Abstract
Context: In obesity, total IGF-I is not reduced to the degree predicted by low GH levels, and free IGF-I levels are normal to high. Total and free IGF-I may not reflect IGF-I biological activity because immunoassays cannot account for the modifying effects of IGF binding proteins on interactions between IGF-I and its receptor.
Objective: The aim of the study was to investigate the biological activity of IGF-I in obesity.
Design and Setting: We conducted a cross-sectional study at a General Clinical Research Center.
Study Participants: Thirty-four healthy women (11 lean, 12 overweight, and 11 obese) of comparable age (overall mean, 30.7 ± 1.3 yr) participated in the study.
Intervention: There were no interventions.
Main Outcome Measures: We measured bioactive IGF-I (as measured by a kinase receptor activation assay), IGFBP-1, and GH using 6-h pools of serum collected every 10 min for 24 h, and fasting IGF-I and IGFBP-3.
Results: Mean 24-h GH (R = −0.76; P < 0.0001), total IGF-I (R = −0.36; P = 0.040), and IGFBP-1 (R = −0.41; P = 0.017) levels were inversely associated with BMI, whereas bioactive IGF-I and IGFBP-3 levels were not. Mean bioactive IGF-I was similar in the groups [0.96 ± 0.09 (lean), 1.08 ± 0.09 (overweight), and 0.84 ± 0.11 (obese) μg/liter; overall P = 0.22]. Percentage bioactive IGF-I [(bioactive/total IGF-I) × 100] was higher in obese subjects than both lean and overweight subjects (P = 0.039).
Conclusions: Despite low GH secretion in obesity and decreasing IGFBP-1 with increasing BMI, 24-h mean bioactive IGF-I levels are not reduced in obese women and do not correlate with BMI or IGFBP-1 levels. This argues against elevated bioactive IGF-I as the etiology of reduced GH secretion through a feedback mechanism in obesity.
Despite a linear decrease in GH secretion with increasing BMI and reduced total IGF-I levels in obese women, bioactive IGF-I levels are not reduced in obesity.
Increasing body mass index (BMI) is associated with a linear decrease in GH secretion in healthy women and men (1,2,3,4,5,6). In syndromes of GH deficiency due to a primary defect in GH secretion, such as pituitary disease, mean population total and free IGF-I levels are lower than in healthy controls, although individual levels may be within the normal range (7,8). However, in obesity, our data and others suggest that total IGF-I is not reduced to the degree predicted by GH levels (1,9), and others have reported normal (10,11) or even high total IGF-I levels (12,13) in obese adults. For example, Gleeson et al. (14) demonstrated a larger increase in IGF-I levels to a single bolus of GH in obese compared with lean subjects. Similarly, free IGF-I levels, as measured by immunoassays, in obesity have been reported to be low (15,16), normal (17,18), and high (19) by different groups, in contrast to findings in adults with GH deficiency in the setting of hypopituitarism, in which mean free IGF-I levels are low (20,21). Therefore, it is unclear whether reduced GH secretion results in reduced IGF-I serum levels in obesity. In addition, total and free IGF-I may not reflect IGF-I biological activity because immunoassays cannot account for the modifying effects of IGF binding proteins (IGFBPs)—both inhibitory and facilitating—on interactions between IGF-I and its receptor.
We hypothesized that: 1) lower GH secretion may lead to decreased bioactive IGF-I in obesity, or 2) elevated bioactive IGF-I in obesity may be a mechanism underlying reduced GH secretion through negative feedback. We therefore measured biological activity of IGF-I in lean, overweight, and obese women. Because IGFBP-1 decreases with increasing weight (13,22), has a diurnal rhythm (23,24), and may be an important determinant of IGF-I bioactivity (22), we measured bioactive IGF-I using 6-h pools of serum collected every 10 min for 24 h with an IGF-I kinase receptor activation (KIRA) assay.
Subjects and Methods
Subjects
Thirty-four healthy volunteers were recruited from the community through advertisements. Inclusion criteria included eumenorrhea and normal female-range serum testosterone level. Exclusion criteria included hypothalamic or pituitary disorders, estrogen or glucocorticoid use, and diabetes mellitus or other chronic illnesses.
