Abstract
The first series of systematically varied C7-functionalized bryostatin analogs (12 members in all) have been synthesized through an efficient and convergent route. A new hotspot for PKC affinity, not present in the natural products, has been discovered, allowing for affinity control and potentially for selective regulation of PKC isozymes. Several analogs exhibit single-digit nanomolar affinity to PKC and display superior activity compared to bryostatin against the leukemia cell line K562.
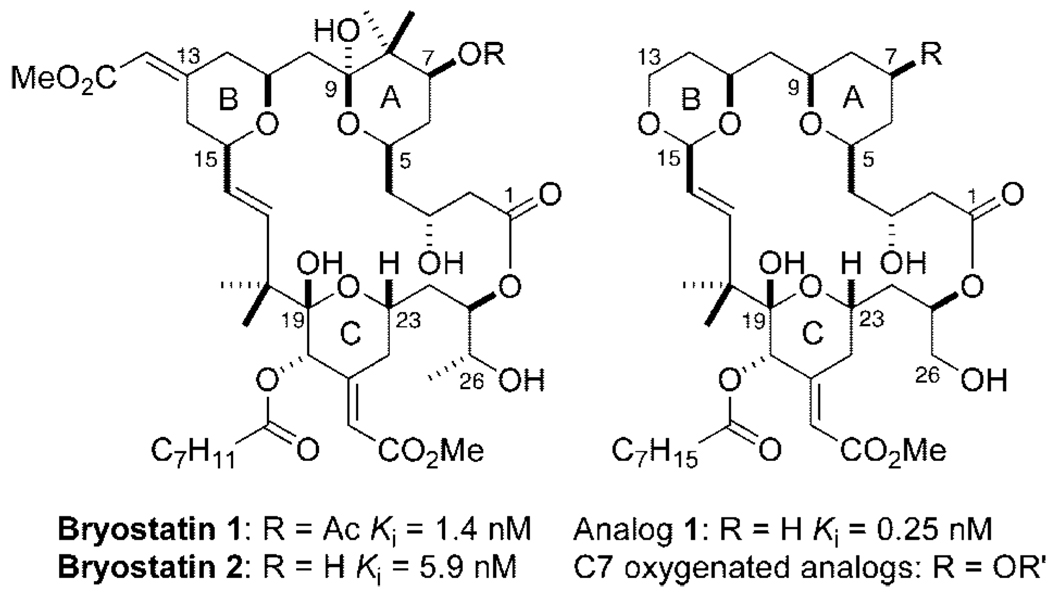
The bryostatins are structurally complex, marine-derived macrolactones (Figure 1)1 with a unique and expanding portfolio of biological activities. Bryostatin 1 is currently in clinical trials for the treatment of cancer. It exhibits exceptional potency, being dosed in humans at ca. 50 µg/m2.2 It restores apoptotic function in cancer cells,3 reverses multidrug resistance,4 and unlike most antineoplastic agents, stimulates the immune system.5 It also acts synergistically with several anticancer agents when used in combination therapy.6 Remarkably, bryostatin also facilitates learning and extends memory in animal models,7 serving as a significant lead for treating cognitive dysfunction including Alzheimer’s disease.8
Figure 1.
Bryostatins 1 and 2, superior lead analog 1, and designed C7 analogs.
There are two major problems that have impeded clinical advancement of the bryostatin leads. Bryostatins are naturally scarce and are isolated in low yields (0.00014%).9 Second, these complex compounds have proven to be difficult to selectively modify. Thus, relatively few bryostatin derivatives have been prepared and fewer still have been evaluated preclinically.10 The importance of accessing new functional and tunable analogs is great as the natural product itself elicits off target effects that limit or preclude clinical applications.
