Abstract
Context: Chemerin is a novel adipokine previously associated with metabolic syndrome phenotypes in a small sample of subjects from Mauritius.
Objective: The aim of the study was to determine whether plasma chemerin levels were associated with metabolic syndrome phenotypes in a larger sample from a second, unrelated human population.
Design, Setting, Patients, and Intervention: Plasma samples were obtained from the San Antonio Family Heart Study (SAFHS), a large family-based genetic epidemiological study including 1431 Mexican-American individuals. Individuals were randomly sampled without regard to phenotype or disease status. This sample is well-characterized for a variety of phenotypes related to the metabolic syndrome.
Main Outcomes: Plasma chemerin levels were measured by sandwich ELISA. Linear regression and correlation analyses were used to determine associations between plasma chemerin levels and metabolic syndrome phenotypes.
Results: Circulating chemerin levels were significantly higher in nondiabetic subjects with body mass index (BMI) greater than 30 kg/m2 compared with those with a BMI below 25 kg/m2 (P < 0.0001). Plasma chemerin levels were significantly associated with metabolic syndrome-related parameters, including BMI (P < 0.0001), fasting serum insulin (P < 0.0001), triglycerides (P < 0.0001), and high-density lipoprotein cholesterol (P = 0.00014), independent of age and sex in nondiabetic subjects.
Conclusion: Circulating chemerin levels were associated with metabolic syndrome phenotypes in a second, unrelated human population. This replicated result using a large human sample suggests that chemerin may be involved in the development of the metabolic syndrome.
The novel adipokine chemerin is associated with metabolic syndrome components in different populations, suggesting it may play a role in pathogenesis and disease risk.
Over the last decade, adipose tissue has been shown to secrete a variety of soluble proteins, termed adipokines, such as leptin, adiponectin, and TNF-α. These factors act locally and/or in other tissues to regulate adipocyte biology and systemic processes including food intake and nutrient metabolism, insulin sensitivity, bone growth, inflammation, and reproduction (1,2). The number of known adipokines secreted from adipose tissue is increasing, and the identification and characterization of these secreted proteins is important in understanding how they may regulate energy homeostasis and contribute to the development of obesity and the metabolic syndrome. Chemerin was recently found to be an adipokine, and its expression was increased in states of obesity (3,4,5,6). We and others have shown that chemerin mRNA expression was increased in adipose tissue of obese and type 2 diabetic animals, was predominantly expressed by adipocytes in adipose tissue, and was significantly induced upon differentiation of 3T3-L1 cells into mature adipocytes (3,4,5,6). Previously, we have identified significant associations between circulating chemerin levels and characteristics of the metabolic syndrome in a relatively small sample of human subjects from Mauritius (3). Here, we have sought to build on these findings by conducting a second survey of circulating chemerin levels in a population that was genetically and geographically distinct from our initial study. In these studies, we have measured circulating chemerin levels in a large sample (n = 1142) of Mexican-American individuals from the San Antonio Family Heart Study (SAFHS) (7,8), and, similar to our previous work, we have found significant associations between plasma chemerin levels and a number of metabolic syndrome phenotypes.
Subjects and Methods
Samples
Plasma samples were obtained from the SAFHS, a study of risk factors for cardiovascular disease in Mexican-Americans living in and around San Antonio, Texas (7,8). The SAFHS is a large family-based genetic epidemiological study including 1431 individuals from 42 extended families at baseline. Individuals from large randomly selected, multigenerational pedigrees were sampled independent of their phenotype or the presence or absence of disease. All subjects participated in an oral glucose tolerance test where they were administered a 75-g glucose load in a fasted state and glucose levels were determined from a blood sample obtained before and at 2 h after the glucose challenge. Diabetes was diagnosed according to the plasma glucose criteria outlined by the World Health Organization (9). Subjects were grouped as being nondiabetic (ND) or having type 2 diabetes (T2D). Those subjects classified as having impaired fasting glucose or impaired glucose tolerance were grouped with the ND subset for the purpose of the analysis performed in this study. The phenotypic characteristics of the SAFHS subjects are outlined in Table 1. All protocols were approved by the Institutional Review Board of the University of Texas Health Science Centre at San Antonio (San Antonio, TX).
