Abstract
Context: Acquisition of ovulatory competence by antral follicles requires development of an adequate vascular supply. Although it is well established that ovarian angiogenesis is cyclically regulated by vascular endothelial growth factor (VEGF), the factors controlling VEGF production by ovarian follicles remain largely unknown. Nerve growth factor (NGF) may be one of these factors, because NGF promotes angiogenesis and synthesis of angiogenic factors in other tissues and is produced by human granulosa cells (hGCs).
Objective: The aim of the study was to determine whether NGF influences the production of VEGF by hGCs and to identify a potential signaling pathway underlying this effect.
Design: We conducted a prospective experimental study.
Patients: hGCs were obtained from 41 women participating in the in vitro fertilization program of our institution.
Methods: Changes in VEGF mRNA after exposure to NGF were evaluated in cultured hGCs by PCR and real-time PCR. The effect of NGF on VEGF secretion was determined by ELISA. The involvement of trkA, the high affinity NGF receptor, was examined by inhibiting the receptor’s tyrosine kinase activity with K252a. The contribution of an ERK1/ERK2-mediated signaling pathway was identified by detecting NGF-dependent phosphorylation of these proteins and by blocking their activity with the inhibitor U0126.
Results: NGF promotes VEGF production in cultured hGCs. Blockade of trkA receptor tyrosine kinase activity blocks this effect. NGF induces MAPK-ERK2 phosphorylation, and blockade of this signaling pathway prevents the NGF-induced increase in VEGF production.
Conclusions: NGF promotes ovarian angiogenesis by enhancing the synthesis and secretion of VEGF from hGCs via a trkA- and ERK2-dependent mechanism.
NGF participates in ovarian angiogenesis by increasing VEGF expression through trkA receptor activation and MAPK-ERK2 signaling.
The NGF is an evolutionary conserved polypeptide of the neurotrophin (NT) family required for the survival and differentiation of sympathetic and sensory neurons of the peripheral nervous system (1). In addition, NGF contributes to the developmental regulation of some peripheral organs, such as the thymus (2), pancreas (3,4), and ovary (5). In the ovary, NGF promotes the development of preantral follicles (6,7,8) and also appears to play a role in ovulation (9,10).
The ovaries from NGF-null infantile mice exhibit a reduced population of primary and secondary follicles, an increased number of oocytes that are not incorporated into a follicular structure, and a reduction in somatic cell proliferation (7). Immunoneutralization of NGF during early postnatal life of the rat leads to stunted growth of antral follicles, delayed puberty, and disrupted estrous cyclicity (8). Although expression of the mRNAs encoding NGF and its high-affinity receptor trkA remains low throughout juvenile development, it increases during the gonadotropin surge, preceding ovulation (9,10), suggesting an involvement of the NGF and its receptor in this process. Accordingly, the administration of a neutralizing antibody to NGF or the pharmacological blockade of tyrosine kinase activity targeted to one ovary resulted in the ipsilateral inhibition of ovulation (9). Importantly, NGF also promotes FSH receptor synthesis in both the rat and the human ovary (11,12).
Another NGF action, less known to endocrinologists, is its ability to promote angiogenesis in tissues as diverse as skin (13), skeletal muscle (14), cornea (15), and the central nervous system (16). NGF accelerates skin wound healing in humans (13,17), and NGF immunoneutralization has been shown to delay the reparative neovascularization of ischemic hindlimbs in mice (14). Noteworthy, NGF triggers the proliferation of human dermal microvascular endothelial cells (18) and human umbilical vein endothelial cells via trkA activation (19,20), suggesting that trkA-dependent signaling underlies the angiogenic effects of NGF in different tissues. NGF induces vascular endothelial growth factor (VEGF) expression in rat superior cervical ganglia neurons and in PC12 cells (16,21), suggesting that the angiogenic, trkA-mediated effects of NGF are mediated by VEGF.
The presence of both NGF and its high-affinity receptors, in addition to VEGF, in the ovary provides an ideal environment for the establishment of a functional relationship between the two growth factors. The ovary undergoes cyclic changes in vascular density and distribution according to the prevailing state of follicular and corpus luteum development (22,23). These vascular changes are highly regulated by VEGF (22,23,24,25). Alternative splicing of the primary VEGF mRNA transcript give rise to four transcripts (26). They encode VEGF proteins that become available to endothelial cells by at least two different mechanisms: 1) diffusion of the shorter isoforms (VEGF121, VEGF165); and 2) after protease activation and cleavage of the longer ones (VEGF189, VEGF206) (26). The mRNAs encoding the isoforms of 121 and 165 amino acids are the most abundantly expressed in normal human ovaries (27,28).
Earlier studies demonstrated a role for VEGF in follicular development (29,30), ovulation, and corpus luteum formation (30). These studies suggest that angiogenesis is an essential component of not only follicular and luteal growth, but also follicular atresia and luteal regression. In fact, the ovary can be revascularized in a dramatically short time after transplantation, and this ability for neovascularization is associated with an increase in VEGF expression (31). Also, VEGF is a central component of the angiogenic switch required for the growth of ovarian tumors, with microvessel density being much higher in VEGF-positive ovarian tumors than in those expressing low levels of the growth factor (32). In addition, ovarian disorders such as polycystic ovary syndrome and ovarian hyperstimulation syndrome have underlying defects in ovarian angiogenesis (33,34,35).