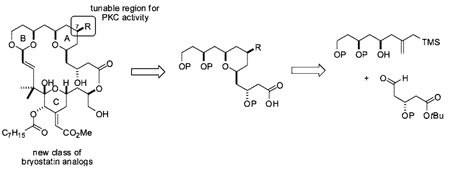
Total synthesis offers a means to address this problem, but current syntheses, while impressive in content, are as yet too long (>70 steps) to impact immediate supply needs.11 Engineered biosynthesis is another notable and promising source under investigation.12 In 1986, we initiated a third approach to addressing this problem based on function-oriented synthesis.13 In this approach, the structural features of the complex bryostatin target that putatively influence function (activity) are recapitulated on a simplified scaffold to produce a functional analog that is designed for rapid, step-economical, and practical synthesis.13a Illustrative of this approach, designed analog 1 (Figure 1) is found to be more potent than bryostatin and can be readily synthesized in a highly convergent fashion (<30 steps, 19 LL) that can be scaled to meet clinical needs.14
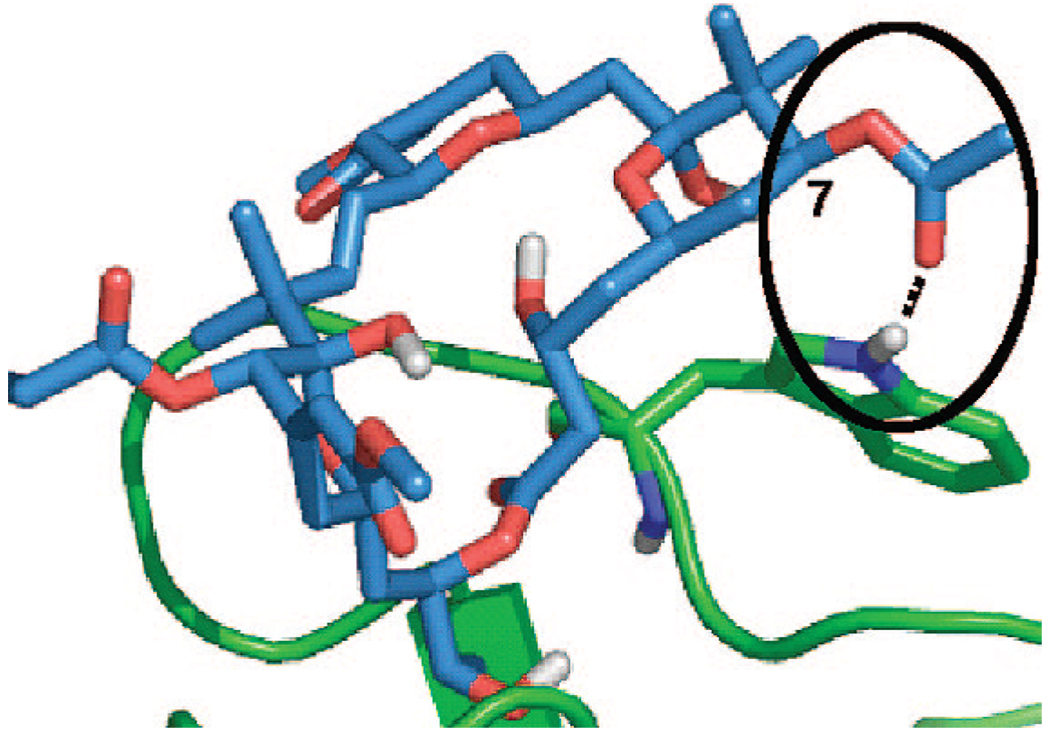
The current study is directed at a hitherto unexplored but fundamentally and clinically significant issue, namely the role of A-ring C7 functionality in the activities of bryostatin and analogs related to 1. Bryostatin 1’s activity is mediated through its potent binding to the C1 domain of various proteins, including kinases such as protein kinase C (PKC). This domain is found in only a small subset of the human kinome, but its functional role could be significant because many of these kinases are implicated in major diseases including cancer, cardiovascular, and cognitive indications.15 Ligands for the selective regulation (activation or inhibition) of C1 domain proteins are not available, adding further importance to the search for new high-affinity, selective agents. Related to the search for ligand structural features that could influence potency, selectivity, and function, it is noteworthy that no systematic studies on the role of C7 functional variations on biological activity have been reported (Figure 1). Significantly, docking studies using PKC sub-domain crystal structures and homology models suggest that the C7 functionality of bryostatin is proximate to a conserved tryptophan residue in the novel class of PKC isozymes and a conserved tyrosine residue in the conventional class of PKC isoforms (Figure 2).16 The biorelevancy of such models has not been tested, but such differences in binding selectivity could have profound therapeutic ramifications. It is note-worthy that these residues of PKC have recently been found to be critical for selective isozyme activation by the endogenous ligand, diacylglycerol.17 We report herein the efficient and convergent synthesis of 12 members of the first class of C7-functionalized bryostatin analogs and their initial biological evaluation.
