Abstract
The objective of this study was to investigate the effects of thalidomide (THD) on interstitial lung fibrosis (ILF). In vitro, human fetal lung fibroblast (HFL-F) to myofibroblast (MF) trans-differentiation was induced by transforming growth factor (TGF)-β1. The effects of THD on trans-differentiation process or differentiated MF were evaluated by measuring hydroxyproline (HYP) content by alkaline hydrolysis colorimetry, α-smooth muscle actin (α-SMA) protein by Western blot and α-SMA and pro-collagen III mRNA expressions by semi-quantitative reverse transcription–polymerase chain reaction; in vivo, a mouse model of ILF was generated by daily subcutaneous injection of bleomycin (BLM) in female C3H mice. Gastric perfusion of THD began 1 week prior to injection and lasted for 8 weeks. Lung specimens were harvested at different time-points (1, 4, 6 and 8 weeks) for pathology and immunohistochemistry examination. The HYP content, α-SMA and pro-collagen III mRNA expressions were also assessed. THD inhibited the up-regulation of HYP protein, pro-collagen III mRNA and α-SMA protein induced by TGF-β1 in HFL-F cells, and additionally inhibited pro-collagen III mRNA expression on trans-differentiated MF. THD reduced HYP synthesis in the lung tissues of BLM-treated mice at week 4, and slightly reduced the numbers of α-SMA-positive cells. THD had an effect on ILF models both in vitro and in vivo.
Keywords: interstitial lung fibrosis, thalidomide
Introduction
Systemic sclerosis (SSc) is a connective tissue disease characterized by over-synthesis of collagen and excessive extracellular matrix (ECM) deposition with unknown aetiology. Multi-organ involvement is extremely common; for example, interstitial lung fibrosis (ILF) is present in approximately one-third of patients with a poor prognosis [1–6]. Treatment of SSc-complicating ILF is far from satisfactory, and remains challenging in clinical practice.
Trans-differentiation of fibroblast to myofibroblast (MF) accompanied by up-regulation of α-smooth muscle actin (α-SMA) and collagen protein expression are thought to be pivotal in the development of ILF [7–9]. Transforming growth factor (TGF)-β1, recognized widely as a crucial fibrogenic cytokine, has been demonstrated as being capable of inducing this trans-differentiation process in vitro and in vivo[10–12].
Thalidomide (THD) exhibits diverse biological activity, including anti-inflammation, immunomodulation and anti-angiogenesis. THD could inhibit tumour necrosis factor (TNF)-α, and in animal models has some effect in preventing liver cirrhosis [13] and lung fibrosis [14]. In patients with idiopathic pulmonary fibrosis, THD could reduce the release of TNF-α, interleukin (IL)-12, IL-18 and IL-8 from alveolar macrophages [15].
To investigate if THD has a therapeutic effect on SSc-ILF, we examined the effects of THD on TGF-β1-induced human fetal lung fibroblast (HFL-F) to MF trans-differentiation [16] and on a bleomycin (BLM)-induced SSc-ILF mouse model [17,18].
Materials and methods
Cell culture
Human fetal lung fibroblasts (13th generation) were purchased from the cell centre at the Chinese Academy of Medical Science and Peking Union Medical College (CAMS&PUMC) (Beijing, China). Cells were cultured in Dulbecco's modified Eagle medium complete culture medium supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), penicillin (100 U/ml) and streptomycin (100 µg/l) under conditions of humidified 5% CO2 air at 37°C. The 15th to 30th generations were used for all experiments. To investigate the effect of THD on HFL-F to MF trans-differentiation, THD (50 µg/l) and TGF-β1 (5 µg/l) were added simultaneously to the culture system and the cells were cultured for 96 h. To investigate the effect of THD on differentiated MF, THD (50 µg/l) was added to the culture system after 96 h incubation of HFL-F with TGF-β1 (5 µg/l), and the cells were then cultured for an additional 48 h before analysis.
