Abstract
Context: Obesity in adolescents is increasingly prevalent and its impact on cardiovascular risk important to determine. Hormonal predictors of cardiovascular risk markers in obese adolescents are not known.
Objective: Our objective was to examine whether relative GH deficiency and cortisol excess are determinants of increased cardiovascular risk markers in obese teenage girls.
Design and Setting: A cross-sectional study was conducted at a clinical research center.
Study participants: Thirty girls (15 obese girls and 15 normal-weight controls) 12–18 years old matched for maturity and race.
Main Outcome Measures: Inflammatory markers of cardiovascular risk including high-sensitivity C-reactive protein (hsCRP), TNF-α receptors 1 and 2, E-selectin, soluble intercellular adhesion molecule-1, and IL-6 were analyzed. Leptin, adiponectin, and 24-h urine free cortisol (UFC) were also measured. A GHRH-arginine stimulation test was performed.
Results: The hsCRP levels were higher in obese girls than controls (4.63 ± 4.81 vs. 0.67 ± 0.72 mg/liter; P = 0.002 after log conversion), as were other markers of cardiovascular risk. Eight of the 15 obese girls but no normal-weight girl had hsCRP higher than 3 mg/liter (P = 0.002). Body mass index sd score was higher than 4.0 in 87.5% of girls with hsCRP higher than 3 mg/liter and no girls with hsCRP less than 3 mg/liter. Girls with hsCRP higher than 3 mg/liter had higher UFC and lower peak GH compared with those with hsCRP less than 3 mg/liter. Peak GH was an important negative predictor of most markers of increased cardiovascular risk. In addition to peak GH, UFC and adiponectin independently predicted hsCRP.
Conclusion: Relative GH deficiency and cortisol excess are significant contributors to increased levels of markers of cardiovascular risk in obese adolescent girls.
Serum markers of cardiovascular risk are increased in obese adolescent girls and are predicted by hormonal and body composition alterations.
Obesity is a well-known contributor to increased cardiovascular risk in adults. With the increasing prevalence of obesity in children and teenagers, understanding its impact on cardiovascular risk in this younger population as well as the body composition and hormonal determinants of cardiovascular risk has become of vital importance. Atherosclerosis is characterized by endothelial dysfunction resulting in inflammatory changes and secretion of procoagulant factors (1,2). Antecedents of atherosclerosis are evident in obese children (3,4) and specific inflammatory markers of cardiovascular risk, such as high-sensitivity C-reactive protein (hsCRP) and IL-6, are elevated in obese children compared with normal-weight controls (5). hsCRP is an acute-phase reactant that may promote procoagulant activity and successfully predicts occurrence of cardiovascular events in adults (2). In addition to CRP, there are a number of other markers of the inflammatory cascade. However, these markers and their hormone determinants have not been systematically investigated in a pediatric population.
High lipid levels promote formation of foam cells and initiate formation of fatty streaks, and subsequent accumulation of macrophages, activated T cells, and mast cells at the site of a plaque triggers the inflammatory cascade (2). Primary cytokines such as IL-1 and TNF-α attract inflammatory cells into vascular tissue, and induce systemic messenger cytokines such as IL-6 (the primary procoagulant cytokine), which increase hepatic secretion of acute-phase reactants such as CRP. IL-1, IL-6, and CRP induce secretion of adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and E-selectin, which facilitate leukocyte adhesion to vessel walls. Of importance, although inflammatory markers of cardiovascular risk have been examined in obese adults (6), there are few data in obese children, particularly with respect to hormonal predictors of these markers. Because early evidence of cardiovascular disease (increased intima-media thickness and fatty streaks) in obese adolescents (3,4) evolves into significant cardiovascular disease by early adult life, a better understanding of the physiological determinants of these markers is particularly important.
Visceral adiposity, insulin resistance, and abnormal lipids are all factors that increase risk for cardiovascular disease in adults (7,8), and obesity is associated with alterations in many metabolically active hormones that affect these variables, including GH, cortisol, and adiponectin (9,10). We have previously reported a relative state of GH deficiency in obese adolescents (9), similar to adults (6). Primary GH deficiency, seen in patients with pituitary or hypothalamic disease, is known to increase visceral fat and risk for cardiovascular disease, both of which improve with GH replacement (11,12). Likewise, obese children are relatively hypercortisolemic (9,13), and hypercortisolemia in Cushing syndrome is associated with truncal adiposity and increased cardiovascular risk (14,15). We have also reported that high cortisol levels in obese adolescents predict the degree of insulin resistance and lipid elevations (9). Finally, adiponectin, a determinant of insulin resistance, is another possible determinant of cardiovascular risk (16), with low adiponectin levels predicting higher risk in adults.
No studies have thus far examined the markers of cardiovascular risk in relation to fat-related hormones such as GH, cortisol, and adiponectin in adolescents. Our objective was to examine whether relative GH deficiency, cortisol excess, and low adiponectin predict inflammatory markers of cardiovascular risk in obese vs. normal-weight adolescents. We hypothesized that low peak GH levels during the GHRH-arginine stimulation test, high cortisol levels, and low adiponectin would predict higher levels of cardiovascular risk markers of the inflammatory cascade including hsCRP, TNF-α receptors 1 and 2, E-selectin, soluble ICAM-1 (sICAM-1) and IL-6.
Subjects and Methods
Subject selection
We screened 17 obese adolescents and 30 normal-weight controls, 12–18 yr old, for this study. Fifteen qualifying obese subjects were matched for race, ethnicity, and bone age (within 1 yr) to 15 normal-weight controls. We have previously reported baseline characteristics, but not levels of cardiovascular risk markers, for these subjects (9). Bone age (a highly reproducible measure of pubertal stage) was assessed using methods of Greulich and Pyle (17) and was used for matching rather than chronological age because many of our hormonal endpoints (such as GH and IGF-I levels) are dependent on pubertal stage, and earlier onset of puberty is reported in overweight girls (18). Our matching process involved enrolling the first available control that fulfilled match criteria for the corresponding overweight subject. Matching criteria included bone age (within 1 yr), race, and ethnicity. To minimize the number of normal-weight subjects screened, we used a telephone prescreen.
