Abstract
The 13q33–34 region harbours a susceptibility locus to Ascaris lumbricoides, although the underlying genes are unknown. Immunoglobulin (Ig)E and IgG confer protective immunity and here we sought to investigate in an endemic population whether LIG4, TNFSF13B and IRS2 genes influence IgE and IgG levels against Ascaris and the ABA-1 allergen as a putative resistance marker. Mite-allergic asthmatic patients were analysed for potential relationships between Ascaris predisposition and allergy. One thousand and sixty-four subjects from Cartagena, Colombia, were included. Single nucleotide polymorphisms (SNPs) were genotyped using TaqMan assays. Antibody levels were measured by enzyme-linked immunosorbent assay. Linear and logistic regressions were used to model effects of genotypes on antibody levels. The GG genotype of LIG4 (rs1805388) was associated with higher IgE levels to Ascaris compared with other genotypes. TNFSF13B (rs10508198) was associated positively with IgG levels against Ascaris extract and IgE levels against ABA-1. In asthmatics, IRS2 (rs2289046) was associated with high total IgE levels. Associations held up after correction by population stratification using a set of 52 ancestry markers, age, sex and disease status. There was no association with asthma or mite sensitization. In a tropical population, LIG4 and TNFSF13B polymorphisms are associated with specific IgE and IgG to Ascaris, supporting previous linkage studies implicating the 13q33 region. Our results suggest that genes protecting against parasite infections can be different to those predisposing to asthma and atopy.
Keywords: ABA-1, Ascaris lumbricoides, genetic association, IgE, mites
Introduction
Ascaris lumbricoides infection is an important health problem in tropical regions. Immunity to this nematode probably involves mechanisms similar to allergic reactions, including specific immunoglobulin (Ig)E synthesis. Studying the genetic regulation of these mechanisms promotes our understanding of both resistance to parasite infections and it potential links with the pathogenesis of allergic diseases [1]. Genetic studies on the immune response to Ascaris have identified several pertinent loci. The role of the major histocompatibility complex (MHC) was studied initially in mice, showing that both IgG and IgE antibody repertoires against a 14·4 kDa antigen/allergen, ABA-1 of A. suum is determined genetically and restricted to the I-As locus and RT1u haplotype of the MHC of mice and rats, respectively [2]. In humans, human leucocyte antigen (HLA) A30/31 and DQw2 alleles have been reported to be associated with resistance to infection [3,4]. Further research showed that Ascaris susceptibility is polygenic and involves other genes, such as STAT6 encoded in a non-MHC region [5–7]. Epidemiological studies have described considerable diversity in Ascaris susceptibility among subjects living under the same conditions; indeed, infection intensity is typically overdispersed, with 10–20% of the population harbouring most of the parasites [8]. Pedigree studies in the Jirel population of Nepal demonstrated a strong genetic component accounting for 30–50% of the variation in worm burden [9], also observed in animal models of infection [10]. Williams-Blanguero et al.[11] performed genomic scans for quantitative trait loci (QTL) influencing A. lumbricoides susceptibility in Jirels, implicating two chromosomal regions with significant effects on variation in egg counts at 13q33–34 [log10 of the odds (LOD) score 4·3] and 1p32 (LOD score 3·01). Recently, the 13q33 region was confirmed as a QTL for Ascaris susceptibility, although underlying genes are still unknown [12].
Experimental data have shown that mechanisms influencing migration of larvae, ability to expel parasites and antibody production determine Ascaris susceptibility [13], specific IgE (sIgE) against Ascaris being one of the best-supported factors determining resistance [14–17]. An up-regulation of IgE synthesis during infection, especially in putatively immune subjects [18], and sIgE to ABA-1 has been observed among people with the lowest worm loads and natural resistance to Ascaris[19]. In addition, a linkage signal at the 13q33–34 region and total IgE levels (tIgE) was detected in the Jirel population [11].
Previous studies have used faecal egg counts as a quantitative phenotype of Ascaris infection, but considering that sIgE is protective and IgE synthesis is thought to be controlled genetically, we sought to investigate whether polymorphisms of genes LIG4 (ligase IV), TNFSF13B (B cell activation factor) and IRS2 (insulin receptor substrate-2), both genes located proximal to the maximum linkage peak within the 13q33–34 region and related mechanistically to antibody production, are associated with tIgE, sIgE and sIgG to Ascaris in a population living in the tropics and exposed endemically to this nematode. Furthermore, considering genetic studies suggesting that Ascaris resistance predisposes to asthma and atopy [7], we included a case–control study to investigate whether those polymorphisms are associated with these traits.
Methods
Study design
The population lived in Cartagena, a tropical city in Colombia, whose genetic background derives from an admixture between Spaniards, Africans and Native Americans [20,21]. All subjects lived in an urban, non-industrialized setting, having access to water and electricity and belonging to the lower three (of six) socio-economic strata in the city. Nematode infection is endemic, and most people are naturally exposed to A. lumbricoides. One thousand and sixty-four subjects participated, including 636 non-related healthy volunteers without history of asthma, allergy or other diseases as evaluated by a questionnaire. To investigate associations between aforementioned factors and asthma by a case–control study, we included 428 asthmatic patients recruited from the Social Security Clinic and public health centres in the years 2002–2005. Asthma was defined according to the Global Initiative for Asthma (GINA) criteria, using a standardized questionnaire tested previously in patients with a history of physician-diagnosed asthma [22,23]. The diagnosis was confirmed by a physician belonging to the research staff, sustained on a clear clinical history with clinical symptoms as described previously [24,25]. The Bioethics Committee of the University of Cartagena approved the study; a full verbal explanation of the investigation was given and written informed consent was obtained from all participants.