Figure 2.
Docking of bryostatin 1 to the C1b domain of novel PKCδ. The distance between the C7 acetate carbonyl and tryptophan hydrogen is 1.9 Å, and the bond angle is ∠O–H–N) 152°.
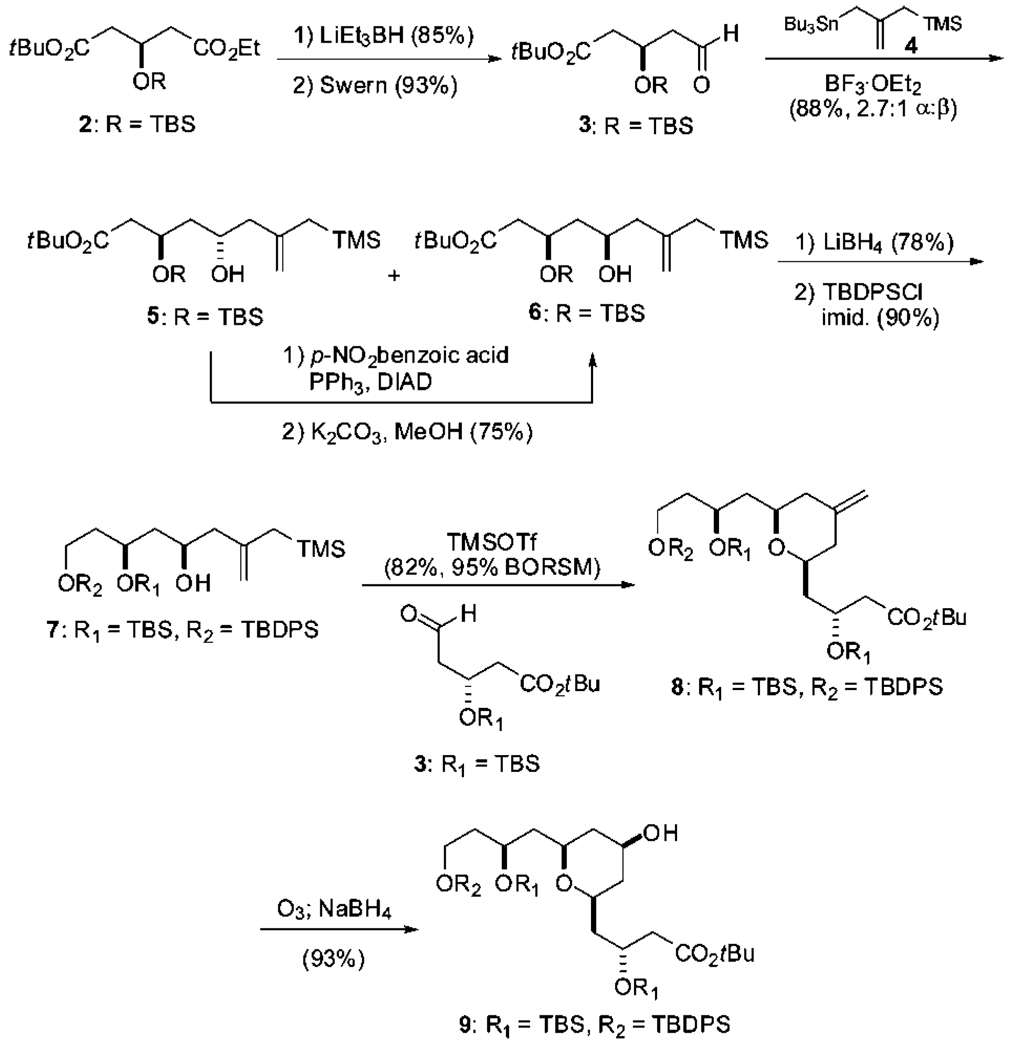
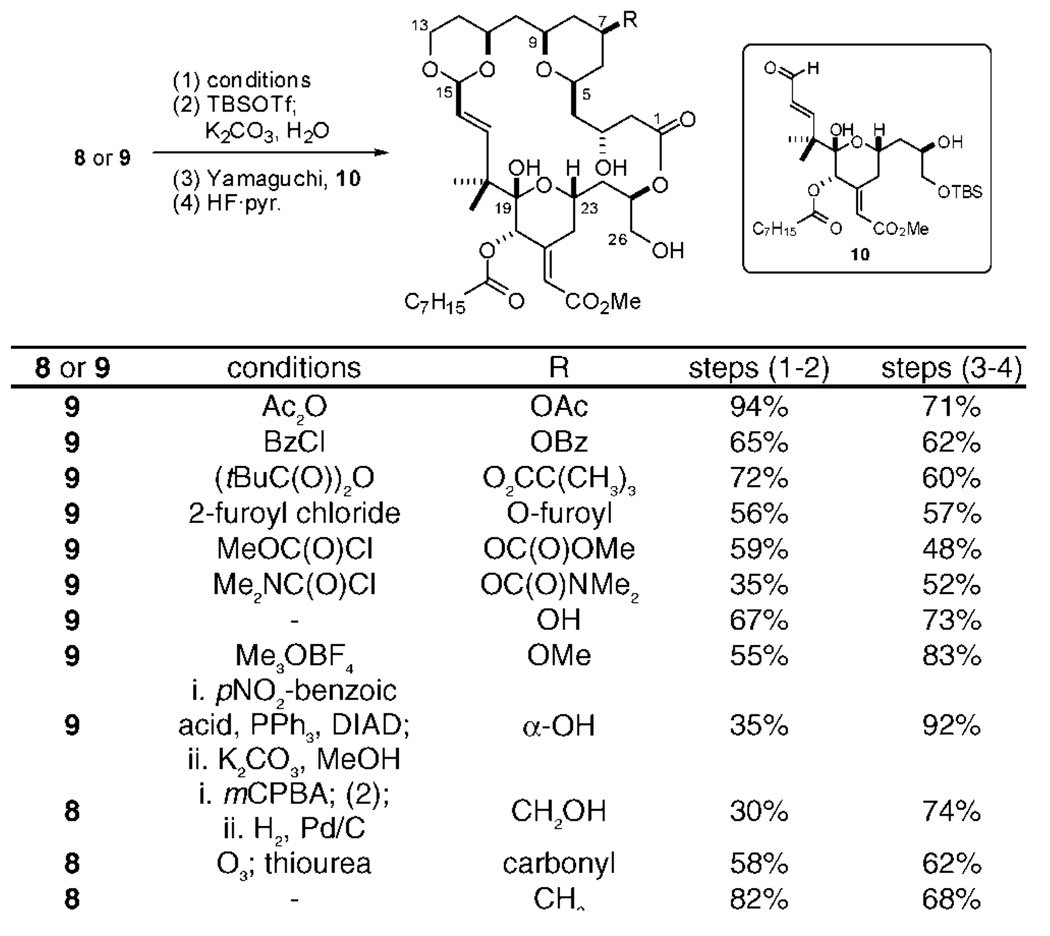
Our overall strategy employs a Yamaguchi esterification followed by a macroacetalization to convergently couple the target recognition (10) and spacer domains generated from intermediates 8 or 9. The synthesis of C7 diversifiable spacer domains draws in turn on a stereospecific Prins cyclization18 to convergently generate the syn-pyran A-ring from bis-nucleophile 4 and 2 equiv of aldehyde 3, a strategy that exploits the pseudosymmetry of the designed spacer domain. The synthesis of spacer domain intermediate 8 began with the selective reduction of commercially available diester 219 and oxidation to the key aldehyde 3 (Scheme 1). Allylation of this aldehyde with stannane 4,20 while efficient, was not highly disatereoselective with various catalytic and stoichiometric Lewis acid systems (Ti/BINOL or Zr/BINOL)21 as well as additives (B(OMe)3 or i-PrSBEt2).22 The undesired α-alcohol 5 was therefore efficiently converted to the desired β-alcohol 6 using Mitsunobu conditions. Reduction of ester 6 and protection of the resultant alcohol led to Prins precursor 7. The Prins cyclization of 7 using aldehyde 3 a second time proceeded smoothly to give pyran 8 in good yield and with high if not complete diastereoselectivity. The use of the more robust TBDPS protecting group for the primary alcohol was critical, as silyl exchange and deprotection was problematic under TMSOTf-promoted conditions using a primary TBS group. Ozonolysis and reductive workup then selectively installed C7 oxygenation as the desired β-alcohol in only seven overall steps from commercial material through 6 or nine steps through 5. Notably, these syntheses of spacer domains through intermediates 8 and 9 are the shortest of any spacer domain synthesis reported thus far.