Girls were classified as obese if they had a body mass index (BMI) greater than the 95th percentile for age. Normal-weight girls were required to have a BMI between the 15th and 85th percentiles. Menarcheal status did not differ between the groups; two normal-weight and three overweight girls were premenarcheal. Subjects were recruited from all racial and ethnic backgrounds through mass mailings to primary care providers, advertisements in community newspapers, and research listings within the Partners HealthCare network. Each study group included 12 Caucasians, two African-Americans, and one subject with multiple racial background. Study groups were also equally matched for ethnic background with 13 non-Hispanic and two Hispanic subjects each. The Institutional Review Board of Partners HealthCare system approved the study, and informed assent and consent were obtained from subjects and parents.
Anthropometric measurements
Subjects were all weighed to the nearest 0.1 kg in a hospital gown on an electronic scale at the General Clinical Research Center of Massachusetts General Hospital. Height was measured to the nearest 0.1 cm using a single stadiometer, and an average of three measurements was taken.
Experimental protocol
Subject inclusion criteria included a normal TSH, fasting glucose less than 126 mg/dl, and hematocrit higher than 30%. Exclusion criteria included pregnancy, use of medications that could affect GH or cortisol levels (such as estrogens, progestins, or glucocorticoids), a weight loss or gain of more than 2 kg within the 3 months preceding the study, diabetes mellitus, and thyroid disorders. Eligible subjects were admitted to the General Clinical Research Center of Massachusetts General Hospital in the fasting state and in the early follicular phase of their cycles to avoid effects of cycle stage on GH levels (19). Fasting levels of hsCRP, IL-6, E-selectin, TNF-α receptors 1 and 2, sICAM, adiponectin, IGF-I, and a lipid panel [total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides] were obtained. Body composition was determined using magnetic resonance imaging (Signa 1.5 Tesla; General Electric Medical Systems, Milwaukee, WI) and dual-energy x-ray absorptiometry (Hologic 4500; Hologic, Waltham, MA). Magnetic resonance imaging assessments were performed in the fasting state and included measurements of sc and visceral adipose tissue (SAT and VAT) at the lumbar 4–5 level (9). Dual-energy x-ray absorptiometry was used to assess total fat mass. A fasting GHRH and arginine stimulation test was performed the following morning to evaluate maximal GH secretory capacity (20,21). GHRH was administered at a dose of 1 μg/kg, followed by 0.5 g/kg (maximum 30 g) of arginine over 30 min. GH levels were obtained at 0, 30, 60, 90, and 120 min. A 24-h urine free cortisol (UFC) level was obtained.
Biochemical analyses
We used RIA to assess adiponectin [Linco Diagnostics, St. Charles, MO; coefficient of variation (CV) 6.4–8.4% and lowest detectable concentration 0.001 ng/ml] and leptin (Linco Diagnostics; CV 3.4–8.3% and sensitivity 0.5 ng/ml) and an immunoradiometric assay to measure GH (Diagnostic Systems Laboratories, Webster, TX; CV 3.1–5.4% and sensitivity 0.01 ng/ml) and IGF-I (Diagnostic Systems Laboratories; CV 3.9–7.0% and sensitivity 2.06 ng/ml). UFC over 24 h was measured in the hospital laboratory by the GammaCoat 125I-RIA (Diasorin, Stillwater, MN; CV 7% and limit of detection 1 μg/dl). hsCRP was determined using a Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, IN) with reagents and calibrators from Equal Diagnostics (Exton, PA) (CV ≤ 10% and sensitivity 0.1 mg/liter). We used ELISA to measure TNF-α receptors 1 and 2 (R&D Systems, Minneapolis, MN; CV 3.6–5.0% and 2.6–4.8%, respectively, and sensitivity 0.77 and 0.60 pg/ml), E-selectin (R&D Systems; CV 5.2–6.6% and sensitivity 0.01 ng/ml), sICAM-1 (R&D Systems; CV 6.0–10.1% and sensitivity 0.35 ng/ml) and IL-6 (R&D Systems; sensitivity 0.094 pg/ml and CV 6.5–9.6%). All samples were stored at −80C until analysis in duplicate.
Statistical analysis
JMP Statistical program version 4 (SAS Institute, Cary, NC) was used for statistical analyses. Results are reported as mean ± sd. Statistical significance was defined as a P value < 0.05. Student’s t test was used for analyzing the differences between means for the two groups. When data were not normally distributed, these were log transformed before t test analysis. This was required for hsCRP, IL-6, peak GH, and GH area under the curve (AUC) (for the GHRH-arginine stimulation test). Pearson correlations were conducted to determine associations between different variables for normally distributed data and after logarithmic transformation of data not normally distributed. We used a stepwise multiple regression model to determine independent hormonal predictors of the various cardiovascular risk markers in obese adolescents and also for the group as a whole. The hormonal predictors that were first entered into this model included log peak GH, adiponectin, and urinary cortisol. Peak GH, GH AUC, and IGF-I all reflect GH status, and we used only peak GH levels in our regression model because this is the clinically used parameter to assess GH sufficiency and because of the dichotomy between GH and IGF-I levels in obesity (please see Discussion). A P value of 0.10 was used to enter and leave the model. We have previously established that GH and cortisol are important predictors of regional fat depots (9), and subsequently, we added SAT and VAT to the regression model to determine whether associations of cardiovascular risk markers with hormonal predictors held after controlling for regional fat depots. Because regional fat depots, especially VAT, are considered important determinants of the metabolic syndrome, we included VAT and SAT, rather than total fat, in the regression model. We tested for and ruled out collinearity (r > 0.90) within covariates entered into the regression model.
