Abstract
Fetal alcohol syndrome(Abel, 2000) is a leading cause of mental retardation. The neuropathology found in fetal alcohol syndrome is similar to the phenotypes expressed in diseases caused by mutations in the gene for L1 cell adhesion molecule. L1 has a crucial role in the developing nervous system, acting in cell-cell adhesion, neuronal guidance, and growth. We have previously shown that L1 mediated neurite outgrowth and L1 activation of ERK1/2 is exquisitely sensitive to ethanol (Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006). One possible mechanism for this effect is through disruption of a tyrosine based sorting signal, Y(1176)RSLE, on the cytoplasmic domain of L1. Our goal was to determine if ethanol inhibited the sorting signal or its phosphorylation state. Ethanol had no effect on L1 distribution to the growth cone or its ability to be expressed on the cell surface. Clustering of L1 resulted in increased dephosphorylation of Y(1176), increased L1 tyrosine phosphorylation, and an increase in the activation of pp60src, all of which were inhibited by 25 mM ethanol. Inhibition of pp60src inhibited increases in L1 tyrosine and ERK1/2 phosphorylation, and Y(1176) dephosphorylation. We conclude that ethanol disrupts L1 trafficking/signaling following its expression on the surface of the growth cone, and prior to its activation of pp60src.
Keywords: Ethanol, L1 cell adhesion molecule, distribution, fetal alcohol syndrome, tyrosine phosphorylation, tyrosine dephosphorylation, pp60src, axonal sorting, lipid rafts
INTRODUCTION
Fetal exposure to ethanol during nervous system development is the leading known cause of mental retardation (Stratton, Howe, and Battaglia, 1996). Many of the anatomic brain anomalies described in fetal alcohol syndrome are also observed in syndromes caused by mutations in the gene for L1, a neural cell adhesion molecule. The neuromigrational defects found in both conditions include hydrocephalus, absence of the corpus callosum, and cerebellar dysplasia (Roebuck, Mattson, and Riley, 1998). The striking correlation between ethanol induced neurological defects and anomalies resulting from defective L1 has led to speculation that disruption of L1 function may be one mechanism by which ethanol exerts teratogenicity on the nervous system (Bearer, 2001; Charness, Safran, and Perides, 1994). Previous work has shown that L1-mediated neurite outgrowth is inhibited by ethanol (Bearer, Swick, O'Riordan, and Cheng, 1999b; Watanabe, Ymazaki, Miyazaki, Arikawa, Itoh, Sasaki, Maehama, Frohman, and Kanaho, 2004). Further, we have shown that L1 activation of ERK1/2 is also inhibited by ethanol (Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006). These results support the hypothesis that ethanol’s effect on L1 function are at least partially responsible for the neurodevelopmental abnormalities observed in infants with fetal alcohol related disorders.
L1, a transmembrane glycoprotein, is a member of the immunoglobulin superfamily of cell adhesion molecules (Moos, Tacke, Schere, Teplow, Fruh, and Schachner, 1988). L1 is expressed on axonal shafts and growth cones of developing neurons (Craig, Wyborski, and Banker, 1995; Kamiguchi and Lemmon, 1998), and has an active role in axonal growth and guidance (Cohen, Taylor, Scott, Guillery, Soriano, and Furley, 1997; Lagenaur and Lemmon, 1987). L1 binds in trans homophilically to itself, or heterophilically to integrins (Yip, Zhao, Montgomery, and Siu, 1998) on adjacent axons. CGN from L1 knock out mice completely lose their ability to extend neurites on L1, indicating the predominance of the L1 to L1 homophilic interaction (Fransen, D'Hooge, Van Camp, Verhoye, Sijbers, Reyniers, Soriano, Kamiguchi, Willemsen, Koekkoek, De Zeeuw, De Deyn, Van der Linden, Lemmon, Kooy, and Willems, 1998).
The gene for L1 is highly conserved among species and is comprised of 28 exons, of which exons 2 and 27 are alternatively spliced (Miura, Kobayashi, Asou, and Uyemura, 1991). Neural cells express an isoform of L1 containing the alternatively spliced exons, whereas non-neuronal cells such as Schwann cells do not (Takeda, Asou, Murakami, Miura, Kobayashi, and Uyemura, 1996). The alternatively spliced exon 27 encodes for the amino acid sequence RSLE (Arg-Ser-Leu-Glu) immediately following a tyrosine residue 1176 (Y(1176)) in the cytoplasmic domain. This sequence conforms to a tyrosine based sorting motif, Yxxø, where Y signifies tyrosine,×stands for any amino acid, and ø represents an amino acid with a bulky hydrophobic side chain (Trowbridge, Collawn, and Hopkins, 1993). Among the important functions of the YRSLE sequence are the regulation of L1 cell surface expression by endocytosis and the sorting of L1 to the growth cone membrane (Kamiguchi and Lemmon, 1998; Kamiguchi, Long, Pendergast, Schaefer, Rapoport, Kirchhausen, and Lemmon, 1998; Wisco, Anderson, Chang, Norden, Boiko, Folsch, and Winckler, 2003). Phosphorylation of Y(1176) inhibits endocytosis of L1 by inhibiting binding to the μ2 chain of AP-2 (Schaefer, Kamei, Kamiguchi, Wong, Rapoport, Kirchhausen, Beach, Landreth, Lemmon, and Lemmon, 2002). DRGs grown on laminin express only constitutively phosphorylated Y1176. Homophilic binding of L1 or cross-linking of L1 causes dramatic and rapid dephosphorylation of Y1176, allowing endocytosis and ERK1/2 activation to occur (Schaefer, Kamei, Kamiguchi, Wong, Rapoport, Kirchhausen, Beach, Landreth, Lemmon, and Lemmon, 2002). Following endocytosis, Y1176 is rephosphorylated by an activated pp60src (Schaefer, Kamei, Kamiguchi, Wong, Rapoport, Kirchhausen, Beach, Landreth, Lemmon, and Lemmon, 2002).
There are three additional tyrosines within the cytoplasmic domain of L1, Y1151, Y1211, andY1229. Y1151 is critical for binding to ERM (Cheng, Itoh, and Lemmon, 2005) and Y1211 is within the ankyrin binding domain (Davis and Bennett, 1994). The third, Y1229, is involved in regulation of ankyrin binding (Garver, Ren, Tuvia, and Bennett, 1997). Dephosphorylation and phosphorylation of this tyrosine regulates ankyrin binding which is involved in the stationary behavior of L1 (Gil, Sakurai, Bradley, Fink, Cassella, Kuo, and Felsenfeld, 2003).
We hypothesize that ethanol inhibits L1-mediated neurite outgrowth by either inhibiting the tyrosine sorting signal and thereby disrupting L1 sorting to the growth cone, or by inhibiting downstream signaling events preceding ERK1/2 activation.
METHODS
Antibodies and Inhibitors
Rabbit polyclonal antibodies against chick NgCAM (8D9) and mouse monoclonal antibodies against chick NCAM (1A6) and the dephosphorylated Y1176 L1 (74-5H7) have been previously described (Schaefer, Kamei, Kamiguchi, Wong, Rapoport, Kirchhausen, Beach, Landreth, Lemmon, and Lemmon, 2002). NgCAM is the chick homolog of L1 and will be referred to as L1 in this paper. Polyclonal goat antibodies to the cytoplasmic domain of L1 (anti-L1CD) were obtained from Santa Cruz. Polyclonal antibodies to phospho-Src family (Tyr416), phospho-p44/42 MAPK (Thr202/Tyr204), and p44/42 MAPK as well as a monoclonal antibody to phosphotyrosine, PY100 were obtained from Cell Signaling. The monoclonal antibody against bovine MAP-2 was purchased from Roche pharmaceuticals and the monoclonal antibody to pp60src from clone GD11 was from Upstate. Goat anti-mouse IgG (H+L), goat anti-rabbit IgG (H+L), fluoroscein (FITC) conjugated affinity purified goat anti-mouse IgG (H+L) chain, rhodamine red X conjugated goat anti-rabbit IgG (H+L) chain, HRP-conjugated goat anti-mouse IgG (H+L) and HRP-conjugated goat anti-Rabbit IgG (H+L) secondary antibodies were obtained from Jackson ImmunoResearch Laboratories. Alexa Fluor 633 dye conjugated donkey anti-goat and Alexa Fluor 488 dye conjugated goat anti-mouse were obtained from Molecular Probes. Monoclonal anti-β tubulin III and the Texas red conjugated secondary antibody raised in goat against mouse IgG was from Sigma. PP2, a selective inhibitor of the Src family of protein tyrosine kinases was obtained from Calbiochem. Sodium vanadate was from Fisher.
Mouse monoclonal antibody to rat L1 (ASCS4) was produced from a hybridoma cell line developed by P.H. Patterson and obtained from the Developmental Studies Hybridoma Bank (The University of Iowa)) as previously described (Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006). Preparation of L1-Fc has been previously described (Bearer, Swick, O'Riordan, and Cheng, 1999a; Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006).
Cell culture
DRGs were dissected from the lumbar region of embryonic day 9–10 chicks, stages 35–36 per the Hamilton and Hamburger system (Hamburger and Hamilton, 1951). The DRGs were dissociated sequentially with 2.4 units/ml dispase II (Roche) and 10 units/ml DNase (Roche) in Ca2+/Mg2+-free PBS. The cells were then washed with 10% FBS in DMEM (Gibco) and centrifuged. The cell layer was added to an uncoated tissue culture dish containing growth media consisting of DMEM 10% FBS with 100 ng/ml mouse β nerve growth factor (Austral Biologicals). After an hour, the cell culture dish was agitated and the growth media with the detached cells in suspension was replated onto glass slides that were prepared as described in the next section. Cell cultures were maintained for 16 to 20 hours in a 37°C humidified 90% air, 10% CO2 incubator.
Rat postnatal day 6 CGN were prepared and plated on poly L-lysine coated tissue culture dishes in DMEM, 10% FBS containing 20 mM HEPES, pH 7.4 and penicillin/streptomycin as previously described (Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006).
