) was examined before, during, and after therapeutic and sub-therapeutic DBS (decreased amplitude or decreased frequency). Therapeutic stimulation parameters for bradykinesia and rigidity were defined using a series of quantitative behavioral metrics (Fig. S2). Microelectrode recordings were performed with the monkey awake and sitting quietly in its chair while the experimenter passively manipulated a joint that resulted in firing rate changes in the recorded spike activity. During this movement, firing patterns were examined with 1) peri-stimulus time histograms (PSTH) triggered to either stimulation pulses during DBS or virtual stimulation epochs before and after DBS (Fig. S3), and 2) peri-event time histograms (PETH) triggered to a particular phase of a passive joint articulation.
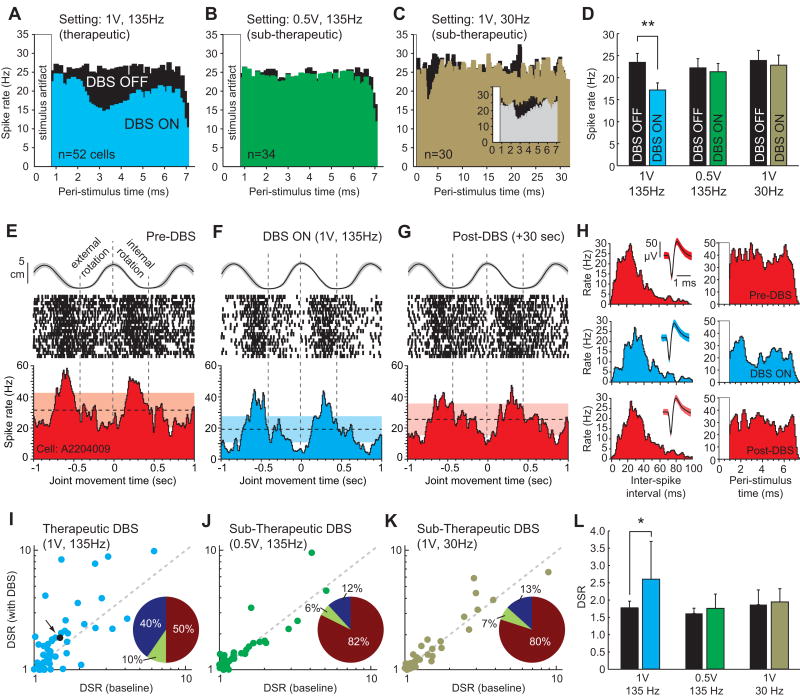
During these movements, neuronal firing patterns in primary motor cortex became time-locked to the stimulation. Therapeutic settings (1V, 135 Hz) elicited a decrease in the firing probability 2-7 ms following each stimulation pulse (n=33 of 52 cells, 63%) (Fig. 1A). The population-averaged trough of this modulation occurred at 3.3 ms, and the population-averaged mean discharge rate did not return to its pre-stimulus level before the onset of the next stimulus pulse (7.4 ms later). When decreasing the amplitude of the DBS waveform (0.5V, 135Hz), improvement in bradykinesia and rigidity was no longer present, and no significant modulation in the population-averaged peri-stimulus time histogram (PSTH) was observed (Fig. 1B). These results emphasize how slightly different DBS parameters can produce markedly different effects on downstream neuronal activity (Hashimoto, et al., 2003, Johnson and McIntyre, 2008, Xu, et al., 2008). When applying sub-therapeutic DBS settings in which the frequency was lowered instead of the voltage (1.0V, 30Hz), the population-averaged PSTH still exhibited the time-locked response (Fig. 1C, inset), but the firing probability returned to its pre-stimulus level before the arrival of the subsequent stimulus pulse (33ms later) (Fig. 1C). Transient entrainment during low-frequency stimulation has also been reported in the pallidum during STN-DBS (Hashimoto, et al., 2003).
Figure 1.