Results
Baseline characteristics
Baseline characteristics of our subjects have been previously reported (9) and are summarized in Table 1. The groups did not differ for maturity as assessed by bone age or pubertal stage, and by design, obese girls had higher BMI, total fat mass, SAT, and VAT than normal-weight girls. Peak GH and AUC from the GHRH-arginine stimulation test were lower in obese girls than in controls, whereas leptin and UFC were higher in the obese group. IGF-I and adiponectin did not differ between groups. Twenty percent of obese girls vs. 0% of normal-weight girls had peak GH levels less than 9 ng/ml (cutoff for GH deficiency), and 69.2% of obese girls vs. 6.7% of normal-weight girls had peak GH levels of less than 24 ng/ml (mean − 1 sd based on values in normal-weight girls).
Table 1.
Baseline characteristics of obese subjects and controls
| Obese (n = 15) | Controls (n = 15) | P | |
|---|---|---|---|
| Age (yr) | 14.0 ± 1.9 | 15.9 ± 1.7 | 0.006 |
| Bone age (yr) | 15.1 ± 1.9 | 15.8 ± 1.8 | 0.26 |
| Tanner stage (breasts) | 4.3 ± 1.0 | 4.5 ± 1.1 | 0.48 |
| BMI (kg/m2) | 34.4 ± 7.1 | 21.7 ± 1.9 | <0.0001 |
| BMI SDS | 3.7 ± 1.5 | 0.1 ± 0.4 | <0.0001 |
| Total lean mass (kg) | 53.4 ± 9.1 | 40.1 ± 4.3 | <0.0001 |
| Total fat mass (kg) | 39.3 ± 13.6 | 16.5 ± 3.4 | <0.0001 |
| Regional fat mass | |||
| SAT (g) | 409.4 ± 159.4 | 137.9 ± 49.3 | <0.0001 |
| VAT (g) | 42.7 ± 17.0 | 19.0 ± 7.5 | 0.0003 |
| Hormones | |||
| GH AUC (ng/ml · 120 min) | 1828 ± 1454 | 2858 ± 1206 | 0.04a |
| Peak GH (ng/ml) | 25.2 ± 21.8 | 40.1 ± 16.1 | 0.03a |
| IGF-I (ng/ml) | 687.5 ± 167.7 | 622.7 ± 130.1 | 0.25 |
| Adiponectin (ng/ml) | 8.34 ± 2.21 | 9.81 ± 2.93 | 0.13 |
| UFC (μg/24 h) | 42.1 ± 13.2 | 31.5 ± 12.2 | 0.03 |
| Leptin (ng/ml) | 42.8 ± 18.2 | 10.0 ± 4.1 | <0.0001 |
Data reported in this table have been previously published (9).
After log conversion to approximate a normal distribution.
Markers of cardiovascular risk
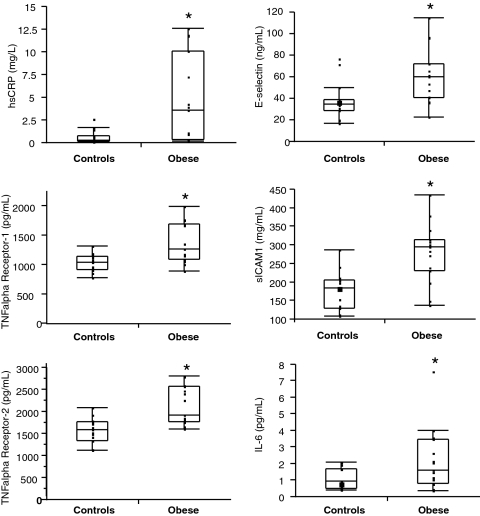
All serum markers of cardiovascular risk (hsCRP, TNF-α receptors 1 and 2, E-selectin, sICAM-1, and IL-6) were elevated in obese girls compared with controls (Table 2). Figure 1 shows the distribution of these markers within the two groups. hsCRP levels higher than 3 mg/liter are considered to indicate increased cardiovascular risk in adults (2), and eight of the 15 obese girls (53.3%) had hsCRP levels higher than 3 mg/liter. In contrast, none of the controls had elevated hsCRP levels. Seven of eight girls with hsCRP higher than 3 mg/liter (87.5%), but none with hsCRP less than 3 mg/liter, had a BMI sd score (SDS) of higher than 4.0. All girls with BMI SDS higher than 4.0 had hsCRP levels higher than 3 mg/liter. When subjects were categorized based on hsCRP levels less than 3 mg/liter or higher than 3 mg/liter, those with hsCRP levels higher than 3 mg/liter had lower peak GH, GH AUC, and adiponectin and higher leptin, UFC, fat mass, SAT, and VAT than those with hsCRP less than 3 mg/liter (Table 3). TNF-α receptors 1 and 2 correlated positively with all other markers of cardiovascular risk. hsCRP correlated with all markers except E-selectin, and sICAM-1 correlated with E-selectin (data not shown).
Table 2.
Markers of cardiovascular risk in obese vs. normal-weight adolescents
| Obese (n = 15) | Controls (n = 15) | P | |
|---|---|---|---|
| hsCRP (mg/liter) | 4.63 ± 4.81 | 0.67 ± 0.72 | 0.002a |
| TNF receptor 1 (pg/ml) | 1360 ± 335 | 1036 ± 144 | 0.003 |
| TNF receptor 2 (pg/ml) | 2142 ± 429 | 1579 ± 275 | 0.0003 |
| E-selectin (ng/ml) | 61.5 ± 25.9 | 38.1 ± 17.0 | 0.007 |
| sICAM-1 (mg/ml) | 279.0 ± 79.6 | 176.6 ± 51.9 | 0.0003 |
| IL-6 (pg/ml) | 2.22 ± 1.88 | 1.08 ± 0.63 | 0.04a |
After log conversion to approximate a normal distribution.
Figure 1.