Glass slides were coated with either poly-L-lysine, protein A, L1-Fc or laminin
Poly-L-Lysine
To prepare slides for DRGs, 500 µl 0.1% poly-L-lysine solution (Sigma) was added to each chamber of a 4 chamber glass slide (Lab Tek, Naperville, IL) for 30 min. The wells were then rinsed with HBSS (Sigma) and allowed to air dry.
Protein A (Sigma)
For L1-Fc experiments, 80 µl of protein A (2mg/ml in 0.1 M potassium phosphate, pH 7.4) was added for 1 hr at 37°C.
L1-Fc
7.3 µg of L1-Fc eluted from protein A columns (Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006)was diluted into 50 µl PBS 1% BSA and incubated for 1 hour at 37°C to achieve 1.2 µg/cm2 L1-Fc per well. Slides prepared for experiments with permeabilized cells were plated with 3.5 µg of L1-Fc in 50 µl of PBS 1% BSA to achieve 0.6 µg/cm2 L1-Fc per well.
Laminin
Slides were treated with laminin (100µg/ml (Sigma) in Ca++, Mg++ free PBS) and incubated overnight at 37°C. Both L1-Fc and laminin coated slides were rinsed with HBSS, and blocked with 10% HS (Gibco) in 0.1 M potassium phosphate, pH 7.4, 0.05% sodium azide.
Ethanol Exposure of DRG cultures
Immediately after transfer of the DRGs in growth media into prepared slide chambers, an equal volume of growth media containing 200 mM ethanol was added, for a final concentration of 100 mM ethanol, to the ethanol exposed DRG slides. Control slides had an equal volume of growth media without ethanol added. The slide chambers were covered and wrapped with parafilm. The ethanol containing slides were placed in a dedicated 37°C 10% CO2 incubator with 100 mM ethanol in the water pan. We have previously shown that ethanol concentrations in the media are constant under these conditions (Bearer, Swick, O'Riordan, and Cheng, 1999a). Prior to fixation or antibody application, the growth media was removed and stored at 4°C for later determination of ethanol concentration by spectrophotometry (Cary 3E UV-Visible Spectrophotometer) with an alcohol dehydrogenase diagnostic kit (Roche).
Immunocytochemistry of permeabilized DRGs
The cells were fixed and permeabilized for 15 minutes in Bouin’s solution (1.2% picric acid, 37% formaldehyde), followed by repeated washes with potassium phosphate 0.1M, pH 7.4, 0.05% azide. The slides were then incubated with blocking solution (10% HS in potassium phosphate 0.1M, pH 7.4, 0.05% azide) for 1 hour at 37° C, rinsed, and kept at 4°C overnight with primary antibodies to NCAM (1A6) 1:500 and L1 (8D9) 1:500 diluted in blocking solution. The slides were rinsed extensively with potassium phosphate buffer, before labeling with the secondary antibodies. Antibodies to L1 were identified with Rhodamine Red-X conjugated goat anti-rabbit and antibodies to NCAM were identified with FITC conjugated goat anti-mouse diluted 1:200 in blocking solution. The secondary antibodies were incubated at 37° C for 2 hours, followed by repeated rinses. The slides were mounted with Slow Fade Light (Molecular Probes) and viewed using a Zeiss LSM 410 Confocal laser scan microscope with an oil immersion 100× objective.
Antibody to MAP-2 was used at 1:600 dilution in blocking solution and labeled with Texas Red goat anti-mouse secondary at 1:500 in blocking solution. Images were captured on a spot digital camera attached to a Nikon Optiphot-2 fluorescence microscope.
Immunocytochemistry of CGN
CGN were placed on glass coverslips coated with L1-Fc. 25 mM ethanol was added to treated cells and cells were incubated overnight. Slide fixation was accomplished through the addition of 4% paraformaldehyde for 20 minutes followed by repeated washes with PBS. The slides were then incubated with blocking solution (3% BSA and 0.2% Triton X-100 in PBS, 0.05% azide) for 1 hour at 37°C or kept at 4°C overnight. Primary antibodies to beta tubulin 1:1,000 and L1CD 1:500 were diluted in blocking solution then incubated for 2 h at 37°C. The slides were rinsed extensively with PBS before labeling with the secondary antibodies. Antibodies to L1 were identified with Alexa Fluor 633 dye conjugated donkey anti-goat antibodies and antibodies to beta tubulin were identified with Alexa Fluor 488 dye conjugated goat anti-mouse antibodies diluted 1:200 in blocking solution. The secondary antibodies were incubated at 37° C for 1 hours, followed by repeated rinses. The slides were mounted with Vectashield (Vector H-1200) containing DAPI and viewed using a Zeiss LSM 410 Confocal laser scan microscope with an oil immersion 63× objective.
Immunocytochemistry of non-permeabilized DRGs
Slides with DRGs were placed on ice. The growth media was carefully removed, and new ice-cold growth media containing primary antibodies to L1 (1:100) and NCAM (1:100) was carefully added to the DRG wells. The primary antibodies were incubated for 30 minutes, followed by three washes with ice cold growth media. The DRG cell cultures were kept at 4°C from the addition of the primary antibodies until fixation to prevent internalization of labeled cell surface adhesion molecules. Slide fixation was accomplished through the addition of 4% paraformaldehyde for 15 minutes. Rhodamine Red X conjugated goat anti-rabbit (1:50 in blocking solution) secondary antibody to visualize L1 and FITC conjugated goat anti-mouse (1:50 in blocking solution) secondary antibody to visualize NCAM were added for 2 hours at 37° C. In experiments designed to ascertain cell membrane integrity, mouse anti-MAP-2 (1:600 in growth media) primary antibody was labeled with Texas red conjugated goat anti-mouse (1:500 in blocking solution) and viewed with a Nikon Optiphot-2 fluorescent microscope.
Imaging analysis and growth cone selection
A slide prepared under identical conditions to the experimental slides, but known to have had no exposure to ethanol was used as a standard for determining the optimal confocal contrast and brightness settings. The microscope parameters were not altered while obtaining images of the ethanol and control slides. The individual selecting the growth cones for this study was blinded to the ethanol exposure status of the slide until the completion of data collection. A growth cone was included in the study if it met the following criteria: extension of at least 50 microns from the cell body, characteristic morphologic structure, and no contact with another neurite (Lagenaur and Lemmon, 1987). The slides were scanned using the fluorescein channel which showed NCAM staining. Growth cones were selected sequentially, starting at the upper left corner of each chamber. L1 was considered present at the growth cone if the neurite outline could be clearly delineated on the rhodamine 568λ channel for which the L1 antibody was immunologically tagged. L1 presence in growth cones was analyzed by chi square. Statistical analysis was performed using Systat software v3.01.
L1 clustering on cerebellar granule cells
Growth medium was removed from CGN cultures and replaced with serum-free medium (DMEM, 20 mM HEPES, penicillin/streptomycin) for two hours prior to L1 clustering. 25 mM ethanol was added to appropriate dishes 1 hour prior to clustering. To form cross-linked ASCS4 antibody complexes, purified ASCS4 was mixed with goat anti-mouse IgG (H+L) (1:2.5 g/g) for 1 hour at 4°C and then added to CGN such that the final concentration was 30 µg/ml of media. Mouse IgG 30 µg/ml final concentration was used as control as previously described (Schmid, Pruitt, and Maness, 2000; Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006). At the indicated times, the tissue culture dishes were placed on ice, the media was removed, and the cells were washed with ice cold HBSS. All procedures were at 4°C.
Immunoblotting and Immunoprecipitation
Cell extracts were made by incubating cells in lysis buffer for 30 min. and then centrifuging the extract at maximum speed in a Beckman Superspeed Centriguge for 10 min at 4°C to remove particulate material. Lysis buffer consisted of: 20 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1mM EDTA, 10% glycerol, 10 mM Na-vanadate, 10 mM NaF, 2 mM aprotinin, 0.1 mM phenylmethylsulfonyl fluoride, 1 µM leupeptin, 1 µg/ml pepstatin, 10 µg/ml turkey trypsin inhibitor, phosphatase inhibitor cocktail I (Sigma) and phosphate inhibitor cocktail II (Sigma).
For immunoprecipitation experiments with L1-CD or Src, the cell extract supernatants were transferred to clean microfuge tubes and precleared with 1 µg rabbit IgG or mouse IgG for 1 hour, then with 30 µl Protein A/G-agarose for 1 hour, followed by low speed centrifugation. Supernatants were collected, and 2 µg of antibody was added. After 1 hr incubation, 30 µl of protein A/G-agarose was added and lysates were incubated overnight. The tubes were centrifuged in a Beckman Superspeed microfuge at 2500 rpm for 4 minutes.
For Western analysis, proteins were precipitated from cell extract supernatants with methanol-chloroform and dried. Precipitants or immunoprecipitants were washed with lysis buffer, and boiled for 5 min in SDS-PAGE sample buffer. The samples were separated by SDS-PAGE (12% gel), transferred to a PDVF membrane. The membrane was blocked in Tris buffered saline containing 2% BSA and 0.1% Tween 20. The membrane was incubated with primary antibodies (74-5H7, phospho-Src (Tyr416)(PY416), PY100 or phospho-p44/42 MAPK (Thr202/Tyr204)(pERK1/2) probed with either HRP-goat anti-mouse IgG or HRP-goat anti-rabbit IgG, and reactive protein bands were visualized using ECL chemiluminescence exposure of x-ray film. Blots were stripped and reprobed with antibodies to either the L1 cytoplasmic domain (L1CD) or p44/p42 MAPK (Total ERK1/2) followed by HRP-conjugated goat antirabbit IgG antibodies or anti-pp60src followed by HRP-conjugated goat anti-mouse IgG antibodies. Blots were analyzed in a semiquantitative manner using Kodak 1D imaging software to measure the density of bands on the x-ray film. The 74-5H7 and PY100 band densities were normalized for the band densities of L1CD protein, band densities of pERK1/2 were normalized for the band densities of total ERK, and PY416 band densities were normalized to total pp60src for all quantitative analyses. Relative densitometric units were calculated by calculating the ratio of the normalized band densities to that of the control.