We previously reported that NGF up-regulates VEGF expression and increases follicular vascularization in the rodent ovary (36) and increases VEGF expression in human ovarian cancer (28). The cellular context within which these interactions may take place in the human ovary is not known, but a likely site is the granulosa cell (GC) compartment because these cells express not only NGF and its receptor trkA, but also the VEGF gene (12,37). Based on these considerations, we sought to determine whether NGF can act on hGCs to increase VEGF expression and to identify a signaling pathway that may mediate this effect.
Patients and Methods
hGC isolation
hGCs were obtained by ultrasound-guided follicular aspiration from 41 patients undergoing in vitro fertilization, due to male-related infertility, at the Hospital Clínico Universidad de Chile. All subjects provided informed consent, and the study protocol was approved by the Institutional Ethics Committee.
Synchronization of ovarian cycles was achieved by administering a GnRH agonist (10 μg/kg, Lupron; Abbott Laboratories, Abbott Park, IL) sc for 7 d before menstruation and continuing until the day before the administration of human Corionic Gonadotropin (hCG), which was given between d 10 and 12 of the menstrual cycle. Superovulation was induced by daily sc administration of 200 IU recombinant FSH (Puregon; Organon, Kenilworth, NJ) for 3 d. This dose was adjusted subsequently according to the individual ovarian response evaluated by daily ultrasound examination. When at least two follicles reached a diameter of 20 mm, a single dose of 10,000 IU hCG (Pregnyl; Organon) was administrated im, and the hGCs were aspirated 34 h later, at the time of oocyte retrieval. GCs were recovered from follicular aspirates and purified as described previously (12). Their number and viability were determined using the trypan blue exclusion method.
Cell culture
Cells were plated at a density of 500,000 cells per well in gelatin-coated wells (Becton Dickinson, Franklin Lakes, NJ) containing 3 ml of serum-free DMEM/Ham’s F-12 medium (Sigma-Aldrich Corp., St. Louis, MO) supplemented with sodium bicarbonate (1.2 g/liter) and antibiotic/antimycotic solution (Hyclone; Thermo Fisher Scientific Inc., Logan, UT) at 37 C in a humidified 5% CO2/95% air environment. Eighteen hours later, the medium was replaced with fresh medium containing NGF (50 ng/ml; Sigma-Aldrich), and the cells were incubated with the NT for 5 and 10 min for protein extraction; for 2, 4, 8, and 18 h for measurement of VEGF mRNA; and for 8 h for measurement of VEGF protein secreted to the culture medium. Two inhibitors were used: K252a (100 nm; Calbiochem, La Jolla, CA), an inhibitor of trk receptor kinase activity (26); and U0126 (10 μm; Cell Signaling Technologies, Beverly, MA), an inhibitor of MAPK activity (38). Both inhibitors were administered 30 min before adding NGF to the cultures and remained in the wells throughout the NGF treatment. Then, the culture medium was collected and stored at −80 C until assayed for VEGF protein. For RNA extraction, Trizol reagent, (Invitrogen, Carlsbad, CA) was added to the cells at the end of culture. For Western blot analysis, the cells were collected by scraping into lysis buffer and were stored at −80 C until protein extraction. Each experiment used a pool of cells from an individual patient, and the cellular viability was evaluated at the end of the experiment by the trypan blue exclusion test.
For the immunocytochemistry (ICC) study, 20,000 cells per well were plated on Lab-Tek chambers (Nunc; Thermo Fisher Scientific Inc.) in DMEM/Ham’s F-12 medium (Sigma-Aldrich) supplemented with sodium bicarbonate (1.2 g/liter), an antibiotic/antimycotic solution (Hyclone), and 10% fetal bovine serum (Hyclone). Twenty-four hours later, the medium was replaced with serum-free DMEM/Ham’s F-12 plus antibiotics, and the cells were exposed to NGF (50 ng/ml) for 8 h. To neutralize the biological effects of NGF, cells were treated with rabbit polyclonal antibodies to NGF (1:1000; provided by Dr. H. F. Urbanski, Oregon National Primate Research Center, Beaverton, OR) or with K252a (100 nm; Calbiochem).
ELISA
VEGF was measured in samples of culture medium using a specific ELISA (Quantikine Human VEGF Immunoassay; R&D Systems, Minneapolis, MN). This assay recognizes VEGF165, as well as VEGF121. An enzyme immunoassay multiwell reader (Sigma-Aldrich) set to read at an emission of 450 nm was used to quantify the results. The interassay coefficient of variation was 8.5%, and the sensitivity of the assay was 5 pg/ml.
Western blotting
Cultured cells (1 × 106) were lysed in 75 μl of lysis buffer containing 150 mm NaCl, 50 mm Tris-HCl, 1% Triton X-100, 1 mm EDTA, 1 mm EGTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 10 mg/ml leupeptin, 1.8 mg/ml aprotinin, 2 mm sodium fluoride, 2 mm sodium pyrophosphate, and 1 m dithiothreitol. Fifty micrograms of protein from each sample were size-fractionated by 12% SDS-PAGE and then transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). Nonspecific binding was blocked by incubating the membranes in PBS (pH 7.4) containing 2% nonfat dried milk and 0.2% Tween-20. Membranes were then incubated with anti-phospho-MAPK p44/42 or anti-p44/42 rabbit polyclonal antibodies (1:1000, 4 C, overnight; Cell Signaling Technologies, Beverly, MA), followed by incubation at room temperature for 2 h with a goat antirabbit antibody conjugated with horseradish peroxidase (1:10,000; Cell Signaling Technologies). In all cases, the same membrane used to detect phosphorylated ERKs was stripped and blotted against total ERKs. Proteins were detected using the enhanced chemiluminescent detection system (PerkinElmer Life Sciences, Wellesley, MA). The films were scanned and analyzed with an automated digitizing system (UN-SCAN-IT gel 4.1; Silk Scientific Corporation, Orem, UT).