Markers of cardiovascular risk in obese vs. normal-weight adolescents. In the obese group significant elevations in hsCRP, TNF-α receptor 1, TNF-α receptor 2, E-selectin, sICAM 1, and IL-6 were observed when compared with the normal-weight group. *, P < 0.05 (hsCRP and IL-6 were log converted for statistical analyses).
Table 3.
Hormones and regional fat mass in girls with hsCRP higher than 3 mg/liter or less than 3 mg/liter
| hsCRP
|
P | ||
|---|---|---|---|
| >3 mg/liter, n = 8 | <3 mg/liter, n = 22 | ||
| Peak GH (ng/ml) | 13.7 ± 7.0 | 39.7 ± 18.7 | <0.0001 |
| GH AUC (ng/ml · 120 min) | 905 ± 536 | 2824 ± 1277 | <0.0001 |
| IGF-I (ng/ml) | 618 ± 144 | 668 ± 154 | 0.43 |
| Adiponectin (ng/ml) | 6.7 ± 1.2 | 9.9 ± 2.5 | 0.002 |
| Leptin (ng/ml) | 52.9 ± 17.8 | 16.7 ± 12.3 | <0.0001 |
| UFC μ g/24 h) | 48.1 ± 12.5 | 32.7 ± 11.8 | 0.004 |
| Total fat (kg) | 48.7 ± 10.6 | 20.3 ± 7.4 | <0.0001 |
| SAT (g) | 534.3 ± 92.6 | 190.2 ± 109.4 | <0.0001 |
| VAT (g) | 51.9 ± 10.5 | 24.0 ± 13.7 | 0.0003 |
Similar data were observed within obese girls, although the P values were higher given the smaller number of subjects.
Predictors of markers of cardiovascular risk
Within obese girls, peak GH and IGF-I were negative predictors of hsCRP, TNF-α receptors 1 and 2, and sICAM-1 but did not predict E-selectin or IL-6 (Table 4). Adiponectin was an inverse predictor of hsCRP, and adiponectin and UFC were negative and positive predictors, respectively, of IL-6. The ratio of peak GH/UFC (an integrated measure of GH and cortisol status) (22) was a strong inverse predictor of hsCRP and sICAM-1. Leptin, total fat, SAT, and VAT were positive predictors of hsCRP. Leptin, total fat, and SAT (but not VAT) predicted levels of IL-6. For the group as a whole, BMI SDS was a strong positive predictor of hsCRP, TNF-α receptors 1 and 2, sICAM, and IL-6 (r ≥ 0.5; P ≤ 0.0006) and a weaker predictor of E-selectin (r = 0.37; P = 0.04). In addition, peak GH was an inverse predictor of hsCRP, TNF-α receptors 1 and 2, sICAM-1, and E-selectin but did not predict IL-6 (Table 4). Adiponectin and UFC were negative and positive predictors, respectively, of both hsCRP and IL-6. The ratio of peak GH/UFC was a strong inverse predictor of hsCRP, TNF-α receptors 1 and 2, and sICAM. Leptin and VAT were positive predictors of all markers of cardiovascular risk, and total fat and SAT predicted all cardiovascular risk markers except E-selectin.
Table 4.
Associations of hormones and regional fat mass with markers of cardiovascular risk
| hsCRPa
|
TNF-α receptor 1
|
TNF-α receptor 2
|
E-selectin
|
sICAM-1
|
IL-6a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Peak GHa | ||||||||||||
| Obese | −0.75 | 0.003 | −0.78 | 0.002 | −0.53 | 0.04 | −0.44 | NS | −0.74 | 0.004 | −0.37 | NS |
| All | −0.46 | 0.01 | −0.58 | 0.001 | −0.60 | 0.0007 | −0.41 | 0.03 | −0.62 | 0.0005 | −0.23 | NS |
| GH AUCa | ||||||||||||
| Obese | −0.71 | 0.004 | −0.46 | 0.09 | −0.19 | NS | −0.23 | NS | −0.74 | 0.004 | −0.52 | 0.05 |
| All | −0.45 | 0.02 | −0.41 | 0.03 | −0.43 | 0.02 | −0.33 | 0.08 | −0.61 | 0.0005 | −0.30 | NS |
| IGF-I | ||||||||||||
| Obese | −0.64 | 0.01 | −0.80 | 0.0004 | −0.61 | 0.02 | −0.36 | NS | −0.62 | 0.01 | −0.35 | NS |
| All | −0.23 | NS | −0.32 | 0.08 | −0.23 | NS | −0.09 | NS | −0.30 | 0.10 | −0.08 | NS |
| Adiponectin | ||||||||||||
| Obese | −0.75 | 0.003 | −0.45 | 0.09 | −0.10 | NS | 0.13 | NS | −0.18 | NS | −0.86 | <0.0001 |
| All | −0.39 | 0.03 | −0.37 | 0.04 | −0.15 | NS | −0.21 | NS | −0.20 | NS | −0.39 | 0.03 |
| UFC | ||||||||||||
| Obese | 0.42 | NS | −0.05 | NS | −0.30 | NS | −0.19 | NS | 0.25 | NS | 0.51 | 0.05 |
| All | 0.50 | 0.005 | 0.18 | NS | 0.17 | NS | −0.02 | NS | 0.21 | NS | 0.55 | 0.002 |
| Peak GH/UFC | ||||||||||||
| Obese | −0.86 | 0.0002 | −0.61 | 0.03 | −0.43 | NS | −0.22 | NS | −0.79 | 0.001 | −0.43 | NS |
| All | −0.57 | 0.002 | −0.44 | 0.02 | −0.50 | 0.007 | −0.22 | NS | −0.53 | 0.004 | −0.32 | 0.09 |
| Leptin | ||||||||||||
| Obese | 0.75 | 0.001 | 0.69 | 0.0005 | 0.58 | 0.02 | −0.16 | NS | 0.09 | NS | 0.61 | 0.02 |
| All | 0.75 | <0.0001 | 0.76 | <0.0001 | 0.72 | <0.0001 | 0.32 | 0.08 | 0.50 | 0.005 | 0.60 | 0.0005 |
| Total fat | ||||||||||||
| Obese | 0.81 | 0.0003 | 0.54 | 0.04 | 0.34 | NS | −0.25 | NS | 0.21 | NS | 0.69 | 0.005 |
| All | 0.78 | <0.0001 | 0.69 | <0.0001 | 0.62 | 0.0003 | 0.23 | NS | 0.57 | 0.001 | 0.65 | 0.0001 |
| SAT | ||||||||||||
| Obese | 0.78 | 0.002 | 0.42 | NS | 0.25 | NS | −0.31 | NS | 0.09 | NS | 0.59 | 0.03 |
| All | 0.74 | <0.0001 | 0.63 | 0.0003 | 0.58 | 0.001 | 0.25 | NS | 0.51 | 0.005 | 0.57 | 0.001 |
| VAT | ||||||||||||
| Obese | 0.63 | 0.02 | 0.50 | 0.08 | 0.30 | NS | 0.41 | NS | 0.59 | 0.03 | 0.13 | NS |
| All | 0.61 | 0.0006 | 0.65 | 0.0002 | 0.55 | 0.003 | 0.52 | 0.005 | 0.68 | 0.0001 | 0.35 | 0.07 |
NS, Not significant.