Scheme 1.
Highly Convergent Synthesis of C7-Functionalized Intermediates
An attractive aspect of this strategy is that alkene 8, alcohol 9, or their derivatives can be converted into bryostatin analogs in only 3–4 steps (Scheme 2). Toward this end, 8, 9, or their derivatives were selectively converted to the C1 carboxylic acid through an efficient TBSOTf-mediated procedure. The resultant spacer domains were then esterified with recognition domain 1014 using Yamaguchi conditions. Our remarkably general14,23 and mild tandem global deprotection and macroacetalization procedure was then employed to form the B-ring which also serves to complete the macrocycle and set the C15 stereocenter under thermodynamic control, providing the analog in good yield. In total, 12 C7-functionalized analogs were efficiently synthesized in this manner, with the spacer domains prepared in as few as seven overall steps.
Scheme 2.
Completion of C7-Functionalized Analogsa
aSee the Supporting Information for experimental details.
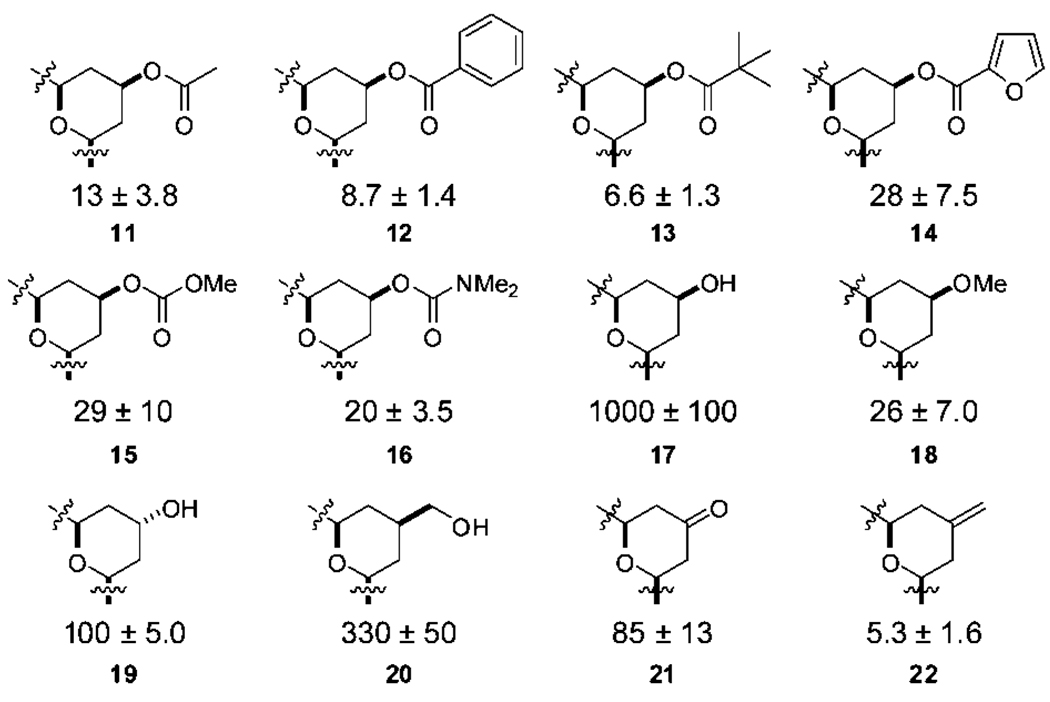
The newly synthesized analogs were tested for their ability to bind to PKC, providing a first-ever benchmark comparison to bryostatin and other analogs as well as to whether C7 modifications influence potency (Figure 3). The C7 acetate analog 11 bound with a Ki of 13 nM while the benzoate and pivaloate analogs (12 and 13) exhibited comparable but higher potency, indicating that steric bulk is tolerated in this region. The more polar furoyl analog 14 displayed a lower affinity, comparable to the carbonate or carbamate analogs 15 and 16. Remarkably, the free hydroxyl analog 17 was found to bind with a Ki = 1000 nM, a 4 orders of magnitude loss in binding potency compared to lead analog 1 simply due to the incorporation of a hydroxyl moiety at C7. This analog was found to be stable to the assay buffer conditions and ROESY NMR studies indicated that the analog adopts a similar conformation to that of other potent bryologs. Interestingly, inversion of this stereocenter to the C7 α-OH analog 19 results in a 10-fold increase in binding potency (Ki = 100 nM) compared to 17, while the homologated hydroxyl analog 20 increases relative affinity by only 3-fold (Ki = 330 nM). It appears that analogs retaining a free hydroxyl in this region of the bryologs lose a significant degree of potency compared to other C7 functionalized analogs, raising the hypothesis that C7 groups are at the protein complex-membrane interface or other hydrophobic region. This hypothesis is further supported by the better binding of methoxy analog 18 relative to 17. Even more dramatically, exomethylene analog 22, nearly isosteric with ketone analog 21, exhibits single digit nanomolar binding.