RNA extraction, reverse transcription, and semiquantitative-PCR
After RNA extraction (see above), RNA concentrations were measured at 260 and 280 nm and reverse transcribed in a 20-μl reaction mixture using 1 μg total RNA, as previously described (12,28).
Conditions for PCR were as follows: 2.5 μl 10 × reaction buffer [200 mm Tris-HCl (pH 8.4), 500 mm KCl], 1 μl MgCl2 (50 mm), 0.5 μl dNTPs (10 mm each), 1.25 μl each primer (10 μm), 0.15 μl DNA polymerase (5 U/μl; Biotools, Madrid, Spain). Reaction mixtures were incubated in a thermal cycler (Eppendorf, Hamburg, Germany) for 2 min at 94 C. Then 25 cycles were performed for β-actin and VEGF including: denaturation at 94 C for 45 sec, annealing at 62 C for 1 min, and extension at 72 C for 1 min. The optimal number of cycles was determined experimentally by determining the linear portion of the amplification reaction, as previously reported (36).
The primers used to detect VEGF and β-actin transcripts (used for signal normalization) were described previously (28). The PCR products were separated on a 2% agarose gel, visualized under UV light, photographed using a capturing program (Ultra Violet Products Doc-It, Upland, CA), and analyzed with an automated digitizing system (UN-SCAN-IT gel 4.1; Silk Scientific Corporation). Their identities were verified by automatic sequencing using an Applied Biosystems DNA Sequencer model 3730 (Applied Biosystems, Foster City, CA).
Real-time PCR
Total RNA was extracted, and 500 ng were reverse-transcribed as outlined above. The resulting cDNAs were amplified by real-time PCR using a procedure previously described (12). The primers and internal oligodeoxynucleotide probe were designed to anneal specifically between exons 5–7 of human VEGF (hVEGF), thereby amplifying only isoform 165 (AF486837), and were selected with the assistance of the program Primer Express (Perkin-Elmer Applied Biosystems, Norwalk, CT). The VEGF primers used were a sense primer (5′-TGT GAA TGC AGA CCA AAG AAA GA-3′) corresponding to nt 382-404 in hVEGF mRNA, and an antisense primer (5′-GCT TTC TCC GCT CTG AGC AA-3′) complementary to nt 454-473. The internal probe (5′-AGA GCA AGA CAA GAA AAT CCC TGT GGG C-3′; Perkin-Elmer Applied Biosystems) corresponds to nt 406-432 in hVEGF mRNA and was covalently linked to the fluorescent dye FAM at the 5′ end and the quencher dye fluorescence emission spectra of carboxytetramethylrhodamine at the 3′end. The 18s ribosomal RNA was used as a normalizing unit for each reaction, using a set of primers purchased as a kit (TaqMan Ribosomal RNA Control Reagents Kit; Perkin-Elmer, Applied Biosystems). The identity of the VEGF product amplified by real-time PCR was verified by sequencing.
Immunocytochemistry
GCs were fixed in ice-cold methanol and treated with Peroxoblock (Zymed LAB-SA Detection System; Invitrogen) for 5 min to block endogenous peroxidase activity. They were incubated for 30 min in serum-blocking solution (Histostain, Zymed LAB-SA Detection System; Invitrogen). The primary VEGF antibody was diluted in 2% BSA in PBS (1:1000, AbCam Inc., Cambridge, MA) and incubated for 24 h at 4 C. The cells were then washed and incubated with biotinylated secondary antibody and enzyme conjugate (Histostain SP; Invitrogen, Camarillo, CA) for 20 min each at room temperature. The signal was detected by using DAB substrate kit (Zymed). Cells were counterstained with Mayer’s hematoxylin and analyzed microscopically. Cells incubated in the absence of primary antibody served as negative controls. The signal intensity was quantified using Image-Pro Plus 6.2 (Media Cybernetics, Inc., Silver Spring, MD). The results were expressed as mean pixels per cell ± sem.
Data analysis
All results expressed as percentage were subjected to an arc-sine transformation to convert their binomial distribution into a normal distribution. The Kruskal Wallis test was used because the data did not follow a normal distribution. A P value less than 0.05 was considered significant. Results are expressed as mean ± sem.
Results
hGCs express VEGF mRNAs encoding three VEGF isoforms
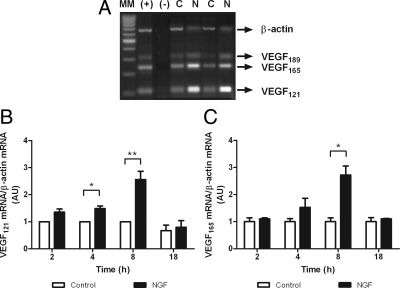
RT-PCR of total RNA extracted from hGC cultures revealed the presence of mRNA transcripts encoding VEGF121, 165, and 189, with the transcript encoding VEGF121 and VEGF165 being the most abundant species (Fig. 1A).
Figure 1.