After log conversion to approximate a normal distribution.
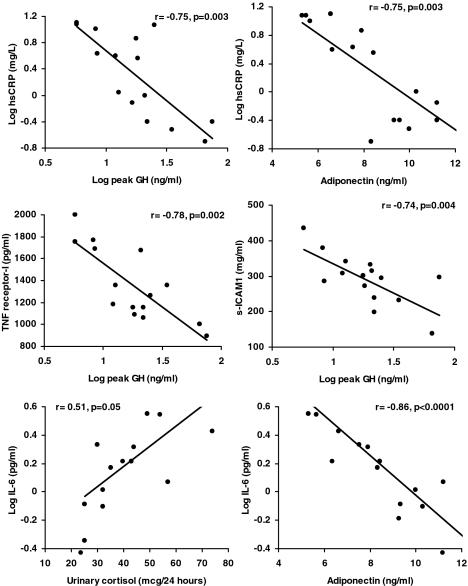
We then performed regression modeling to determine independent hormonal predictors of these cardiovascular risk markers, with peak GH, adiponectin, and UFC included in this model. IGF-I was a weaker predictor of markers of cardiovascular risk than peak GH on correlational analysis for most parameters and was not included in the regression model. Leptin was not included because it is a reflection of fat mass, and we have previously reported that peak GH and UFC are important predictors of regional fat mass (9). Results from regression modeling are shown in Table 5. Peak GH remained an important independent predictor of hsCRP, TNF-α receptors 1 and 2, and sICAM1 on regression modeling but did not predict levels of IL-6. In addition to peak GH, levels of hsCRP were predicted by adiponectin in obese girls and by UFC for the group as a whole. IL-6 levels were independently predicted by UFC for the group as a whole and by adiponectin and UFC in obese girls. Figure 2 shows correlational analyses between inflammatory markers of cardiovascular risk and their independent predictors.
Table 5.
Regression modeling (log peak GH, adiponectin, and UFC included in the model)
| Parameter | Parameter estimate | F ratio | P | R2 | Cumulative R2 |
|---|---|---|---|---|---|
| Log hsCRP | |||||
| Obese | |||||
| Intercept | 2.75 | ||||
| Log peak GH | −1.01 | 8.2 | 0.02 | 0.56 | |
| Adiponectin | −0.14 | 7.3 | 0.02 | 0.19 | 0.75 |
| All subjects | |||||
| Intercept | 0.266 | ||||
| UFC | 0.019 | 6.9 | 0.01 | 0.24 | |
| Log peak GH | −0.728 | 5.5 | 0.03 | 0.14 | 0.38 |
| TNF-α receptor 1 | |||||
| Obese | |||||
| Intercept | 2346.0 | ||||
| Log peak GH | −777.4 | 16.8 | 0.002 | 0.60 | 0.60 |
| All subjects | |||||
| Intercept | 1905.1 | ||||
| Log peak GH | −507.8 | 12.7 | 0.001 | 0.33 | 0.33 |
| TNF-α receptor 2 | |||||
| Obese | |||||
| Intercept | 3701.2 | ||||
| Log peak GH | −808.0 | 7.5 | 0.02 | 0.33 | |
| UFC | −12.8 | 3.4 | 0.09 | 0.17 | 0.50 |
| All subjects | |||||
| Intercept | 3004.8 | ||||
| Log peak GH | −821.5 | 14.7 | 0.0007 | 0.36 | 0.36 |
| E-selectin | |||||
| Obese | |||||
| None | |||||
| All subjects | |||||
| Intercept | 94.4 | ||||
| Log peak GH | −31.0 | 5.3 | 0.03 | 0.17 | 0.17 |
| sICAM-1 | |||||
| Obese | |||||
| Intercept | 500.8 | ||||
| Log peak GH | −160.9 | 13.5 | 0.004 | 0.55 | 0.55 |
| All subjects | |||||
| Intercept | 459.5 | ||||
| Log peak GH | −160.3 | 16.1 | 0.0005 | 0.38 | 0.38 |
| Log IL-6 | |||||
| Obese | |||||
| Intercept | 0.93 | ||||
| Adiponectin | −0.11 | 99.4 | <0.0001 | 0.91 | |
| UFC | 0.004 | 5.6 | 0.04 | 0.03 | 0.94 |
| All subjects | |||||
| Intercept | −0.375 | ||||
| UFC | 0.012 | 14.3 | 0.0008 | 0.35 | 0.35 |
Figure 2.
Associations between hormonal parameters and inflammatory markers of cardiovascular risk in obese adolescent girls. hsCRP was predicted by peak GH and adiponectin levels in a regression model that included peak GH, urinary cortisol, and adiponectin. Using the same model, TNF-α receptor 1 and sICAM were predicted by peak GH, and IL-6 by urinary cortisol and adiponectin. The figure shows simple correlational analysis of these variables for the independent predictors of the respective cardiovascular risk marker.
