Abstract
An innate immune response to bacterial components is speculated to be involved in the pathogenesis of primary biliary cirrhosis (PBC). Recently, CD4-positive T helper type 17 (Th17) cells, characterized by the secretion of interleukin (IL)-17, have been implicated in the pathogenesis of autoimmune diseases. Human Th17 cells are generated from Th0 cells by IL-6 and IL-1β and maintained by IL-23. In this study, the role of IL-17 in PBC and its association with biliary innate immunity were examined. Using cultured human biliary epithelial cells (BECs), the expression of Th17-related cytokines and chemokines and changes therein on treatment with pathogen-associated molecular patterns (PAMPs) and IL-17 were examined. Immunohistochemistry for IL-17 and Th17-related cytokines was performed using tissue samples of human liver. Consequently, the expression of IL-6, IL-1β, IL-23p19 and IL-23/IL-12p40 mRNAs, and their up-regulation by PAMPs, were found in BECs. Moreover, BECs possessed IL-17-receptors and stimulation with IL-17 induced production of IL-6, IL-1β, IL-23p19 and chemokines. Several IL-17-positive cells had infiltrated damaged bile ducts and the expression of IL-6 and IL-1β was enhanced in the bile ducts of PBC patients. In conclusion, IL-17-positive cells are associated with the chronic inflammation of bile ducts in PBC which is associated causally with the biliary innate immune responses to PAMPs.
Keywords: biliary epithelial cells, innate immunity, interleukin 17, primary biliary cirrhosis, Th17
Introduction
The aetiopathogenesis of primary biliary cirrhosis (PBC) remains speculative. However, the high prevalence of vaginal and urinary tract infections, the presence of bacterial and viral components in bile and liver and the cross-reaction of human and bacterial pyruvate dehydrogenase complex-E2 (PDC-E2) with anti-mitochondrial antibody (AMA) and autoreactive T cells in PBC patients suggest that the presence of bacterial components, including bacterial infections and innate immune responses to bacterial components, are involved in the pathogenesis of PBC, particularly the damage to bile ducts [1,2]. We have reported previously that human biliary epithelial cells (BECs) possess an innate immune system consisting of Toll-like receptors (TLRs), which recognize various pathogen-associated molecular patterns (PAMPs), suggesting biliary innate immunity to be associated closely with PBC [3–5].
CD4-positive helper T cells are essential regulators of immune responses and inflammatory diseases. Immunoreactivity to intra- and extracellular antigens is regulated mainly by two different types of memory CD4-positive T helper type cells, namely Th1 and Th2 cells, which are distinguished principally by their production of different cytokines as well as their ability to induce either cellular (Th1) or humoral (Th2) immune reactions. Recently, a third pathogenic type, Th17 cells, and its association with the chronic inflammation of autoimmune diseases, have been noted [6–8]. Human Th17 cells are characterized by the production of interleukin (IL)-17 (IL-17A and IL-17F) and differentiate from naive T cells (Th0). Studies in mice have identified transforming growth factor (TGF)-β and IL-6 as the critical cytokines driving the differentiation of naive T cells into Th17 cells [9], while a subsequent study indicated that IL-6 and IL-1β, but not TGF-β1, are required for the differentiation in human cells [10]. Recently, the differentiation of human Th17 cells has been reported to require the activity of TGF-β[11–13], but the importance of TGF-β is still controversial. IL-23 is required for maintaining or stabilizing the cellular functions and for the survival, but not the differentiation, of Th17 cells [6]. Recent studies have identified the subset of T cells producing IL-17 (Th17) as playing a predominant role in the pathogenesis of experimental autoimmune encephalomyelitis [6] and autoimmune arthritis [7,8]. Moreover, Elson et al. reported that enteric bacteria-reactive CD4-positive Th17 cells expanded in number in colitic mice and that the relative expression of the IL-17 mRNA transcript in colonic lesions was increased greatly [14]. Leppkes et al. reported that the transcriptional factor of Th17 cells, retinoic acid receptor-related organ receptors (ROR) gamma, controls IL-17A and IL-17F production, and these cytokines have a crucial pathogenic role in chronic intestinal inflammation [15]. Because the receptor for IL-17 (IL-17R), a heterodimer of IL-17RA and IL-17RC, is expressed by many cells, IL-17 has the ability to induce the production of several cytokines including IL-6, IL-8 and IL-1, and chemokines such as CXCL (CXC-chemokine ligand)-1 (GRO-α), CXCL2, CXCL3, CXCL6(GCP-2), CXCL8(IL-8), CCL2(MCP-1) and CCL (CC-chemokine ligand)-20 from various cells, including epithelial and vascular endothelial cells [16–20]. These cytokines and chemokines are associated with a continuous (chronic) inflammation and the activation of nuclear factor-κB (NF-κB) and C-Jun N-terminal kinases (JNK) [19]. Although details of the signalling mechanism of the IL-17 pathway remain elusive, Act1 (transcription factor NF-κB activator 1) has been demonstrated recently to be an essential adaptor protein in IL-17 receptor signalling in autoimmune and inflammatory diseases [21].
In chronic hepatitis, particularly chronic viral hepatitis C (CVH-C), the non-suppurative damage of interlobular bile ducts known as hepatitis-associated bile duct damage, or hepatitic duct lesions, is not infrequent [22,23]. However, the difference in the histogenesis of cholangiopathies between PBC and CVH-C remains unclear.
In this study, we found a marked intrahepatic distribution of IL-17-positive cells and speculated that, in the presence of PAMPs, biliary epithelial cells are sufficient sources of IL-6, IL-1β and IL-23 for the generation and stabilization of Th17 cells at sites of periductal antigen-presenting cells such as dendritic cells.