Table 1.
Physical and metabolic characteristics of SAFHS subjects
| Phenotype | ND (n = 969) | T2D (n = 173) | P value |
|---|---|---|---|
| Sex distribution (females/males) | 569/400 | 109/64 | |
| Age (yr) | 36.2 ± 15.3 | 54.6 ± 14.6 | < 0.0001 |
| Fasting glucose (mg/dl) | 86.9 ± 11.0 | 179.0 ± 70.7 | < 0.0001 |
| Fasting insulin (U/ml) | 14.0 ± 15.5 | 26.5 ± 29.5 | < 0.0001 |
| HOMA_IR | 3.1 ± 4.4 | 11.3 ± 13.3 | < 0.0001 |
| Total serum cholesterol (mg/dl) | 185.9 ± 39.4 | 204.2 ± 44.9 | < 0.0001 |
| HDL cholesterol (mg/dl) | 50.6 ± 13.2 | 47.6 ± 13.4 | 0.0053 |
| Triglycerides (mg/dl) | 138.9 ± 125.5 | 220.1 ± 160.3 | < 0.0001 |
| BMI (kg/m2) | 28.6 ± 6.7 | 31.8 ± 8.1 | < 0.0001 |
| Total body fat (%) | 24.2 ± 15.2 | 30.5 ± 16.5 | < 0.0001 |
| Chemerin (ng/ml) | 180.5 ± 61.8 | 191.3 ± 61.7 | 0.026 |
Data are presented as mean ± sd. HOMA_IR (homeostasis model of assessment for insulin), Quantitative estimate of insulin resistance calculated as previously described (13). Values in bold represent significance where P < 0.05.
ELISA
Chemerin levels in human plasma from the SAFHS were determined using a sandwich ELISA developed with commercially available unlabeled and biotinylated polyclonal antihuman chemerin antibodies (R&D Systems, Minneapolis, MN) as previously described (3). The interassay coefficient of variation was less than 10%, and the within-assay coefficient of variation was less than 5%. The sensitivity of the ELISA assay was 0.5–10 ng/ml, and the midrange of the assay was 5 ng/ml. The least detectable concentration of human chemerin was 0.5 ng/ml.
Statistical analysis
Statistical analysis of the ELISA data was performed using the SPSS for Windows 14.1 software package (SPSS Inc., Chicago, IL). Normality of the distribution of the data was determined using a Kolmogorov-Smirnov test. A Student’s t test (for data that was normally distributed) or a Mann-Whitney test (for data that was not normally distributed) was used to compare the ND and T2D samples. Associations between circulating chemerin levels and various phenotypes of the metabolic syndrome were determined using Pearson’s correlation (for normally distributed data) or Spearman’s correlation (for nonnormally distributed data). Linear regression analyses were used to control for covariates such as sex and age. The correlations of the residuals were then recalculated to determine whether the correlations persisted. Differences between groups were assessed by one-way ANOVA, followed by Fisher’s least significance difference or Games-Howell post hoc test. Associations were considered significant if P < 0.05.