Methods
The study was approved by the Partners Healthcare Inc. Institutional Review Board, and written informed consent was obtained from each study participant. Each participant was admitted to the General Clinical Research Center at the Massachusetts General Hospital during the follicular phase of her menstrual cycle. Fasting blood was obtained at 0800 h for measurement of total IGF-I and IGFBP-3. Serum was collected every 10 min for 24 h under standardized conditions, starting at 0800 h. Equal aliquots of serum were combined in 6-h pools of sample as follows: 1) 0900 h to 1450 h; 2) 1500 h to 2050 h; 3) 2100 h to 0250 h; and 4) 0300 h to 0750 h. Mean bioactive IGF-I, IGFBP-1, and GH levels were calculated as the mean of the values from the four time intervals. Serum samples were stored at −80 C. Baseline clinical characteristics, mean 24-h GH levels, total IGF-I levels, and IGFBP-3 levels have been previously published (1,25).
Biochemical analyses
Serum GH was measured using an immunoradiometric assay (IRMA) kit (Diagnostic Systems Laboratories, Inc., Webster, TX), with a minimum detection limit of 0.01 μg/liter, an intraassay coefficient of variation (CV) of 3.1-5.4%, and an interassay CV of 5.9–11.5%. Serum IGFBP-1 was measured using an in-house RIA, with intraassay CV of less than 5% and interassay CV of 12% (26). IGFBP-3 was measured by IRMA kit (Diagnostic Systems Laboratories, Inc.) with an intraassay CV of 1.8–3.9%, an interassay CV of 0.5–1.9%, and a minimum limit of detection of 0.5 μg/liter. Serum total IGF-I levels were measured using an Immulite 2000 automated immunoanalyzer (Diagnostic Products Corporation, Inc., Los Angeles, CA), by a solid-phase enzyme-labeled chemiluminescent immunometric assay, with an interassay CV of 3.7–4.2%. Bioactive IGF-I was measured using an IGF-I KIRA assay, as previously described (27). The assay detection limit is 0.08 μg/liter, and the maximum cross-reactivity of the assay is 0.7% for insulin analogs, 0.8% for human insulin, 0.06% for proinsulin, and 12% for IGF-II (27).
Statistical analysis
JMP Statistical Discoveries (version 4.0.2; SAS Institute, Inc., Cary, NC) was used for statistical analyses. All variables were tested for normality using the Shapiro-Wilk test. Means were compared with ANOVA for variables with normal distributions and with the Wilcoxon test for those with nonnormal distributions. Corrections for multiple comparisons were made by Tukey-Kramer (after log transformation for variables that were not normally distributed). Univariate regression models were constructed, and Pearson coefficients are reported. Results are expressed as mean ± sem.
Results
Clinical characteristics of study subjects
Eleven lean (BMI <25 kg/m2), 12 overweight (25 ≤ BMI <30 kg/m2), and 11 obese (BMI ≥30 kg/m2) young women of comparable age (lean, 30.7 ± 2.3 yr; overweight, 28.1 ± 2.5 yr; obese, 33.8 ± 1.8 yr; P = not significant) were studied. As designed, mean BMI differed between the groups (lean, 22.3 ± 0.6 kg/m2; overweight, 27.6 ± 0.4 kg/m2; and obese, 35.4 ± 0.8 kg/m2; P < 0.05).
GH, total and bioactive IGF-I levels, and IGFBPs
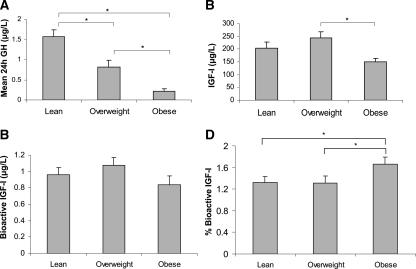
Mean 24-h GH (R = −0.76; P < 0.0001) was strongly inversely associated, whereas total IGF-I levels were only weakly, inversely associated with BMI (R = −0.36; P = 0.040). Mean total IGF-I was lower in obese than overweight subjects; there was no significant difference when comparing either overweight or obese subjects with lean subjects [204 ± 23 (lean), 243 ± 25 (overweight), 150 ± 13 (obese) μg/liter; P < 0.05 for obese vs. overweight subjects] (Fig. 1).
Figure 1.
A, Mean 24-h GH was significantly lower in overweight than lean women and lowest in obese women. B, Mean IGF-I levels were lower in obese than overweight women. C, Mean bioactive IGF-I levels were comparable in lean, overweight, and obese women. D, Mean percentage bioactive IGF-I was higher in obese women than overweight and lean controls. *, P < 0.05.