Materials and methods
Cultured human BECs
Three cultured cell lines of human BECs (BEC1–BEC3) were used in this study. BEC1 and BEC2 were newly established from the explanted livers of PBC patients according to methods reported previously [24,25]. BEC3 was established from background liver showing a normal histology far from metastatic foci in surgically resected liver with a metastatic liver tumour. Informed consent for human research was obtained from all patients prior to surgery. This study was approved by the Kanazawa University Ethics Committee. The cultured BECs were incubated with a culture medium composed of Dulbecco's modified Eagle medium (DMEM)/F-12 (Invitrogen, Tokyo, Japan), 5% newborn calf serum (Invitrogen), 0·18 mM adenine (Sigma, St Louis, MO, USA), hydrocortisone (0·4 µg/ml), cholera toxin (10 ng/ml), tri-iodo-thyronine (1·3 µg/l), insulin transferrin selenium-positive (ITS+) (Becton Dickinson, Franklin Lakes, NJ, USA), 25 mM sodium bicarbonate (Sigma), 1% antibiotics anti-mycotic, 20 ng/ml of human epidermal growth factor (Invitrogen) and 10 ng/ml of human hepatocyte growth factor (Invitrogen). The cells were grown as monolayers in a 5% CO2-humidified incubator at 37°C. These BECs had been confirmed to be biliary epithelial cells by the expression of biliary-type cytokeratins (CK7 and CK19) and a marker of polarity (cystic fibrosis transmembrane conductance regulator, CFTR) [26]. All the cultured BECs were used between passages 4 and 9.
Patients and tissue preparations
Liver tissue specimens were used from eight patients with PBC (all positive for AMA by immunofluorescence at least once during follow-up; average age, 57 years; all female; histological stages I/II = 3/5) and, as controls, from nine patients with CVH-C (average age, 58 years; six male/three female; histological stages F1/F2 = 4/5) and five normal livers (average age, 62 years; three male/two female, non-cancerous hepatic regions showing no histological abnormalities from surgically resected livers for metastatic liver tumour). These specimens were selected from registered files in our laboratory, which contained at least three interlobular bile ducts including damaged bile ducts, and were obtained prior to treatments. The pathological diagnoses were based on established criteria including clinical and laboratory data and confirmed by histological review by an independent observer. More than 10 4-µm-thick sections were prepared from each paraffin-embedded block; several were stained with haematoxylin and eosin (H&E) and Gomori's reticulum stain for histological diagnosis and the others were used for immunohistochemistry. In addition to fixed specimens, fresh surgical specimens were available in three cases each of PBC and CVH-C, and used for immunohistochemistry.
Isolation of RNA and real-time polymerase chain reaction (PCR)
For the evaluation of mRNAs of IL-17(A), IL-6, IL-1β, IL-23 (IL-23 p19 and IL-23/IL-12 p40), caspase 1 (IL-1β converting enzyme), chemokines (CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, CCL2 and CCL20), IL-17 receptor (IL-17RA and IL-17RC) and Act1 in BECs, total RNA was isolated from cultured cells using the RNeasy Total RNA System (Qiagen, Hilden, Germany), following the manufacturer's instructions. Then, 1 µg of total RNA was reverse-transcribed with an oligodeoxythymidylic acid (oligo)-(dT) primer and ReverTra Ace (Toyobo, Osaka, Japan) to synthesize a cDNA template for PCR. With regard to caspase 1, IL-17RA, IL-17RC and Act1, conventional PCR was performed in order to examine the presence of these molecules. The reaction profile consisted of initial denaturation at 94°C for 3 min followed by 28–35 cycles with 30 s of denaturation at 94°C, 30 s of annealing of primers at 55°C and 60 s of extension at 72°C. After the PCR, 5 µl aliquots of the products were subjected to 1·5% agarose gel electrophoresis. As for the other molecules, to carry out relative quantification, real-time quantitative PCR was performed for measurements according to a standard protocol using the SYBR Green PCR Master Mix and ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Tokyo, Japan), and relative gene expression was calculated using the comparative cycle threshold method. The primers used in this study are listed in Table 1.
Table 1.
Primer sequences used in this study.
| Target gene | Forward | Reverse |
|---|---|---|
| IL-17(A) | 5′-TGTCCACCATGTGGCCTAAGAG-3′ | 5′-GTCCGAAATGAGGCTGTCTTTGA-3′ |
| IL-6 | 5′-AGTGAGGAACAAGCCAGAGC-3′ | 5′-AAAGCTGCGCAGAATGAGAT-3′ |
| IL-1β | 5′-CCAGGGACAGGATATGGAGCA-3′ | 5′-TTCAACACGCAGGACAGGTACAG-3′ |
| IL-23 p19 | 5′-TACTGGGCCTCAGCCAACTC-3′ | 5′-TACAGCCACAAAGGCCTGGA-3′ |
| IL-23/IL-12 p40 | 5′-GGAGCGAATGGGCATCTGT-3′ | 5′-TGGGTCTATTCCGTTGTGTCTTTA-3′ |
| CXCL1 | 5′-GAAAGCTTGCCTCAATCCTG-3′ | 5′-CACCAGTGAGCTTCCTCCTC-3′ |
| CXCL2 | 5′-CTTCTATTTATTTATTTATTTATTTATTTGTTTGTTTT-3′ | 5′-GAACTAACTTGGGTTTGACCTAAA-3′ |
| CXCL3 | 5′-TGAAAAAGAGAACAGCAGCTTTCT-3′ | 5′-AGGACTGAGCTATGTTTGATGAAACA-3′ |
| CXCL6 | 5′-GCTCCAAGGTGGAAGTGGTA-3′ | 5′-AGAAAACTGCTCCGCTGAAG-3′ |
| CXCL8 | 5′-ACACTGCGCCAACACAGAAATTA-3′ | 5′-TTTGCTTGAAGTTTCACTGGCATC-3′ |
| CCL2 | 5′-CTGAATTTTGTTTGTTGATGTGAAA-3′ | 5′-GCAATTTCCCCAAGTCTCTG-3′ |
| CCL20 | 5′-GCGCAAATCCAAAACAGACT-3′ | 5′-CAAGTCCAGTGAGGCACAAA-3′ |
| IL-17RA | 5′-CCAGATCCCAGCTTTGAGAG-3′ | 5′-AAATGCCCGCCACATAGTAG-3′ |
| IL-17RC | 5′-CTATGGGACGATGACTTGGGAG-3′ | 5′-AGCGCAGCGGCAAAGAGTA-3′ |
| Act1 | 5′-AACAAGGAAGCATGAATTTCAGA-3′ | 5′-ATTCTTGGGCCAGCTGTAGA-3′ |
| GAPDH | 5′-GGCCTCCAAGGAGTAAGACC-3′ | 5′-AGGGGTCTACATGGCAACTG-3′ |
IL, interleukin; CXCL, CXC-chemokine ligand; CCL, CC-chemokine ligand; GAPDH, glyceraldehyde-3-phosphate-dehydrogenase.