Results
Plasma chemerin levels were significantly higher in overweight and obese subjects compared with lean subjects
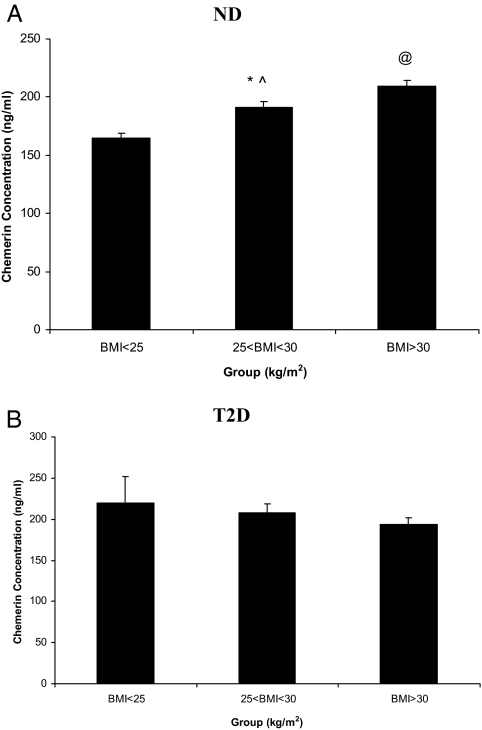
Circulating chemerin levels were measured in plasma samples from 1142 individuals, of whom 969 were ND and 173 had T2D. The physical and metabolic characteristics of the subjects included in this study are detailed in Table 1. Plasma chemerin levels were significantly different between ND and T2D subjects in the SAFHS samples. In addition, we found a significant difference in circulating chemerin levels between ND subjects with a body mass index (BMI) of more than 30 kg/m2 (obese; n = 349), those with a BMI between 25 and 30 kg/m2 (overweight; n = 351), and those with a BMI less than 25 kg/m2 (lean; n = 289; Fig. 1A). Plasma chemerin levels were significantly higher in ND individuals with a BMI greater than 30 kg/m2 compared with those with a BMI less than 25 kg/m2. Furthermore, plasma chemerin levels in overweight individuals were significantly higher than in lean subjects, but lower than those in obese individuals. In contrast to these results, plasma chemerin levels were not significantly different in T2D subjects that were overweight (n = 46) or obese (n = 110) compared with lean subjects (n = 18; Fig. 1B).
Figure 1.
Plasma chemerin levels in different BMI categories in SAFHS subjects. Plasma chemerin levels in different BMI categories in ND subjects (A) and T2D subjects (B). Data are expressed as mean plasma chemerin levels ± sem. Differences between groups were assessed by one-way ANOVA followed by Fisher’s least significance difference or Games-Howell post hoc test for the comparisons of the different BMI groups. Differences were considered significant if P < 0.05. *, P = 0.00015 compared with subjects with a BMI less than 25 kg/m2; ^, P = 0.00042 compared with subjects with a BMI greater than 30 kg/m2; @, P < 0.0001, compared with subjects with a BMI less than 25 kg/m2.
Plasma chemerin levels are associated with characteristics of the metabolic syndrome in ND subjects
Although chemerin levels were significantly different between individuals who were ND and those that had T2D, no significant correlations between chemerin levels and quantitative diabetes endophenotypes were observed in subjects with T2D (data not shown). However, in ND SAFHS subjects, plasma chemerin levels were significantly correlated with a number of phenotypes tested, including BMI, fasting glucose, and fasting serum insulin (Table 2). Strong correlations with age and sex were also observed due to higher chemerin levels in females compared with males (188.5 ± 65.3 and 168.2 ± 55.7 ng/ml, respectively; P < 0.0001) and older individuals compared with younger individuals (P = 0.005). After adjustment for age and sex, chemerin levels remained significantly associated with metabolic syndrome-related parameters, including BMI, fasting serum insulin, high-density lipoprotein (HDL) cholesterol, and plasma triglycerides. When the data were adjusted further for age, sex, and BMI, significant associations were observed with HDL cholesterol, total serum cholesterol, and plasma triglycerides. These results clearly show that chemerin was associated with metabolic syndrome-related phenotypes independent of age, sex, and BMI in ND individuals.
Table 2.