Genotyping
Three SNPs in LIG4 (rs1805388), TNFSF13B (rs10508198) and IRS2 (rs2289046) were chosen (Table 1) according to the following criteria: (i) a minor allele frequency higher than 10%; (ii) experimentally confirmed or suspected functional effect; and (iii) and availability of tag SNPs for African and European panels in the HapMap database. Genomic DNA was extracted from peripheral white blood cells using the salting-out method described by Miller et al.[26]. Polymorphisms were genotyped using validated TaqMan 5′-exonuclease assays, designed and manufactured by Applied Biosystems (Foster City, CA, USA). After amplification, end-point detection of fluorescence was performed at 60°C and automatic genotype calling was achieved with a quality value above 98% using the 7300-system sds software in a 7300-real time–polymerase chain reaction (PCR) system (Applied Biosystems).
Table 1.
Polymorphisms analysed in this study.
| Gene name | Protein encoded | dbSNP | SNP | Genomic location | Transcript location | Functional effect |
|---|---|---|---|---|---|---|
| LIG4 | Ligase-IV | rs1805388 | G/A | 13q33.3 | G299A | Missense substitution (Thr9Ile) |
| 107661592 | Exon 2 | Enzymatic activity affected | ||||
| TNFSF13B | B cell activating factor (BAFF) | rs10508198 | C/G | 13q33.3 | Intron 3 | Unknown |
| 107740789 | ||||||
| IRS-2 | Insulin receptor substrate-2 | rs2289046 | T/C | 13q34 | 5276 | Unknown |
| 109205907 | 3′UTR |
SNP, single nucleotide polymorphism; 3′UTR, three-prime untranslated region.
Ascaris and mite extracts
Ascaris extract was prepared by an acetone–saccharose precipitation method [27]. Because it is the source employed currently for in vitro testing, and because of its almost identical protein profile with A. lumbricoides[28], Ascaris suum extract was used in this study. Adult worms were washed in 0·85% sterile saline with 0·01% of penicillin and streptomycin and homogenized in 4 ml of 0·25 M saccharose per gram of parasite. Extraction was performed by shaking the homogenate several times in cold acetone (–80°C), centrifuged at 9500 g, lyophilized and reconstituted in phosphate-buffered saline (PBS) pH 7·2 in a proportion of 0·4 ml per initial volume of homogenate. Finally, the supernatant was dialyzed against PBS pH 7·2 using a 3500 molecular weight cut-off membrane. Protein concentration (1·8 mg/ml) was determined by the Lowry method. The lyophilized extract was kept at −20°C until used. Extracts of Dermatophagoides pteronyssinus and Blomia tropicalis were produced as described previously [29].
Purification of recombinant ABA-1 (rABA-1)
cDNA encoding ABA-1 from A. suum was cloned into pGEX-1λT, and expressed as a glutathione-S-transferase (GST) fusion protein in Escherichia coli BL21. The protein represents a single A-type repeat unit of the ABA-1 polyprotein (ABA-1A), with only a few amino acid differences from the ABA-1 of A. lumbricoides and fully immunologically cross-reactive [30–32]. Expression was induced by adding isopropyl β-D-thiogalactopyranoside (0·1 mM) and incubating 4 h at 37°C. The ABA-1-GST fusion protein was isolated by affinity chromatography on glutathione-Sepharose beads and rABA-1 was released from the GST fusion partner by digestion with thrombin, collected, dialyzed and kept at −20°C.
Total IgE and mite-specific IgE
Total IgE was determined in duplicate using an enzyme-linked immunosorbent assay (ELISA) kit (RIDASCREEN; R-Biopharm, Darmstadt, Germany), according to the manufacturer's instructions. Levels above and below 343 IU/ml [mean tIgE in the control group + 1 standard deviation (s.d.)] were considered as high and low, respectively. Because B. tropicalis and D. pteronyssinus are the main source of sensitization in tropical environments [33–35] and the prevalence of IgE sensitization to other aeroallergens is very low in Cartagena [36], sIgE against these two mite extracts was determined by indirect ELISA, as described previously [24]. Atopy was defined as positive sIgE to at least one of the two mite extracts tested.
Specific IgE and IgG to Ascaris extract and rABA-1
This was detected by ELISA using microtitre plates (Immulon-4 Dynatech, Chantilly, VA, USA) following the same protocol as for the mite antibody ELISA. The appropriate concentrations of antigen and secondary antibody were obtained by titration. Ascaris extract and rABA-1 (1 µg/well) were diluted in 64 mM sodium carbonate/bicarbonate buffer (pH 9·6) incubated overnight, washed with 0·1% Tween-20/PBS (TPBS) and blocked with 3% bovine serum albumin (BSA)/PBS. Serum samples diluted 1 : 5 in blocking buffer were added to the wells and incubated overnight, washed and incubated with 100 µl of anti-human IgE alkaline-phosphatase conjugate (Sigma, St Louis, MO, USA) diluted 1 : 500. The assay was developed with p-nitrophenylphosphate chromogen (15 mg/ml; Sigma) and the absorbance measured at 405 nm using a Spectrophotometer (Spectra MAX 250 Molecular Device; Sunnyvale, CA, USA). IgE levels above 0·113 optical density (OD) (mean OD of six negative, non-allergic, non-parasitized controls + 3 s.d.) was considered positive. To determine specific IgG (sIgG) against Ascaris, sera samples were diluted 1 : 200 and for detecting sIgG against rABA-1 diluted 1 : 50. The secondary antibody was anti-human IgG alkaline phosphatase conjugate diluted 1 : 10 000. For both nematode antigens, a level above 0·65 OD (mean OD of four sera of non-parasitized subjects + 3 s.d.) was considered as positive. All samples were assayed in duplicate, and interassay and intra-assay variation coefficients were lower than 15% and 10%, respectively.