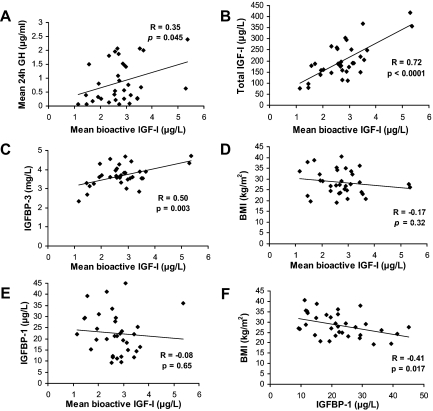
Mean bioactive IGF-I was similar in the groups [0.96 ± 0.09 (lean), 1.08 ± 0.09 (overweight), 0.84 ± 0.11 (obese) μg/liter; overall P = 0.22] (Fig. 1). Percentage bioactive IGF-I [(bioactive/total IGF-I) ∗ 100] was higher in obese than in both lean and overweight subjects [1.66 ± 0.13% (obese), 1.32 ± 0.11% (lean), and 1.31 ± 0.13% (overweight); overall P = 0.039] (Fig. 1). Bioactive IGF-I levels were associated with total IGF-I levels (R = 0.72; P < 0.0001) (Fig. 2) and mean 24-h GH (R = 0.35; P = 0.045) (Fig. 2), and peak stimulated GH (R = 0.37; P = 0.037) but did not correlate with BMI (Fig. 1). Bioactive IGF-I did not vary diurnally (data not shown).
Figure 2.
Mean 24-h bioactive IGF-I levels were positively associated with mean 24-h GH secretion (A), total IGF-I levels (B), and IGFBP-3 levels (C) but were not associated with BMI (D) or IGFBP-1 levels (E), despite the fact that IGFBP-1 levels were inversely associated with BMI (F).
Mean IGFBP-1 over 24 h was lower in obese than lean subjects [26.64 ± 2.67 μg/liter (lean) vs. 23.53 ± 3.38 μg/liter (overweight) vs. 17.14 ± 1.73 μg/l (obese); P = 0.022 obese vs. lean], but this difference was not significant after controlling for multiple comparisons. In univariate regression analysis, mean IGFBP-1 levels were inversely associated with BMI (R = −0.41; P = 0.017) (Fig. 2) but were not associated with bioactive IGF-I levels (R = −0.08; P = 0.65) (Fig. 2). IGFBP-1 varied diurnally with highest mean levels between 0300 h and 0850 h [22.67 ± 1.86 μg/liter (0900 h to 1450 h) vs. 17.15 ± 1.39 μg/liter (1500 h to 2050 h) vs. 18.41 ± 1.48 μg/liter (1200 h to 1750 h) vs. 31.32 ± 2.51 μg/liter (0300 h to 0850 h), P < 0.05 vs. all other time points after correction for multiple comparisons]. Mean IGFBP-1 during this period was lower in obese than lean study subjects (P = 0.002) and remained significant after controlling for multiple comparisons, but it did not differ between overweight and lean subjects.
Mean fasting IGFBP-3 levels did not differ between the groups [3.63 ± 0.14 mg/liter (lean) vs. 3.89 ± 0.11 mg/liter (overweight) vs. 3.53 ± 0.22 mg/liter (obese); P = not significant]. IGFBP-3 levels were positively associated with bioactive IGF-I levels (R = 0.50; P = 0.003) (Fig. 2) but did not correlate with BMI (R = −0.13; P = 0.46).
Discussion
We report that although total IGF-I in obesity is reduced, IGF-I bioactivity in obese women is normal. Therefore, our data argue against both of our hypotheses, including that lower GH secretion may lead to decreased bioactive IGF-I in obesity and that elevated bioactive IGF-I may cause reduced GH secretion through a negative feedback mechanism in obesity. Rather, our results support the hypothesis that in obesity normal IGF-I activity is maintained and mediated by enhanced sensitivity to GH at the level of the liver.
