Abstract
Context: Vitamin D deficiency is common among older adults, but the association between 25-hydroxyvitamin D [25(OH)D] levels and rates of bone loss is uncertain.
Objective: Our aim was to test the hypothesis that lower 25(OH)D levels are associated with higher rates of hip bone loss in older men.
Design and Setting: We conducted a prospective cohort study in six U.S. centers.
Participants: A total of 1279 community-dwelling men aged 65 yr or older with 25(OH)D levels (liquid chromatography-tandem mass spectroscopy) and hip bone mineral density (BMD) (dual-energy x-ray absorptiometry) at baseline and repeat hip BMD an average of 4.4 yr later participated in the study.
Main Outcome Measure(s): We measured the annualized percentage rate of change in hip BMD.
Results: After adjustment for multiple potential confounders, the average rate of decline in total hip BMD was −0.59%/yr among men with 25(OH)D levels below 15.0 ng/ml, −0.54%/yr among men with 25(OH)D levels 15.0–19.9 ng/ml, −0.35%/yr among men with 25(OH)D levels 20.0–29.9 ng/ml, and −0.37%/yr among men with 25(OH)D levels of at least 30 ng/ml (P trend = 0.008 for multivariable model). Evidence was strong to support an association among men aged 75 yr and older (P trend <0.001), but not among younger men (P trend = 0.55). Findings were similar when 25(OH)D level was expressed in quintiles and when BMD at hip subregions was substituted for total hip BMD.
Conclusions: In this cohort of community-dwelling older men, men with 25(OH)D levels below 20 ng/ml had greater subsequent rates of hip bone loss, but rates of loss were similar among men with higher levels. These results lend support to the view that low 25(OH)D levels are detrimental to BMD in older men.
Older community-dwelling men with total 25-hydroxyvitamin D levels <20 ng/mL have greater rates of hip bone loss, while rates of loss are similar among men with higher levels.
Lower levels of circulating 25-hydroxyvitamin D [25(OH)D] are increasingly prevalent with advancing age (1). It has been postulated that lower levels of 25(OH)D among older people due to limited sun exposure plus impaired rates of formation of vitamin D3 in the skin, as well as lower dietary vitamin D intake, lead to compensatory secondary hyperparathyroidism with greater bone resorption, higher rates of bone loss, and an increased risk of fractures (2). Although the level of 25(OH)D is widely accepted as the best indicator of vitamin D status, there is no uniform consensus on what cutpoints of serum 25(OH)D concentrations to use in defining vitamin D status for bone health (3). Possible endpoints might include the 25(OH)D concentration below which rates of bone loss are increased for vitamin D deficiency and the 25(OH)D concentration above which bone loss is minimized for adequate or sufficient vitamin D status. However, there is inconsistent evidence to support an association between baseline 25(OH)D level and subsequent rate of change in bone mineral density (BMD) (4,5,6,7,8,9,10). In particular, data in older men are lacking. Prior investigations have been limited by small sample sizes, lack of control of potential confounders, or choice of analytical methods used to measure 25(OH)D.
To test the hypothesis that lower 25(OH)D levels are associated with higher rates of hip bone loss among community-dwelling older men, we measured 25(OH)D and hip BMD in a cohort of 1279 community-dwelling men at least 65 yr of age enrolled in the Osteoporotic Fractures in Men (MrOS) study and followed them prospectively for an average of 4.4 yr for changes in hip BMD.
Subjects and Methods
Participants
From March 2000 through April 2002, 5995 men who were at least 65 yr of age were recruited for participation in the baseline examination of MrOS (11), a study of healthy aging in older men with a focus on osteoporosis. Men were recruited from population-based listings in six regions of the United States (12). Men with a history of bilateral hip replacement and men who were unable to walk without the assistance of another person were excluded from MrOS. The Institutional Review Board at each center approved the study protocol, and written informed consent was obtained from all participants.