Treatment with PAMPs and IL-17
Cultured BECs were stimulated with Pam3CSK4 (TLR-1/2 ligand, 100 ng/ml; Invivogen, San Diego, CA, USA), polyinosinic–polycytidylic acid [poly(I:C), a synthetic analogue of viral dsRNA, TLR-3 ligand, 25 µg/ml; Invivogen], lipopolysaccharide (LPS) (ultrapure grade, TLR-4 ligand, 1 µg/ml; Invivogen), or recombinant human IL-17 (1000 U/ml; PeproTech, London, UK), for 3 h and used for the preparation of samples for PCR analysis.
Immunohistochemistry
The deparaffinized and rehydrated sections for studying IL-17, IL-6, IL-1β, IL-23 p19 and caspase 1 were microwaved in 10 mM citrate buffer for 20 min in a microwave oven. Following the blocking of endogenous peroxidase, these sections were incubated at 4°C overnight with antibodies against IL-17 (goat polyclonal immunoglobulin (Ig)G, 1 µg/ml; R&D, Minneapolis, MN, USA) or caspase 1 (mouse monoclonal, 2·5 µg/ml; Imgenex, San Diego, CA, USA), and then at room temperature for 1 h with anti-goat immunoglobulins conjugated to a peroxidase-labelled dextran polymer (Simple Staining Kit; Nichirei, Tokyo, Japan) or anti-mouse immunoglobulins conjugated to a peroxidase labelled-dextran polymer (Envision System; Dako, Tokyo, Japan), respectively. As for IL-6, IL-1β and IL-23 p19, the sections were incubated for 15 min with anti-IL-6 antibody (rat monoclonal IgG1κ, 10 µg/ml; Abcam, Cambridge, UK), anti-IL-1β antibody (mouse monoclonal IgG2b, 5 µg/ml; Abcam) or IL-23 p19 antibody (mouse monoclonal IgG1κ, 10 µg/ml; BioLegend, San Diego, CA, USA), and then the catalysed signal amplification (CSA) system (Dako) was used. After a benzidine reaction, sections were counterstained lightly with haematoxylin. As a negative control, normal mouse IgG1κ or IgG2b or goat IgG was used as the primary antibody.
Among IL-17-induced chemokines, antibodies against CCL2 and CXCL6 were commercially available for immunohistochemistry using fresh sections. After fixation of frozen 5 µm sections in cold acetone for 10 min and the blocking of endogenous peroxidase, these sections were incubated with human CCL2 antibody (mouse monoclonal; 10 µg/ml; R&D Systems Inc.) or human CXCL6 antibody (goat polyclonal; 4 µg/ml; Santa Cruz, Santa Cruz, CA, USA) at room temperature for 60 min.
Histological examination
For the semi-quantitative evaluation of the immunohistochemistry, three to five representative portal tracts containing interlobular bile ducts including damaged bile ducts were chosen in each section for assessment. A total of 37 bile ducts in PBC and 44 in CVH-C were evaluated. IL-17-positive infiltrating mononuclear cells were counted around bile ducts in a high-power field (×400). For IL-6, IL-1β and IL-23 p19, immunoreactivity in bile ducts was graded semiquantitatively as follows: 0, absence of expression; 1, low constitutive expression; 2, intermediate expression; and 3, high expression. The final score indices of PBC and CVH-C were defined as the mean of individual cases.
Statistical analysis
Data were analysed using the paired t-test and Mann–Whitney U-test; P < 0·05 was considered statistically significant.
Results
Intrahepatic distribution of IL-17-positive cells in liver tissue
Representative images of the immunohistochemistry with anti-IL-17 antibody are shown in Fig. 1. IL-17-positive infiltrating cells were scattered mainly within the inflammed portal area of PBC and CVH-C, especially in interface areas. No epithelial and mesenchymal elements other than infiltrating cells expressed IL-17 in livers. Moreover, in PBC, IL-17-positive infiltrating mononuclear cells were accumulated around the damaged interlobular bile ducts such as chronic non-suppurative destructive cholangitis (CNSDC). In CVH-C, several interlobular bile ducts showing hepatitis-associated bile duct damage were found, but IL-17-positive cells were not accumulated around these bile ducts, including the undamaged ones. In normal livers, IL-17-positive mononuclear cells were very few or absent around interlobular bile ducts. Semi-quantitative evaluation revealed that number of IL-17-positive mononuclear cells around bile ducts to be significantly greater in PBC (5·7 ± 0·5) [mean ± standard error of the mean (s.e.m.)] than in CVH-C (1·3 ± 0·3) (P < 0·05).