Figure 3.
PKC binding affinities of C7 functionalized analogs (nM). Only A-rings are shown.
Interestingly, bryostatin 2 (Figure 1), containing a C7 hydroxyl group as in analog 17, is only about 4-fold less potent than bryostatin 1 with a C7 acetate. The effect of the neighboring gem-dimethyl groups in the natural series could enhance membrane association by offsetting or shielding the C7 differences (Figure 2). The absence of a crystal structure of full length PKC and more significantly, of a solution structure involving membrane association, makes this ligand-based probe of the complex structure a singularly critical means of detailing molecular level interactions. It is clear from the binding data that PKC and the lipid cofactors required for proper protein folding24 represent a more complex system than the hypothetical binding model of the analogs docked to the C1 domain would indicate. These studies do show that, in contrast to the natural bryostatins, the C7 region of the bryologs can play a significant role in binding affinity and could be potentially exploited for improved pharmacological function such as PKC selectivity.
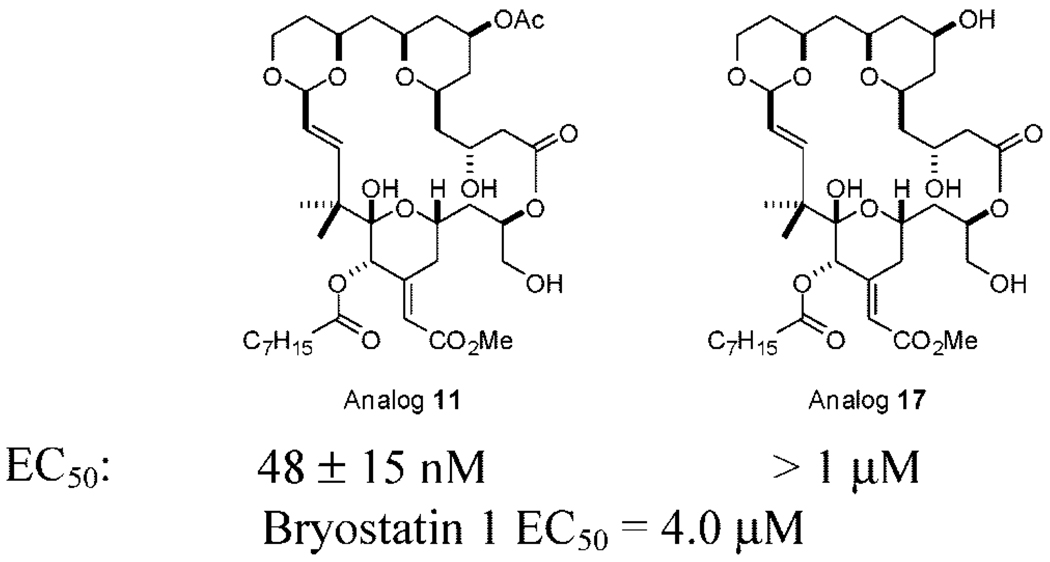
To further compare the cellular effects of these analogs, 11 and 17 were tested for their effectiveness against the human leukemia cell line K562 (Figure 4). Analog 1 is effective against this line (EC50 = 32 nM), while bryostati 1 is significantly less active (EC50 = 4.0 µM).14 In line wit their binding potencies, analog 11 was more effective that analog 17 against this cell line. Importantly, the synthesis of the first C7 acetate bryolog allows for retention of activity when compared with lead analog 1 and superior activity when compared to bryostatin 1.