A cell creep plate was made after treatment, then fixed with 4% paraformaldehyde and permeabilized with 0·2% Triton X-100. Mouse anti-human α-SMA monoclonal antibody (mAb) and fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G were dripped consecutively onto the cell creep plate, with intensive washing with phosphate-buffered saline (PBS) after each step.
Animals
Six-week-old female C3H mice weighing 20–22 g were purchased from Beijing Vital River Laboratory Animal Technology and maintained under specific pathogen-free conditions in the experimental animal centre at the Institute of Basic Medical Sciences, CAMS. BLM (0·1 ml), dissolved in PBS at a concentration of 1 mg/ml, was injected subcutaneously each day for 3 consecutive weeks while negative control mice were injected with PBS only [17,18]. Gastric perfusion of 0·5 ml/day THD at a concentration of 10 mg/ml dissolved in carboxymethyl cellulose or carboxymethyl cellulose only began 1 week prior to BLM injection and lasted for 8 weeks. Mice were killed at the end of weeks 1, 4, 6 and 8 and lung specimens were harvested.
Antibodies and reagents
Recombined human TGF-β1 was purchased from Peprotech (London, UK). Mouse anti-human α-SMA mAb, mouse anti-human β-actin mAb and anti-mouse α-SMA mAb were all obtained from Sigma (St Louis, MO, USA). THD was obtained from Changzhou Pharmacy Ltd (Jiangsu, China). BLM was the product of Nippon Kayaku Company Ltd (Tokyo, Japan).
Hydroxyproline quantitative analysis
Cells were collected and counted accurately. Lung tissue was harvested and weighed; 1–2 × 104 cells or 30–100 mg lung tissue were used for hydroxyproline (HYP) quantitative analysis by alkaline hydrolysis colorimetry. In brief, cells or tissues were hydrolyzed in basic solution, and chloramines-T, perchloric acid and dimethylaminobenzaldehyde were added consecutively, following incubation at 60°C for 15 min for colour-based oxidative reaction, and the optical density 550 was read with a spectrophotometer. HYP concentrations of specimens were determined according to standard curve.
Reverse transcription–polymerase chain reaction
Total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed to cDNA using a first-strand cDNA synthesis kit (Fermentas, Burlington, ON, Canada). Primer pairs and amplification reaction parameters for semi-quantitative polymerase chain reaction are shown in Table 1. Amplification products were detected by 2% agarose electrophoresis. Results are expressed as the ratio of the target gene and glyceraldehyde-3-phosphate dehydrogenase.
Table 1.
Primers for polymerase chain reaction.
| cDNA | Primers | Cycles | Annealing temperature (?) | Product (bp) |
|---|---|---|---|---|
| Collagen (human) | 5′-CAGGGGCCCCAGGACTTAGAG-3′ 5′-GGGCCAGGAGGACCAATAGGA-3′ | 30 | 60 | 250 |
| α-SMA (human) | 5′-CCAGCTATGTGAAGAAGAAGAGG-3′ 5′-GTGATCTCCTTCTGCATTCGGT-3′ | 30 | 56 | 928 |
| GAPDH (human) | 5′-CCCATCACCATCTTCCAGGA-3′ 5′-TTGTCATACCAGGAAATGAGC-3′ | 30 | 56 | 731 |
| Collagen (mice) | 5′-GCTCAGAGTAGCACCATCAG-3′ 5′-GGCTGATGTACACATGCTCC-3′ | 30 | 58 | 220 |
| α-SMA (mice) | 5′-CTGGAGAAGAGCTACGAACTGC-3′ 5′-CTGATCCACATCTGCTGGAAGG-3′ | 30 | 64 | 368 |
| GAPDH (mice) | 5′-AAGCCCATCACCATCTTCCA-3′ 5′-CCTGCCTCACCACCTTCTTG-3′ | 30 | 60 | 580 |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; bp, base pairs; α-SMA, α-smooth muscle actin.