We determined IGF-I bioactivity directly using a highly sensitive and specific cell-based IGF-I KIRA assay (27,28). The IGF-I KIRA assay has been validated against the [3H]thymidine incorporation assay, a classic bioassay (28), and in patients with low, normal, and high total IGF-I levels (27). KIRA assays have an advantage over immunoassays, which cannot accurately capture the complex interactions between IGF-I, IGFBPs, proteases, and the IGF-I receptor, in that they directly measure IGF-I receptor activation. Approximately 0.5 to 1% of IGF-I is free, with the majority being bound in the serum by acid-labile subunit and IGFBPs, including IGFBP-3, the most ubiquitous of the binding proteins. A smaller fraction of IGF-I is bound to other IGF binding proteins, including IGFBP-1, which is low in obesity and varies diurnally (with lowest levels during the day, as a result of the effects of insulin) (13,22,23,24,29,30). IGFBP-2 is also BMI-dependent (31). The bioavailability of IGF-I to its receptor is thought in part to be dependent on the actions of binding protein proteolysis, with increased local binding protein cleavage resulting in increased IGF-I bioactivity (32,33). Therefore, immunoassay measurement of total or free IGF-I may not accurately reflect IGF-I bioactivity. In contrast, the KIRA assay technique directly measures IGF-I receptor activation. IGF-I binding causes IGF-I receptor conformational changes resulting in stimulation of the intracellular kinase domain. This is followed by autophosphorylation of tyrosine residues, initiating the intracellular signaling cascade (34,35,36). Thus, the KIRA measures IGF-I bioactivity directly, providing a more accurate measure of IGF-I action than any model based on immunoassays of blood levels, which do not incorporate complex interactions with binding proteins and proteases. Our data suggest that despite conflicting data regarding immunoreactive levels of total and free IGF-I in obesity, IGF-I bioactivity is normal in such patients.
The mechanisms underlying the lack of a reduction in bioactivity IGF-I, despite reduced endogenous GH secretion, in obesity warrant further study. Our results do not support a significant role for reduced IGFBP-1 levels. Our data are consistent with other reports of lower IGFBP-1 in subjects with higher BMIs (19), which is likely due to the effects of relative hyperinsulinemia in obesity (22,37,38). However, in contrast to published data demonstrating an inverse relationship between IGFBP-1 and free IGF-I levels (19) and data that IGFBP-1 inhibits IGF-I binding to and activation of IGF cell surface receptors in vitro (37), we did not find an association between IGFBP-1 and bioactive IGF-I levels, at least in this cross-sectional study. This may reflect the complexity of IGF-I, IGFBP, and IGF receptor interactions and result from the relatively low levels of IGFBP-1 present in humans. IGFBP-1 concentrations are a fraction of those of IGFBP-3, which we and others have shown to be present in normal concentrations in obesity (1,11,13). Therefore, our data suggest that lower IGFBP-1 levels are not likely major contributors to the elevation in the fraction of bioactive IGF-I in obese compared with lean women. Whether increased binding protein cleavage is present and contributes to the bioactivity of IGF-I in obesity is not known. We have recently demonstrated that elevated serum free testosterone levels, in contrast to estrogens, are important determinants of normal total IGF-I levels in obese women not receiving oral contraceptives (1), and testosterone has been shown to independently stimulate IGF-I in men (39), but these effects are not strong enough such that the relative preservation of IGF-I in obesity can be attributed entirely to the effects of gonadal steroids. In a human hepatoma cell line, insulin has been shown to up-regulate GH receptor biosynthesis, resulting in increased GH binding, which may be a mechanism underlying increased sensitivity to GH at the level of the liver in obesity (40). Hyperinsulinemia may also mediate increased pituitary sensitivity to IGF-I, as has been shown in mouse models of obesity (41). As GH binding protein levels correlate positively with BMI and visceral adipose tissue mass (42), a relative elevation of free compared with total GH levels is unlikely to be the explanation for preserved bioactive IGF-I levels in obesity. However, direct measurement of free GH in obesity to investigate this hypothesis would be informative.
Our data also do not support the hypothesis that increased IGF-I bioactivity contributes through a feedback mechanism to the reduction in GH secretion observed in obesity. This hypothesis was proposed because of published data demonstrating elevated free IGF-I levels in obesity (19). Free IGF-I assays attempt to take into account binding. However, the free IGF-I assay used was an immunoassay and therefore measures levels, not activity, which is a limitation.