Figure 4.
Effective concentrations of analogs 11 and 17 against human leukemia cell line K562 after 48 h incubation.
The first C7 A-ring functionalized bryologs have been synthesized in a scalable and step-economical fashion, with spacer domains available in 7–10 steps (overall 26–29 steps). These 12 analogs represent the first in a series of C7 modified bryologs, some of which retain potent, single-digit nanomolar affinity to PKC. A critical hotspot for PKC affinity has been discovered at the C7 position of the bryostatin analogs. The discovery of this hotspot adds weight to prior data17 implicating this region of PKC as critical for potency and potentially for controlling selectivity and optimizing pharmacological function. Indeed, the C7 acetate analog 11 exhibits superior in vitro antileukemic activity when compared to bryostatin 1. Further biological evaluation of these analogs, including isozyme selective activity, is currently underway and will be reported in due course.
Acknowledgment
Support of this work through a grant (CA31845) provided by the NIH is gratefully acknowledged. Fellowship support from Amgen (V.A.V.) is also recognized. We thank the Daria Mochly-Rosen group (Department of Chemical and Systems Biology) for support with biological studies.
Footnotes
Supporting Information Available: Available experimental conditions and spectral data for compounds reported in this paper. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. J. Am. Chem. Soc. 1982;104:6846–6848. [Google Scholar]
- 2.For current information, see http://clinicaltrials.gov.
- 3.Wall NR, Mohammad RM, Al-Katib AM. Leuk. Res. 1999;23:881–888. doi: 10.1016/s0145-2126(99)00108-3. [DOI] [PubMed] [Google Scholar]
- 4.Elgie AW, Sargent JM, Alton P, Peters GJ, Noordhuis P, Williamson CJ, Taylor CG. Leuk. Res. 1998;22:373–378. doi: 10.1016/s0145-2126(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 5.Oz HS, Hughes WT, Rehg JE, Thomas EK. Microb. Pathog. 2000;29:187–190. doi: 10.1006/mpat.2000.0374. [DOI] [PubMed] [Google Scholar]
- 6.For a recent clinical trial, see: Ajani JA, Jiang Y, Faust J, Chang BB, Ho L, Yao JC, Rousey S, Dakhil S, Cherny RC, Craig C, Bleyer A. Invest. New Drugs. 2006;24:353–357. doi: 10.1007/s10637-006-6452-1.
- 7.Hongpaisan J, Alkon DL. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19571–19576. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van der Auwera I, Wera S, Qiao LX, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11141–11146. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaufelberger DE, Koleck MP, Beutler JA, Vatakis AM, Alvarado AB, Andrews P, Marzo LV, Muschik GM, Roacch J, Ross JT, Lebherz WB, Reeves MP, Eberwein RM, Rodgers LL, Testerman RP, Snader KM, Forenza S. J. Nat. Prod. 1991;54:1265–1270. doi: 10.1021/np50077a004. [DOI] [PubMed] [Google Scholar]
- 10.(a) Wender PA, DeBrabander J, Harran PG, Jimenez JM, Koehler MFT, Lippa B, Park CM, Siedenbiedel C, Pettit GR. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6624–6629. doi: 10.1073/pnas.95.12.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kortmansky J, Schwartz GK. Cancer Invest. 2003;21:924–936. doi: 10.1081/cnv-120025095. [DOI] [PubMed] [Google Scholar]