Western blot analysis
Harvested cells or tissues were lysed in lysis buffer [Tris 20 mM, sodium dodecyl sulphate 0·1%, Trixon X-100 1%, NaCl 250 mM, Na3VO4 100 uM, phenylmethylsulphonyfluoride 50 µg/ml, dithiothreitol 1 mM, pepstatin 0·8 µg/ml, leupeptin 0·5 µg/ml, aprotinin 1 µg/ml] to extract total protein. Protein concentrations were measured by Bradford protein assay kit (Applygen Technologies Inc., Beijing, China). Twenty-five µg cell protein was electrophoresed on 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and wet-transferred onto nitrocellulose membranes, then blocked in Tris-buffered saline Tween 20 (TBST) with 5% defatted milk overnight at 4°C. The membranes containing protein were incubated with α-SMA mAb or β-actin mAb (both diluted at 1:400), respectively, for 2 h at 37°C. After intensive washing, incubation with horseradish peroxidase goat anti-mouse secondary antibody (diluted at 1 : 5000) was carried out for 1 h at room temperature. Antibody binding was detected by enhanced chemiluminescence reagent (GE Healthcore, UK) and results were analysed using the Fluorescence Chemiluminescence and Visible Imaging System (Alpha Innotech, San Leandro, CA, USA).
Pathology and immunohistochemistry
Tissue specimens were fixed in 10% formalin solution and embedded in paraffin. Routine haematoxylin and eosin staining were performed. As for immunohistochemistry of α-SMA, specimen sections were deparaffinized, treated with 3% hydrogen peroxide to block endogenous peroxidase activity and were then treated with citric acid (pH 7·6) and heated in a microwave for antigen retrieval. Slides were then incubated with anti-α-SMA mAb overnight at room temperature, washed and incubated with biotin-conjugated secondary antibody and horseradish peroxidase-conjugated streptavidin sequentially with intensive washing steps after each incubation step. Diaminobenzidine was used as the colour-developing substrate.
Statistical analysis
Data were expressed as means ± standard error of the mean. For immunohistochemistry experiments, Image ProPlus version 6·0 software (Media Cybernetics, Bethesda, MD, USA) was used to calculate mean density or integrated optical density; a t-test was used for analysing the difference between two groups and one-way analysis of variance for three or more groups with spss version 11·0 software (spss Inc., Chicago, IL, USA). P < 0·05 was considered statistically significant.
Results
In vitro
The THD inhibited TGF-β1-induced up-regulation of HYP protein, pro-collagen III mRNA as well as α-SMA protein in HFL-F cells (Figs 1 and 2).
Fig. 1.
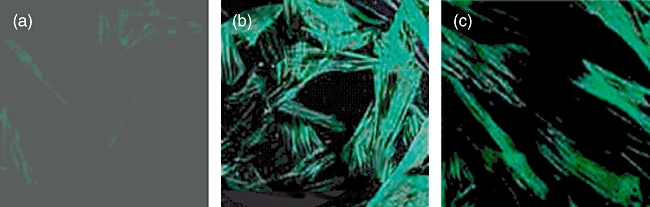
Effects of thalidomide (THD) on morphological transformation of myofibroblast (MF) induced by transforming growth factor (TGF)-β1. Human fetal lung fibroblast (HFL-F) were incubated for 4 days with 0·1% fetal bovine serum (a), TGF-β1 5 µg/l (b), TGF-β1 5 µg/l and THD 50 µg/l (c). Fluorescein isothiocyanate stain (α-smooth muscle actin) ×200.
Fig. 2.