RESULTS
The total L1 present at the growth cone is not changed by the presence of ethanol
A target ethanol concentration of 100 mM was chosen as it approaches the maximum concentration of ethanol realistically achieved in vivo before lethal toxicity is likely to occur. Individuals who have developed a chronic tolerance to ethanol, however, may have blood ethanol levels as high as 245–300 mM (Deitrich and Harris, 1996). An in vitro ethanol level of 100 mM does not significantly decrease DRG survival but does negatively impact neurite outgrowth (Bradley, Paiva, Tonjes, and Heaton, 1995). Prior work in our lab has shown a half maximal inhibitory effect at 400 mM ethanol on laminin mediated cerebellar neurite outgrowth and a half maximal inhibitory effect from 3 – 5 mM ethanol for L1-Fc mediated cerebellar neurite outgrowth (Bearer, Swick, O'Riordan, and Cheng, 1999a).
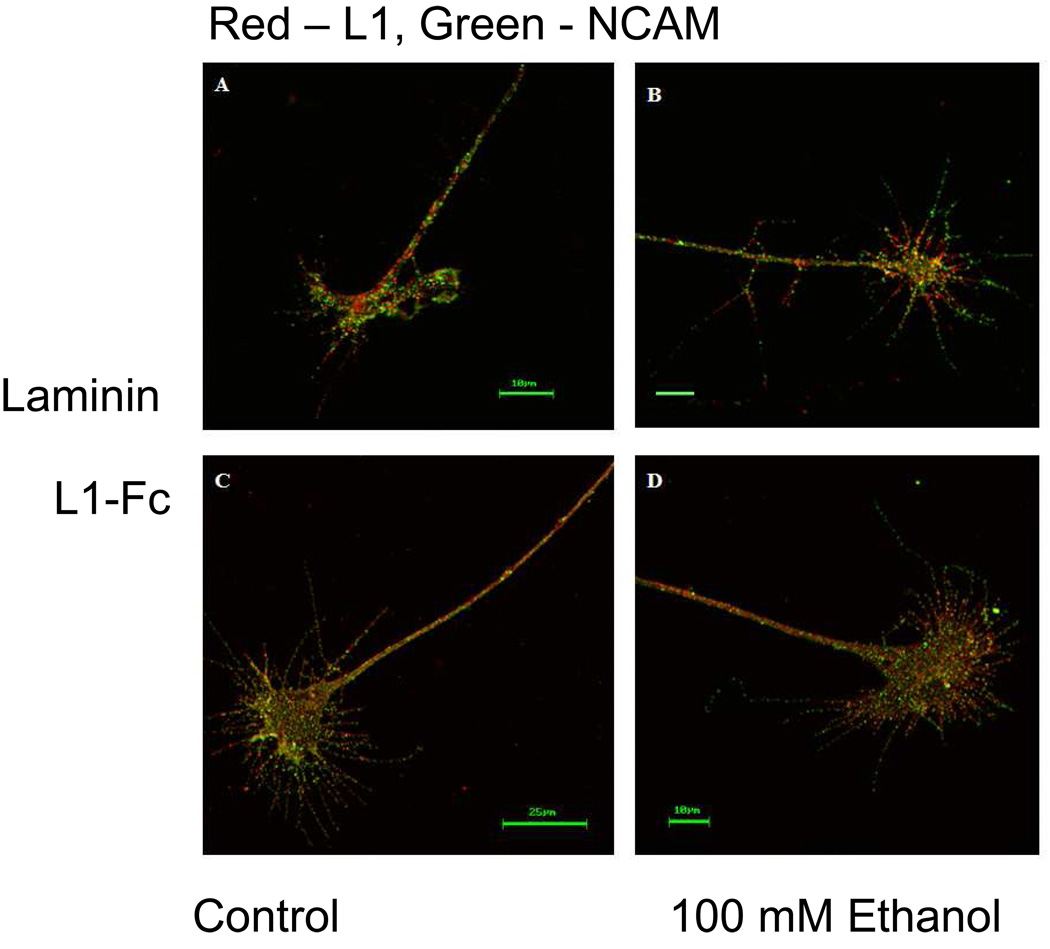
To determine if ethanol disrupts intracellular transport of L1 from the cell body to the growth cone, permeabilized DRGs were labeled with a rabbit anti-L1 antibody. L1 distribution was first examined in DRGs propagated on laminin substrate. Neurites growing on the extracellular matrix protein laminin depend upon integrin mediated signaling for neurite extension (Drazba and Lemmon, 1990). Neurites extending upon laminin were highly fasciculated and exhibited a characteristic growth cone morphology (Burden-Gulley, Payne, and Lemmon, 1995; Nakai and Kamiguchi, 2002) of small lamellipodial domains from which long filopodia extend. L1 was similarly distributed within the growth cone lamellipodia and filopodia in both the no ethanol control group (Figure 1A) and the ethanol group (Figure 1B). L1 was present in all 111 control and 152 ethanol exposed growth cones. The results pooled from 11 separate experiments are summarized in Table 1.
Fig. 1.
Exposure to ethanol does not affect growth cone distribution of total L1. (A and B) Representative DRG growth cones adherent to laminin substrate. Confocal images show the total L1 present at the growth cone in red and the total NCAM in green. L1 distribution in the no ethanol control growth cone (A) is similar to the L1 distribution found in the growth cone exposed to 100 mM ethanol (B). (C and D) DRG growth cones adherent to L1-Fc substrate exhibit a different morphology compared to those propagated on laminin. Despite this difference, the distribution of total L1 is the same for the no ethanol control growth cone (C) and the 100 mM exposed growth cone (D).
Table 1.
Ethanol does not alter the distribution of L1 at the growth cone.
| Experiment Type | Control | Ethanol | ||
|---|---|---|---|---|
| Cells counted | Cells with L1 in growth cone (%) |
Cells counted | Cells with L1 in growth cone (%) |
|
|
Total L1/ Laminin Substrate n=11 |
111 | 111 (100%) | 152 | 152 (100%) |
|
Total L1/ L1-Fc Substrate n=3 |
91 | 90 (98.9%) | 97 | 96 (99.0%) |
|
Extracellular L1/ L1-Fc Substrate n=3 |
85 | 85 (100%) | 94 | 94 (100%) |
Next, the distribution of L1 on growth cones of DRGs cultured on L1-Fc substrate was examined. These neurites extended in a defasciculated pattern and growth cones exhibited broader lamellipodia and shorter filopodia compared to DRG grown on laminin, as previously reported (Burden-Gulley, Payne, and Lemmon, 1995; Nakai and Kamiguchi, 2002). Once again, L1 was distributed throughout all areas of nearly all growth cones in both the control (Figure 1C) and ethanol exposed groups (Figure 1D) (Table 1).
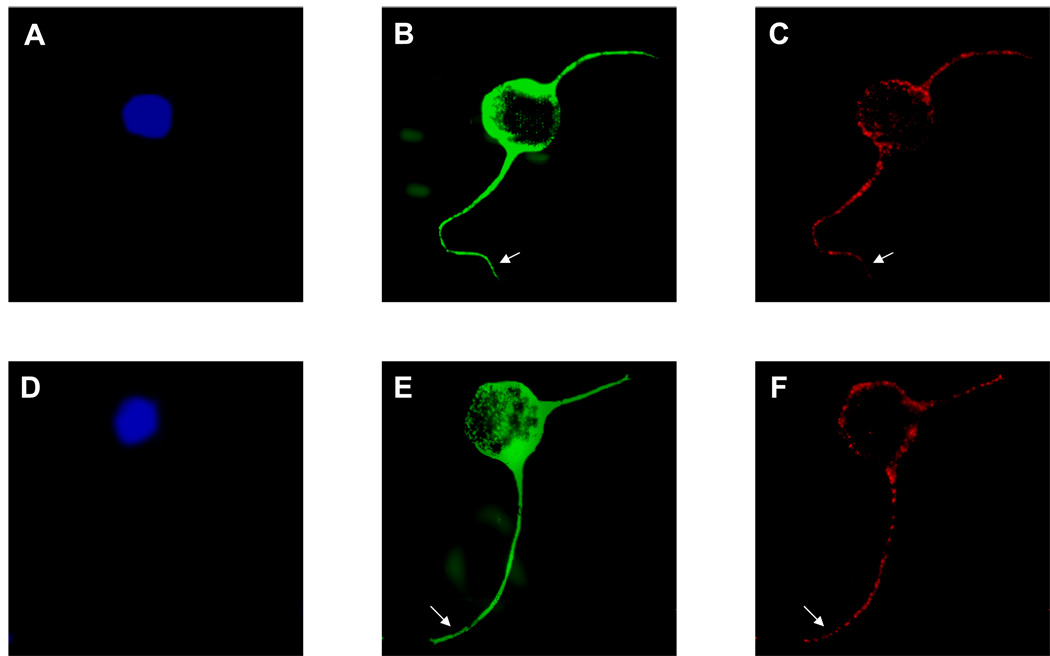
To confirm these results in CGN, CGN were plated on L1-Fc, and exposed to 25mM ethanol for 1h. Growing tips of neurites were identified by anti-beta tubulin. L1 was present in all tips of all neurites examined (Figure 2).
Fig. 2.
Supplemental Exposure to ethanol does not affect growth cone distribution of total L1 in CGN. A representative cell from ethanol exposed and control experiments is shown. Cell bodies were identified by DAPI (A, D). The immunocytochemical distribution of L1 (red, B, E) and beta tubulin (C, F) was examined using confocal images of CGN cultured on L1-Fc overnight. No difference between L1 distribution along the tips of the neuritis (arrows) of CGN exposed to 25mM ethanol (A, B and C) and that of control (D, E and F) can be detected.
Although these results show that ethanol did not disrupt the transportation of L1 to the growth cone, it is unclear whether ethanol disrupted the insertion of L1 into the cell membrane. Proper membrane insertion is critical for L1-mediated growth cone motility which involves endocytosis and recycling of L1 back to the leading edge of the growth cone (Kamiguchi and Lemmon, 2000).