NGF increases mRNA expression in hGCs. A, Ethidium bromide-stained gel showing the presence of VEGF mRNA transcripts encoding the VEGF isoforms 121 and 165 in cultured hGCs, as measured by RT-PCR. MM, Molecular markers; (+), positive control (ovarian cancer); (−), negative control (PCR mix without cDNA); C, hGCs cultured under basal conditions; N, hGCs cultured for 8 h in the presence of 50 ng/ml NGF. This gel is representative of seven independent experiments. B, Effect of NGF on VEGF121 mRNA levels measured by semiquantitative PCR after different times of treatment (2, 4, 8, and 18 h). Each column represents the mean ± sem of seven independent experiments, each assayed in duplicate. C, Effect of NGF on VEGF165 mRNA levels. *, P < 0.05; **, P < 0.01 vs. untreated controls. AU, Arbitrary units.
NGF increases VEGF protein and mRNA abundance in hGCs
Based on the results of preliminary experiments, we selected for these studies a 50 ng/ml NGF dose. Both VEGF121 and VEGF165 transcripts were significantly increased by 8-h exposure to NGF (Fig. 1, B and C). In the case of VEGF121 mRNA, the increase was also statistically significant at 4 h (Fig. 1B). In a subsequent experiment, VEGF protein secreted into the culture medium was measured by ELISA after 8-h incubation with NGF. Because of the variability in the amount of VEGF secreted by hGCs derived from the eight patients studied, the results were expressed as percentage change from basal values. In each case, NGF elicited a relatively small, but highly significant increase in VEGF output (25 ± 6.5%, mean ± sem; P < 0.001).
NGF increases VEGF expression via activation of trkA receptors
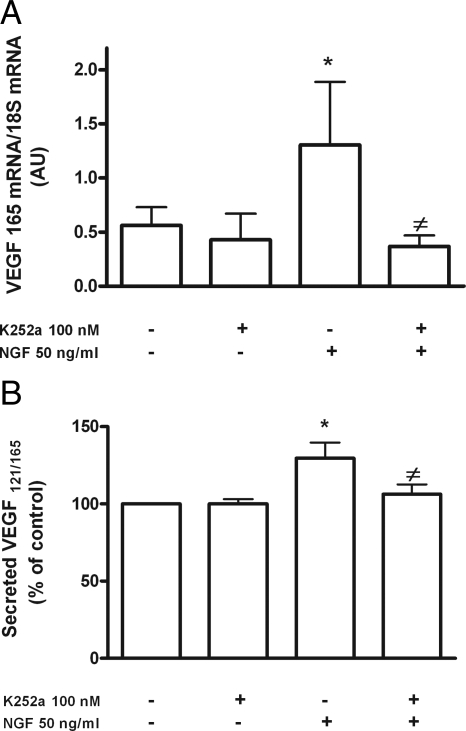
Previous reports indicate that the only receptors for NGF in GCs of the adult human ovary are trkA receptors (12,39). To determine whether these receptors mediate the effect of NGF on VEGF mRNA levels, we preincubated the cells with the trk receptor tyrosine kinase inhibitor K252a for 30 min before adding NGF and then during treatment with the NT. At the end of this treatment, we measured VEGF165 mRNA levels, in addition to secreted VEGF. We selected VEGF165 mRNA as a representative mRNA isoform for measurement because NGF is similarly effective in increasing the expression of both VEGF121 and VEGF165 mRNA after an 8-h treatment (Fig. 1, B and C).
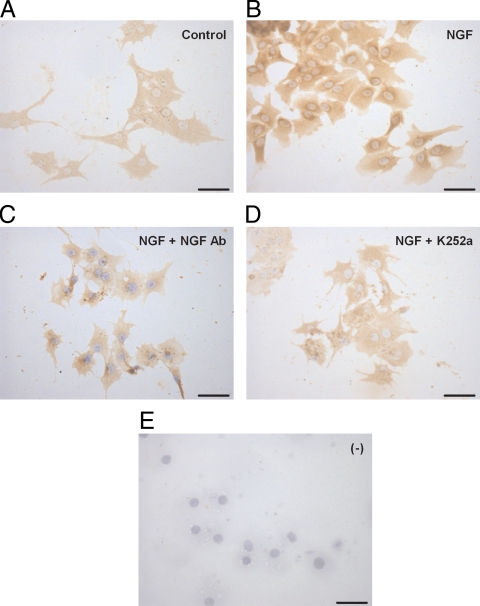
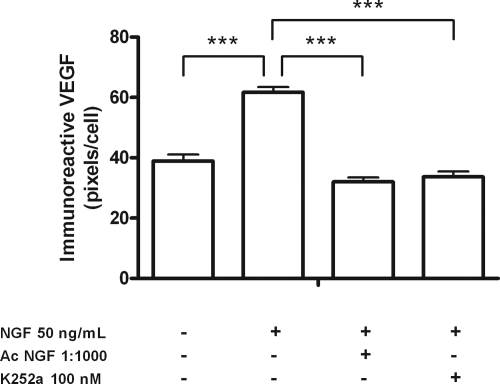
The inhibitor prevented the stimulatory effect of NGF on both VEGF165 mRNA abundance (Fig. 2A) and VEGF secretion (Fig. 2B), without affecting basal mRNA or protein secretion levels. These results were confirmed by semiquantitative ICC of cultured hGCs (Figs. 3 and 4). The inhibitor significantly reduced the amount of immunoreactive VEGF detected in hGCs (Fig. 3) to levels similar to those of controls incubated in the absence of NGF (Fig. 4). The specificity of the NGF effect was further documented by the ability of neutralizing antibodies to NGF to abolish the NGF-dependent increase in VEGF immunoreactivity (Figs. 3 and 4). These results indicate that activation of trkA receptors is required for NGF to increase VEGF expression.
Figure 2.