Fig. 1.
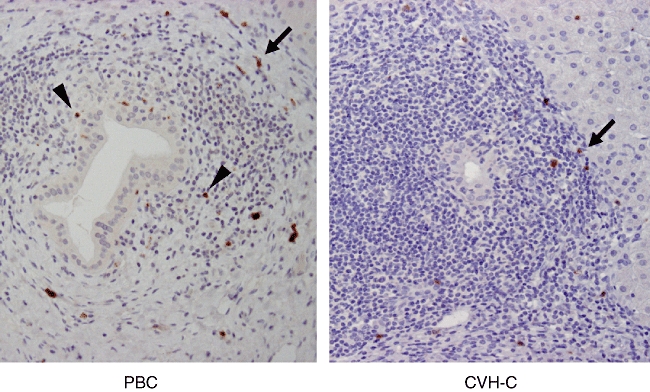
Immunohistochemistry for interleukin (IL)-17 using tissue sections of primary biliary cirrhosis (PBC) and chronic viral hepatitis C (CVH-C). Several IL-17-positive mononuclear cells are scattered in the interface area of inflamed portal tracts in PBC and CVH-C (arrows). Moreover, IL-17-positive cells are found around chronic non-suppurative destructive cholangitis (CNSDC) in PBC (arrowheads), but not around interlobular bile ducts showing hepatitis-associated bile duct injury in CVH-C.
Expression of IL-6, IL-1β, IL-23 and caspase 1 mRNAs and their regulation by PAMPs in cultured BECs
Real-time PCR analysis revealed that LPS, Pam3CSK4 and poly(I:C) enhanced the mRNA expression of both Th17-inducible cytokines, IL-6 and IL-1β in cultured BECs, with the increases being statistically significant (Fig. 2). Regarding the Th17-maintaining cytokine (IL-23), the expression of IL-23 p19 mRNA was up-regulated by stimulation with all these PAMPs, but that of the IL-23/IL-12 p40 component was induced by LPS and Pam3CSK4, but not poly(I:C) (Fig. 2). Cultured BECs constantly expressed caspase 1 mRNA, which is necessary for the production of activated IL-1β (Fig. 3).
Fig. 2.
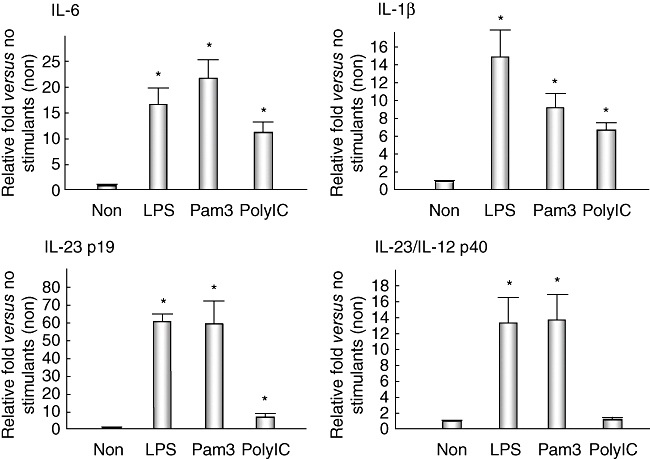
Quantitative analysis of T helper 17 (Th17)-inducible cytokines [interleukin (IL)-6 and IL-1β] and Th17-maintaining cytokines (IL-23 p19 and IL-23/IL-12 p40) in cultured human biliary epithelial cells (BECs). Expression of IL-6 mRNA was up-regulated 16·6 ± 4·3 [mean ± standard error of the mean (s.e.m.)]-fold by stimulation with lipopolysaccharide (LPS) [Toll-like receptor (TLR)-4 ligand], 21·7 ± 2·5-fold with Pam3CSK4 (TLR-1/2 ligand) and 11·3 ± 1·5-fold with poly(I:C) (TLR-3 ligand). The relative fold-increase in the expression of IL-1β caused by LPS, Pam3CSK4 and poly(I:C), compared with no stimulant (non), was 14·9 ± 4·1, 9·2 ± 1·6 and 6·7 ± 0·6, respectively; that of IL-23 p19 was 60·7 ± 3·0, 60·0 ± 15·7 and 7·3 ± 0·6, and that of IL-23/IL-12 p40 was 13·4 ± 3·3, 13·7 ± 3·6 and 1·2 ± 0·3, respectively. LPS and Pam3CSK4 up-regulated the production of all these cytokines significantly (P < 0·05). Poly(I:C) also up-regulated the production of IL-6, IL-1β and IL-23 p19 (P < 0·05), but not IL-23/IL-12 p40. Duplicate experiments were performed using three lines of cultured biliary epithelial cells (BECs) (BEC1–BEC3) and data are shown as the mean ± s.e.m.
Fig. 3.
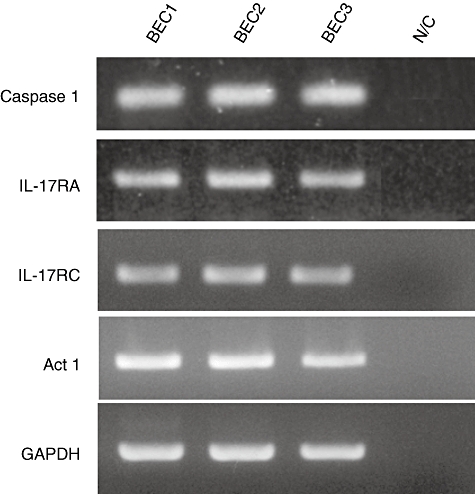
Gel images of reverse transcription–polymerase chain reaction for caspase 1, interleukin (IL)-17RA, IL-17RC, Act1 and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) [internal control for reverse transcription–polymerase chain reaction (RT–PCR)] using three cultured human biliary epithelial cell lines (BECs) (BEC1–BEC3). The BECs constantly expressed all these molecules, including GAPDH. N/C, negative control without cDNA.