Chemerin is associated with obesity and metabolic syndrome-related phenotypes in ND SAFHS subjects
| Phenotype | Unadjusted | Plasma chemerin levels adjusted for
|
|
|---|---|---|---|
| Age/sex | Age/sex/BMI | ||
| Age | − | − | |
| BMI | P < 0.0001, r = 0.286 | P < 0.0001, r = 0.217 | − |
| Fasting glucose (mm) | P < 0.0001, r = 0.104 | P = 0.007, r = 0.086 | P = 0.17, r = 0.044 |
| Fasting insulin (mm) | P < 0.0001, r = 0.164 | P < 0.0001, r = 0.251 | P = 0.05, r = 0.055 |
| HOMA_IR | P < 0.0001, r = 0.147 | P < 0.0001, r = 0.165 | P = 0.04, r = 0.071 |
| Total body fat (%) | P < 0.0001, r = 0.265 | P < 0.0001, r = 0.142 | P = 0.42, r = 0.026 |
| HDL (mm) | P < 0.0001, r = −0.142 | P = 0.00014, r = −0.121 | P = 0.007, r = −0.083 |
| Total serum cholesterol (mm) | P = 0.00084, r = 0.101 | P = 0.002, r = 0.082 | P = 0.01, r = 0.065 |
| Triglycerides (mm) | P < 0.0001, r = 0.177 | P < 0.0001, r = 0.148 | P = 0.0004, r = 0.111 |
| 2-h Glucose (mm) | P < 0.0001, r = 0.125 | P = 0.16, r = 0.046 | P = 0.81, r = 0.007 |
| Systolic BP (mm Hg) | P = 0.37, r = 0.035 | P = 0.33, r = 0.032 | P = 0.25, r = 0.039 |
| Diastolic BP (mm Hg) | P = 0.85, r = 0.006 | P = 0.53, r = 0.021 | P = 0.44, r = 0.026 |
Associations were performed using Spearman’s correlation analyses. The data were adjusted for age, sex, and BMI using linear regression, and the correlations were recalculated on the residual from the regression results. BP, Blood pressure; HOMA_IR (homeostasis model of assessment for insulin), quantitative estimate of insulin resistance calculated using formula as previously described (13). Values were significant if P < 0.05 (shown in bold).
Discussion
Chemerin was recently described as being an adipokine that was highly expressed by adipocytes in adipose tissue (3,4,5,6). In addition, chemerin expression in adipose tissue was increased in animal models of obesity and T2D. We have previously shown that circulating chemerin levels were associated with obesity and the characteristics of the metabolic syndrome including BMI, plasma triglycerides, and blood pressure in ND subjects from Mauritius (3). Here we present data from a separate study of a second unrelated population of Mexican-Americans that also identified significant associations between plasma chemerin levels and the metabolic syndrome phenotypes such as BMI and plasma triglycerides in ND subjects. These findings support the possibility that the observed associations between circulating chemerin levels and a number of metabolic syndrome phenotypes may represent a widespread phenotypic relationship that is not population-specific. Similar observations have been found with other adipokines including monocyte chemoattractant protein-1, IL-8, and IL-18, where circulating levels of these factors were associated with obesity and other metabolic syndrome phenotypes in ND subjects (10,11,12). Because these factors clearly play a role in these conditions, it is tempting to speculate that chemerin may also play a role in obesity and the metabolic syndrome; however, whether chemerin is a cause or consequence of these disorders remains to be elucidated and requires further investigation.
There were several differences between findings obtained in the current study and those observed previously in the Mauritian samples (3). For example, plasma chemerin levels were lower in the SAFHS subjects compared with the Mauritius population, despite the subjects being heavier. In addition, plasma chemerin levels in ND individuals were significantly associated with blood pressure, independent of age, sex, and BMI in the Mauritius population; however, this was not replicated in ND subjects from the SAFHS population. The reason for these differences is unclear at present, but it could possibly be attributable to the environmental and ethnic differences between the populations or differences in sample collection and storage. In addition, because age and sex were found to be significantly associated with plasma chemerin levels in both studies, it was notable that the SAFHS subjects were generally younger (36 vs. 46 yr of age) and had a higher female/male ratio compared with the Mauritius population (569 females to 400 males vs. 129 females to 171 males, respectively), and this may have influenced the findings in each study.