NGF increases VEGF mRNA content in hGCs via activation of trk receptors. A, Inhibitory effect of the tyrosine kinase receptor inhibitor K252a (100 nm) on NGF-induced increase in VEGF165 mRNA abundance. The cells were pretreated with the inhibitor for 30 min before adding NGF (50 ng/ml), and the mRNA levels were measured after 8 h of NGF treatment. The inhibitor was maintained in the culture medium during this time. *, P < 0.05 vs. untreated control group; ≠, P < 0.05 vs. NGF-treated group. Each column (expressed in arbitrary units) represents the mean ± sem of five independent observations analyzed in triplicate by real-time PCR. B, K252a also abolishes the increase in VEGF secretion elicited by NGF, as measured by ELISA. Each value represents the mean ± sem (expressed as percentage of the mean value observed in untreated controls) of five independent observations measured in triplicate. *, P < 0.05 vs. control group; ≠, P < 0.05 vs. NGF-treated group.
Figure 3.
Increase in immunoreactive VEGF levels in cultured hGCs treated with NGF (50 ng/ml) for 24 h. Representative microphotographs from five experiments are shown. A, hGCs contain VEGF immunoreactive material in the absence of NGF treatment. B, NGF treatment increases the content of VEGF in hGCs. C, Immunoneutralization of NGF actions with rabbit polyclonal antibodies blocks the effect of NGF on VEGF immunoreactive levels. D, Blockade of trk receptors with K252a also prevents the effect of NGF on VEGF immunoreactive levels. E, hGCs incubated in the absence of primary VEGF antibodies (ICC negative control). Scale bars, 50 μm.
Figure 4.
Semiquantitative analysis of VEGF protein levels detected by ICC in cultured hGCs. VEGF protein levels are increased by NGF (***, P < 0.0001), and this effect was blocked by either neutralizing NGF antibodies (NGF-Ab; ***, P < 0.0001) or K252a, a tyrosine kinase receptor inhibitor (***, P < 0.0001). Each bar represents the mean ± se of five independent experiments.
NGF-induced VEGF expression requires activation of the MAPK pathway
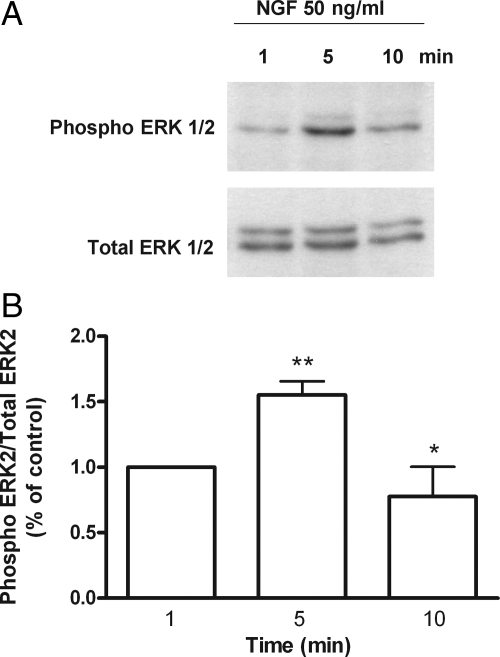
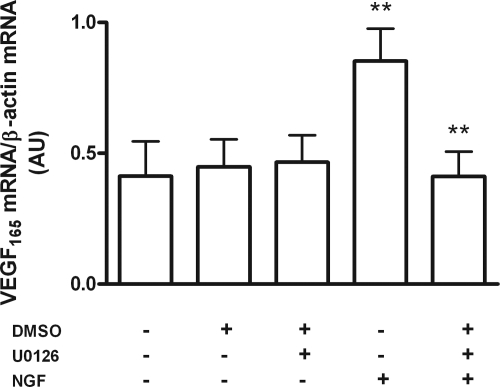
In other cellular systems, activation of trkA receptors results in activation of the MAPK pathway (40). To determine whether NGF also activates this pathway in hGCs, we treated the cells with NGF (50 ng/ml). Phosphorylation of ERK2 (and to a much lesser extent that of ERK1) was transiently increased within 5 min of treatment, with the effect no longer detected after 10 min (Fig. 5, A and B). Moreover, blockade of MAPK signaling with the inhibitor U0126 abolished the effect of NGF on VEGF mRNA content (Fig. 6), indicating that activation of this pathway is necessary for NGF to increase VEGF expression.
Figure 5.
NGF induces phosphorylation of MAPK ERK1/2 in hGCs. A, A representative Western blot showing that NGF induces a rapid (5 min), but transient, phosphorylation of ERK2, and to a lesser extent, ERK1, in cultured hGCs. B, Densitometric analysis of the blot shown in panel A. Each value represents the mean ± sem of eight experiments expressed as the ratio of phosphorylated ERK2/total ERK2 relative to untreated controls. *, P < 0.05 vs. t = 5 min and t = 10 min; **, P < 0.01 vs. t = 5 min and t = 1 min.
Figure 6.
MAPK pathway activation mediates NGF-induced VEGF expression. Human GCs were treated with the MAPK inhibitor U 0126 for 8 h in the presence or absence of NGF (50 ng/ml), and VEGF165 mRNA levels were measured by semiquantitative PCR at the end of the treatment. Each column represents the mean ± sem of VEGF165 mRNA levels from four independent experiments, expressed as arbitrary units. **, P < 0.01 vs. NGF + U 0126. DMSO, Dimethylsulfoxide.