Among the overall cohort of 5995 participants, 5908 men had foil-wrapped vials to prevent UV exposure and were initially eligible for participation in this analysis. A random sample comprised of 1608 men was selected from the cohort of 5908 men for measurement of 25(OH)D. We excluded one participant with insufficient serum and another with a 25(OH)D level more than 3 sd values above the mean (75.6 ng/ml), leaving 1606 men eligible for this analysis. Of these, a total of 1279 men (80%) with baseline and repeat hip BMD measurements are the subject of this analysis.
Measurement of 25(OH)D
Fasting morning blood was collected; serum was prepared immediately after phlebotomy and then was stored at −70 C. All samples remained frozen until assay in foil-wrapped vials to prevent UV exposure. Measures for 25(OH)D2 (derived from ergocalciferol) and 25(OH)D3 (derived from cholecalciferol) were performed at the Mayo Clinic using mass spectrometry as previously described (13). Deuterated stable isotope (d3-25-hydroxyvitamin D) was added to a 0.2-ml serum sample as internal standard. 25(OH)D2, 25(OH)D3, and the internal standard were extracted using acetonitrile precipitation. The extracts were then further purified online and analyzed by liquid chromatography tandem mass spectroscopy using multiple reaction monitoring. 25(OH)D2 and 25(OH)D3 were quantified, reported individually, and summed for total 25(OH)D. The minimum detectable limit for 25(OH)D2 was 4 ng/ml, and for 25(OH)D3 was 2 ng/ml. Duplicate pooled serum controls were included in every other assay run. Using the pooled serum, the interassay coefficient of variation (CV) was 4.4%, and the intraassay CV was 4.9%.
BMD
BMD at the total hip and two subregions (femoral neck and trochanter) was measured at baseline and follow-up (latest available) examinations [mean (sd), 4.4 (0.8) yr between examinations] using dual-energy x-ray absorptiometry (DXA) with Hologic QDR-4500W scanners (Hologic, Inc., Bedford, MA). Repeat measurements were performed on the same instruments used for the initial measurements. A central quality control laboratory, certification of DXA technicians, and standardized procedures for scanning were implemented to ensure reproducibility of DXA measurements. At baseline, a hip phantom was circulated and scanned at the six clinical centers. Cross-calibration studies indicated no linear differences across scanners, and the interscanner CV was 0.9%. Each clinic scanned a hip phantom throughout the study to monitor longitudinal changes, and correction factors were applied to participant data as appropriate. In addition, multivariable models included an indicator variable for the individual center to adjust for interclinic differences. The rate of change in hip BMD was expressed as an annualized percentage of the initial value as percentage change in BMD per year.
Other measurements
Participants completed a questionnaire, were interviewed at the baseline examination, and were asked about health status, smoking status, and alcohol use. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE) (14). Total calcium and vitamin D intake (foods and supplements) was estimated by using a modified Block food-frequency questionnaire (Block Dietary Systems, Berkley, CA). Participants were asked to bring all current medications with them to the clinic for verification of use. Tests of lower extremity performance included the ability to rise up from a chair (without using the arms) five times. Body weight and height measurements were used to calculate a standard body mass index (BMI). Using baseline stored sera, serum creatinine was measured (n = 1200 participants among the 1279 men in the cohort) using a variation of the Jaffe enzymatic method. The interassay CV was 5.3%. Renal function was expressed as estimated glomerular filtration rate (eGFR) in ml/min/1.73 m2 using a standardized serum creatinine-based formula (15).
Statistical analysis
Characteristics of participants at the baseline examination by quintile of total 25(OH)D level were compared using χ2 for categorical variables and ANOVA for continuous variables.