Applying primary antibody to living cells allows localization of cell surface antigens
To examine the effect of ethanol on the proper insertion of L1 into plasma membranes, antibodies were applied to living cells to determine whether the extracellular domain of L1 was present on the surface of growth cones.
To differentiate between an external or internal location of an antigen, primary antibodies were applied to living cells prior to fixation. The cell cultures were maintained at 4°C over a 30 minute incubation period to suspend cellular activity thereby preventing internalization of cell surface molecules. Membrane integrity was tested using antibodies against the intracellular protein MAP-2. If the cell membrane is not disrupted, MAP-2 will not be visualized by immunohistochemistry.
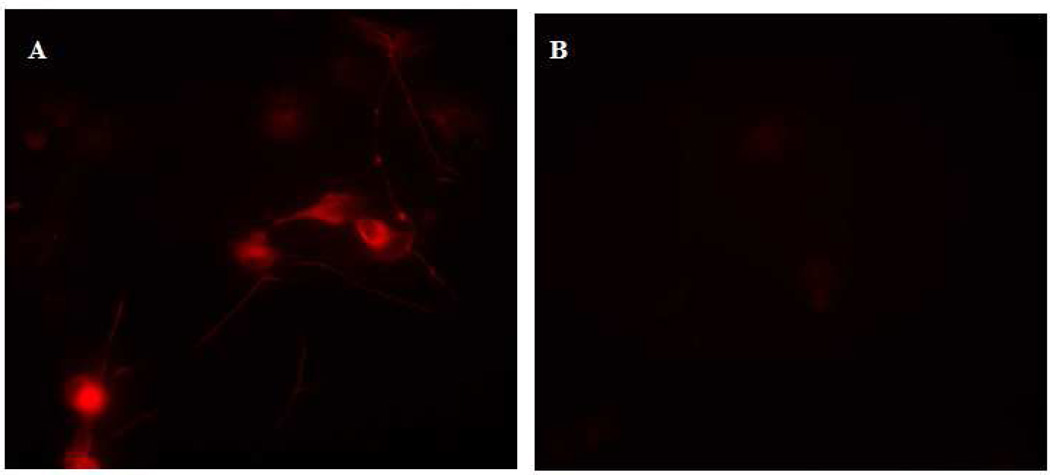
Control DRG cell cultures were fixed with 4% paraformaldehyde for 15 minutes, followed by application of mouse anti-MAP-2. The fixation process caused enough disruption of the cell membrane to allow binding of the primary antibody to MAP-2 antigen, as visualized after the application of Texas red goat anti-mouse (Figure 3A). The DRG cell cultures undergoing primary antibody application while still alive were cooled to 4°C, incubated with primary antibody, washed repeatedly with cold growth media, fixed with 4% paraformaldehyde, and finally labeled with Texas red goat anti-mouse secondary antibody. In contrast to the DRG cultures that were fixed prior to antibody application, anti-MAP-2 could not bind to internal antigen when applied to living DRG cells. Since MAP-2 is solely intracellular, it was not visualized by this method (Figure 3B).
Fig. 3.
Supplemental Application of antibody prior to cell fixation selectively targets extracellular antigen. Slides of DRG cells were prepared by the standard immunocytochemistry method of fixation with 4% paraformaldehyde followed by antibody application. (A) Intracellular MAP-2 is present in the DRG cell bodies and proximal axons. Fixation caused enough disruption of the cell membrane to enable antibody attachment to MAP-2. (B) The application of antibody to cooled DRG cells before fixation labels only proteins with extracellular domains. Because antibody-antigen binding occurred before fixation perturbed cell membrane integrity, intracellular MAP-2 is not labeled and cannot be visualized.
The L1 present at the growth cone surface is not changed by the presence of ethanol
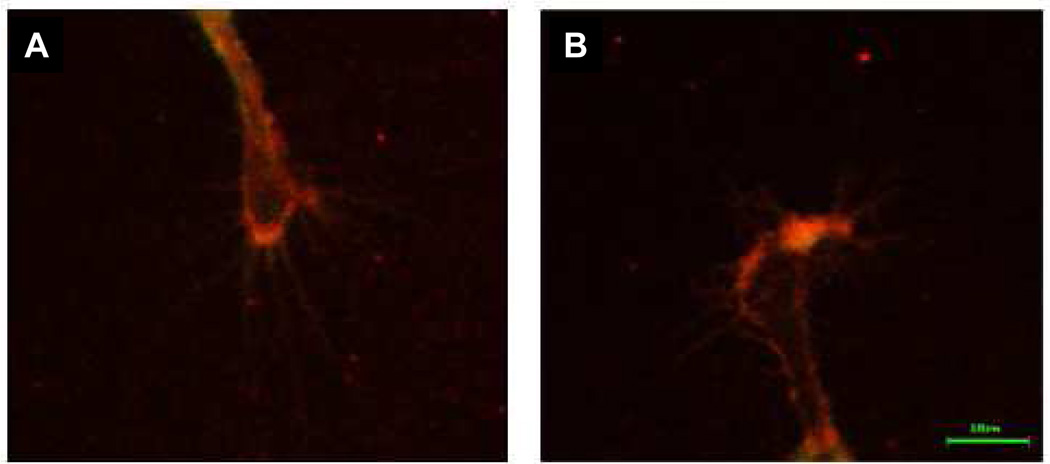
When DRG cultures grown on L1-Fc substrate were labeled at 4°C with rabbit anti-L1 (8D9) L1 was found to be present on the surface of growth cones. DRGs were grown on 0.6 µg/cm2 L1-Fc to minimize background interference. The distribution pattern of cell surface L1 was similar in both ethanol and control samples (Table 1). Immunofluorescence was prominent at the P domain of the growth cone, or leading edge, where L1 is active in neuronal guidance (Kamiguchi and Lemmon, 1998; Nakai and Kamiguchi, 2002). L1 was observed at the growth cone in the control cells as well as in the ethanol exposed cells (Figure 4A, B).
Fig. 4.
L1 is present at the cell surface of DRG growth cones propagated on L1-Fc substrate. Cell surface L1 was prominent at the leading edge of the growth cone during L1 mediated neurite outgrowth. There was no difference in surface L1 distribution between the control growth cones (A) and the 100 mM ethanol exposed growth cones (B).
Ethanol inhibits the dephosphorylation of the YRSL domain of L1
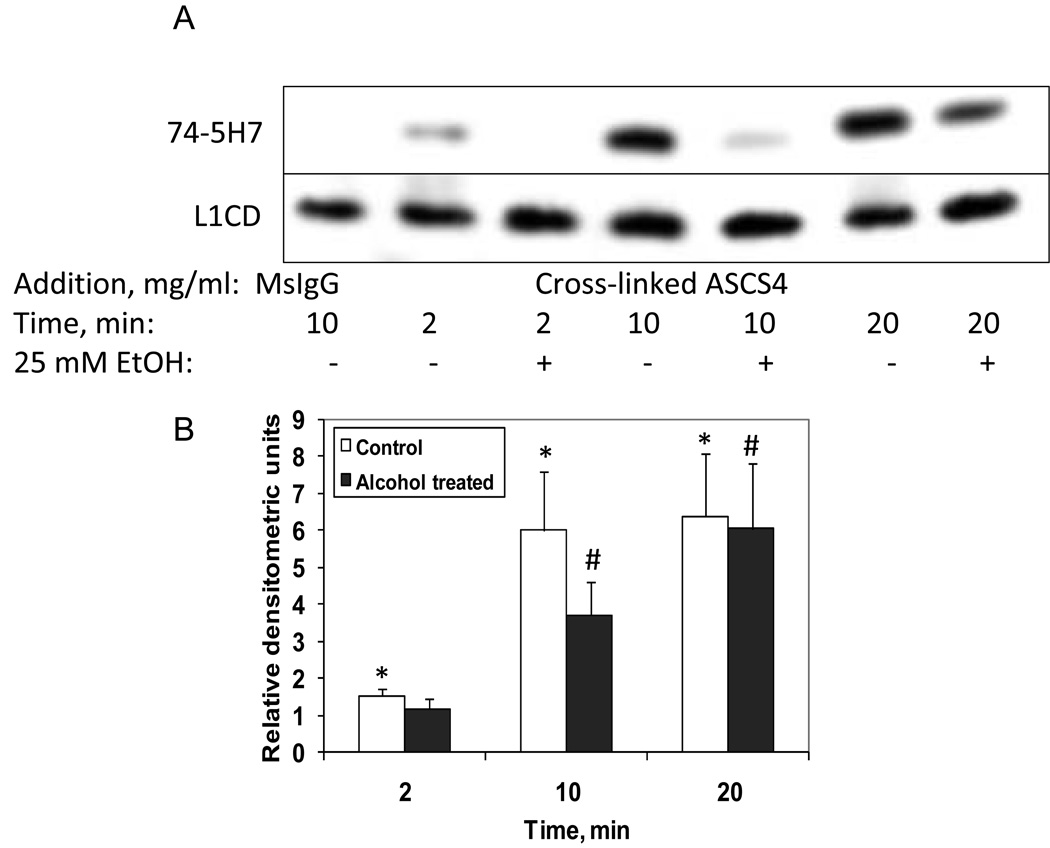
The targeting of L1 to the plasma membrane is regulated by the dephosphorylation of the YRSL domain of L1, which can be identified by the monoclonal antibody, 74-5H7 (Schaefer, Kamei, Kamiguchi, Wong, Rapoport, Kirchhausen, Beach, Landreth, Lemmon, and Lemmon, 2002). In DRGs sparsely cultured on laminin, no 74-5H7 immunoreactivity was observed. However, following L1 clustering on the cell surface using a polyclonal anti-L1 antibody, a rapid appearance of 74-5H7 immunoreactivity was observed (Schaefer, Kamei, Kamiguchi, Wong, Rapoport, Kirchhausen, Beach, Landreth, Lemmon, and Lemmon, 2002). These results indicate that the tyrosine of the YRSL is constitutively phosphorylated for axonal sorting to the plasma membrane since no 74-5H7 immunoreactivity is seen prior to L1 clustering. However, following L1 activation on the cell surface, a dephosphorylation of Y1176 occurs. To test whether ethanol interferes with this dephosphorylation, CGN were grown on poly L-lysine overnight in serum containing media, then serum starved for 2 hours prior to clustering of cell surface L1. In some cases, 25 mM ethanol was added for 1 hour prior to L1 clustering. As can be seen in Figure 5A, B, in the absence of L1 clustering, CGN had minimal 74-5H7 immunoreactivity (Mouse IgG control), similar to that reported for DRGs (Schaefer, Kamei, Kamiguchi, Wong, Rapoport, Kirchhausen, Beach, Landreth, Lemmon, and Lemmon, 2002). However, following addition of cross-linked ASCS4 to induce clustering of L1, 74-5H7 immunoreactivity was initially observed at 2 min with a sustained peak at 10 – 20 min, a time course similar to that seen in DRGs (Schaefer, Kamei, Kamiguchi, Wong, Rapoport, Kirchhausen, Beach, Landreth, Lemmon, and Lemmon, 2002). In the cells pretreated for 1 hour with 25 mM ethanol, a reduction in 74-5H7 immunoreactivity was observed at both 10 and 20 min, but more marked at 10 min (Figure 5A, B). At 20 min, the difference between 74-5H7 immunoreactivity in control and ethanol exposed cells was less, indicating that ethanol’s effect was primarily a delay in the dephosphorylation of Y1176.