Within obese girls, after adding SAT and VAT to the regression model, hsCRP was predicted by VAT and adiponectin (contributing to 87% of the variability; P = 0.0005 and 0.03). In this model, peak GH was the sole independent predictor of TNF-α receptors 1 and 2 in obese girls contributing to 59 and 32% of the variability, respectively (P = 0.006 and 0.07), VAT predicted sICAM-1 (72% variability explained, P = 0.001), and adiponectin and UFC predicted IL-6 (94% of variability explained, P < 0.0001 and 0.06). For the group as a whole, after adding SAT and VAT to the regression model, SAT was the sole predictor of hsCRP (54% of variability explained, P < 0.0001), whereas VAT predicted TNF-α receptors 1 and 2, E-selectin, and sICAM-1 accounting for 54, 33, 33, and 63% of the variability, respectively (P = 0.0001, <0.0001, 0.002, 0.002, and <0.0001), and SAT with UFC contributed to 44% of the variability of IL-6 (P = 0.03 and 0.04 for SAT and UFC). Importantly, we have previously demonstrated in this group that in a regression model including peak GH, cortisol, and BMI SDS, peak GH is an important independent predictor of VAT, and cortisol predicts SAT (9).
Discussion
We demonstrate elevations of markers all along the inflammatory cascade in obese adolescent girls and that all girls with BMI SDS greater than 4.0 have hsCRP levels that are more than 3 mg/liter. We also show that peak GH is an important negative predictor of a number of markers of increased cardiovascular risk including hsCRP. In addition, UFC and adiponectin predict levels of hsCRP and IL-6. Our data indicate a possible increase in cardiovascular risk with obesity even at this young age and suggest the need to intervene early to ameliorate this risk.
Obese adolescents have high triglyceride and low HDL levels, both of which are known traditional markers of increased risk of cardiovascular events and atherosclerosis in adults (6,23,24,25). We have previously reported that peak GH and IGF-I correlate positively with HDL and inversely with triglycerides and LDL, whereas cortisol directly predicts triglycerides and LDL (9). However, markers of inflammatory pathways leading to increased atherogenic risk (26,27) are less well characterized, especially in obese adolescents.
In this study, we demonstrate marked elevations in inflammatory markers of cardiovascular risk at different steps of the inflammatory cascade including primary cytokines, systemic cytokines, and adhesion molecules in obese adolescent girls compared with normal-weight girls. This is consistent with and builds on data from one other published study in obese adolescents in which hsCRP and IL-6 were shown to be elevated, but no other markers were examined (5). We also show that BMI SDS of higher than 4.0 is highly predictive of hsCRP levels in the range indicative of high vascular risk (>3 mg/liter) in adults (26). In addition to being an acute-phase reactant, CRP is produced by smooth muscle cells of coronary vessels and preferentially in diseased vessels and also affects expression of adhesion molecules, fibrinolysis, and endothelial function (26). Because antecedents of atherosclerosis have been reported in children with obesity (3,4) and hsCRP is a predictor of atherosclerosis risk in adults (25), the occurrence of hsCRP levels greater than 3 mg/liter in as many as 53% of obese girls in this study is of great concern.
To the best of our knowledge, this is the first study examining hormonal predictors of inflammatory markers of cardiovascular risk in obese adolescent girls. We show that peak GH (as assessed by the GHRH-arginine stimulation test) is an important independent predictor of inflammatory risk markers including hsCRP, TNF-α receptors 1 and 2, and sICAM-1 but not E-selectin or IL-6. In this study, even after controlling for regional fat mass, peak GH levels remained a significant predictor of TNF-α receptors 1 and 2 in obese girls. It is well known that primary GH deficiency (as in patients with hypopituitarism) is associated with increased visceral fat and cardiovascular risk, and replacement with GH is associated with a reduction in both visceral fat and cardiovascular risk (11,12). More recently, relatively low GH levels have been demonstrated to predict increased levels of traditional and inflammatory markers of cardiovascular risk in adult premenopausal obese and overweight women (6). We now report that peak GH levels predict inflammatory cardiovascular risk markers in obese adolescent girls. This is particularly interesting in that GH levels peak in the adolescent years and then gradually decline to adult levels, and relatively low GH levels in the adolescent years in obese girls may still be as high as or higher than levels seen in normal adults. In one study, mean peak GH on the GHRH-arginine stimulation test in overweight and obese premenopausal women was 15 ng/ml (6), whereas the mean peak GH in obese girls in our study was much higher at 25 ng/ml. Of importance, the GH levels in obese adolescent girls were still significantly lower compared with normal-weight adolescent girls matched for maturity and were strong predictors of inflammatory cardiovascular risk markers. Therefore, relative GH deficiency, rather than absolute GH deficiency, predicts levels of these markers in obese adolescent girls.
Of importance, there is a dichotomy in GH and IGF-I levels in obesity with studies reporting low, normal, or high IGF-I levels in overweight and obese adults (28,29,30,31,32). It is possible that IGF-I levels vary based on the degree of weight gain, with overweight people having higher IGF-I levels than controls from nutritional effects, whereas those with marked obesity have lower IGF-I levels as a consequence of lower GH, rather than nutritional effects, driving IGF-I secretion (6). Importantly, effects of the GH-IGF-I axis on fat metabolism appear to be mediated by direct effects of GH (33), and less so by IGF-I. This is best exemplified by conditions of GH deficiency and resistance, both of which are associated with marked increases in body fat (11,12,34,35). Whereas GH replacement in GH-deficient states is associated with a decrease in fat mass (11,12), these effects are not seen with IGF-I replacement in GH-resistant states (as in Laron’s syndrome) (34). Reports indicate that adipocytes may affect regulation of serum IGF-I concentrations; however, IGF-I does not affect the development and differentiation of adipocytes (36). Our previously published data are consistent with these known effects of GH vs. IGF-I on fat metabolism and indicate that GH, but not IGF-I, best predicts specific body fat depots (9). Similarly, GH is posited to be a mediator of inflammatory mechanisms underlying atherogenesis through direct effects (37). Our data are consistent in that primarily GH concentrations, and not IGF-I, best predicted various markers of cardiovascular risk and are also consistent with data from obese adults (6).