The bioassay also has limitations and these include its complexity and the length of time that is required to perform the assay. In addition, the cells used in the assay are transfected with the human IGF-I receptor, and there is controversy over whether such cell lines are representative of the in vivo situation because, for example, they lack vascular and connective tissue. In addition, it should be noted that we do not advocate replacing total IGF-I and IGFBP assays with the KIRA. There may be situations in which total IGF-I may be high or normal due to elevated binding proteins, for example in certain cancers in which IGFBP-2 levels may be elevated (43,44,45,46), with normal bioactive IGF-I.
In summary, we demonstrate normal IGF-I bioactivity in obese women despite severely reduced endogenous GH secretion. This argues against both of our hypotheses that: 1) lower GH secretion may lead to decreased bioactive IGF-I in obesity; and 2) elevated bioactive IGF-I in obesity may be a mechanism underlying reduced GH secretion through negative feedback. The mechanisms underlying the preservation of IGF-I bioactivity despite low GH levels in obesity do not appear to include reduced IGFBP-1 levels. Effects of the dissociation of GH levels and IGF-I bioactivity in obese women and men warrant further studies.
Acknowledgments
We thank Lone Svendsen of the Medical Research Laboratory, Aarhus University Hospital, Denmark, for her excellent technical assistance with the IGF-I bioassay. We also thank the nurses and bionutritionists of the Massachusetts General Hospital General Clinical Research Center and the subjects who participated in the study.
Footnotes
This work was supported by National Institutes of Health Grants HL077674, MO1 RR01066, and UL1 RR025758 and the Danish Research Council for Health and Disease.
Disclosure Summary: The authors have no conflicts to declare.
First Published Online May 26, 2009
Abbreviations: BMI, Body mass index; CV, coefficient of variation; IGFBP, IGF binding protein; KIRA, kinase receptor activation assay.
References
- Utz AL, Yamamoto A, Sluss P, Breu J, Miller KK 2008 Androgens may mediate a relative preservation of IGF-I levels in overweight and obese women despite reduced growth hormone secretion. J Clin Endocrinol Metab 93:4033–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijl H, Langendonk JG, Burggraaf J, Frölich M, Cohen AF, Veldhuis JD, Meinders AE 2001 Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab 86:5509–5515 [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Hvidberg A, Juul A, Main KM, Gotfredsen A, Skakkebaek NE, Hilsted J, Skakkebae NE 1995 Massive weight loss restores 24-hour growth hormone release profiles and serum insulin-like growth factor-I levels in obese subjects. J Clin Endocrinol Metab 80:1407–1415 [DOI] [PubMed] [Google Scholar]
- Ross RJ, Buchanan CR 1990 Growth hormone secretion: its regulation and the influence of nutritional factors. Nutr Res Rev 3:143–162 [DOI] [PubMed] [Google Scholar]
- Strobl JS, Thomas MJ 1994 Human growth hormone. Pharmacol Rev 46:1–34 [PubMed] [Google Scholar]
- Thissen JP, Ketelslegers JM, Underwood LE 1994 Nutritional regulation of the insulin-like growth factors. Endocr Rev 15:80–101 [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Vahl N, Hansen TB, Skjaerbaek C, Fisker S, Orskov H, Hagen C, Christiansen JS 1998 Determinants of serum insulin-like growth factor I in growth hormone deficient adults as compared to healthy subjects. Clin Endocrinol (Oxf) 48:479–486 [DOI] [PubMed] [Google Scholar]
- Aimaretti G, Corneli G, Baldelli R, Di Somma C, Gasco V, Durante C, Ausiello L, Rovere S, Grottoli S, Tamburrano G, Ghigo E 2003 Diagnostic reliability of a single IGF-I measurement in 237 adults with total anterior hypopituitarism and severe GH deficiency. Clin Endocrinol (Oxf) 59:56–61 [DOI] [PubMed] [Google Scholar]
- Maccario M, Ramunni J, Oleandri SE, Procopio M, Grottoli S, Rossetto R, Savio P, Aimaretti G, Camanni F, Ghigo E 1999 Relationships between IGF-I and age, gender, body mass, fat distribution, metabolic and hormonal variables in obese patients. Int J Obes Relat Metab Disord 23:612–618 [DOI] [PubMed] [Google Scholar]