Fig. 5.
Y1176 is dephosphorylated by addition of cross-linked ASCS4 and is inhibited by ethanol. (A) Serum starved CGN were treated with 25 mM ethanol for 1 hour, then either mouse IgG, or ASCS4 was added for 2, 10 or 20 minutes. Dephosphorylation of Y1176 was assayed by Western blot analysis. Western blots showed that addition of cross-linked ASCS4 significantly increased dephosphorylation of Y1176 (74-5H7) at 2, 10 and 20 minutes in the absence of ethanol (No Alcohol). Significant inhibition of ASCS4 mediated Y1176 dephosphorylation occurred in ethanol pretreated cells (1 of 4 representative blots shown) at 10 and 20 minutes (Alcohol treated). (B) Dephosphorylation of Y1176 is significantly increased at 2, 10 and 20 min following addition of cross-linked ASCS4. One hour pretreatment with 25 mM ethanol significantly reduces dephosphorylation of Y1176 at 10 and 20 minutes, although it remains significantly increased over MsIgG at 10 and 20 minutes (Alcohol treated). Densitometric quantification of Y1176 dephosphorylation corrected for total L1 shown in A is plotted as relative densitometric units relative to the MsIgG control. The values of four separate experiments are shown. The bar indicates the mean of the values +/− S.E. The single asterisk (*) indicates a statistically significant increase in dephosphorylated Y1176 following addition of cross-linked ASCS4 (No Alcohol) versus MsIgG (p<0.05, paired t test). The number sign (#) indicates a statistically significant decrease in Y1176 dephosphorylation following alcohol pretreatment (Alcohol treated) (p<0.05, paired t test).
Ethanol inhibits L1 tyrosine phosphorylation
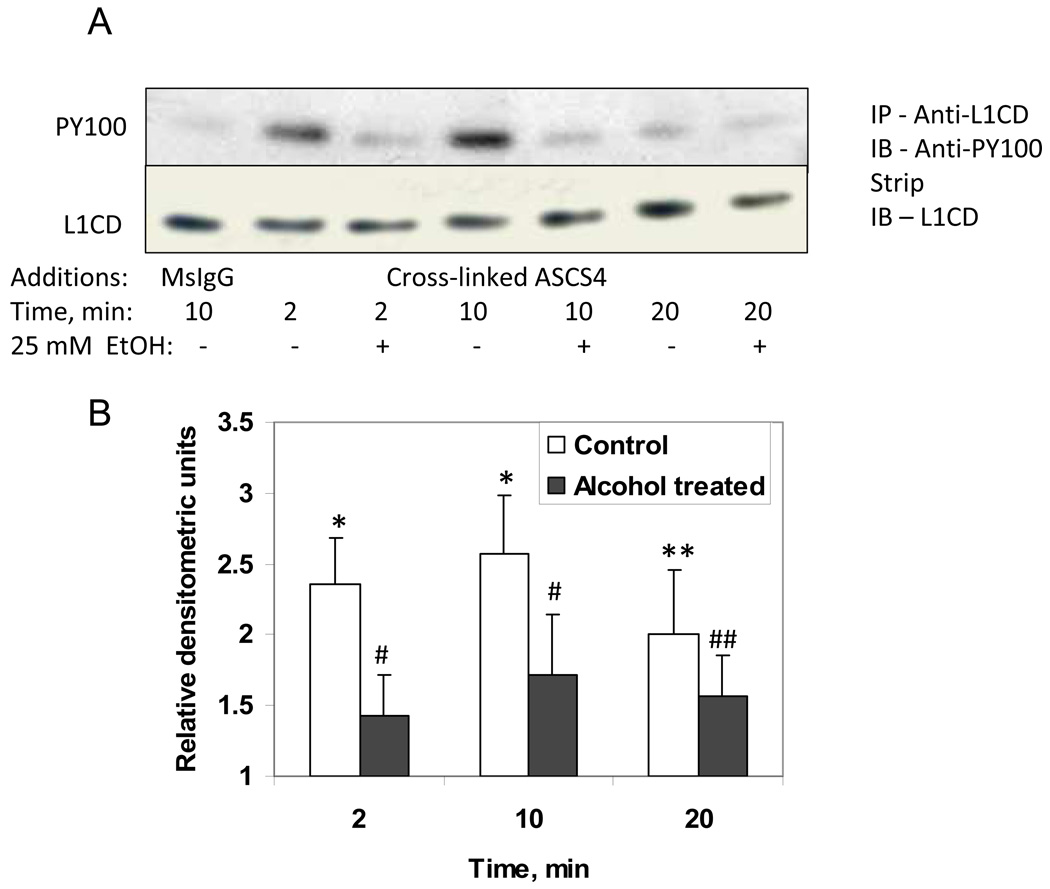
Tyrosine Y1229 of L1 is also phosphorylated/dephosphorylated to regulate binding of L1 to ankyrin (Garver, Ren, Tuvia, and Bennett, 1997). Regulation of L1 binding to ERM and ankyrin may be regulated by phosphorylation/dephosphorylation of tyrosines 1151 and 1211, respectively. To determine whether ethanol influences tyrosine phosphorylation following clustering of L1, we added cross-linked ASCS4 to serum starved CGN, and measured tyrosine phosphorylation by using anti-phosphotyrosine monoclonal antibodies on immunoblots of immunoprecipitated L1. Tyrosine phosphorylation was quantified by stripping blots and reprobing for total L1 using anti-L1CD. Values were normalized to controls. The results in Figure 6 show that the addition of cross-linked ASCS4 significantly increased tyrosine phosphorylation of L1, and that the addition of 25 mM ethanol 1 hour prior to addition of ASCS4 blocked this response. Interestingly, the tyrosine phosphorylation appears to occur prior to the dephosphorylation of Y1176. It also appears that the anti-PY antibody does not recognize Y1176 since in controls, Y1176 is constitutively phosphorylation (i.e. is not recognized by 74-5H7, see Figure 5) but there is little phosphotyrosine detected by anti-PY in control cells (see Figure 6).
Fig. 6.
Tyrosine phosphorylation is increased by addition of cross-linked ASCS4 and is blocked by ethanol. (A) Serum starved CGN were treated with 25 mM ethanol for 1 hour, then ASCS4 was added for 2, 10 or 20 minutes. Control cells were harvested 10 min after addition of MsIgG. Tyrosine phosphorylation was assayed by immunoprecipitation of L1 followed by Western blot analysis for phosphotyrosine (PY100). Blots were stripped and reprobed with anti-L1CD as a loading control. Western blots showed that addition of cross-linked ASCS4 significantly increased tyrosine phosphorylation at 2, 10 and 20 minutes in the absence of ethanol (No Alcohol). No increase in tyrosine phosphorylation was seen in the presence of ethanol (Alcohol treated). (B) Densitometric quantification of tyrosine phosphorylation corrected for total L1 shown in A is plotted as relative densitometric units relative to the MsIgG control. The values of four separate experiments are shown. The bar indicates the mean of the values +/− S.E. The single and double asterisks indicate statistically significant increase in tyrosine phosphorylation following addition of cross-linked ASCS4 versus the MsIgG control (*, p<0.02; **, p<0.05, paired t test). The single and double number signs indicate statistically significant decreases in tyrosine phosphorylation following alcohol pretreatment (Alcohol treated) (#,p<0.005; ##, p<0.05, paired t test). There was no significant difference in tyrosine phosphorylation at any time point from the MsIgG control in the presence of ethanol (Alcohol treated).