Discussion
NTs are expressed in the human ovary (12,28,39,41). Studies in rodents and humans have made it clear that NTs, including NGF, contribute to the control of ovarian physiology by promoting oocyte survival (42) and facilitating follicular development (5,6,7). NGF and its trkA receptor have also been implicated in the process of ovulatory rupture (9). In addition to these functions, NGF acting via trkA receptors has been shown to regulate the steroidogenic output of cultured hGCs by increasing the production of estradiol and reducing that of progesterone (12).
By showing that NGF can stimulate the production of VEGF in hGCs via activation of trkA tyrosine kinase receptors and using MAPK/ERK2 for signal transduction, our results suggest a new role for NGF in ovarian physiology as a factor able to promote angiogenesis. The intermediacy of ERK2 in mediating this NGF effect is in harmony with a recent study showing that NGF activates the ERK1/2 pathway in pancreatic β-cells to regulate transcription of the insulin gene in response to glucose (3,40).
VEGF is an angiogenic molecule that enhances blood vessel permeability and stimulates endothelial cell proliferation and migration (26,43). Although VEGF is produced by hGCs of both preantral and antral follicles, it is particularly abundant in hGCs during the late preovulatory and early luteal phases of human ovarian cycle (38), a pattern of expression that correlates well with the increased vascularization observed in the thecal component of follicles approaching ovulation (25,27,36). It is now well established that this angiogenic process is mainly driven by VEGF (25,27) and that VEGF is required not only for follicle growth and ovulation, but also for the development and maintenance of the corpus luteum (44,45).
The ability of NGF to increase the synthesis of VEGF in cultured hGCs, considered in conjunction with the presence of both growth factors in addition to trkA receptors in GC of growing follicles, suggests that NGF acts in the in situ follicle in an autocrine/paracrine manner to promote VEGF production and thereby stimulate ovarian angiogenesis (12,36,37). Notwithstanding the importance of this function, our results do not rule out the possibility of a direct action of NGF on ovarian endothelial cells to promote cell migration and proliferation (18,19,20). In fact, both NGF and VEGF share the ability to activate MAPK/ERK- and PI3K/Akt-mediated intracellular signaling cascades in endothelial cells, suggesting that NGF may act in concert with VEGF to directly promote angiogenesis (28,36,45). Further studies are required to explore this possibility.
Our results show that not only the expression of a small, readily diffusible VEGF isoform (VEGF121) is modulated by NGF, but also that of VEGF165, which is a partially secreted form (26). Because the antibody used for ICC detects the nonsecreted VEGF189 form, in addition to VEGF165 and VEGF121 (36), it appears that the abundance of larger, tissue-associated isoforms of VEGF is also regulated by the NT, as previously shown in epithelial ovarian cancer (28). Considering that secreted and nonsecreted VEGF isoforms control different aspects of angiogenesis (26), it seems reasonable to conclude that NGF may contribute to regulating these features of angiogenesis in the ovary by virtue of its ability to increase the expression of all VEGF isoforms. It must be acknowledged, however, that cultured hGCs might not faithfully reflect the response to NGF of GCs in situ because cultured GCs have been luteinized by exposure to high levels of hCG before retrieval.
The increased synthesis of the diffusible forms VEGF121 and VEGF165 elicited by NGF also suggests that the NT contributes dynamically to regulating ovarian angiogenesis. According to this view, NGF would act within a short time-frame to replenish the supply of VEGF forms required for the vascularization of growing preovulatory follicles (and perhaps newly formed corpora lutea). Inferential support for this idea is provided by the demonstration that overexpressing the VEGF gene in the rat ovary enhances antral follicle development by increasing the density of the thecal vasculature (46). It is likely that NGF acts cooperatively with other factors, such as insulin and the IGFs, to stimulate VEGF production by hGCs (32,47,48,49).
By enhancing the production of VGEF from GCs, NGF supports the vascularization of the thecal compartment of growing follicles, a compartment that is functionally connected, but morphologically separated from GCs. The present results further highlight the capacity of NGF to affect GC function regardless of its site of production, because in both rats and humans, NGF is able to induce the formation of functional FSH receptor (11,12), and yet in rats NGF is mostly produced in thecal-interstitial cells of the ovary (7).
The ability of NGF to stimulate VEGF production raises the possibility of an involvement of the NT in ovarian disorders associated with defects in angiogenesis. For instance, serum VEGF concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries (33), and serum VEGF levels appear to have a predictive value in the case of ovarian hyperstimulation syndrome (34). In fact, excessive VEGF production has been implicated as a factor contributing to the pathogenesis of ovarian hyperstimulation syndrome and polycystic ovaries (33,34,47,48). A potential link with a deranged production of NGF is provided by a recent report showing that GCs from human polycystic ovaries produce excessive amounts of NGF (49).
In conclusion, our results identify NGF as an intraovarian factor able to promote VEGF production by follicular cells of the human ovary and suggest that this functional relationship may be relevant for the dynamic control of angiogenesis during antral follicle growth.
Footnotes
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico Grants 1030661 and 1071036 and partially by Universidad de Chile Grant DI 2006 ENL 06/03 (to C.R.); Comisión Nacional de Investigación Científica y Tecnológica Grant 4040059; Departamento de Postgrado y Postítulo Universidad de Chile Grants PG/90/2003 and PG/67/2005 (to M.J.-P.); National Institutes of Health Grant HD24870; the National Institute of Child Health and Human Development through Cooperative Agreement U54 HD18185, as part of the Specialized Cooperative Centers Program in Reproduction Research; and Grant RR000163-49 for the operation of the Oregon National Primate Research Center (to S.R.O.). M.J.-P., a Ph.D. student of the Biochemistry Postgraduate Program of Universidad de Chile, is supported by a fellowship from the Comisión Nacional de Investigación Científica y Tecnológica (Conicyt no. 102241).