Statistics
Statistical analyses were performed using the Statistical Package for the Social Sciences software (spss version 16 for Windows; SPSS Inc., Chicago, IL, USA). Comparison of demographic characteristics between asthmatics and controls were performed by χ2 tests and Student's t-test, as needed. The phenotypes included in the analyses were asthma as a dichotomous trait (presence/absence), tIgE, sIgE against Ascaris extract, rABA-1, D. pteronyssinus and B. tropicalis, sIgG against Ascaris extract and rABA-1. Comparisons of allele and genotype frequencies between asthmatic and controls were performed using Fisher's exact test. Data for tIgE and sIgE were log10-transformed (IgElog) to be used in the regression models. IgG data were used directly in the analysis as they were distributed normally. Logistic regression was used to model the effect of genotypes and age and sex on asthma. In the total sample, linear regression was used to model the effect of genotypes on antibody levels, including age, sex and asthma as covariates. Departures from Hardy–Weinberg equilibrium and LD (D') between SNPs were assessed with arlequin software. The significance level was set at P < 0·05 and, because multiple testing was performed, P values were corrected using the formula Pc = 1 − (1 − P)n. To detect gene–gene interactions we applied the Multifactor Dimensionality Reduction (mdr) software, version 1·1·0 (Computational Genetics Laboratory, Dartmouth Medical School, Hanover, NH, USA).
Correction by population stratification
A panel of 52 ancestry informative markers (AIMs) was also genotyped in the Cartagena sample (37). These markers show large differences in frequency between the parental populations and were used to control for the presence of genetic structure due to admixture. To test for association of the SNPs in the candidate genes within the phenotypes correcting by genetic population structure, we used the program admixmap (available at http://sourceforge.net/projects/admixmap). This is a general-purpose program for modelling population admixture with genotype and phenotype data, based on a combination of Bayesian and classical methods. For this analysis, the Cartagena population was modelled as formed by admixture between three subpopulations: European, Native American and West African. Score tests for allelic association with the traits, conditional on individual admixture and age and sex as covariates were constructed by testing the coefficient b for the effect of the allele under study (coded as 0, 1 or 2 copies) in a regression model. An approximation to the maximum likelihood estimate of the effect size was obtained from the score test by dividing the score by the observed information. P < 0·05 was considered statistically significant.
Results
Demographic data concerning the population studied are summarized in Table 2. Both total and specific IgE levels against nematode and mite allergens were significantly higher in asthmatics, while IgG against Ascaris extract and rABA-1 were significantly higher in controls. Neither total nor sIgE levels were significantly different between smokers and non-smokers, both in patients and controls (data not shown). Genotype distributions of SNPs were in Hardy–Weinberg equilibrium in asthmatics, controls and the total sample. Allele and genotype frequencies are shown in Table 3; we found no association between any polymorphism and asthma. In the total sample, D' values were not statistically significant, indicating that they were not in linkage disequilibrium, and remained similar when analysing either asthmatics or controls individually.
Table 2.
Demographic characteristics of the population.
| Study population (n = 1064) |
|||
|---|---|---|---|
| Variables | Asthmatics (n = 428) | Healthy controls (n = 636) | P value |
| Age‡ | 36·2 ± 18·2 (8–84 years) | 35·7 ± 18·0 (8–89 years) | 0·68 |
| Gender, female (%) | 266 (62·1) | 359 (56·4) | 0·07 |
| Duration of asthma | 17·9 ± 14·7 | – | – |
| Total IgE, IU/ml† | 754·2 (251–1075) | 129·7 (47–326·3) | < 0·001 |
| IgE to Blomia tropicalis (OD)‡ | 0·886 ± 1·1 | 0·160 ± 0·2 | < 0·001 |
| IgE to Dermatophagoides pteronyssinus (OD)‡ | 0·575 ± 0·8 | 0·146 ± 0·2 | < 0·001 |
| Mite sensitized (%) | 339 (79·2) | 207 (32·5) | < 0·001 |
| Nematode Ig levels (OD)‡ | |||
| IgE to Ascaris | 0·194 ± 0·26 | 0·137 ± 0·11 | < 0·001 |
| IgE to rABA-1 | 0·184 ± 0·17 | 0·152 ± 0·11 | < 0·001 |
| IgG to Ascaris | 2·01 ± 0·54 | 2·10 ± 0·62 | 0·01 |
| IgG to ABA-1 | 1·49 ± 0·50 | 1·73 ± 0·58 | < 0·001 |
Median (interquartile range).
Mean ± standard deviation; OD, optical density units; Ig, immunoglobulin.
Table 3.
Allele and genotype distribution among asthmatic and controls.
| Asthma risk |
|||||
|---|---|---|---|---|---|
| Gene | Genotype | Asthmatic patients | Healthy controls | OR (95% CI)† | P-value |
| LIG4 (rs1805388) | G | 717 (84·2%) | 1068 (84·9%) | 0·95 (0·74–1·21) | 0·68 |
| A | 135 (15·8%) | 192 (15·2%) | |||
| GG | 302 (70·9%) | 450 (71·4%) | |||
| GA | 113 (26·5%) | 168 (26·7%) | |||
| AA | 11 (2·6%) | 12 (1·9%) | |||
| Total Genotyped | 426 | 630 | |||
| TNFSF13B (rs10508198) | G | 702 (82·8%) | 1058 (83·7%) | 0·93 (0·73–1·18) | 0·93 |
| C | 146 (17·2%) | 206 (16·3%) | |||
| GG | 290 (68·4%) | 440 (69·6%) | |||
| GC | 122 (28·8%) | 178 (28·2%) | |||
| CC | 12 (2·8%) | 14 (2·2%) | |||
| Total Genotyped | 424 | 632 | |||
| IRS2 (rs2289046) | T | 573 (67·3%) | 852 (67·7%) | 0·98 (0·82–1·18) | 0·88 |
| C | 279 (32·7%) | 406 (32·3%) | |||
| TT | 196 (46·0%) | 296 (47·1%) | |||
| TC | 181 (42·5%) | 260 (41·3%) | |||
| CC | 49 (11·5%) | 73 (11·6%) | |||
| Total Genotyped | 426 | 629 |
Logistic regression (age- and sex-adjusted); CI, confidence interval; OR, odds ratio.