Expression of IL-6, IL-1β, IL-23 p19 and caspase 1 in interlobular bile ducts
Immunohistochemistry revealed that the expression of IL-1β, IL-6 and IL23p19 were basically restricted in biliary epithelial cells and several infiltrating inflammatory cells. Hepatocytes, excluding several periportal hepatocytes, were negative for these cytokines. IL-23 p19 and caspase 1 were expressed constantly in biliary epithelial cells lining all intrahepatic bile ducts, irrespective of anatomical levels of diseased livers and normal livers (Fig. 4). However, the expression of IL-6 and IL-1β was prominent in the damaged bile ducts in PBC, but faint or lacking in CVH-C and normal livers (Fig. 4). As shown in Fig. 5, the semiquantitative evaluation also showed the expression of IL-6 and IL-1β to be significant in PBC, compared with CVH-C (P < 0·05).
Fig. 4.
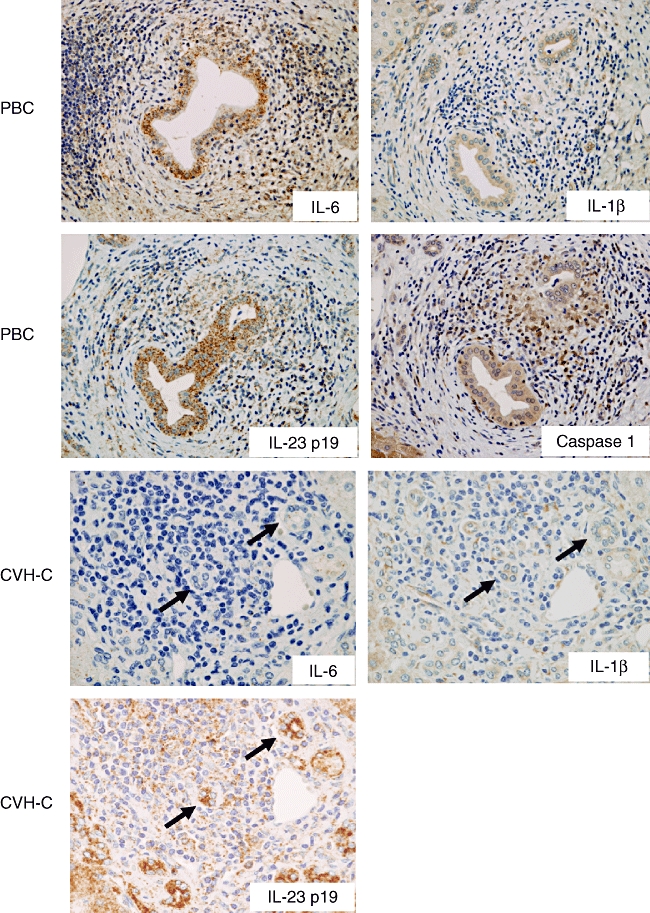
Immunohistochemistry for T helper type 17 (Th17)-inducible cytokines [interleukin (IL)-6 and IL-1β], a component of IL-23 (IL-23 p19), and caspase 1. IL-6 and IL-1β are expressed in bile ducts and infiltrating cells to various degrees; prominently in the damaged bile ducts of primary biliary cirrhosis (PBC), but faintly or not at all in interlobular bile ducts of chronic viral hepatitis C (CVH-C). Caspase 1, which cleaves IL-1β for its secretion, is also expressed in the damaged bile ducts of PBC. In contrast, IL-23 p19 is constantly expressed in interlobular bile ducts, irrespective of PBC and CVH-C.
Fig. 5.
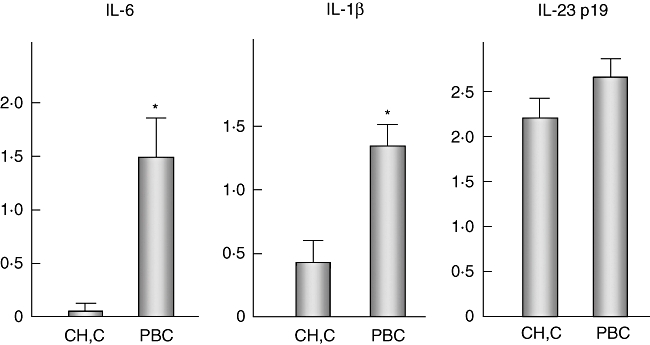
Semiquantitative evaluation for the expression of interleukin (IL)-6, IL-1β and IL-23 p19 in interlobular bile ducts including damaged bile ducts. The scores for IL-6 were 0·02 ± 0·02 (mean ± standard error of the mean) and 1·5 ± 0·28 in chronic viral hepatitis C (CVH-C) and primary biliary cirrhosis (PBC), respectively; those for IL-1β were 0·48 ± 0·10 and 1·34 ± 0·12; and those for IL-23 p19 were 2·2 ± 0·2 and 2·6 ± 0·08, respectively. Statistical analysis revealed the expression of IL-6 and IL-1β to be significantly up-regulated in PBC, compared with CVH-C (P < 0·05).