In primary motor cortex (M1), single-unit responses during passive joint articulation differed between therapeutic and sub-therapeutic DBS settings. A, For therapeutic settings (1V, 135Hz), population-averaged firing patterns became time-locked to the stimulation, which B, was not present for attenuated DBS voltages (0.5V, 135Hz) that no longer produced a therapeutic effect. C, Lowering the DBS frequency (1V, 30Hz) resulted in a similar initial firing pattern to that observed during therapeutic DBS (inset), but the overall firing probability returned to baseline within 5 ms. The black histograms show population-averaged responses to virtual stimulation epochs, whereas colored histograms correspond to population-averaged responses to actual stimulation pulses. D, A significant decrease in overall rate was observed for therapeutic, but not for sub-therapeutic settings (**p<0.001, *p<0.05). E-H, Therapeutic DBS also modulated the specificity of neuronal discharge to passive movement as shown for a representative cell (responsive to shoulder internal/external rotation, n=20 cycles) before, during, and after stimulation. Horizontal dotted lines indicate mean firing rate, whereas shaded boxes indicate 95% confidence intervals. The curve above the raster scan shows the movement profile compressed into 2D space. I-K, The neuronal discharge specificity ratio, quantified as the ratio of average firing rates between opposing joint articulations, increased during therapeutic but less so during sub-therapeutic DBS. Pie chart inserts represent the proportion of units with DSR changes above +20% (blue), below -20% (yellow), or no change (maroon). The black dot in I (indicated by the arrow) represents the example cell in E-H. L, A statistically significant increase in the recorded population's DSR was only present during therapeutic DBS (*p=0.019, n=55, paired t-test).
The propagation of modulatory effects into primary motor cortex is not that surprising given the strong direct and indirect connections between pallidal-receiving areas of thalamus and the motor cortices (Akkal, et al., 2007, McFarland and Haber, 2002). The stimulation-induced suppression of spike activity at a 3.3 ms latency – a pattern also observed during therapeutic DBS for spontaneously active cells in periods of no movement (data not shown) – appears to be too short to reflect poly-synaptic pallido-thalamocortical activity from the same inter-pulse interval. It is possible, however, that the modulation could reflect recurrent activity patterns through the basal ganglia – thalamocortical network (Montgomery, 2004). Another possible explanation for the observed modulation in M1 is that the effects stem from refractory responses to direct stimulation of corticospinal tract fibers (Ashby, et al., 1998) that course along the medial edge of both pallidal segments (Schmahmann and Pandya, 2006). The therapeutic voltage amplitude used in our experiments was typically <66% of that necessary to elicit overt muscle contraction, identified by both visual inspection and surface EMG activity. In addition, short-latency excitation (identified at ∼1.5 ms after each pulse at higher DBS voltages) was not observed across the recorded population at the therapeutic voltage. Nevertheless, sub-threshold effects or activation of a small minority of fibers and subsequent intracortical dynamics cannot be ruled out.
Because of the entrained decrease in firing probability during therapeutic stimulation, the population mean discharge rate of M1 neurons decreased significantly during passive movement (23.5 to 17.2 Hz, n=52, p<0.001), but no significant difference in mean discharge rate was evident for either sub-therapeutic settings (p>0.05) (Fig. 1D). These findings appear to be consistent with studies showing a decrease in metabolic activity in motor cortex that correlated with the therapeutic efficacy of DBS (Haslinger, et al., 2005) or with the administration of levodopa (Asanuma, et al., 2006). It should be noted, however, that single-unit recordings of spontaneous activity in primate primary motor cortex before and after injection of MPTP have not shown evidence for increased firing rates with the appearance of parkinsonian symptoms (Doudet, et al., 1990, Goldberg, et al., 2002, Watts and Mandir, 1992). This observation does not necessarily contradict the finding of decreased firing rates in M1 during therapeutic DBS as the therapeutic mechanisms of action may not necessarily entail reversing M1 firing rates to a ‘normal’ state.
Besides a decrease in mean discharge rate during passive movement, therapeutic DBS also modulated the specificity of M1 responses to opposing joint articulations as quantified by a discharge specificity ratio (DSR) of average firing rates between each phase of the movement (Fig. 1E-H). Prior to stimulation, the articulation that generated the largest audible change in the recorded cell's firing rate was selected for analysis. Across the recorded population of cells, the DSR increased during therapeutic stimulation (p<0.001), but not for sub-therapeutic settings (p>0.05) (Fig. 1I-K). For those cells with DSR changes >20% during therapeutic DBS, 40% of the units showed an increase and 10% showed a decrease. These effects were nominal for subtherapeutic settings. Finally, across the recorded population, there was a statistically significant increase in the DSR during therapeutic DBS (paired t-test, p=0.019, n=55), which was not observed for either sub-therapeutic setting (p>0.05) (Fig. 1L).