Ethanol inhibits L1 mediated activation of p60src
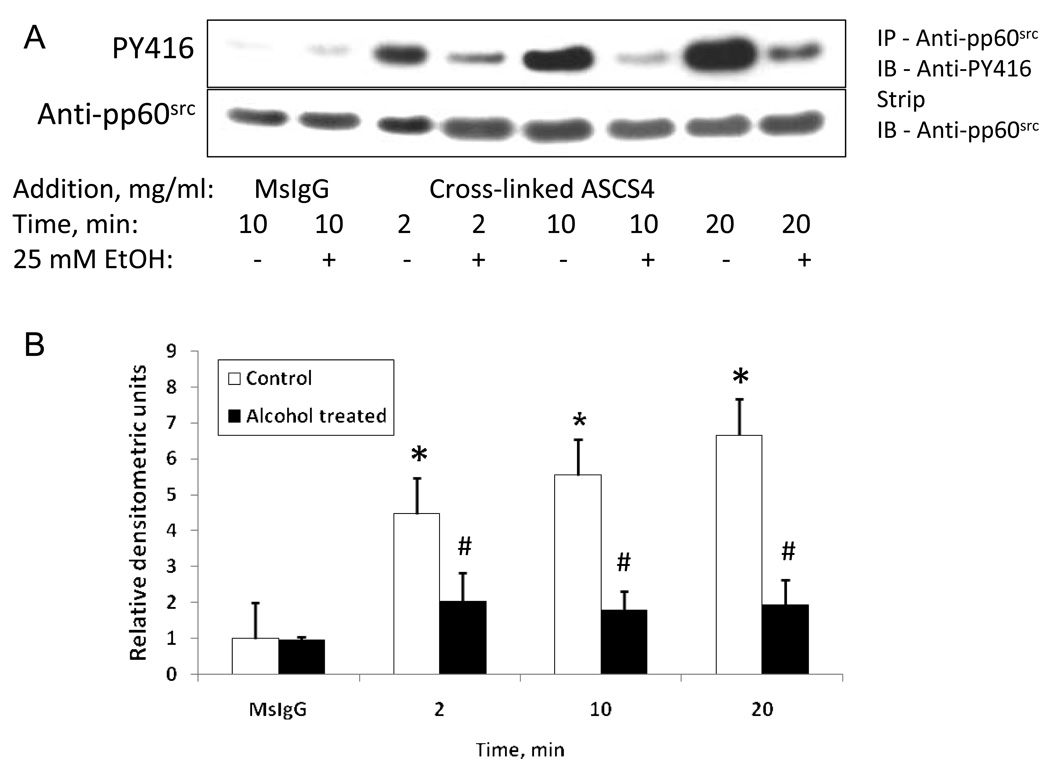
pp60src is activated following the clustering of L1 (Schmid, Pruitt, and Maness, 2000) which leads to rephosphorylation of Y1176 (Schmid, Pruitt, and Maness, 2000). To determine whether ethanol influences pp60src activation following clustering of L1, we added cross-linked ASCS4 to serum starved CGN, and measured activated pp60src by using polyclonal antibodies to phospho-src (Tyr416) on immunoblots of immunoprecipitated pp60src. Activated pp60src was quantified by stripping blots and reprobing for total pp60src. Values were normalized to controls. Results in Figure 7 show that the addition of cross-linked ASCS4 significantly increased tyrosine 416 phosphorylation of pp60src, and that the addition of 25 mM ethanol 1 hour prior to addition of ASCS4 blocked this response. The activation of pp60src, like L1 tyrosine phosphorylation, appears to occur prior to the dephosphorylation of Y1176, suggesting that pp60src is activated prior to the endocytosis of L1. These findings are consistent with those previously reported (Schmid, Pruitt, and Maness, 2000).
Fig. 7.
pp60src is activated by addition of cross-linked ASCS4 and is blocked by ethanol. (A) Serum starved CGN were treated with 25 mM ethanol for 1 hour, then ASCS4 was added for 2, 10 or 20 minutes. Control cells were harvested 10 min after addition of MsIgG. Activated pp60src was assayed by immunoprecipitation of pp60src followed by Western blot analysis for phospho-Src family (Tyr416) (PY416). Blots were stripped and reprobed with anti- pp60src as a loading control. Western blots showed that addition of cross-linked ASCS4 significantly increased activation of pp60src at 2, 10 and 20 minutes in the absence of ethanol (No Alcohol). No increase in activated pp60src was seen in the presence of ethanol (Alcohol treated). One of 3 representative blots shown. (B) Densitometric quantification of PY416 corrected for total pp60src shown in A is plotted as relative densitometric units relative to the MsIgG control. The values of three separate experiments are shown. The bar indicates the mean of the values +/− S.E. The single asterisks indicate statistically significant increases in pp60src tyrosine phosphorylation following addition of crosslinked ASCS4 versus MsIgG control (* p<0.05, paired t test). The number signs indicate statistically significant decreases in pp60src PY416 following alcohol pretreatment (# p<0.05, paired t test). There was no significant difference between pp60src tyrosine phosphorylation at any time point compared to the MsIgG control in the presence of ethanol.
Order of signaling cascade downstream from L1
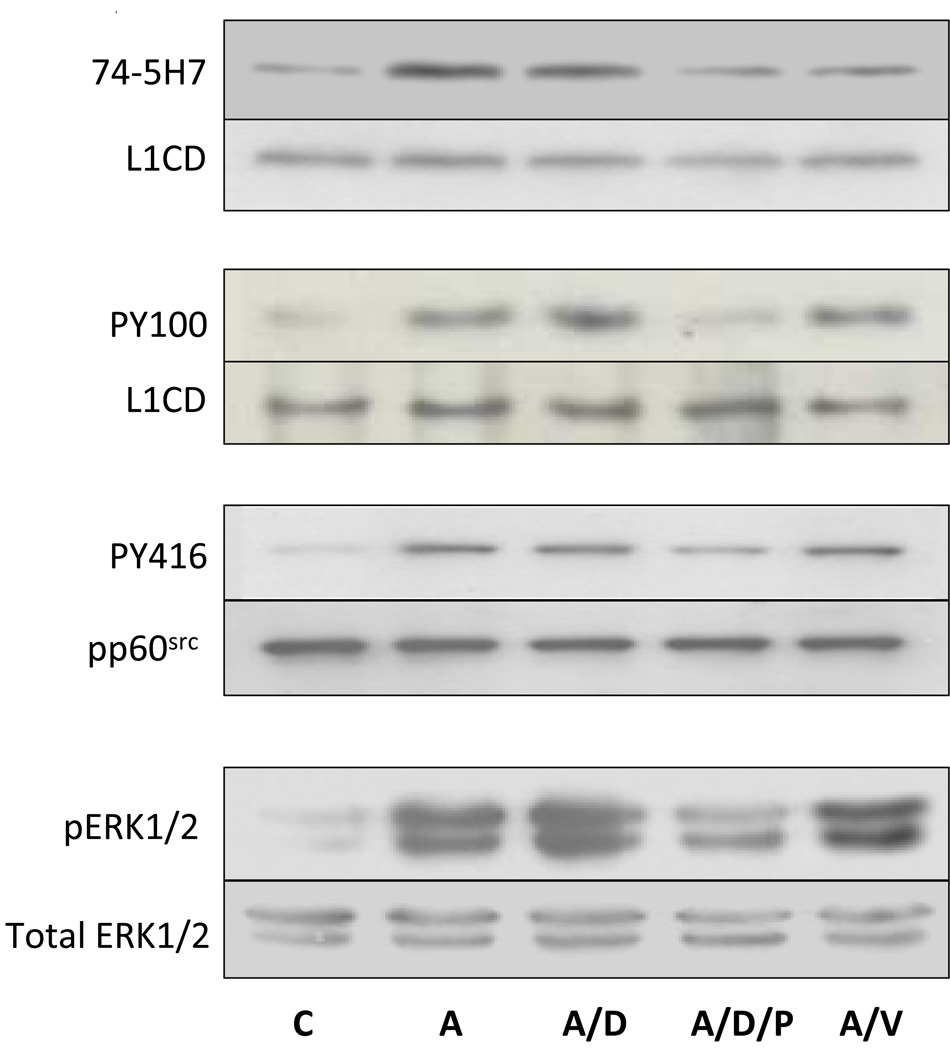
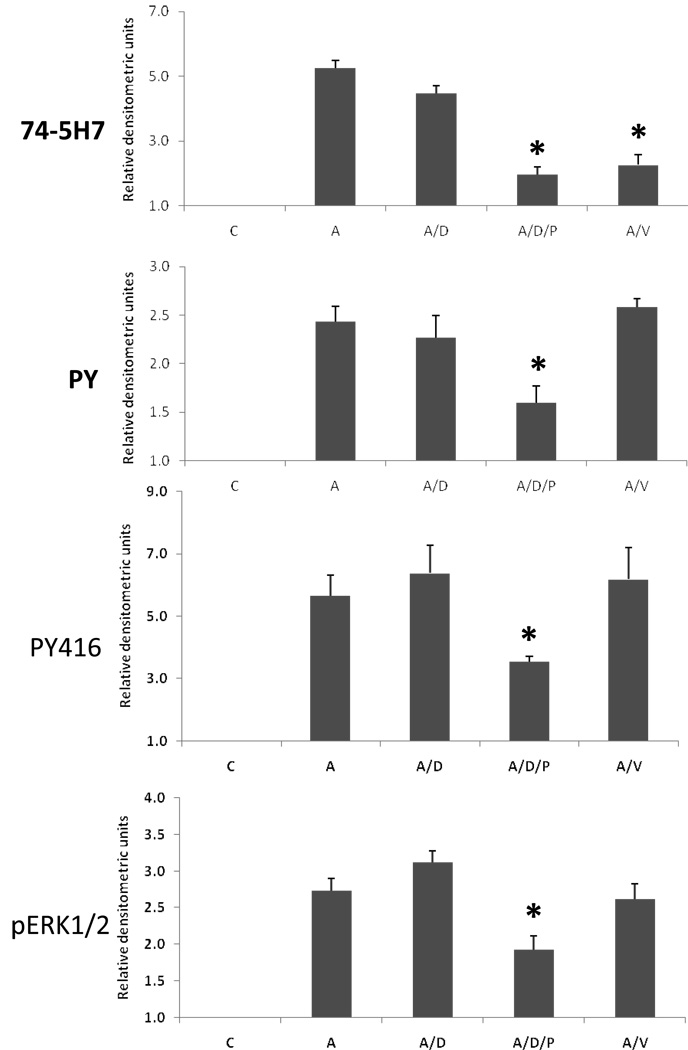
We have now shown that ethanol inhibits L1 mediated dephosphorylation of Y1176, tyrosine phosphorylation of L1 and activation of both pp60src and ERK1/2 (Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006). In order to delineate what was a direct effect of ethanol rather than a secondary effect, we sought to determine the order of these signaling events. The data presented in Figure 5, Figure 6, and Figure 7 and our previous findings with ERK1/2(Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006) suggest that pp60src activation and tyrosine phosphorylation of L1 precede dephosphorylation of L1 and ERK1/2 activation. Using PP2 to selectively inhibit src kinases and sodium vanadate to inhibit phosphatases, L1 was activated by cross-linked ASCS4. The dephosphorylation of Y1176, tyrosine phosphorylation of L1, and the activation of pp60src and ERK1/2 were measured. The results are shown in Figure 8. Addition of DMSO, the vehicle for PP2, had no effect on any reaction. As expected, the addition of DMSO/PP2 and vanadate resulted in significant inhibition of activation of pp60src and dephosphorylation of Y1176 respectively. Addition of DMSO/PP2 also inhibited the dephosphorylation of Y1176, tyrosine phosphorylation of L1, and activation of ERK1/2. In contrast, addition of vanadate had no effect on activation of pp60src, tyrosine phosphorylation of L1, and activation of ERK1/2. However, lack of inhibition of tyrosine kinase activities in the presence of a phosphatase inhibitor is difficult to interpret as vanadate may increase phosphorylation by itself. These results are consistent with the activation of pp60src as the proximal step in L1 downstream signaling. Hence, inhibition of the L1 activation of pp60src by ethanol could account for the results presented here.