- 11.(a) Evans DA, Carter PH, Carreira EM, Charette AB, Prunet JA, Lautens M. J. Am. Chem. Soc. 1999;121:7540–7552. [Google Scholar]; (b) Kageyama M, Tamura T, Nantz MH, Roberts JC, Somfai P, Whritenour DC, Masamune S. J. Am. Chem. Soc. 1990;112:7407–7408. [Google Scholar]; (c) Ohmori K, Ogawa Y, Obitsu T, Ishikawa Y, Nishiyama S, Yamamura S. Angew. Chem., Int. Ed. 2000;39:2290–2294. [PubMed] [Google Scholar]
- 12.(a) Lopanik N, Lindquist N, Targett N. Oecologia. 2004;139:131–139. doi: 10.1007/s00442-004-1487-5. [DOI] [PubMed] [Google Scholar]; (b) Sudek S, Lopanik NB, Waggoner LE, Hildebrand M, Anderson C, Liu HB, Patel A, Sherman DH, Haygood MG. J. Nat. Prod. 2007;70:67–74. doi: 10.1021/np060361d. [DOI] [PubMed] [Google Scholar]
- 13.(a) Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc. Chem. Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]; (b) Wender PA, Koehler KF, Sharkey NA, Dellaquila ML, Blumberg PM. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4214–4218. doi: 10.1073/pnas.83.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wender PA, Baryza JL, Bennett CE, Bi FC, Brenner SE, Clarke MO, Horan JC, Kan C, Lacôte E, Lippa BS, Nell PG, Turner TM. J. Am. Chem. Soc. 2002;124:13648–13649. doi: 10.1021/ja027509+. For other recent analog work, see: Wender PA, DeChristopher BA, Schrier AJ. J. Am. Chem. Soc. 2008;130:6658–6659. doi: 10.1021/ja8015632. Keck GE, Kraft MB, Truong AP, Li W, Sanchez CC, Kedei N, Lewin NE, Blumberg PM. J. Am. Chem. Soc. 2008;130:6660–6661. doi: 10.1021/ja8022169. Wender PA, Horan JC, Verma VA. Org. Lett. 2006;8:5299–5302. doi: 10.1021/ol0620904. and references therein. (e) For a review of our bryostatin analog program, see: Wender PA, Baryza JL, Hilinski MK, Horan JC, Kan C, Verma VA. Huang Z. Drug Discovery Research: New Frontiers in the Post-Genomic Era. Hoboken, NJ: Wiley-VCH; 2007. Beyond Natural Products: Synthetic Analogues of Bryostatin 1; pp. 127–162.
- 15.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 16.Wender PA, Baryza JL, Brenner SE, Clarke MO, Craske ML, Horan JC, Meyer T. Curr. Drug Discov. Tech. 2004;1:1–11. doi: 10.2174/1570163043484888. [DOI] [PubMed] [Google Scholar]
- 17.Dries DR, Gallegos LL, Newton AC. J. Biol. Chem. 2007;282:826–830. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- 18.(a) Keck GR, Covel JA, Schiff T, Yu T. Org. Lett. 2002;4:1189–1192. doi: 10.1021/ol025645d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mekhalfia A, Marko´ IE, Adams H. Tetrahedron Lett. 1991;32:4783–4786. [Google Scholar]
- 19.Item 49.60 from Ju¨lich Chiral Solutions (www.codexis.com) for 33 euros/g.
- 20.Available in one step: Clive DLJ, Paul CC, Wang Z. J. Org. Chem. 1997;62:7028–7032.
- 21.(a) Yu CM, Lee JY, So B, Hong J. Angew. Chem., Int. Ed. 2002;41:161–163. doi: 10.1002/1521-3773(20020104)41:1<161::aid-anie161>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; (b) Kurosu M, Lorca M. Tetrahedron Lett. 2002;43:1765–1769. [Google Scholar]
- 22.(a) Yu CM, Choi HS, Yoon SK, Jung WH. Synlett. 1997:889–890. [Google Scholar]; (b) Yu CM, Choi HS, Jung WH, Kim HJ, Shin J. Chem. Commun. 1997:761–762. [Google Scholar]
- 23. Wender PA, Verma VA. Org. Lett. 2006;8:1893–1896. doi: 10.1021/ol060457z. For a related application, see: Smith AB, Razler TM, Meis RM, Pettit GR. Org. Lett. 2006;8:797–799. doi: 10.1021/ol060014v.
- 24.Wender PA, Irie K, Miller BL. Proc. Natl. Acad. Sci. U.S.A. 1995;92:239–243. doi: 10.1073/pnas.92.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]