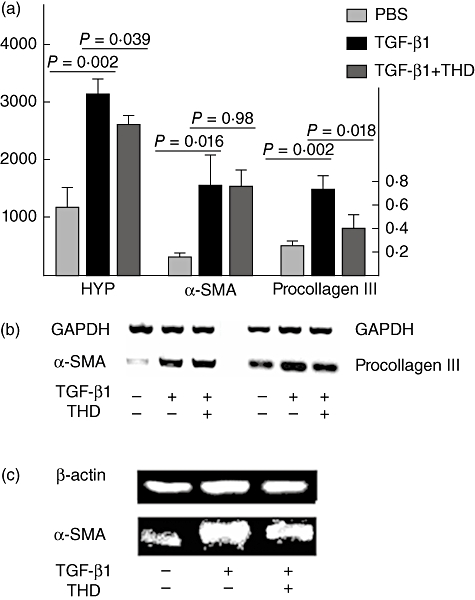
Hydroxyproline (HYP), α-smooth muscle actin (α-SMA) protein, α-SMA and pro-collagen III mRNA expressions in human fetal lung fibroblast (HFL-F) treated by transforming growth factor (TGF)-β1 with or without thalidomide (THD) or phosphate-buffered saline only (each experiment was repeated at least three times, data were shown as mean ± standard deviation). (a) Relative levels of HYP protein, α-SMA mRNA and pro-collagen III mRNA; (b) reverse transcription–polymerase chain reaction: α-SMA and pro-collagen III mRNA; (c) Western blot: α-SMA protein.
Compared with PBS control, TGF-β1 up-regulated significantly the levels of HYP protein, α-SMA mRNA and protein as well as pro-collagen III mRNA in HFL-F (P < 0·05), indicating the trans-differentiation of HFL-F to MF. When THD was added to the culture system simultaneously with TGF-β1, it inhibited the up-regulation of HYP protein, pro-collagen III mRNA and α-SMA protein (P < 0·05), although it had no significant effect on α-SMA mRNA expression (P= 0·98) (Fig. 2). Indirect immunofluorescence staining also showed fewer α-SMA-positive cells in HFL-F cells treated with both THD and TGF-β1 (mean density 0·0346 ± 0·002) compared with HFL-F cells stimulated with TGF-β1 only (mean density 0·0979 ± 0·003, P < 0·05) (Fig. 1).
The THD additionally inhibited pro-collagen III mRNA expression on trans-differentiated MF
Forty-eight hours after removal of TGF-β1, trans-differentiated MF continued to express higher levels of HYP protein and pro-collagen III mRNA (P < 0·05), but α-SMA mRNA, which was up-regulated during TGF-β1 stimulation, returned to the baseline. To investigate whether THD had an effect on trans-differentiated MF, 50 µg/l THD was added to the culture system after 96-h incubation of HFL-F with 5 µg/l TGF-β1. Results showed that THD additionally inhibited pro-collagen III mRNA expression (P = 0·017), while it had no effect on levels of HYP and α-SMA protein (P > 0·05) (Fig. 3).
Fig. 3.
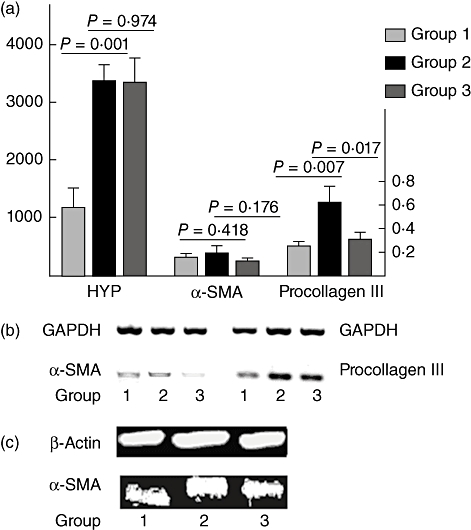
Relative levels of hydroxyproline (HYP), α-smooth muscle actin (α-SMA) protein, α-SMA and pro-collagen III mRNA expressions in trans-differentiated myofibroblast (MF) treated by thalidomide (THD) or phosphate-buffered saline (PBS) (each experiment was repeated at least three times, data were shown as mean ± standard deviation). Group 1: human fetal lung fibroblast (HFL-F) was cultured with PBS for 144 h; group 2: HFL-F was cultured with 5 µg/l transforming growth factor (TGF)-β1 for 96 h, then TGF-β1 was removed and cells were incubated with PBS for additional 48 h; group 3: HFL-F was cultured with 5 µg/l TGF-β1 for 96 h, then TGF-β1 was removed and cells were incubated with 50 µg/l THD for another 48 h. (a) Relative levels of HYP protein, α-SMA mRNA and pro-collagen III mRNA; (b) reverse transcription–polymerase chain reaction: α-SMA and pro-collagen III mRNA; (c) Western blot: α-SMA protein.