A key observation in both the present study and the previous study of the Mauritius population was that a significant association between plasma chemerin levels and phenotypes of the metabolic syndrome was not observed in T2D subjects. The reason for the associations observed in ND but not T2D subjects is not clear from the present study; however, it may be due to the difference in sample size between ND and T2D individuals (n = 969 vs. n = 179, respectively). To demonstrate this, power calculations were performed in the individuals with T2D and found that there was 80% power to detect differences in mean values of 14.5 mg/dl for fasting glucose, 2.7 U for homeostasis model of assessment for insulin resistance, and 6.1 U/ml for fasting insulin at a P value of 0.05, which indicates a reduced power in this subset. It is also possible that the dysregulation of metabolic processes associated with the development of T2D disrupts the association between circulating chemerin levels and metabolic syndrome phenotypes. In addition, it is conceivable that other factors such as the level of glycemic control, medication history, duration of diabetes, and the presence of complications such as renal disease may impact on the relationship between circulating chemerin levels and metabolic syndrome phenotypes. These are clearly important questions and will need to be addressed in future work.
In summary, we have shown that plasma chemerin levels were significantly associated with characteristics of the metabolic syndrome in a large family-based population of Mexican-American individuals. This current study confirms a number of the associations we previously observed in our study of the Mauritian population, suggests that these associations are not population-specific, and raises the possibility that chemerin may be involved in the development of the metabolic syndrome.
Acknowledgments
We are grateful to the participants of the San Antonio Family Heart Study for their continued involvement.
Footnotes
This work was supported by Diabetes Australia Research Trust. Data collection for the San Antonio Family Heart Study was supported by a program project grant from the National Heart, Lung and Blood Institute, National Institutes of Health (HL045222).
Disclosure Summary: K.B., K.A.S., N.C., J.E.C., A.G.C., M.C.M., D.L.R., J.L.V., J.W.M., and J.B. have nothing to declare. D.S., G.C., K.W., and J.J. have equity interest in ChemGenex Pharmaceuticals. K.W. consults for ChemGenex Pharmaceuticals.
First Published Online May 26, 2009
Abbreviations: BMI, Body mass index; HDL, high-density lipoprotein; ND, nondiabetic; T2D, type 2 diabetes.
References
- Wisse BE 2004 The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 15:2792–2800 [DOI] [PubMed] [Google Scholar]
- Coppack SW 2001 Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 60:349–356 [DOI] [PubMed] [Google Scholar]
- Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D 2007 Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148:4687–4694 [DOI] [PubMed] [Google Scholar]
- Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ 2007 Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282:28175–28188 [DOI] [PubMed] [Google Scholar]
- Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sasaki S 2007 Chemerin—a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun 362:1013–1018 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Takahashi Y, Takahashi K, Zolotaryov FN, Hong KS, Kitazawa R, Iida K, Okimura Y, Kaji H, Kitazawa S, Kasuga M, Chihara K 2008 Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3–L1 adipocytes. FEBS Lett 582:573–578 [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Almasy LA, Rainwater DL, Schneider JL, Blangero J, Stern MP, MacCluer JW 1999 Diabetes and hypertension in Mexican American families: relation to cardiovascular risk. Am J Epidemiol 149:1047–1056 [DOI] [PubMed] [Google Scholar]
- MacCluer JW, Stern MP, Almasy L, Atwood LA, Blangero J, Comuzzie AG, Dyke B, Haffner SM, Henkel RD, Hixson JE, Kammerer CM, Mahaney MC, Mitchell BD, Rainwater DL, Samollow PB, Sharp RM, VandeBerg JL, Williams JT 1999 Genetics of atherosclerosis risk factors in Mexican Americans. Nutr Rev 57:S59–S65 [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ 1998 Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553 [DOI] [PubMed] [Google Scholar]
- Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R 2006 Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 30:1347–1355 [DOI] [PubMed] [Google Scholar]
- Straczkowski M, Kowalska I, Nikolajuk A, Otziomek E, Adamska A, Karolczuk-Zarachowicz M, Gorska M 2007 Increased serum interleukin-18 concentration is associated with hypoadiponectinemia in obesity, independently of insulin resistance. Int J Obes (Lond) 31:221–225 [DOI] [PubMed] [Google Scholar]
- Straczkowski M, Dzienis-Straczkowska S, Stêpieñ A, Kowalska I, Szelachowska M, Kinalska I 2002 Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-α system. J Clin Endocrinol Metab 87:4602–4606 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]