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 19, 2009
Abbreviations: GC, Granulosa cell; hCG, human Corionic Gonadotropin; hGC, human GC; hVEGF, human VEGF; ICC, immunocytochemistry; NGF, nerve growth factor; NT, neurotrophin; VEGF, vascular endothelial growth factor.
References
- Levi-Montalcini R 1987 The nerve growth factor 35 years later. Science 237:1154–1162 [DOI] [PubMed] [Google Scholar]
- García-Suárez O, Germanà A, Hannestad J, Ciriaco E, Laurà R, Naves J, Esteban I, Silos-Santiago I, Vega JA 2000 TrkA is necessary for the normal development of the murine thymus. J Neuroimmunol 108:11–21 [DOI] [PubMed] [Google Scholar]
- Polak M, Scharfmann R, Seilheimer B, Eisenbarth G, Dressler D, Verma IM, Potter H 1993 Nerve growth factor induces neuron-like differentiation of an insulin-secreting pancreatic β-cell line. Proc Natl Acad Sci USA 90:5781–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfmann R, Tazi A, Polak M, Kanaka C, Czernichow P 1993 Expression of functional nerve growth factor receptors in pancreatic β-cell lines and fetal rat islets in primary culture. Diabetes 42:1829–1836 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Paredes A, Romero C, Dees WL, Ojeda SR 2004 Neural and neurotrophic control of ovarian development. In: Leung P, Adashi E, eds. The ovary. 2nd ed. San Diego: Academic Press; 3–23 [Google Scholar]
- Dissen GA, Hirshfield AN, Malamed S, Ojeda SR 1995 Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinology 136:4681–4692 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Hirshfield AN, Ojeda SR 2001 Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology 142:2078–2086 [DOI] [PubMed] [Google Scholar]
- Lara HE, Hill DF, Katz KH, Ojeda SR 1990 The gene encoding nerve growth factor is expressed in the immature rat ovary: effect of denervation and hormonal treatment. Endocrinology 126:357–363 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Les Dees CW, Lara HE, Ojeda SR 1996 A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology 137:198–209 [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Dissen GA, Parrott JA, Hill DF, Mayerhofer D, Garfield RE, Costa ME, Skinner MK, Ojeda SR 1996 Involvement of nerve growth factor in the ovulatory cascade: trkA receptor activation inhibits gap junctional communication between thecal cells. Endocrinology 137:5662–5670 [DOI] [PubMed] [Google Scholar]
- Romero C, Paredes A, Dissen GA, Ojeda SR 2002 Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology 143:1485–1494 [DOI] [PubMed] [Google Scholar]
- Salas C, Julio-Pieper M, Valladares M, Pommer R, Vega M, Mastronardi C, Kerr B, Ojeda SR, Lara HE, Romero C 2006 Nerve growth factor-dependent activation of trkA receptors in the human ovary results in synthesis of follicle-stimulating hormone receptors and estrogen secretion. J Clin Endocrinol Metab 91:2396–2403 [DOI] [PubMed] [Google Scholar]
- Chiaretti A, Piastra M, Caresta E, Nanni L, Aloe L 2002 Improving ischaemic skin revascularisation by nerve growth factor in a child with crush syndrome. Arch Dis Child 87:446–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P 2002 Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106:2257–2262 [DOI] [PubMed] [Google Scholar]
- Seo K, Choi J, Park M, Rhee C 2001 Angiogenesis effects of nerve growth factor (NGF) on rat corneas. J Vet Sci 2:125–130 [PubMed] [Google Scholar]
- Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R 2001 Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci USA 98:4160–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Aloe L, Russo A, Cesari M, Onder G, Bonini S, Carbonin PU, Bernabei R 2003 Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann Intern Med 139:635–641 [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SK, Raychaudhuri SP, Weltman H, Farber EM 2001 Effect of nerve growth factor on endothelial cell biology: proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch Dermatol Res 293:291–295 [DOI] [PubMed] [Google Scholar]
- Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappalà G, Pafumi C, Bernardini R 2002 Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J 16:1307–1309 [DOI] [PubMed] [Google Scholar]
- Park MJ, Kwak HJ, Lee HC, Yoo DH, Park IC, Kim MS, Lee SH, Rhee CH, Hong SI 2007 Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol-3 kinase/Akt signaling pathway and AP-2 transcription factor. J Biol Chem 282:30485–30496 [DOI] [PubMed] [Google Scholar]
- Middeke M, Hoffmann S, Hassan I, Wunderlich A, Hofbauer LC, Zielke A 2002 In vitro and in vivo angiogenesis in PC12 pheochromocytoma cells is mediated by vascular endothelial growth factor. Exp Clin Endocrinol Diabetes 110:386–392 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A, Nagura H 1998 Cyclic changes of vasculature and vascular phenotypes in normal human ovaries. Hum Reprod 13:953–959 [DOI] [PubMed] [Google Scholar]
- Fraser HM, Wulff C 2001 Angiogenesis in the primate ovary. Reprod Fertil Dev 13:557–566 [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kawahara M, Abe Y, Yokoo M, Sasada H, Sato E 2003 Follicular microvasculature and angiogenic factors in the ovaries in domestic animals. J Reprod Dev 49:181–192 [DOI] [PubMed] [Google Scholar]