In vivo
The THD reduced HYP synthesis in the lung tissues of BLM-treated mice at week 4
HYP content was detected at weeks 1, 4, 6 and 8 for each group of mice (six to eight mice at each time-point for each group). BLM-treated mice had significantly higher HYP content in their lung tissues than PBS control mice (P < 0·05). Treatment with THD suppressed HYP synthesis markedly in BLM-treated mice at the end of week 4, and this effect was attenuated gradually at the end of weeks 6 and 8 (Table 2).
Table 2.
Hydroxyproline contents in the lung tissues of mice at different time points (shown as mean ± standard deviation).
| Group | 1st week | 4th week | 6th week | 8th week |
|---|---|---|---|---|
| n | 626·5 ± 57·2 | 637·2 ± 80·2 | 650·3 ± 82·7 | 896·2 ± 67·1 |
| BLM | 627·4 ± 65·1 | 878·4 ± 104·6* | 941·3 ± 168·5* | 1054·1 ± 29·9* |
| THD | 636·7 ± 101·7 | 724·0 ± 40·7*,** | 842·8 ± 68·3* | 1046·3 ± 127·3* |
Significant difference compared with group n (P < 0·05);
significant difference compared with group bleomycin (BLM) (P < 0·05); group n: mice injected with phosphate-buffered saline subcutaneously and gastric perfused with carboxymethyl cellulose (CMC); group BLM: mice injected with BLM subcutaneously and gastric perfused with CMC; group thalidomide (THD): mice injected with BLM subcutaneously and gastric perfused with THD.
The THD treatment had no significant effect on the lung histopathology in BLM-treated mice and slightly reduced the numbers of α-SMA-positive cells
At the end of weeks 4, 6 and 8, large amounts of mononuclear cells infiltration with hyperplasia of fibroblasts and thickening of small vessel wall and alveolar septum were observed in the lung tissues of BLM-treated mice, indicating successful establishment of the SSc-ILF mice model. THD treatment had no significant effect in improving the lung histopathology in BLM-treated mice either at week 4 or week 8 (Fig. 4a–c). Immunohistochemistry staining showed that α-SMA-positive cells were scattered in the lung matrix of BLM-treated mice, and THD treatment reduced the number of positive cells slightly (integrated optical density of group BLM and THD were 23 515 ± 492 and 20 677 ± 454 respectively, P = 0·204) (Fig. 4d–f).
Fig. 4.
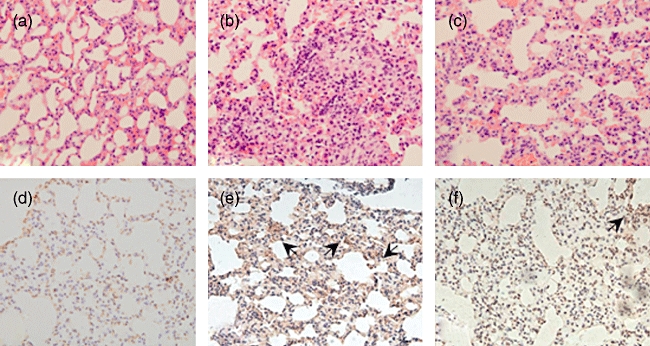
Haematoxylin and eosin staining and immunohistochemistry staining of the lung tissues in mice (×100). (a–c) Haematoxylin and eosin staining; (d–f) immunohistochemistry staining of α-smooth muscle actin (α-SMA). (a,d) Group n; (b,e) group bleomycin (BLM); (c,f) group thalidomide (THD). Group n: mice injected with phosphate-buffered saline subcutaneously and gastric perfused with carboxymethyl cellulose (CMC); group BLM: mice injected with BLM subcutaneously and gastric perfused with CMC; group THD: mice injected with BLM subcutaneously and gastric perfused with THD.