Effect of IL-17 on biliary epithelial cells and chemokine expression in bile ducts
Conventional reverse transcription (RT)–PCR revealed that cultured BECs possess receptors for IL-17 (IL-17RA and IL-17RC) and also Act1 (an essential adaptor protein in IL-17 receptor signalling) (Fig. 3). Therefore, biliary epithelial cells could be affected by the periductal cytokine milieu associated with IL-17; consequently, we examined the effect of IL-17 in cultured BECs. The expression of IL-6, IL-1β and IL-23 p19, but not IL-23/IL-12 p40, was up-regulated by IL-17 (Fig. 6). Moreover, expression of all the IL-17-induced chemokines, CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, CCL2 and CCL20, was also up-regulated by IL-17 (Fig. 6). The expression of CCL2 and CXCL6 was examined in intrahepatic bile ducts using fresh liver sections. The damaged bile ducts expressed CCL2 and CXCL6 in PBC, but not or only faintly in CVH-C (Fig. 7).
Fig. 6.
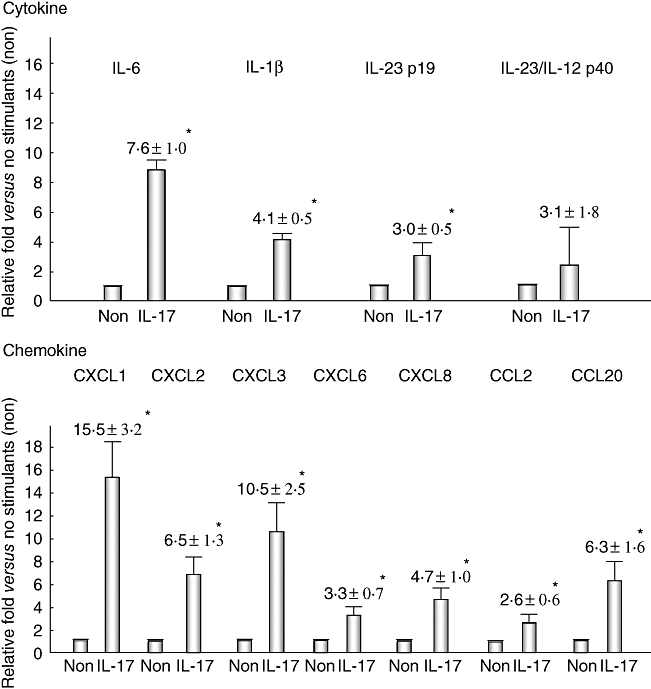
The effect of interleukin (IL)-17 on the production of cytokines and chemokines in cultured human biliary epithelial cells (BECs). Real-time polymerase chain reaction revealed that the expression of Th17-inducible cytokines (IL-6 and IL-1β), Th17-maintaining cytokines (IL-23 p19 and IL-23/IL-12 p40) and IL-17-induced chemokines [epithelial-derived neutophil activating protein (CXCL)-1, CXCL2, CXCL3, CXCL6, CXCL8, monocyte chemoattractant protein (CCL)-2 and CCL20] is basically up-regulated by stimulation with IL-17 and there are significant differences except for IL-23/IL-12 p40 between no stimulants (non) and IL-17 (P < 0·05). Duplicate experiments were performed using three lines of BECs, and data are shown as the mean ± standard error of the mean.
Fig. 7.
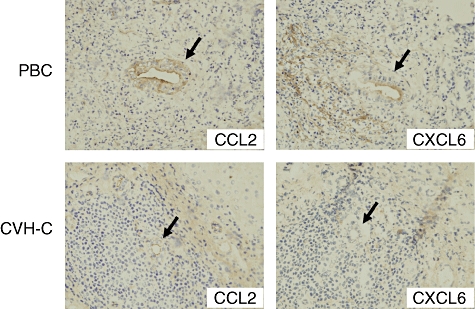
Immunohistochemistry for monocyte chemoattractant protein (CCL)-2 and epithelial-derived neutophil activating protein (CXCL)-6 using frozen sections of primary biliary cirrhosis (PBC) and chronic viral hepatitis C (CVH-C). In PBC, damaged bile ducts show aberrant expression of CCL2 and CXCL6, but interlobular bile ducts in CVH-C lack expression of these chemokines.
Discussion
Th17 cells are part of the mucosal host defence system and their major role seems to be protection against infections sustained by extracellular bacteria. Moreover, since the discovery that under certain conditions, Th17 cells can also be involved in the pathogenesis of chronic inflammatory disorders including models of some autoimmune diseases, there has been intense interest in the relative contributions of Th17 and Th1/Th2 cells to the pathogenesis of these diseases. Th17 cells are characterized by the production of primarily IL-17(A), known formerly as CTLA8. Lan et al. have already examined the IL-17-positive cells in PBC and concluded no differerence in intraportal IL-17-positive cells between PBC and chronic active hepatitis including CVH-C, non-alcoholic steatohepatitis and autoimmune hepatitis [27]. However, in the present study we noted interlobular bile ducts showing bile duct damage and cholangitis, including CNSDC, which is characteristic histology of PBC, and evaluated IL-17-positive mononuclear cells around these bile ducts in PBC. Our immunohistochemical examination revealed that IL-17-positive infiltrating mononuclear cells were present mainly at the interface of inflammed portal tracts in cases of PBC and CVH-C, and also in PBC, accumulated around the damaged interlobular bile ducts including CNSDC. In CVH-C, hepatitis-associated bile duct damage is seen frequently, but there is no periductal accumulation of IL-17-positive infiltrating cells. These findings suggest that Th17 cells are associated with interface hepatitis in chronic liver diseases and that the Th17-related peribiliary cytokine milieu is enhanced in PBC and implicated in the histogenesis of the sustained cholangitis of PBC. Although some epithelial cells such as synovial cells in rheumatoid arthritis patients and glandular cells in salivary glands have been reported to be a source of IL-17 [28,29], no epithelial elements within livers expressed IL-17 and cultured BECs also lacked IL-17 mRNA (data not shown).