Quantifying sensorimotor receptive fields during DBS is a technically challenging endeavor because stimulation artifacts hamper listening to real-time changes in firing activity during passive manipulation. To the best of our knowledge this study, which used a combination of 3D motion capture and post-hoc stimulation artifact suppression, accounts for the first examination of sensorimotor receptive fields during DBS. The observed increase in neuronal specificity to passive joint articulation in M1 resulted in part from an overall decrease in firing rate during both phases of the opposing joint articulations, which may suggest a restoration of cortico-cortical interactions (Silberstein, et al., 2005). Although the precise mechanisms by which these effects occur remain uncertain, the data provide further evidence that deep brain stimulation produces its beneficial effects via a global change in neuronal firing activity throughout the pallido-thalamocortical sensorimotor circuit.
Supplementary Material
Acknowledgments
This study was funded by the National Institutes of Health through grants NS061541, NS047388, and NS037019. We thank Dr. Weidong Xu, Dr. Jianyu Zhang, Jennie Minnich, and Erin Bynum for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson's disease. Brain. 2006;129:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashby P, Strafella A, Dostrovsky JO, Lozano A, Lang AE. Immediate motor effects of stimulation through electrodes implanted in the human globus pallidus. Stereotact Funct Neurosurg. 1998;70:1–18. doi: 10.1159/000029593. [DOI] [PubMed] [Google Scholar]
- 4.Boraud T, Bezard E, Bioulac B, Gross CE. Ratio of inhibited-to-activated pallidal neurons decreases dramatically during passive limb movement in the MPTP-treated monkey. J Neurophysiol. 2000;83:1760–1763. doi: 10.1152/jn.2000.83.3.1760. [DOI] [PubMed] [Google Scholar]
- 5.Dejean C, Hyland B, Arbuthnott G. Cortical Effects of Subthalamic Stimulation Correlate with Behavioral Recovery from Dopamine Antagonist Induced Akinesia. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn149. [DOI] [PubMed] [Google Scholar]
- 6.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 7.Doudet DJ, Gross C, Arluison M, Bioulac B. Modifications of precentral cortex discharge and EMG activity in monkeys with MPTP-induced lesions of DA nigral neurons. Exp Brain Res. 1990;80:177–188. doi: 10.1007/BF00228859. [DOI] [PubMed] [Google Scholar]
- 8.Elder CM, Hashimoto T, Zhang J, Vitek JL. Chronic implantation of deep brain stimulation leads in animal models of neurological disorders. J Neurosci Methods. 2005;142:11–16. doi: 10.1016/j.jneumeth.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Filion M, Tremblay L, Bedard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res. 1988;444:165–176. doi: 10.1016/0006-8993(88)90924-9. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haslinger B, Kalteis K, Boecker H, Alesch F, Ceballos-Baumann AO. Frequency-correlated decreases of motor cortex activity associated with subthalamic nucleus stimulation in Parkinson's disease. Neuroimage. 2005;28:598–606. doi: 10.1016/j.neuroimage.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MD, McIntyre CC. Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol. 2008;100:2549–2563. doi: 10.1152/jn.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson MD, Vitek JL, McIntyre CC. Motor cortex activity during pallidal deep brain stimulation in a parkinsonian monkey. Soc Neurosci Abs. 2008;641:644. [Google Scholar]
- 15.Leblois A, Boraud T, Meissner W, Bergman H, Hansel D. Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. J Neurosci. 2006;26:3567–3583. doi: 10.1523/JNEUROSCI.5050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- 17.McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery E. Dynamically coupled, high-frequency reentrant, non-linear oscillators embedded in scale-free basal ganglia-thalamic-cortical networks mediating function and deep brain stimulation effects. Nonlinear Studies. 2004;11:385–421. [Google Scholar]
- 20.Montgomery EB., Jr Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol. 2006;117:2691–2702. doi: 10.1016/j.clinph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci. 2005;25:1523–1531. doi: 10.1523/JNEUROSCI.4056-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford University Press; Oxford; New York: 2006. [Google Scholar]
- 23.Silberstein P, Pogosyan A, Kuhn AA, Hotton G, Tisch S, Kupsch A, Dowsey-Limousin P, Hariz MI, Brown P. Cortico-cortical coupling in Parkinson's disease and its modulation by therapy. Brain. 2005;128:1277–1291. doi: 10.1093/brain/awh480. [DOI] [PubMed] [Google Scholar]
- 24.Watts RL, Mandir AS. The role of motor cortex in the pathophysiology of voluntary movement deficits associated with parkinsonism. Neurol Clin. 1992;10:451–469. [PubMed] [Google Scholar]
- 25.Xu W, Russo GS, Hashimoto T, Zhang J, Vitek JL. Subthalamic nucleus stimulation modulates thalamic neuronal activity. J Neurosci. 2008;28:11916–11924. doi: 10.1523/JNEUROSCI.2027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.