Discussion
SSc-complicating ILF has a poor prognosis and remains a challenge to both rheumatologists and pulmonologists. Collagen synthesis and ECM deposition are characteristics of the fibrosis process [19,20]. MF expressing α-SMA play a pivotal role in ECM deposition, leading to a decrease of pulmonary compliance and apoptosis of pulmonary epithelial cells. Moreover, by producing multiple cytokines, including TGF-β1, MF are capable of sustaining and aggravating local inflammation and fibrosis [8,9]. TGF-β1 is regarded as one of the key mediators of the fibrosis process; it is known to induce HFL-F to MF trans-differentiation, inhibit MF apoptosis [21], up-regulate the expression of α-SMA and ECM proteins and stimulate protease inhibitors that prevent enzymatic breakdown of ECM [12].
In this work, C3H mice were chosen for in vivo study because C3H mice could reproduce human SSc disease better in comparison with BALB/c mice with not only ILF, but also skin thickening and collagen deposition under BLM stimulation (data not shown), which has also been demonstrated in previous reports [17,22].
Consistent with previous observations, our in vitro study showed that HYP content, α-SMA protein and mRNA, as well as pro-collagen III mRNA, were up-regulated significantly after stimulation with TGF-β1, indicating successful trans-differentiation of HFL-F to MF. Unlike HYP protein and pro-collagen III mRNA, after removal of TGF-β1 α-SMA mRNA expression decreased gradually to the baseline, suggesting that transformed MF may down-regulate α-SMA mRNA expression in vitro. Also, reduced α-SMA mRNA expression may be the early presentation of increased apoptosis of transformed MF, with the removal of the anti-apoptotic function of TGF-β1.
Our study shows that THD could inhibit TGF-β1-induced HFL-F to MF trans-differentiation by down-regulating HYP synthesis and pro-collagen III mRNA expression. Moreover, it had some affect on collagen synthesis in transformed MF by down-regulating pro-collagen III mRNA expression. In agreement with in vitro observations, in vivo study showed that THD treatment could reduce HYP synthesis in the lung tissues of BLM-treated mice during the early stage of fibrotic process, although the effect attenuated gradually with time. We also noted that THD only slightly reduced the numbers of α-SMA-positive cells in the lung tissues treated by BLM; one explanation may be that this is due to the interference of large amounts of small smooth muscle vessels in the lung tissues. The above data provide experimental evidence suggesting that THD could be effective in treating SSc-ILF. ILF is a complicated progressive process, the underlying mechanism of which remains to be explored. While the clinical need for effective treatment is yet to be fulfilled, THD may be one of the candidates for SSc-complicating ILF, at least as a choice of adjuvant drug.
Acknowledgments
This work was supported by New Century Excellent Talents, Ministry of Education of China (NCET-04-0191), National Natural Sciences Foundation of China (30400410), Natural Sciences Foundation of Beijing (7052052) and National Program for Key Basic Research Project (2007CB512405 for Immunology), Ministry of Science and Technology, China.
Disclosures
The authors state that they have no conflict of interest.