So far, studies on the Th1/Th2 balance in liver diseases have been based mainly on the profile of cytokines using peripheral blood lymphocytes or liver tissues, and both PBC and CVH-C have been thought to belong to Th1-predominant diseases [4,30–32]. In this study, in situ detection of IL-17 by immunohistochemistry revealed that the number of IL-17-positive mononuclear cells in the periductal area of inflamed portal tracts was larger in PBC than in CVH-C. These findings suggest that Th17 cells as well as Th1 cells are important in the pathogenesis of the cholangiopathy of PBC. Th1 cells are associated with the pathogenesis of granulomatous lesions and immune reactions to intracellular microorganisms, in which Th17 cells are associated with sustained inflammation including autoimmunity. In this study, we could not reach a final conclusion as to whether Th1 or Th17 is the most important in the histopathogenesis of PBC. In IL-10(–/–) mice, a model of Crohn's disease, a peroxisome proliferator-activated receptor (PPAR) α ligand, fenofibrate, has been reported to repress both IL-17 (Th17) and interferon (IFN)-γ (Th1) expression and improve colitis [33]. This finding suggests the sequential involvement and different functions of Th17 and Th1 cells rather than an exclusive role for these Th cells during the development of inflammatory and autoimmune diseases. Therefore, we speculate that also in PBC, both Th1 and Th17 cytokine milieus are important in the pathogenesis of cholangiopathy.
As Th17-inducible cytokines, TGF-β and IL-6 were identified in mice. However, a later study demonstrated that, in humans, IL-1β but not TGF-β is needed together with IL-6 to induce Th17 cells. IL-23 was also identified initially as a Th17-inducible cytokine, but is now recognized as a functional cytokine of Th17 cells. In contrast, IL-27, IL-12p35 and IFN-gamma play roles in the regulation of Th1 cell differentiation and also act as anti-Th17 differentiation cytokines [34]. Recently, a role of innate immunity via TLR signalling in the differentiation of Th17 cells has been described in inflammatory bowel diseases and multiple sclerosis; TLR signalling in B cells and T cells is responsible for the induction of Th17 [35,36] and TLR signalling in T cells is responsible for the induction of Th17, but not Th1 cells [37]. The present study demonstrated that bacterial PAMPs (LPS and Pam3CSK4) induced the production of Th17-inducible cytokines (IL-6 and IL-1β) and a Th17-maintaining cytokine (IL-23) in cultured BECs. Because the expression of IL-1β accompanied that of caspase 1 in cultured BECs, IL-1β could be released into the culture medium in a functional cleaved form. As for anti-Th17 differentiation cytokines, cultured BECs did not express mRNAs of IL27 nor IFN-gamma, irrespective of the presence of PAMPs. Although IL-12 p35 mRNA was constantly detected, significant changes by the treatments with bacterial PAMPs (LPS and Pam3CSK4) were not found (data not shown). Immunohistochemistry also revealed that interlobular bile ducts, in particular, damaged bile ducts in PBC, expressed IL-6 and IL-1β. IL-23 p19 and caspase 1 were expressed constitutively in intrahepatic bile ducts including interlobular bile ducts of normal livers as well as PBC and CVH-C. These results suggest that the biliary innate immune response to bacterial components involves the production of Th17-inducible and -maintaining cytokines in biliary epithelial cells and the differentiation into Th17 cells of periductal dendritic cells and macrophages; that is, biliary innate immunity plays a role in the induction and maintenance of Th17 cells in the periductal area in cases of PBC.
There remain a number of unanswered questions, such as the role of Th17 cells in host defence and how Th17 cells cause tissue damage. It seems likely that Th17 cells are important in the host response to commensal bacteria as well as enteric pathogens. An alternative view is that Th17 cells do not have a specific role in host defence against pathogens, but that Th17 and regulatory T cells (Treg) cells work together to elicit or restrain tissue inflammation. Because heterodimeric receptors for IL-17 (IL-17RA and IL-17RC) are expressed in many cells, IL-17 has the ability to induce the production of cytokines and chemokines by epithelial and vascular endothelial cells. Recently, the presence of a transcription factor, Act1, has reported to be imperative in IL-17 receptor signalling in autoimmune and inflammatory diseases [20,21]. This study has demonstrated that biliary epithelial cells also constantly possess IL-17RA, IL-17RC and Act1, and produced Th1-inducible cytokines (IL-6 and IL-1β) on treatment with IL-17, suggesting that periductal IL-17 itself could enhance the induction of the Th17-dominant milieu. Moreover, we have confirmed that the production of all IL-17-induced chemokines (CXCL1, CXCL2, CXCL3, CXCL6, CXCL8, CCL2 and CCL20), reported previously in several cells [16–20], were expressed in cultured BECs stimulated with IL-17 and also that the expression of CCL2 and CXCL6 was enhanced in damaged bile ducts of PBC. Because Th17 cells possess chemokine receptors, CCR2, CCR4 and CCR6 [10,38,39], CCL2 and CCL20 among IL-17-induced chemokines in cultured BECs could play a role in the attraction of Th17 cells. These findings suggest that IL-17 takes part in the chronicity of cholangiopathy in PBC via the production of cytokines and chemokines. Moreover, in IL-10(–/–) mice, a model of Crohn's disease, the expression of chemokines (CCL2, CCL20 and CXCL10) as well as of IL-17 (Th17) and IFN-γ (Th1) has been reported to be attenuated by the PPAR α ligand fenofibrate [33], supporting that CCL2 and CCL20 are important in the pathogenesis of Th17-related chronic inflammation. Therefore, we speculate that these chemokines are important in sustaining the chronic inflammation accompanying several immune cells including effecter cells and dendritic cells. Further study is needed to clarify the mechanism of Th17 cell-mediated bile duct injury in PBC.