States of cortisol excess are associated with truncal adiposity and increased cardiovascular risk (14,15), and in our study, high cortisol was an independent and direct predictor of hsCRP, TNF-α receptor 2, and IL-6 levels. Urinary cortisol remained an independent predictor of IL-6 even after controlling for regional fat mass. Although urinary cortisol levels were above the upper limit of normal for our laboratory in only one subject, mean levels in obese girls were significantly higher than in normal-weight girls, indicative of a state of relative cortisol excess. However, higher urinary cortisol levels in obese adolescents may also indicate reduced GH effects on 11-β hydroxysteroid dehydrogenase type 1 activity at the level of the kidneys causing higher cortisone to cortisol conversion (38). Of note, consistent with our data, elevated morning serum cortisol levels have been reported in obese children with the metabolic syndrome compared with those who do not have this syndrome (13,39). Therefore, relative cortisol excess compared with normal-weight girls may be an important indicator of increased levels of inflammatory cardiovascular risk markers in obese adolescent girls. Finally, adiponectin, a determinant of insulin resistance, is another possible determinant of cardiovascular risk (16), and in our study, adiponectin was an independent predictor of hsCRP and IL-6 in obese adolescents. Adiponectin and IL-6 are both produced by adipocytes and may explain the strong inverse association between these hormones. However, adiponectin remained an independent predictor of IL-6 even after controlling for regional fat mass.
The association of regional fat mass and particularly truncal fat in predicting cardiovascular risk in adults is well established. In this study, addition of regional fat mass to the regression model indicated that SAT and particularly VAT are important independent predictors of the various inflammatory markers of cardiovascular risk. Therefore, the impact of relative GH deficiency and cortisol excess on cardiovascular risk markers in obesity may be mediated through the impact of these hormonal alterations on regional fat mass. However, in obese girls, peak GH, cortisol, and adiponectin remained independent predictors of some important markers even after controlling for VAT and SAT.
Limitations of this study include its cross-sectional design and associative nature. However, these data provide important preliminary data for prospective studies and will need to be validated in such studies. In addition, we did not assess structural markers of cardiovascular risk (such as carotid intima-media thickness) in this study, and it will be important to determine whether hormonal changes in obesity predict changes in these structural parameters. This is particularly important because studies have demonstrated that structural antecedents of atherosclerosis are already evident in obese adolescents (3,4).
We show significant elevations in inflammatory markers of cardiovascular risk in obese adolescents are predicted by a state of relative GH deficiency and cortisol excess. In addition, adiponectin is an independent predictor of certain inflammatory cardiovascular risk markers. Studies are necessary to determine whether these hormonal predictors of cardiovascular risk remain significant in a prospective design. Another important future direction for investigation includes evaluation of prepubertal children to better understand the role that puberty may play in these pathways.
Acknowledgments
We thank the Bionutrition Core of the Clinical Research Center of Massachusetts General Hospital for its help with the protocol. We also thank our patients without whom this study would not have been possible.
Footnotes
This work was supported by Grants M01-RR-01066, 1 UL1 RR025758-01, K23 RR018851, F32-DK072816, and P30 DK46200.
Disclosure Summary: The authors have no conflict of interest to declare.
First Published Online May 12, 2009
Abbreviations: AUC, Area under the curve; CV, coefficient of variation; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule-1; LDL, low-density lipoprotein; SAT, sc adipose tissue; SDS, sd score; sICAM-1, soluble ICAM-1; UFC, urine free cortisol; VAT, visceral adipose tissue.
References
- Ross R 1999 Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM 2004 Inflammation as a cardiovascular risk factor. Circulation 109:II2–I10 [DOI] [PubMed] [Google Scholar]
- Freedman DS, Newman 3rd WP, Tracy RE, Voors AE, Srinivasan SR, Webber LS, Restrepo C, Strong JP, Berenson GS 1988 Black-white differences in aortic fatty streaks in adolescence and early adulthood: the Bogalusa Heart Study. Circulation 77:856–864 [DOI] [PubMed] [Google Scholar]
- McGill Jr HC, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP 2000 Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 72:1307S–1315S [DOI] [PubMed] [Google Scholar]
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S 2004 Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350:2362–2374 [DOI] [PubMed] [Google Scholar]
- Utz AL, Yamamoto A, Hemphill L, Miller KK 2008 Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab 93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB 2007 Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT). Stroke 38:2422–2429 [DOI] [PubMed] [Google Scholar]
- Grundy SM 2007 Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab 92:399–404 [DOI] [PubMed] [Google Scholar]
- Misra M, Bredella MA, Tsai P, Mendes N, Miller KK, Klibanski A 2008 Lower growth hormone and higher cortisol are associated with greater visceral adiposity, intramyocellular lipids, and insulin resistance in overweight girls. Am J Physiol Endocrinol Metab 295:E385–E392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JC, Zern TL, Taksali SE, Dziura J, Cali AM, Wollschlager M, Seyal AA, Weiss R, Burgert TS, Caprio S 2006 Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab 91:4415–4423 [DOI] [PubMed] [Google Scholar]