References
- 1.Renzoni EA. Interstitial lung disease in systemic sclerosis. Monaldi Arch Chest Dis. 2007;67:217–28. doi: 10.4081/monaldi.2007.478. [DOI] [PubMed] [Google Scholar]
- 2.Fujisawa T, Suda T, Nakamura Y, et al. Differences in clinical features and prognosis of interstitial lung diseases between polymyositis and dermatomyositis. J Rheumatol. 2005;32:58–64. [PubMed] [Google Scholar]
- 3.Kim DS. Interstitial lung disease in rheumatoid arthritis: recent advances. Curr Opin Pulm Med. 2006;12:346–53. doi: 10.1097/01.mcp.0000239552.55326.ee. [DOI] [PubMed] [Google Scholar]
- 4.Hoyles RK, Ellis RW, Wellsbury J, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 5.Clements PJ, Roth MD, Elashoff R, et al. Scleroderma Lung Study (SLS): differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann Rheum Dis. 2007;66:1641–7. doi: 10.1136/ard.2007.069518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanna D, Yan X, Tashkin DP, et al. Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum. 2007;56:1676–84. doi: 10.1002/art.22580. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138:1257–65. [PMC free article] [PubMed] [Google Scholar]
- 8.Phan SH, Zhang K, Zhang HY, et al. The myofibroblast as an inflammatory cell in pulmonary fibrosis. Curr Top Pathol. 1999;93:173–82. doi: 10.1007/978-3-642-58456-5_18. [DOI] [PubMed] [Google Scholar]
- 9.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(6 Suppl):286S–9S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 10.Gauldie J, Sime PJ, Xing Z, et al. Transforming growth factor-beta gene transfer to the lung induces myofibroblast presence and pulmonary fibrosis. Curr Top Pathol. 1999;93:35–45. doi: 10.1007/978-3-642-58456-5_5. [DOI] [PubMed] [Google Scholar]
- 11.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 12.Ihn H. Autocrine TGF-beta signaling in the pathogenesis of systemic sclerosis. J Dermatol Sci. 2008;49:103–13. doi: 10.1016/j.jdermsci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Lv P, Paul SC, Xiao Y, et al. Effects of thalidomide on the expression of adhesion molecules in rat liver cirrhosis. Mediators Inflamm. 2006;2006:93253. doi: 10.1155/MI/2006/93253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabata C, Tabata R, Kadokawa Y, et al. Thalidomide prevents bleomycin-induced pulmonary fibrosis in mice. J Immunol. 2007;179:708–14. doi: 10.4049/jimmunol.179.1.708. [DOI] [PubMed] [Google Scholar]
- 15.Ye Q, Chen B, Tong Z, et al. Thalidomide reduces IL-18, IL-8 and TNF-alpha release from alveolar macrophages in interstitial lung disease. Eur Respir J. 2006;28:824–31. doi: 10.1183/09031936.06.00131505. [DOI] [PubMed] [Google Scholar]
- 16.Malmstrom J, Westergren-Thorsson G, Marko-Varga G. A proteomic approach to mimic fibrosis disease evolvement by an in vitro cell line. Electrophoresis. 2001;22:1776–84. doi: 10.1002/1522-2683(200105)22:9<1776::AID-ELPS1776>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Takagawa S, Katayama I, et al. Animal model of sclerotic skin. I. Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999;112:456–62. doi: 10.1046/j.1523-1747.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Nishioka K. Animal model of sclerotic skin. V. Increased expression of alpha-smooth muscle actin in fibroblastic cells in bleomycin-induced scleroderma. Clin Immunol. 2002;102:77–83. doi: 10.1006/clim.2001.5138. [DOI] [PubMed] [Google Scholar]
- 19.Selman M, Thannickal VJ, Pardo A, et al. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs. 2004;64:405–30. doi: 10.2165/00003495-200464040-00005. [DOI] [PubMed] [Google Scholar]
- 20.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol. 2003;201:343–54. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1) Am J Respir Cell Mol Biol. 1999;21:658–65. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Kuroda M, Nishioka K. Animal model of sclerotic skin. III. Histopathological comparison of bleomycin-induced scleroderma in various mice strains. Arch Dermatol Res. 2000;292:535–41. doi: 10.1007/s004030000183. [DOI] [PubMed] [Google Scholar]