The Thr9Ile variant of LIG4 is associated with specific IgE levels to Ascaris
In the total sample, using a linear regression model including age, sex and asthma as covariates, the genotypes of Thr9Ile were associated with IgElog against Ascaris extract (P = 0·01, Pc = 0·03). This association was driven by a higher level of IgE in those individuals carrying the wild-type genotype (average IgElog Thr/Thr = 0·279 versus Thr/Ile + Ile/Ile = 0·240, P = 0·008, Pc = 0·024). This genotypic association was also detected when only asthmatic patients were considered in the analysis, and similar Thr/Thr genotype carriers had higher levels of sIgE against Ascaris (average IgElog Thr/Thr = 0·336 versus Thr/Ile + Ile/Ile = 0·266, P = 0·009, Pc = 0·027). These associations were also observed when individual admixture estimates were included in the analysis (P = 0·01, Pc = 0·03); sIgE to rABA-1 and sIgG levels (Ascaris and rABA-1) were not affected by this polymorphism. There was no association of this polymorphism of LIG4 with asthma, tIgE or sIgE levels to the mites B. tropicalis and D. pteronyssinus when analysed as quantitative or dichotomous variables.
Specific IgG levels to Ascaris are strongly influenced by TNFSF13B
The intronic variant in TNFSF13B (G3980>C, rs.10508198) was not associated with sIgE against Ascaris, neither as a quantitative nor a dichotomous trait. The results were similar when stratifying according to disease status and after correcting by population stratification. Interestingly, in the total population, G3980>C was associated significantly with sIgG levels against Ascaris (P = 0·001, Pc = 0·003) after adjusting for age, sex and asthma. Levels of sIgG to Ascaris were significantly higher in carriers of the wild-type GG genotype (average OD ± s.d. = 2·11 ± 0·5) compared to the GC + CC carriers (1·97 ± 0·58; P = 0·001, Pc = 0·003 Fig. 1a). After correcting for population structure, the G3980>C polymorphism remains associated consistently with sIgG against Ascaris (P = 0·004, Pc = 0·012).
Fig. 1.
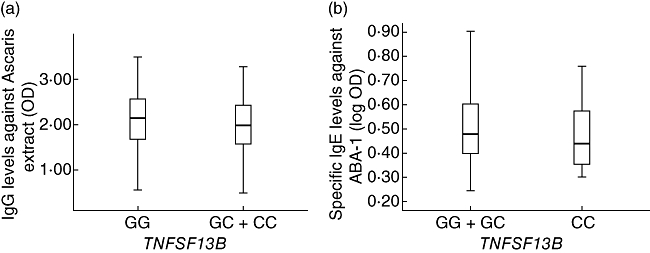
(a) Effects of B cell activation factor (TNFSF13B) (rs10508198) polymorphism on specific immunoglobulin (Ig)G levels against Ascaris. Homozygotes for the wild-type genotype have higher levels of IgG to Ascaris than carriers of one or two doses of the variant allele C. (b) Effects of TNFSF13B (rs10508198) polymorphism on specific IgE levels to ABA-1 in asthmatics. CC homozygotes have lower levels of specific IgE against ABA-1 compared with carriers of the other genotypes.
We found no association of any genotype of G3980>C with sIgE or sIgG against rABA-1 in the total sample (P = 0·23 and P = 0·61, respectively). However, when including only asthmatic patients, we found a significant relationship between genotypes and sIgE levels against rABA-1 (P = 0·02, Pc = 0·06), and this effect was driven by lower levels in CC homozygotes (P = 0·003, Pc = 0·009, Fig. 1b). This association was maintained after adjusting by individual admixture estimates in the asthmatic group (P = 0·031, Pc = 0·09). There was no association of any allele or genotype of this polymorphism with asthma, tIgE and sIgE levels to B. tropicalis or D. pteronyssinus.
The IRS2 gene is associated with total IgE levels in asthmatics
In the total sample, the T5276>C (rs.2289046) polymorphism of IRS2 was not associated with any of the specific antibody isotypes to Ascaris extract or rABA-1. In addition, no association was observed with asthma, tIgE levels or sIgE to mites. However, when only asthmatics were included in the analysis, an association between genotypes and tIgElog levels was found (P = 0·009, Pc = 0·027); carriers of the mutant allele C were grouped (average tIgE ± s.d. = 2·69 ± 0·47) and compared with those individuals with the TT genotype (2·81 ± 0·42, Fig. 2). The effect of age and sex was corrected in the model (P = 0·01, Pc = 0·03) and this association was still observed after correcting by population structure (P = 0·01, Pc = 0·03). There was no significant prediction of phenotypes with any of the genotype combinations when looking for gene–gene interactions, considering all possible models.
Fig. 2.