- Caufriez A, Golstein J, Lebrun P, Herchuelz A, Furlanetto R, Copinschi G 1984 Relations between immunoreactive somatomedin C, insulin and T3 patterns during fasting in obese subjects. Clin Endocrinol (Oxf) 20:65–70 [DOI] [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R 2004 Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol 150:161–171 [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Hertz P, Colin V, Ish-Shalom S, Yeshurun D, Youdim MB, Amit T 1992 The distal axis of growth hormone (GH) in nutritional disorders: GH-binding protein, insulin-like growth factor-I (IGF-I), and IGF-I receptors in obesity and anorexia nervosa. Metabolism 41:106–112 [DOI] [PubMed] [Google Scholar]
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB 1997 Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord 21:355–359 [DOI] [PubMed] [Google Scholar]
- Gleeson HK, Lissett CA, Shalet SM 2005 Insulin-like growth factor-I response to a single bolus of growth hormone is increased in obesity. J Clin Endocrinol Metab 90:1061–1067 [DOI] [PubMed] [Google Scholar]
- Gómez JM, Maravall FJ, Gómez N, Navarro MA, Casamitjana R, Soler J 2004 The IGF-I system component concentrations that decrease with ageing are lower in obesity in relationship to body mass index and body fat. Growth Horm IGF Res 14:91–96 [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Juul A, Hilsted J 2007 Effect of weight loss on free insulin-like growth factor-I in obese women with hyposomatotropism. Obesity (Silver Spring) 15:879–886 [DOI] [PubMed] [Google Scholar]
- Onder G, Liperoti R, Russo A, Soldato M, Capoluongo E, Volpato S, Cesari M, Ameglio F, Bernabei R, Landi F 2006 Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab 291:E829–E834 [DOI] [PubMed] [Google Scholar]
- Ricart W, Fernández-Real JM 2001 No decrease in free IGF-I with increasing insulin in obesity-related insulin resistance. Obes Res 9:631–636 [DOI] [PubMed] [Google Scholar]
- Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H 1995 Free insulin-like growth factors in human obesity. Metabolism 44:37–44 [DOI] [PubMed] [Google Scholar]
- Musolino NR, Da Cunha Neto MB, Marino Junior R, Giannella-Neto D, Bronstein MD 1999 Evaluation of free insulin-like growth factor-I measurement on the diagnosis and follow-up treatment of growth hormone-deficient adult patients. Clin Endocrinol (Oxf) 50:441–449 [DOI] [PubMed] [Google Scholar]
- Skjaerbaek C, Vahl N, Frystyk J, Hansen TB, Jørgensen JO, Hagen C, Christiansen JS, Orskov H 1997 Serum free insulin-like growth factor-I in growth hormone-deficient adults before and after growth hormone replacement. Eur J Endocrinol 137:132–137 [DOI] [PubMed] [Google Scholar]
- Conover CA, Lee PD, Kanaley JA, Clarkson JT, Jensen MD 1992 Insulin regulation of insulin-like growth factor binding protein-1 in obese and nonobese humans. J Clin Endocrinol Metab 74:1355–1360 [DOI] [PubMed] [Google Scholar]
- Baxter RC, Cowell CT 1987 Diurnal rhythm of growth hormone-independent binding protein for insulin-like growth factors in human plasma. J Clin Endocrinol Metab 65:432–440 [DOI] [PubMed] [Google Scholar]
- Hall K, Brismar K, Grissom F, Lindgren B, Povoa G 1991 IGFBP-1. Production and control mechanisms. Acta Endocrinol (Copenh) 124(Suppl 2):48–54 [PubMed] [Google Scholar]
- Utz AL, Yamamoto A, Hemphill L, Miller KK 2008 Growth hormone deficiency by GHRH/arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassas GE, Pontikides N, Kaltsas T, Dumas A, Frystyk J, Chen JW, Flyvbjerg A 2003 Free and total insulin-like growth factor (IGF)-I, -II, and IGF binding protein-1, -2, and -3 serum levels in patients with active thyroid eye disease. J Clin Endocrinol Metab 88:132–135 [DOI] [PubMed] [Google Scholar]
- Chen JW, Ledet T, Orskov H, Jessen N, Lund S, Whittaker J, De Meyts P, Larsen MB, Christiansen JS, Frystyk J 2003 A highly sensitive and specific assay for determination of IGF-I bioactivity in human serum. Am J Physiol Endocrinol Metab 284:E1149–E1155 [DOI] [PubMed] [Google Scholar]