In conclusion, the present study demonstrated that IL-17-positive cells were accumulated around the damaged bile ducts in PBC and that biliary epithelial cells possessed the ability to produce Th17-inducible cytokines (IL-6 and IL-1β) and Th17-maintaining cytokine (IL-23) as a result of the innate immune response. These results suggest that periductal IL-17-secreting cells facilitate the migration of inflammatory cells including Th17 cells around the bile ducts in PBC, which could further aggravate the chronic cholangitis, and also that the differentiation into Th17 cells is associated closely with biliary innate immunity. It seems likely that periductal Th17 cells propagate and modulate the chronic cholangitis and bile duct damage in PBC, although further studies are necessary to establish the exact roles of Th17 cells in the aetiopathogenesis of cholangiopathy in PBC.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Health, Labour and Welfare of Japan and Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Acknowledgments
The authors declare no conflicts of interest.
References
- 1.Parikh-Patel A, Gold EB, Worman H, Krivy KE, Gershwin ME. Risk factors for primary biliary cirrhosis in a cohort of patients from the United States. Hepatology. 2001;33:16–21. doi: 10.1053/jhep.2001.21165. [DOI] [PubMed] [Google Scholar]
- 2.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835–45. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harada K, Ohira S, Isse K, et al. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab Invest. 2003;83:1657–67. doi: 10.1097/01.lab.0000097190.56734.fe. [DOI] [PubMed] [Google Scholar]
- 4.Harada K, Isse K, Kamihira T, Shimoda S, Nakanuma Y. Th1 cytokine-induced downregulation of PPARgamma in human biliary cells relates to cholangitis in primary biliary cirrhosis. Hepatology. 2005;41:1329–38. doi: 10.1002/hep.20705. [DOI] [PubMed] [Google Scholar]
- 5.Harada K, Isse K, Nakanuma Y. Interferon gamma accelerates NF-kappaB activation of biliary epithelial cells induced by Toll-like receptor and ligand interaction. J Clin Pathol. 2006;59:184–90. doi: 10.1136/jcp.2004.023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–7. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 9.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 10.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 11.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgamma t. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–7. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–2. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elson CO, Cong Y, Weaver CT, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 15.Leppkes M, Becker C, Ivanov II, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–67. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–43. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 17.Kao CY, Huang F, Chen Y, et al. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–85. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 18.Prause O, Laan M, Lotvall J, Linden A. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol. 2003;462:193–8. doi: 10.1016/s0014-2999(03)01341-4. [DOI] [PubMed] [Google Scholar]
- 19.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–13. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–54. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 21.Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–56. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 22.Kaji K, Nakanuma Y, Sasaki M, et al. Hepatitic bile duct injuries in chronic hepatitis C: histopathologic and immunohistochemical studies. Mod Pathol. 1994;7:937–45. [PubMed] [Google Scholar]
- 23.Lefkowitch JH, Schiff ER, Davis GL, et al. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. The hepatitis interventional therapy group. Gastroenterology. 1993;104:595–603. doi: 10.1016/0016-5085(93)90432-c. [DOI] [PubMed] [Google Scholar]
- 24.Kamihira T, Shimoda S, Harada K, et al. Distinct costimulation dependent and independent autoreactive T-cell clones in primary biliary cirrhosis. Gastroenterology. 2003;125:1379–87. doi: 10.1016/j.gastro.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Harada K, Ohba K, Ozaki S, et al. Peptide antibiotic human beta-defensin-1 and -2 contribute to antimicrobial defense of the intrahepatic biliary tree. Hepatology. 2004;40:925–32. doi: 10.1002/hep.20379. [DOI] [PubMed] [Google Scholar]
- 26.Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857–64. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- 27.Lan RY, Salunga TL, Tsuneyama K, et al. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun. 2009;32:43–51. doi: 10.1016/j.jaut.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chabaud M, Durand JM, Buchs N, et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42:963–70. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren's syndrome: findings in humans and mice. Arthritis Rheum. 2008;58:734–43. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada K, Van de Water J, Leung PS, et al. In situ nucleic acid hybridization of cytokines in primary biliary cirrhosis: predominance of the Th1 subset. Hepatology. 1997;25:791–6. doi: 10.1002/hep.510250402. [DOI] [PubMed] [Google Scholar]
- 31.Dienes HP, Lohse AW, Gerken G, et al. Bile duct epithelia as target cells in primary biliary cirrhosis and primary sclerosing cholangitis. Virchows Arch. 1997;431:119–24. doi: 10.1007/s004280050077. [DOI] [PubMed] [Google Scholar]
- 32.Bertoletti A, D'Elios MM, Boni C, et al. Different cytokine profiles of intraphepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology. 1997;112:193–9. doi: 10.1016/s0016-5085(97)70235-x. [DOI] [PubMed] [Google Scholar]
- 33.Lee JW, Bajwa PJ, Carson MJ, et al. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology. 2007;133:108–23. doi: 10.1053/j.gastro.2007.03.113. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida H, Yoshiyuki M. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–47. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 35.Marta M, Andersson A, Isaksson M, Kampe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur J Immunol. 2008;38:565–75. doi: 10.1002/eji.200737187. [DOI] [PubMed] [Google Scholar]
- 36.Lampropoulou V, Hoehlig K, Roch T, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–73. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 37.Fukata M, Breglio K, Chen A, et al. The myeloid differentiation factor 88 (MyD88) is required for CD4+ T cell effector function in a murine model of inflammatory bowel disease. J Immunol. 2008;180:1886–94. doi: 10.4049/jimmunol.180.3.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato W, Aranami T, Yamamura T. Cutting edge: human Th17 cells are identified as bearing CCR2+CCR5– phenotype. J Immunol. 2007;178:7525–9. doi: 10.4049/jimmunol.178.12.7525. [DOI] [PubMed] [Google Scholar]
- 39.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–21. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]