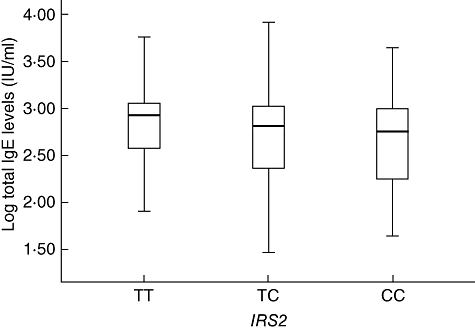
Association between insulin receptor substrate-2 (IRS2) (rs2289046) and total immunoglobulin E levels in asthmatics. This association was driven by higher levels in TT genotype carriers.
Discussion
Two genome-wide linkage scans have previously detected a QTL in the 13q33 region influencing Ascaris susceptibility [11,12]. Here, we show that variants of LIG4 and TNFSF13B genes are associated with IgE and IgG levels against Ascaris. Our results were obtained in a sex- and age-matched population, being the largest sample size yet for an association study of IgE response to Ascaris, with a power of 0·90 to detect an odds ratio of 1·5 for asthma. Although the genetic backgrounds of ours and the Jirel population will differ in many respects, the 13q33 region effect on two related phenotypes (namely, faecal egg-counts and specific antibodies against Ascaris) was detected, supporting the idea that this region harbours an important locus or loci controlling susceptibility to this nematode.
The environmental conditions make our population particularly suitable for genetic studies on the IgE response for two main reasons. First, Ascaris infection is endemic in the region [38] and, despite the fact that the population under study live in an urban setting, limited sanitary conditions prevail, and Ascaris exposure will occur early in life. Secondly, the city is in a tropical area and the population is exposed perennially to high concentrations of mite allergens [39–41], with about 30% of the population exhibiting IgE sensitization to mites but without allergic symptoms [42]. Under these conditions, particular gene–environment interactions could emerge that make it easier to detect the effects of genetic polymorphisms on the strength and specificity of antibody responses.
The associations we have observed clearly prompt elucidation by direct experimentation. In addition, as we employed tag SNPs, we cannot define whether the associated variants are causal or proxies of other polymorphisms, and therefore it will very important to analyse additional SNPs in the same genes in order to define variants associated primarily with the phenotypes included in the study. However, those genes participate in different pathways of IgE synthesis. We found a dose relationship between Thr9Ile of LIG4 and sIgE levels against Ascaris, and ligase-IV is an essential enzyme of the non-homologous end-joining pathway and class-switch recombination [43,44]. The substitution of threonine by isoleucine decreased to around 50% the adenylation and double-strand ligation activity of ligase-IV [45]. It is possible that this variant could influence the immunoglobulin recombination rate and IgE isotype levels during infection.
The G3980C polymorphism of TNFSF13B was associated with the IgG responses to Ascaris and the IgE response to rABA-1. This gene encodes the cytokine B cell activating factor (BAFF), a member of the ligand tumour necrosis factor superfamily that controls B cell development and induces immunoglobulin secretion, B cell proliferation, differentiation and survival [46]. BAFF is expressed in myeloid-derived cells and alternative splicing generates four isoform variants [47]. Previous studies have demonstrated that an isoform lacking exon 3 is a dominant negative inhibitor of the full-length BAFF, negating its function by forming homomultimers and heteromers [48]. Although the effect of this SNP is unknown, it could influence alternative splicing and transcriptional regulation of TNFSF13B.
IRS2 encodes for insulin receptor substrate 2, an adaptor molecule in the interleukin (IL)-4Rα signalling pathway [49,50]. Here we found an association between the TT genotype and higher levels of total IgE in asthmatics. This effect may probably be observed only in the context of an asthmatic genetic background, where additional variants predisposing to atopy are expressed simultaneously.
In previous linkage studies, Ascaris infection was assessed by worm load and expressed as eggs per gram of faeces, but others have evaluated genetic associations and linkages based on IgE antibody responses to Ascaris[6,51]. Egg identification seems to be more specific, but it has been described that positive serological tests are more frequent than detection of eggs in stool samples [52,53], which has been confirmed in animals [54], and recently the use of serological tests for studying the impact of parasitic diseases on other immunologically related conditions such as human immunodeficiency virus (HIV) and allergic diseases has been well supported and strongly recommended [28]. Because IgE response to helminth infections is long-lasting [55] and specific antibodies against Ascaris are associated with resistance, our results support the idea of a direct causal relationship between 13q33 locus and Ascaris susceptibility. In our present survey, however, we were unable to obtain samples for parasite egg counts at the same time we collected blood samples, but collection of parallel parasitological information in future studies would be advantageous.
In order to analyse the antibody response in more detail, we evaluated IgE and IgG antibodies to the rABA-1 allergen of Ascaris. The IgE response to this protein is associated with resistance to infection, and our data show that there is no cross-reactivity between this nematode-specific molecule and allergens from mite extracts [56]. It is worth noting that the GG genotype of TNFSF13B was associated not only with high levels of sIgG to Ascaris extract, in agreement with reports suggesting that the IgG response is protective [57,58], but also with elevated sIgE levels to rABA-1 in asthmatics. The association of those phenotypes with the wild-type allele G, which is present in about 70% of the general population, suggests strongly that they are quantitative traits for susceptibility, conferring a selective advantage. Unfractionated extracts of Ascaris contain many different antigenic components, some of them cross-reactive with mites [56] and other nematodes, and IgE targeting irrelevant allergens could therefore confound the detection of any protective effect of sIgE. Therefore, the inclusion of rABA-1 in this investigation allowed us to identify clearly an important relationship of TNFSF13B with the protective antibody response to Ascaris.