Fig. 8.
pp60src activation is the proximal step in downstream signaling of L1. (A) Serum starved CGN were activated with crosslinked ASCS4 for 10 minutes following a 1 hour treatment with nothing (A), DMSO (A/D), DMS with PP2 (A/D/P) or sodium vanadate (A/V). Control cells (C) were harvested 10 min after addition of MsIgG. Dephosphorylation of Y1176 (74-5H7), tyrosine phosphorylation of L1 (PY100) and activation of pp60src (PY416) were assayed as described in Fig. 5, Fig. 6 and Fig. 7. The MAPK ERK1/2 was assayed by immunoblot of cell lysates with phosphop44/42 MAP kinase (Thr202/Tyr204) (pERK1/2). Blots were stripped and reprobed with p44/42 MAP kinase (total ERK1/2) as a loading control. One of 3 representative blots shown. (B) Densitometric quantification of immunoblots shown in A are plotted as relative densitometric units relative to the control. The values of three separate experiments are shown. The bar indicates the mean of the values +/− S.E. The single asterisks indicate statistically significant decreases the ASCS4 alone control (A) (* p<0.05, paired t test).
DISCUSSION
This study yielded several important new findings: 1) In DRGs, ethanol had no effect on either sorting of L1 to the growth cone, or the insertion of L1 into the growth cone membrane; 2) L1 in CGN is constitutively phosphorylated on the tyrosine of the YRSL sorting signal as observed in DRGs; 3) Clustering of L1 on the surface of CGN results in a dephosphorylation of Y1176 with a time course similar to that of DRGs. 4) Clustering of L1 increases L1 tyrosine phosphorylation and activation of pp60src; 5) 25 mM ethanol dramatically reduces these signaling events; 6) pp60src is proximal in the downstream signaling cascade of L1. These results are consistent with our previous findings that ethanol inhibits the phosphorylation of ERK1/2 induced by L1 clustering (Tang, He, O'Riordan, Farkas, Buck, Lemmon, and Bearer, 2006). Transfection with dominant negative src inhibits L1 endocytosis and L1 mediated activation of ERK1/2(Schmid, Pruitt, and Maness, 2000). L1 mediated activation of ERK1/2(Schmid, Pruitt, and Maness, 2000) and L1 mediated neurite outgrowth(Ignelzi, Miller, Soriano, and Maness, 1994) are also inhibited in cerebellar granule cells homozygous for src gene knockout. PP1, a src inhibitor, has been shown to inhibit L1 activation of ERK1/2(Schaefer, Kamiguchi, Wong, Beach, Landreth, and Lemmon, 1999). If ethanol inhibits L1 activation of pp60src, then dephosphorylation of the Y1176, endocytosis and MAPK activation would decrease. These effects should dramatically inhibit L1-mediated neurite outgrowth and produce the the types of neurodevelopmental abnormalities observed in fetal alcohol syndrome.
Neurite outgrowth on laminin was not inhibited by ethanol (Bearer, Swick, O'Riordan, and Cheng, 1999a). A disruption by ethanol of L1 mediated activation of pp60src may be the underlying reason for the specificity of the ethanol effect on neurite outgrowth. CGN from wild type or src−/− mice have the same neurite outgrowth when plated on laminin(Ignelzi, Miller, Soriano, and Maness, 1994) showing the independence from pp60src of laminin mediated neurite outgrowth. These observations are consistent with the hypothesis that disruption by ethanol of pp60src activation by L1 is the critical site of ethanol action.
The inhibition by ethanol of pp60src may occur by several different mechanisms: 1) By an inhibition of pp60src independent of L1, or 2) by disrupting the protein-protein interactions necessary for L1 signaling to activate pp60src.
Ethanol has been previously shown to prevent src activation leading to increased endocytosis of the NR2 subunit of the NMDA receptor in a hippocampal slice preparation (Suvarna, Borgland, Wang, Phamluong, Auberson, Bonci, and Ron, 2005). The inhibition was felt to be secondary to activation of H-Ras by ethanol. However, in a hippocampal slice, many signaling events could be occurring at baseline. Inhibition of any of these pathways may also reduce src activation. H-Ras is not involved in L1 signaling (Schmid, Pruitt, and Maness, 2000) and ethanol treatment alone did not affect levels of activated pp60src in our CGN preparation. Ethanol may act directly on the enzymes catalyzing tyrosine phosphorylation-dephosphorylation of tyrosine 416 and tyrosine 529 of src. Ethanol alters tyrosine phosphorylation in a number of systems examined including the NMDA receptor (Alvestad, Grosshans, Coultrap, Nakazawa, Yamamoto, and Browning, 2003; Ferrani-Kile, Randall, and Leslie, 2003), the insulin receptor (Seiler, Henderson, and Rubin, 2000), the insulin-like growth factor-1 receptor (Hallak, Seiler, Green, Henderson, Ross, and Rubin, 2001), and both Cas and Cbl (Nishio and Suzuki, 2002; Nishio, Otsuka, Kinoshita, Tokuoka, Nakajima, Noda, Fukuyama, and Suzuki, 2002). For the NMDA receptor, ethanol both increased the activity of tyrosine phosphatases as well as increased tyrosine kinases, whereas ethanol inhibited the tyrosine kinase activity of the insulin-like growth factor-1 receptor.
Secondly, ethanol may act by inhibiting the events between L1 activation and activation of pp60src such as trafficking to a compartment containing both L1 and src. Lipid rafts may be one such compartment. Lipid rafts are platforms for bringing together signaling molecules. Recently, L1 has been found associated with lipid rafts (Nakai and Kamiguchi, 2002). Localized perturbation of the lipid rafts in the P domain, but not the C domain of the growth cone reduced neurite outgrowth on an L1 substrate. It may be that ethanol inhibits L1 from trafficking through lipid rafts, and hence not interacting with the pp60src, a known resident of lipid rafts (Encinas, Crowder, Milbrandt, and Johnson, 2004). Three papers have recently been published examining the effect of ethanol on the partitioning of specific proteins in lipid rafts. Ethanol blocked the LPS redistribution of CD14 to a lower density fraction of the lipid raft (Dai, Zhang, and Pruett, 2005). Ethanol inhibited the LPS mediated redistribution of toll-like receptor 4 to the lipid raft, but had no effect on the peptidoglycan mediated redistribution of toll-like receptor 2 (Dolganiuc, Bakis, Kodys, Mandrekar, and Szabo, 2006). Insulin treatment of adipocytes from rats recruited cCbl and TC10 to lipid rafts. However, in adipocytes from rats chronically fed ethanol, insulin treatment did not translocate cCbl and TC10 to the lipid raft (Sebastian and Nagy, 2005). L1-mediated neurite outgrowth may be dependent on protein-protein interactions in the lipid raft because recent data suggests that the cytoplasmic domain of L1 may not be critical for this function (Cheng and Lemmon, 2004).
Third, ethanol may inhibit the direct interaction of proteins such as that of receptors with scaffolding proteins. One recently reported example is that of Fyn kinase, a src family member, and the NMDA receptor. Fyn kinase is targeted to the NMDA receptor by the scaffolding protein RACK1. During acute exposure to ethanol, RACK1 dissociates from the Fyn kinase/NMDA receptor complex, facilitating tyrosine phosphorylation of the receptor (Yaka, Phamluong, and Ron, 2003). In our system, ethanol may be disrupting the protein-protein interactions necessary to phosphorylate tyrosines in src.
Our results implicate that the major effect of ethanol on L1 is not on the sorting signal itself, but on its activation of pp60src. Inhibition of pp60src activation by L1 would inhibit subsequent L1 dephosphorylation, endocytosis, ERK activation, and, therefore, neurite outgrowth. These results suggest that the neurodevelopmental abnormalities observed in fetal alcholol syndrome could result from ethanol’s effects on L1-mediated axon outgrowth.
ACKNOWLEDGEMENTS
We wish to thank H. Kamiguchi, M. Pendergast, and D. Major for their assistance in performing these studies. This work was supported by National Institutes of Health Grants AA-11839 and AA-016398 to C.F. Bearer and HD-039884 to V. Lemmon. C.F. Bearer holds the Cobey Professorship in Neonatology. V. Lemmon holds the Walter G. Ross Chair in Developmental Neuroscience at the Univ. of Miami.