- Beauregard C, Utz AL, Schaub AE, Nachtigall L, Biller BM, Miller KK, Klibanski A 2008 Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab 93:2063–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich JN, Huerta MG, Sundaresan SM, Rogol AD 2001 Alterations in body composition and fat distribution in growth hormone-deficient prepubertal children during growth hormone therapy. Metabolism 50:537–547 [DOI] [PubMed] [Google Scholar]
- Weigensberg MJ, Toledo-Corral CM, Goran MI 2008 Association between the metabolic syndrome and serum cortisol in overweight Latino youth. J Clin Endocrinol Metab 93:1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiger N, Testa RM, Almoto B, Ferrari M, Bilora F, Petrobelli F, Pagnan A, Mantero F, Scaroni C 2006 Patients with Cushing’s syndrome have increased intimal media thickness at different vascular levels: comparison with a population matched for similar cardiovascular risk factors. Horm Metab Res 38:405–410 [DOI] [PubMed] [Google Scholar]
- Faggiano A, Pivonello R, Spiezia S, De Martino MC, Filippella M, Di Somma C, Lombardi G, Colao A 2003 Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab 88:2527–2533 [DOI] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB 2004 Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291:1730–1737 [DOI] [PubMed] [Google Scholar]
- Greulich W, Pyle S 1959 Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press [Google Scholar]
- Lee JM, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH, Lumeng JC 2007 Weight status in young girls and the onset of puberty. Pediatrics 119:e624–e630 [DOI] [PubMed] [Google Scholar]
- Faria AC, Bekenstein LW, Booth Jr RA, Vaccaro VA, Asplin CM, Veldhuis JD, Thorner MO, Evans WS 1992 Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol (Oxf) 36:591–596 [DOI] [PubMed] [Google Scholar]
- Aimaretti G, Baffoni C, Bellone S, Di Vito L, Corneli G, Arvat E, Benso L, Camanni F, Ghigo E 2000 Retesting young adults with childhood-onset growth hormone (GH) deficiency with GH-releasing-hormone-plus-arginine test. J Clin Endocrinol Metab 85:3693–3699 [DOI] [PubMed] [Google Scholar]
- Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, Stavrou S, Kleinberg DL, Chipman JJ, Hartman ML 2002 Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 87:2067–2079 [DOI] [PubMed] [Google Scholar]
- Nass R, Thorner MO 2002 Impact of the GH-cortisol ratio on the age-dependent changes in body composition. Growth Horm IGF Res 12:147–161 [DOI] [PubMed] [Google Scholar]
- Maccario M, Grottoli S, Aimaretti G, Gianotti L, Endrio Oleandri S, Procopio M, Savio P, Tassone F, Ramunni J, Camanni F, Ghigo E 1999 IGF-1 levels in different conditions of low somatotrope secretion in adulthood: obesity in comparison with GH deficiency. Minerva Endocrinol 24:57–61 [PubMed] [Google Scholar]
- Weltman A, Despres JP, Clasey JL, Weltman JY, Wideman L, Kanaley J, Patrie J, Bergeron J, Thorner MO, Bouchard C, Hartman ML 2003 Impact of abdominal visceral fat, growth hormone, fitness, and insulin on lipids and lipoproteins in older adults. Metabolism 52:73–80 [DOI] [PubMed] [Google Scholar]
- Stein EA 2008 Additional lipid lowering trials using surrogate measurements of atherosclerosis by carotid intima-media thickness: more clarity or confusion? J Am Coll Cardiol 52:2206–2209 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL 2004 Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation 109:IV6–IV19 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N 2000 C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342:836–843 [DOI] [PubMed] [Google Scholar]
- Copeland KC, Colletti RB, Devlin JT, McAuliffe TL 1990 The relationship between insulin-like growth factor-I, adiposity, and aging. Metabolism 39:584–587 [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Hertz P, Colin V, Ish-Shalom S, Yeshurun D, Youdim MB, Amit T 1992 The distal axis of growth hormone (GH) in nutritional disorders: GH-binding protein, insulin-like growth factor-I (IGF-I), and IGF-I receptors in obesity and anorexia nervosa. Metabolism 41:106–112 [DOI] [PubMed] [Google Scholar]
- Maccario M, Ramunni J, Oleandri SE, Procopio M, Grottoli S, Rossetto R, Savio P, Aimaretti G, Camanni F, Ghigo E 1999 Relationships between IGF-I and age, gender, body mass, fat distribution, metabolic and hormonal variables in obese patients. Int J Obes Relat Metab Disord 23:612–618 [DOI] [PubMed] [Google Scholar]
- Mårin P, Kvist H, Lindstedt G, Sjöström L, Björntorp P 1993 Low concentrations of insulin-like growth factor-I in abdominal obesity. Int J Obes Relat Metab Disord 17:83–89 [PubMed] [Google Scholar]
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB 1997 Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord 21:355–359 [DOI] [PubMed] [Google Scholar]
- Dietz J, Schwartz J 1991 Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3–F442A adipocytes. Metabolism 40:800–806 [DOI] [PubMed] [Google Scholar]
- Laron Z, Ginsberg S, Lilos P, Arbiv M, Vaisman N 2006 Long-term IGF-I treatment of children with Laron syndrome increases adiposity. Growth Horm IGF Res 16:61–64 [DOI] [PubMed] [Google Scholar]
- Laron Z, Ginsberg S, Lilos P, Arbiv M, Vaisman N 2006 Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity). Clin Endocrinol (Oxf) 65:114–117 [DOI] [PubMed] [Google Scholar]
- Klöting N, Koch L, Wunderlich T, Kern M, Ruschke K, Krone W, Brüning JC, Blüher M 2008 Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes 57:2074–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath S, Morris M, Bouloux PM 1999 Growth hormone deficiency and atherosclerosis: is there a link? Growth Horm IGF Res 9(Suppl A):9–13 [DOI] [PubMed] [Google Scholar]
- Agha A, Monson JP 2007 Modulation of glucocorticoid metabolism by the growth hormone-IGF-1 axis. Clin Endocrinol (Oxf) 66:459–465 [DOI] [PubMed] [Google Scholar]
- Sen Y, Aygun D, Yilmaz E, Ayar A 2008 Children and adolescents with obesity and the metabolic syndrome have high circulating cortisol levels. Neuro Endocrinol Lett 29:141–145 [PubMed] [Google Scholar]