The potential relationships between these polymorphisms and allergy are also interesting. Some authors consider that IgE antibodies to Ascaris are an independent risk factor for asthma and allergic sensitization [52,59], and genetic variants conferring resistance to Ascaris could predispose to atopy in non-parasitized populations [7]. Our findings, when adjusting for covariates including IgE to mites, contrast with these results. Remarkably, the associations found in this study were only to sIgE against Ascaris, and not against mites. How LIG4 and TNFSF13B polymorphisms regulate IgE and IgG responses in such a particular way remains to be defined experimentally.
In summary, we found that polymorphisms of LIG4, TNFSF13B and IRS-2 modulate antibody levels. These results support previous linkage studies, and suggest that genes underlying the QTL for Ascaris susceptibility at the 13q33 region regulate IgE and IgG responses against Ascaris antigens and, by promoting a protective humoral response, could influence Ascaris susceptibility. IRS2 was associated with high tIgE levels among asthmatics, suggesting a relationship between genes controlling responses to Ascaris and atopy in this region. There was no association with asthma, and experimental studies are needed to reveal the molecular mechanisms defining how these polymorphisms may influence specific IgE reactivity to Ascaris antigens, but not to mite allergens.
Acknowledgments
We thank all subjects for their voluntary participation in the study. This work was supported by Colciencias grant numbers 331-2004 and 093-2007. M. W. K. was supported by the Wellcome Trust and the Medical Research Council (UK). N. A. was supported by Fundemeb.
Disclosure
None of the authors have any conflicts to declarre.
References
- 1.Cooper PJ, Chico ME, Sandoval C, Nutman TB. Atopic phenotype is an important determinant of immunoglobulin E-mediated inflammation and expression of T helper cell type 2 cytokines to Ascaris antigens in children exposed to ascariasis. J Infect Dis. 2004;190:1338–46. doi: 10.1086/423944. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy MW, Fraser EM, Christie JF. MHC class II (I-A) region control of the IgE antibody repertoire to the ABA-1 allergen of the nematode Ascaris. Immunology. 1991;72:577–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Holland CV, Crompton DW, Asaolu SO, Crichton WB, Torimiro SE, Walters DE. A possible genetic factor influencing protection from infection with Ascaris lumbricoides in Nigerian children. J Parasitol. 1992;78:915–16. [PubMed] [Google Scholar]
- 4.Bundy DA. Population ecology of intestinal helminth infections in human communities. Phil Trans R Soc Lond B Biol Sci. 1988;321:405–20. doi: 10.1098/rstb.1988.0100. [DOI] [PubMed] [Google Scholar]
- 5.Peisong G, Yamasaki A, Mao XQ, et al. An asthma-associated genetic variant of STAT6 predicts low burden of Ascaris worm infestation. Genes Immun. 2004;5:58–62. doi: 10.1038/sj.gene.6364030. [DOI] [PubMed] [Google Scholar]
- 6.Ramsay CE, Hayden CM, Tiller KJ, et al. Association of polymorphisms in the beta2-adrenoreceptor gene with higher levels of parasitic infection. Hum Genet. 1999;104:269–74. doi: 10.1007/s004390050947. [DOI] [PubMed] [Google Scholar]
- 7.Moller M, Gravenor MB, Roberts SE, Sun D, Gao P, Hopkin JM. Genetic haplotypes of Th-2 immune signalling link allergy to enhanced protection to parasitic worms. Hum Mol Genet. 2007;16:1828–36. doi: 10.1093/hmg/ddm131. [DOI] [PubMed] [Google Scholar]
- 8.Forrester JE, Scott ME, Bundy DA, Golden MH. Predisposition of individuals and families in Mexico to heavy infection with Ascaris lumbricoides and Trichuris trichiura. Trans R Soc Trop Med Hyg. 1990;84:272–6. doi: 10.1016/0035-9203(90)90284-l. [DOI] [PubMed] [Google Scholar]
- 9.Williams-Blangero S, Subedi J, Upadhayay RP, et al. Genetic analysis of susceptibility to infection with Ascaris lumbricoides. Am J Trop Med Hyg. 1999;60:921–6. doi: 10.4269/ajtmh.1999.60.921. [DOI] [PubMed] [Google Scholar]
- 10.Nejsum P, Roepstorff A, Jorgensen CB, et al. High heritability for Ascaris and Trichuris infection levels in pigs. Heredity. 2009;102:357–64. doi: 10.1038/hdy.2008.131. [DOI] [PubMed] [Google Scholar]
- 11.Williams-Blangero S, VandeBerg JL, Subedi J, et al. Genes on chromosomes 1 and 13 have significant effects on Ascaris infection. Proc Natl Acad Sci USA. 2002;99:5533–8. doi: 10.1073/pnas.082115999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams-Blangero S, Vandeberg JL, Subedi J, Jha B, Correa-Oliveira R, Blangero J. Localization of multiple quantitative trait loci influencing susceptibility to infection with Ascaris lumbricoides. J Infect Dis. 2008;197:66–71. doi: 10.1086/524060. [DOI] [PubMed] [Google Scholar]
- 13.Lewis R, Behnke JM, Cassidy JP, Stafford P, Murray N, Holland CV. The migration of Ascaris suum larvae, and the associated pulmonary inflammatory response in susceptible C57BL/6j and resistant CBA/Ca mice. Parasitology. 2007;134:1301–14. doi: 10.1017/S0031182007002582. [DOI] [PubMed] [Google Scholar]
- 14.Hagel I, Cabrera M, Buvat E, et al. Antibody responses and resistance against Ascaris lumbricoides infection among Venezuelan rural children: the influence of ethnicity. J Trop Pediatr. 2008;54:354–6. doi: 10.1093/tropej/fmn032. [DOI] [PubMed] [Google Scholar]