The abbreviations used are
- CGN
cerebellar granule neurons
- L1
L1 cell adhesion molecule
- ERK1/2
extracellular receptor kinases 1/2
- NCAM
neural cell adhesion molecule
- MAP-2
microtubule associated protein-2
- L1CD
the cytoplasmic domain of L1
- DRG
dorsal root ganglions
- PBS
phosphate buffered saline
- FBS
fetal bovine serum
- NICHD
National Institute of Child Health and Development
- DMEM
Dulbecco’s Modified Eagle’s Medium
- BSA
bovine serum albumin
- HBSS
Hank’s balanced salt solution
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- HS
horse serum
- L1-Fc
a chimeric protein consisting of the extracellular domain of L1 and the Fc domain of IgG
- HRP
horse radish peroxidase
- MAPK
mitogen activated protein kinases
- PY416
phospho-src family (Tyr416) antibody
- ERM
ezrin-radixin-moesin
- NMDA
N-methyl-D-aspartic acid
- RACK1
a Receptor for Activated C Kinase 1
References
- Abel EL. The role of dietary fat in alcohol's prenatal effects. Alcohol. 2000;20:83–86. doi: 10.1016/s0741-8329(99)00059-2. [DOI] [PubMed] [Google Scholar]
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. Tyrosine dephosphorylation and ethanol inhibition of N-Methyl-D-aspartate receptor function. Journal of Biological Chemistry. 2003;278:11020–11025. doi: 10.1074/jbc.M210167200. [DOI] [PubMed] [Google Scholar]
- Bearer CF. Mechanisms of brain injury: L1 cell adhesion molecule as a target for ethanol-induced prenatal brain injury. Seminars in Pediatric Neurology. 2001;8(2):100–107. doi: 10.1053/spen.2001.25227. [DOI] [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. Journal of Biological Chemistry. 1999a;274(19):13264–13270. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. Journal of Biological Chemistry. 1999b;274(19):13264–13270. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DM, Paiva M, Tonjes LA, Heaton MB. In vitro comparison of the effects of ethanol and acetaldehyde on dorsal root ganglion neurons. Alcoholism: Clinical and Experimental Research. 1995;19:1345–1350. doi: 10.1111/j.1530-0277.1995.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Burden-Gulley SM, Payne HR, Lemmon V. Growth cones are actively influenced by substrate-bound adhesion molecules. Journal of Neuroscience. 1995;15:4370–4381. doi: 10.1523/JNEUROSCI.15-06-04370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness M, Safran R, Perides G. Ethanol inhibits neural cell-cell adhesion. Journal of Biological Chemistry. 1994;269:9304–9309. [PubMed] [Google Scholar]
- Cheng L, Itoh K, Lemmon V. L1-mediated branching is regulated by two ezrin-radixin-moesin (ERM)-binding sites, the RSLE region and a novel juxtamembrane ERM-binding region. J. Neurosci. 2005;25(2):395–403. doi: 10.1523/JNEUROSCI.4097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Lemmon V. Pathological missense mutations of neural cell adhesion molecule L1 affect neurite outgrowth and branching on an L1 substrate. Mol. Cell. Neurosci. 2004;27(4):522–530. doi: 10.1016/j.mcn.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Cohen NR, Taylor JSH, Scott LB, Guillery RW, Soriano P, Furley AJW. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Current Biology. 1997;8(1):26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- Craig AM, Wyborski RJ, Banker G. Preferential addition of newly synthesized membrane protein at axonal growth cones. Nature. 1995;375:592–594. doi: 10.1038/375592a0. [DOI] [PubMed] [Google Scholar]
- Dai Q, Zhang J, Pruett SB. Ethanol alters cellular activation and CD14 partitioning in lipid rafts. Biochem. Biophys. Res. Commun. 2005;332(1):37–42. doi: 10.1016/j.bbrc.2005.04.088. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. Journal of Biological Chemistry. 1994;269(44):27163–27166. [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcoholism: Clinical and Experimental Research. 1996;20(1):1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol. Clin. Exp. Res. 2006;30(1):76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Drazba J, Lemmon V. The role of cell adhesion molecules in neurite outgrowth on Muller cells. Developmental Biology. 1990;138:82–93. doi: 10.1016/0012-1606(90)90178-l. [DOI] [PubMed] [Google Scholar]
- Encinas M, Crowder RJ, Milbrandt J, Johnson EM., Jr Tyrosine 981, a novel ret autophosphorylation site, binds c-Src to mediate neuronal survival. J. Biol. Chem. 2004;279(18):18262–18269. doi: 10.1074/jbc.M400505200. [DOI] [PubMed] [Google Scholar]
- Ferrani-Kile K, Randall PK, Leslie SW. Acute ethanol affects phosphorylation state of the NMDA receptor complex: implication of tyrosine phosphatases and protein kinase A. Brain Research: Molecular Brain Research. 2003;115:78–86. doi: 10.1016/s0169-328x(03)00186-4. [DOI] [PubMed] [Google Scholar]
- Fransen E, D'Hooge R, Van Camp G, et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Human Molecular Genetics. 1998;7:999–1009. doi: 10.1093/hmg/7.6.999. [DOI] [PubMed] [Google Scholar]
- Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. Journal of Cell Biology. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil OD, Sakurai T, Bradley AE, Fink MY, Cassella MR, Kuo JA, Felsenfeld DP. Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface. J. Cell Biol. 2003;162(4):719–730. doi: 10.1083/jcb.200211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak H, Seiler AE, Green JS, Henderson A, Ross BN, Rubin R. Inhibition of insulin-like growth factor-I signaling by ethanol in neuronal cells. Alcoholism: Clinical and Experimental Research. 2001;25:1058–1064. [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Ignelzi M, Miller D, Soriano P, Maness P. Impaired neurite outgrowth of src-minus cerebellar neurons on the cell adhesion molecule L1. Neuron. 1994;12:873–884. doi: 10.1016/0896-6273(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Recycling of the cell adhesion molecule L1 in axonal growth cones. Journal of Neuroscience. 2000;20(10):3676–3686. doi: 10.1523/JNEUROSCI.20-10-03676.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. A neuronal form of the cell adhesion molecule L1 contains a tyrosine-based signal required for sorting to the axonal growth cone. Journal of Neuroscience. 1998;18:3749–3756. doi: 10.1523/JNEUROSCI.18-10-03749.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Long KE, Pendergast M, Schaefer AW, Rapoport I, Kirchhausen T, Lemmon V. The neural cell adhesion molecule L1 interacts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway. Journal of Neuroscience. 1998;18:5311–5321. doi: 10.1523/JNEUROSCI.18-14-05311.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proceedings of the National Academy of Science U S A. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Kobayashi M, Asou H, Uyemura K. Molecular cloning of cDNA encoding the rat neural cell adhesion molecule L1. Two L1 isoforms in the cytoplasmic region are produced by differential splicing. FEBS Letters. 1991;289:91–95. doi: 10.1016/0014-5793(91)80915-p. [DOI] [PubMed] [Google Scholar]
- Moos M, Tacke R, Schere H, Teplow D, Fruh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Kamiguchi H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. Journal of Cell Biology. 2002;159(6):1097–1108. doi: 10.1083/jcb.200209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio H, Otsuka M, Kinoshita S, Tokuoka T, Nakajima M, Noda Y, Fukuyama Y, Suzuki K. Phosphorylation of c-Cbl protooncogene product following ethanol administration in rat cerebellum: possible involvement of Fyn kinase. Brain Research. 2002;950:203–209. doi: 10.1016/s0006-8993(02)03038-x. [DOI] [PubMed] [Google Scholar]
- Nishio H, Suzuki K. Ethanol-induced Cas tyrosine phosphorylation and Fyn kinase activation in rat brain. Alcoholism: Clinical and Experimental Research. 2002;26 Suppl 8:38S–43S. doi: 10.1097/01.ALC.0000026832.06336.A1. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1998;22:339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Kamei Y, Kamiguchi H, Wong EV, Rapoport I, Kirchhausen T, Beach CM, Landreth G, Lemmon SK, Lemmon V. L1 endocytosis is controlled by a phosphorylation-dephosphorylation cycle stimulated by outside-in signaling by L1. Journal of Cell Biology. 2002;157(7):1223–1232. doi: 10.1083/jcb.200203024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Kamiguchi H, Wong EV, Beach CM, Landreth G, Lemmon V. Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. Journal of Biological Chemistry. 1999;274(53):37965–37967. doi: 10.1074/jbc.274.53.37965. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Pruitt WM, Maness PF. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. Journal of Neuroscience. 2000;20(11):4177–4188. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian BM, Nagy LE. Decreased insulin-dependent glucose transport by chronic ethanol feeding is associated with dysregulation of the Cbl/TC10 pathway in rat adipocytes. Am. J. Physiol. Endocrinol. Metab. 2005;289(6):E1077–E1084. doi: 10.1152/ajpendo.00296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler AE, Henderson A, Rubin R. Ethanol inhibits insulin receptor tyrosine kinase. Alcoholism: Clinical and Experimental Research. 2000;24:1869–1872. [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- Suvarna N, Borgland SL, Wang J, Phamluong K, Auberson YP, Bonci A, Ron D. Ethanol alters trafficking and functional N-methyl-D-aspartate receptor NR2 subunit ration via H-Ras. Journal of Biological Chemistry. 2005;36:31450–31459. doi: 10.1074/jbc.M504120200. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Asou H, Murakami Y, Miura M, Kobayashi M, Uyemura K. A nonneuronal isoform of cell adhesion molecule L1: tissue-specific expression and functional analysis. Journal of Neurochemistry. 1996;66:2338–2349. doi: 10.1046/j.1471-4159.1996.66062338.x. [DOI] [PubMed] [Google Scholar]
- Tang N, He M, O'Riordan MA, Farkas C, Buck K, Lemmon L, Bearer CF. Ethanol inhibits L1 cell adhesion molecule activation of mitogen-activated protein kinases. Journal of Neurochemistry. 2006;96:1480–1490. doi: 10.1111/j.1471-4159.2006.03649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I, Collawn J, Hopkins C. Signal-dependent membrane protein trafficking in the endocytic pathway. Annual Review of Cell Biology. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Ymazaki M, Miyazaki H, Arikawa C, Itoh K, Sasaki T, Maehama T, Frohman MA, Kanaho Y. Phospholipase D2 functions as a downstream signaling molecule of MAP kinase pathway in L1-stimulated neurite outgrowth of cerebellar granule neurons. Journal of Neurochemistry. 2004;89:142–151. doi: 10.1111/j.1471-4159.2004.02308.x. [DOI] [PubMed] [Google Scholar]
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Folsch H, Winckler B. Uncovering multiple axonal targeting pathways in hippocampal neurons. Journal of Cell Biology. 2003;162:1317–1328. doi: 10.1083/jcb.200307069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. Journal of Neuroscience. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip PM, Zhao X, Montgomery AM, Siu CH. The Arg-Gly-Asp motif in the cell adhesion molecule L1 promotes neurite outgrowth via interaction with the. Molecular and Cellular Biology. 1998;9:277–290. doi: 10.1091/mbc.9.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]