- Kaczmarek MM, Schams D, Ziecik AJ 2005 Role of vascular endothelial growth factor in ovarian physiology—an overview. Reprod Biol 5:111–136 [PubMed] [Google Scholar]
- Ferrara N 2001 Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280:C1358–C1366 [DOI] [PubMed] [Google Scholar]
- Geva E, Jaffe RB 2000 Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril 74:429–438 [DOI] [PubMed] [Google Scholar]
- Campos X, Muñoz Y, Selman A, Yazigi R, Moyano L, Weinstein-Oppenheimer C, Lara HE, Romero C 2007 Nerve growth factor and its high-affinity receptor trkA participate in the control of vascular endothelial growth factor expression in epithelial ovarian cancer. Gynecol Oncol 104:168–175 [DOI] [PubMed] [Google Scholar]
- Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, Kitajewski J, Ferin M 2001 Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J Clin Endocrinol Metab 86:768–772 [DOI] [PubMed] [Google Scholar]
- Hazzard TM, Xu F, Stouffer RL 2002 Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod 67:1305–1312 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Lara HE, Fahrenbach WH, Costa ME, Ojeda SR 1994 Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology 134:1146–1154 [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Kodama J, Yoshinouchi M, Tokumo K, Kamimura S, Okuda H, Kudo T 1997 The expression of vascular endothelial growth factor and transforming growth factor-β associates with angiogenesis in epithelial ovarian cancer. Int J Gynecol Pathol 16:256–262 [DOI] [PubMed] [Google Scholar]
- Agrawal R, Sladkevicius P, Engmann L, Conway GS, Payne NN, Bekis J, Tan SL, Campbell S, Jacobs HS 1998 Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum Reprod 13:651–655 [DOI] [PubMed] [Google Scholar]
- Ludwig M, Jelkmann W, Bauer O, Diedrich K 1999 Prediction of severe ovarian hyperstimulation syndrome by free serum vascular endothelial growth factor concentration on the day of human chorionic gonadotrophin administration. Hum Reprod 14:2437–2441 [DOI] [PubMed] [Google Scholar]
- Albert C, Garrido N, Mercader A, Rao CV, Remohí J, Simón C, Pellicer A 2002 The role of endothelial cells in the pathogenesis of ovarian hyperstimulation syndrome. Mol Hum Reprod 8:409–418 [DOI] [PubMed] [Google Scholar]
- Julio-Pieper M, Lara HE, Bravo JA, Romero C 2006 Effects of nerve growth factor (NGF) on blood vessels area and expression of the angiogenic factors VEGF and TGF-β 1 in the rat ovary. Reprod Biol Endocrinol 4:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, Giordano T, Peale F 2003 Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol 162:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf-Colby A, Eichhorn J, Webster NJ, Olefsky JM 1999 Inhibition of PLC-γ1 activity converts nerve growth factor from an anti-mitogenic to a mitogenic signal in CHO cells. Oncogene 18:4908–4919 [DOI] [PubMed] [Google Scholar]
- Abir R, Fisch B, Jin S, Barnnet M, Ben-Haroush A, Felz C, Kessler-Icekson G, Feldberg D, Nitke S, Ao A 2005 Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol Hum Reprod 11:229–236 [DOI] [PubMed] [Google Scholar]
- Lawrence MC, McGlynn K, Shao C, Duan L, Naziruddin B, Levy MF, Cobb MH 2008 Chromatin-bound mitogen-activated protein kinases transmit dynamic signals in transcription complexes in β-cells. Proc Natl Acad Sci USA 105:13315–13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Robinson LL, Brooks J, Spears N 2002 Neurotropins and their receptors are expressed in the human fetal ovary. J Clin Endocrinol Metab 87:890–897 [DOI] [PubMed] [Google Scholar]
- Spears N, Molinek MD, Robinson LL, Fulton N, Cameron H, Shimoda K, Telfer EE, Anderson RA, Price DJ 2003 The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development 130:5481–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papetti M, Herman IM 2002 Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol 282:C947–C970 [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Martínez-Chequer JC, Molskness TA, Xu F, Hazzard TM 2001 Regulation and action of angiogenic factors in the primate ovary. Arch Med Res 32:567–575 [DOI] [PubMed] [Google Scholar]
- Nico B, Mangieri D, Benagiano V, Crivellato E, Ribatti D 2008 Nerve growth factor as an angiogenic factor. Microvasc Res 75:135–141 [DOI] [PubMed] [Google Scholar]
- Shimizu T, Iijima K, Miyabayashi K, Ogawa Y, Miyazaki H, Sasada H, Sato E 2007 Effect of direct ovarian injection of vascular endothelial growth factor gene fragments on follicular development in immature female rats. Reproduction 134:677–682 [DOI] [PubMed] [Google Scholar]
- Neulen J, Yan Z, Raczek S, Weindel K, Keck C, Weich HA, Marmé D, Breckwoldt M 1995 Human chorionic gonadotropin-dependent expression of vascular endothelial growth factor/vascular permeability factor in human granulosa cells: importance in ovarian hyperstimulation syndrome. J Clin Endocrinol Metab 80:1967–1971 [DOI] [PubMed] [Google Scholar]
- Lee A, Christenson LK, Stouffer RL, Burry KA, Patton PE 1997 Vascular endothelial growth factor levels in serum and follicular fluid of patients undergoing in vitro fertilization. Fertil Steril 68:305–311 [DOI] [PubMed] [Google Scholar]
- Dissen GA, Garcia-Rudaz C, Paredes A, Mayer C, Mayerhofer A, Ojeda SR 2009 Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinology 150:2906–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]