- 15.McSharry C, Xia Y, Holland CV, Kennedy MW. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect Immun. 1999;67:484–9. doi: 10.1128/iai.67.2.484-489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagel I, Lynch NR, Perez M, Di Prisco MC, Lopez R, Rojas E. Relationship between the degree of poverty and the IgE response to Ascaris infection in slum children. Trans R Soc Trop Med Hyg. 1993;87:16–18. doi: 10.1016/0035-9203(93)90401-b. [DOI] [PubMed] [Google Scholar]
- 17.Hagel I, Lynch NR, Puccio F, et al. Defective regulation of the protective IgE response against intestinal helminth Ascaris lumbricoides in malnourished children. J Trop Pediatr. 2003;49:136–42. doi: 10.1093/tropej/49.3.136. [DOI] [PubMed] [Google Scholar]
- 18.Hagel I, Lynch NR, Di Prisco MC, Rojas E, Perez M, Alvarez N. Ascaris reinfection of slum children: relation with the IgE response. Clin Exp Immunol. 1993;94:80–3. doi: 10.1111/j.1365-2249.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner JD, Faulkner H, Kamgno J, et al. IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 2005;7:990–6. doi: 10.1016/j.micinf.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Caraballo LR, Marrugo J, Erlich H, Pastorizo M. HLA alleles in the population of Cartagena (Colombia) Tissue Antigens. 1992;39:128–33. doi: 10.1111/j.1399-0039.1992.tb01922.x. [DOI] [PubMed] [Google Scholar]
- 21.Builes JJ, Martinez B, Gomez A, et al. Y chromosome STR haplotypes in the Caribbean city of Cartagena (Colombia) Forensic Sci Int. 2007;167:62–9. doi: 10.1016/j.forsciint.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Caraballo L, Cadavid A, Mendoza J. Prevalence of asthma in a tropical city of Colombia. Ann Allergy. 1992;68:525–9. [PubMed] [Google Scholar]
- 23.Dennis R, Caraballo L, Garcia E, et al. Asthma and other allergic conditions in Colombia: a study in 6 cities. Ann Allergy Asthma Immunol. 2004;93:568–74. doi: 10.1016/S1081-1206(10)61265-3. [DOI] [PubMed] [Google Scholar]
- 24.Martinez B, Barrios K, Vergara C, et al. A NOS1 gene polymorphism associated with asthma and specific immunoglobulin E response to mite allergens in a Colombian population. Int Arch Allergy Immunol. 2007;144:105–13. doi: 10.1159/000103221. [DOI] [PubMed] [Google Scholar]
- 25.Acevedo N, Vergara C, Mercado D, Jimenez S, Caraballo L. The A-444C polymorphism of leukotriene C4 synthase gene is associated with IgE antibodies to Dermatophagoides pteronyssinus in a Colombian population. J Allergy Clin Immunol. 2007;119:505–7. doi: 10.1016/j.jaci.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–73. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 28.Abebe W, Tsuji N, Kasuga-Aoki H, et al. Species-specific proteins identified in Ascaris lumbricoides and Ascaris suum using two-dimensional electrophoresis. Parasitol Res. 2002;88:868–71. doi: 10.1007/s00436-002-0640-5. [DOI] [PubMed] [Google Scholar]
- 29.Puerta L, Fernandez-Caldas E, Lockey RF, Caraballo LR. Mite allergy in the tropics: sensitization to six domestic mite species in Cartagena, Colombia. J Invest Allergol Clin Immunol. 1993;3:198–204. [PubMed] [Google Scholar]
- 30.Christie JF, Dunbar B, Kennedy MW. The ABA-1 allergen of the nematode Ascaris suum: epitope stability, mass spectrometry, and N-terminal sequence comparison with its homologue in Toxocara canis. Clin Exp Immunol. 1993;92:125–32. doi: 10.1111/j.1365-2249.1993.tb05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia Y, Spence HJ, Moore J, et al. The ABA-1 allergen of Ascaris lumbricoides: sequence polymorphism, stage and tissue-specific expression, lipid binding function, and protein biophysical properties. Parasitology. 2000;120:211–24. doi: 10.1017/s0031182099005363. [DOI] [PubMed] [Google Scholar]
- 32.Moore J, McDermott L, Price NC, Kelly SM, Cooper A, Kennedy MW. Sequence-divergent units of the ABA-1 polyprotein array of the nematode Ascaris suum have similar fatty-acid- and retinol-binding properties but different binding-site environments. Biochem J. 1999;340:337–43. [PMC free article] [PubMed] [Google Scholar]
- 33.Puerta Llerena L, Fernandez-Caldas E, Caraballo Gracia LR, Lockey RF. Sensitization to Blomia tropicalis and Lepidoglyphus destructor in Dermatophagoides spp-allergic individuals. J Allergy Clin Immunol. 1991;88:943–50. doi: 10.1016/0091-6749(91)90252-j. [DOI] [PubMed] [Google Scholar]
- 34.Chew FT, Lim SH, Goh DY, Lee BW. Sensitization to local dust-mite fauna in Singapore. Allergy. 1999;54:1150–9. doi: 10.1034/j.1398-9995.1999.00050.x. [DOI] [PubMed] [Google Scholar]
- 35.Ferrandiz R, Casas R, Dreborg S. Sensitization to Dermatophagoides siboney, Blomia tropicalis, and other domestic mites in asthmatic patients. Allergy. 1996;51:501–5. doi: 10.1111/j.1398-9995.1996.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 36.Caraballo L, Puerta L, Fernandez-Caldas E, Lockey RF, Martinez B. Sensitization to mite allergens and acute asthma in a tropical environment. J Invest Allergol Clin Immunol. 1998;8:281–4. [PubMed] [Google Scholar]
- 37.Vergara C, Caraballo L, Mercado D, et al. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet. 2009 doi: 10.1007/s00439-009-0649-2. in press. [DOI] [PubMed] [Google Scholar]