- Sadick MD 1999 Kinase receptor activation (KIRA): a rapid and accurate alternative to endpoint bioassays. Dev Biol Stand 97:121–133 [PubMed] [Google Scholar]
- Frystyk J, Skjaerbaek C, Dinesen B, Orskov H 1994 Free insulin-like growth factors (IGF-I and IGF-II) in human serum. FEBS Lett 348:185–191 [DOI] [PubMed] [Google Scholar]
- Juul A, Flyvbjerg A, Frystyk J, Muller J, Skakkebaek NE 1996 Serum concentrations of free and total insulin-like growth factor-I, IGF binding proteins -1 and -3 and IGFBP-3 protease activity in boys with normal or precocious puberty. Clin Endocrinol (Oxf) 44:515–523 [DOI] [PubMed] [Google Scholar]
- Mattsson A, Svensson D, Schuett B, Osterziel KJ, Ranke MB 2008 Multidimensional reference regions for IGF-I, IGFBP-2 and IGFBP-3 concentrations in serum of healthy adults. Growth Horm IGF Res 18:506–516 [DOI] [PubMed] [Google Scholar]
- Baxter RC 2000 Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab 278:E967–E976 [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR 1995 Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 [DOI] [PubMed] [Google Scholar]
- De Meyts P, Christoffersen CT, Ursø B, Wallach B, Grønskov K, Yakushiji F, Shymko RM 1995 Role of the time factor in signaling specificity: application to mitogenic and metabolic signaling by the insulin and insulin-like growth factor-I receptor tyrosine kinases. Metabolism 44:2–11 [DOI] [PubMed] [Google Scholar]
- Kenner KA, Heidenreich KA 1991 Insulin and insulin-like growth factors stimulate in vivo receptor autophosphorylation and tyrosine phosphorylation of a 70K substrate in cultured fetal chick neurons. Endocrinology 129:301–311 [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A 2001 The somatomedin hypothesis: 2001. Endocr Rev 22:53–74 [DOI] [PubMed] [Google Scholar]
- Lee PD, Conover CA, Powell DR 1993 Regulation and function of insulin-like growth factor-binding protein-1. Proc Soc Exp Biol Med 204:4–29 [DOI] [PubMed] [Google Scholar]
- Suikkari AM, Koivisto VA, Koistinen R, Seppälä M, Yki-Järvinen H 1989 Dose-response characteristics for suppression of low molecular weight plasma insulin-like growth factor-binding protein by insulin. J Clin Endocrinol Metab 68:135–140 [DOI] [PubMed] [Google Scholar]
- Gibney J, Wolthers T, Johannsson G, Umpleby AM, Ho KK 2005 Growth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary men. Am J Physiol Endocrinol Metab 289:E266–E271 [DOI] [PubMed] [Google Scholar]
- Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK 2000 Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab 85:4712–4720 [DOI] [PubMed] [Google Scholar]
- Luque RM, Kineman RD 2006 Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology 147:2754–2763 [DOI] [PubMed] [Google Scholar]
- Roelen CA, Koppeschaar HP, de Vries WR, Snel YE, Doerga ME, Zelissen PM, Thijssen JH, Blankenstein MA 1997 Visceral adipose tissue is associated with circulating high affinity growth hormone-binding protein. J Clin Endocrinol Metab 82:760–764 [DOI] [PubMed] [Google Scholar]
- Becher OJ, Peterson KM, Khatua S, Santi MR, MacDonald TJ 2008 IGFBP2 is overexpressed by pediatric malignant astrocytomas and induces the repair enzyme DNA-PK. J Child Neurol 23:1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl D, Hessel E, Oesterle D, Renner-Müller I, Elmlinger M, Langhammer M, Göttlicher M, Wolf E, Lahm H, Hoeflich A 2009 IGFBP-2 overexpression reduces the appearance of dysplastic aberrant crypt foci and inhibits growth of adenomas in chemically induced colorectal carcinogenesis. Int J Cancer 124:2220–2225 [DOI] [PubMed] [Google Scholar]
- Kitszel A, Krawczuk-Rybak M 2007 Are elevated serum levels of IGFBP-2 after intensive chemotherapy of childhood acute lymphoblastic leukemia a risk factor of relapse? Adv Med Sci 52:147–153 [PubMed] [Google Scholar]
- Wang H, Arun BK, Wang H, Fuller GN, Zhang W, Middleton LP, Sahin AA 2008 IGFBP2 and IGFBP5 overexpression correlates with the lymph node metastasis in T1 breast carcinomas. Breast J 14:261–267 [DOI] [PubMed] [Google Scholar]