- 38.Agudelo-Lopez SG-RL, Coronado X, Orozco A, et al. Prevalence of intestinal parasitism and associated factors in a village on the Colombian Atlantic Coast. Rev Salud Publica. 2008;10:633–42. doi: 10.1590/s0124-00642008000400013. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Caldas E, Puerta L, Mercado D, Lockey RF, Caraballo LR. Mite fauna, Der p I, Der f I and Blomia tropicalis allergen levels in a tropical environment. Clin Exp Allergy. 1993;23:292–7. doi: 10.1111/j.1365-2222.1993.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 40.Puerta L, Fernandez-Caldas E, Mercado D, Lockey RF, Caraballo LR. Sequential determinations of Blomia tropicalis allergens in mattress and floor dust samples in a tropical city. J Allergy Clin Immunol. 1996;97:689–91. doi: 10.1016/s0091-6749(96)70315-9. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Caldas E, Puerta L, Caraballo L, Mercado D, Lockey RF. Sequential determinations of Dermatophagoides spp. allergens in a tropical city. J Invest Allergol Clin Immunol. 1996;6:98–102. [PubMed] [Google Scholar]
- 42.Caraballo L, Mercado D, Vergara C, Fernández A, Gutiérrez M. High prevalence of IgE antibodies to Ascaris in asthmatic patients living in a tropical environment. J Allergy Clin Immunol. 2007;119:S210. [Google Scholar]
- 43.Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber MR. DNA ligase IV is essential for V(D)J recombination and DNA double-strand break repair in human precursor lymphocytes. Mol Cell. 1998;2:477–84. doi: 10.1016/s1097-2765(00)80147-1. [DOI] [PubMed] [Google Scholar]
- 44.Pan-Hammarstrom Q, Jones AM, Lahdesmaki A, et al. Impact of DNA ligase IV on nonhomologous end joining pathways during class switch recombination in human cells. J Exp Med. 2005;201:189–94. doi: 10.1084/jem.20040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girard PM, Kysela B, Harer CJ, Doherty AJ, Jeggo PA. Analysis of DNA ligase IV mutations found in LIG4 syndrome patients: the impact of two linked polymorphisms. Hum Mol Genet. 2004;13:2369–76. doi: 10.1093/hmg/ddh274. [DOI] [PubMed] [Google Scholar]
- 46.Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–3. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 47.Smirnova AS, Andrade-Oliveira V, Gerbase-DeLima M. Identification of new splice variants of the genes BAFF and BCMA. Mol Immunol. 2008;45:1179–83. doi: 10.1016/j.molimm.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Gavin AL, Duong B, Skog P, et al. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J Immunol. 2005;175:319–28. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 49.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–9. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Kelly-Welch AE, Wang HY, Wang LM, et al. Transgenic expression of insulin receptor substrate 2 in murine B cells alters the cell density-dependence of IgE production in vitro and enhances IgE production in vivo. J Immunol. 2004;172:2803–10. doi: 10.4049/jimmunol.172.5.2803. [DOI] [PubMed] [Google Scholar]
- 51.Hunninghake GM, Lasky-Su J, Soto-Quiros ME, et al. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177:830–6. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dold S, Heinrich J, Wichmann HE, Wjst M. Ascaris-specific IgE and allergic sensitization in a cohort of school children in the former East Germany. J Allergy Clin Immunol. 1998;102:414–20. doi: 10.1016/s0091-6749(98)70129-0. [DOI] [PubMed] [Google Scholar]
- 53.Karadag B, Ege M, Bradley JE, et al. The role of parasitic infections in atopic diseases in rural schoolchildren. Allergy. 2006;61:996–1001. doi: 10.1111/j.1398-9995.2006.01107.x. [DOI] [PubMed] [Google Scholar]
- 54.Roepstorff A. Natural Ascaris suum infections in swine diagnosed by coprological and serological (ELISA) methods. Parasitol Res. 1998;84:537–43. doi: 10.1007/s004360050444. [DOI] [PubMed] [Google Scholar]
- 55.Mitre E, Nutman TB. IgE memory: persistence of antigen-specific IgE responses years after treatment of human filarial infections. J Allergy Clin Immunol. 2006;117:939–45. doi: 10.1016/j.jaci.2005.12.1341. [DOI] [PubMed] [Google Scholar]
- 56.Acevedo N, Sanchez J, Fernandez A, et al. IgE cross-reactivity between Ascaris and domestic mites: the role of fatty acid binding proteins allergens and the panallergen tropomyosin. Allergy. 2007;62:300. [Google Scholar]
- 57.van Riet E, Wuhrer M, Wahyuni S, et al. Antibody responses to Ascaris-derived proteins and glycolipids: the role of phosphorylcholine. Parasite Immunol. 2006;28:363–71. doi: 10.1111/j.1365-3024.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 58.Frontera E, Carron A, Serrano FJ, Roepstorff A, Reina D, Navarrete I. Specific systemic IgG1, IgG2 and IgM responses in pigs immunized with infective eggs or selected antigens of Ascaris suum. Parasitology. 2003;127:291–8. doi: 10.1017/s003118200300355x. [DOI] [PubMed] [Google Scholar]
- 59.Obihara CC, Beyers N, Gie RP, et al. Respiratory atopic disease, Ascaris-immunoglobulin E and tuberculin testing in urban South African children. Clin Exp Allergy. 2006;36:640–8. doi: 10.1111/j.1365-2222.2006.02479.x. [DOI] [PubMed] [Google Scholar]