Because there is controversy regarding cutpoints for defining vitamin D deficiency, the primary predictor variable, 25(OH)D level, was expressed as quintiles and as categories based on vitamin D status defined as 25(OH)D level less than 15.0 ng/ml, 15.0–19.9 ng/ml, 20.0–29.9 ng/ml, and 30.0 ng/ml or greater (3). Linear regression was used to calculate the mean annualized rate of change in BMD at the total hip and subregions by quintile and category of 25(OH)D level. Tests for linear trend were performed. Initial models were adjusted for age, race, site, season, BMI, and baseline hip BMD (base model); then for multiple potential confounders (multivariable model). Factors previously associated with the rate of change in hip BMD in the MrOS cohort or those related to 25(OH)D level at P ≤ 0.20 were considered for inclusion in multivariable models; covariates included age, race, site, season, BMI, hip BMD, health status, physical activity level, smoking status, alcohol intake, and a measure of lower extremity strength (inability to rise from a chair five times without using the arms).
Because any association between 25(OH)D level and rate of change in hip BMD might be modified by baseline BMD, physical activity, or age, we evaluated for the presence of interactions between 25(OH)D level (expressed as an ordinal variable using quintiles and as categories) and baseline total hip BMD [expressed as a continuous variable and dichotomous variable (<0.954, median, vs. ≥0.954 g/cm2)], PASE score [expressed as a continuous variable and dichotomous variable (<147, median, vs. ≥147)], and age [expressed as a continuous and dichotomous variable (<75 vs. ≥75 yr)] for the prediction of rate of change in BMD. We performed secondary analyses stratifying participants by median BMD value, median PASE score, and age group. In the 1200 participants who had baseline serum creatinine, we also examined the effect of renal function on the association between 25(OH)D and rate of change in hip BMD and performed an analysis stratifying participants by level of eGFR (≤60 vs. >60 ml/min/1.73 m2). Because 25(OH)D levels may vary by race/ethnicity, we determined the association between 25(OH)D level and rate of change in total hip BMD, limiting our analysis to Caucasian men. To examine whether the use of pharmacological agents affecting bone metabolism accounted for our results, we also performed analyses excluding men (n = 27) taking bone active agents (androgens, testosterone, or pharmacological treatments for osteoporosis). Finally, we examined the association between 25(OH)D3 level and rate of change in total hip BMD and evaluated whether any association was dependent on whether 25(OH)D2 was detectable or not.
Results
Study population
Of the 1606 men with measurement of 25(OH)D level at the baseline exam, 1279 had baseline and follow-up measurements of hip BMD. Compared with the 327 men missing follow-up hip BMD measurements, the 1279 men in the analytical cohort were younger (73.1 vs. 76.7 yr; P < 0.001) and had higher mean values of total hip BMD (0.96 vs. 0.92 g/cm2; P < 0.001) and 25(OH)D (25.6 vs. 23.2 ng/ml; P < 0.001). The most common reason for missing the follow-up examination was death (43%). The median (interquartile range) for 25(OH)D was 25.4 (20.4–30.1) ng/ml. Using categories based on vitamin D status, 110 men (9%) had a 25(OH)D level below 15.0 ng/ml, 184 (14%) had a 25(OH)D level of 15.0–19.9 ng/ml, 605 (47%) had a 25(OH)D level of 20.0–29.9 ng/ml, and 376 (29%) had a 25(OH)D level of at least 30.0 ng/ml. Characteristics of the 1279 participants by quintile of 25(OH)D level are shown in Table 1.
Table 1.
Baseline characteristics of 1279 participants by quintile of total 25(OH)D level
| Variable | Quintile of 25(OH)D (ng/ml)a
|
||||||
|---|---|---|---|---|---|---|---|
| Overall (n = 1279) | Q1 (n = 257) | Q2 (n = 253) | Q3 (n = 258) | Q4 (n = 256) | Q5 (n = 255) | P value | |
| Age (yr) | 73.1 (5.6) | 73.8 (6.3) | 73.0 (5.5) | 73.1 (5.4) | 73.2 (5.5) | 72.1 (5.2) | 0.01 |
| Age group (%) | 0.02 | ||||||
| <75 yr | 61 | 58 | 62 | 59 | 59 | 70 | |
| ≥75 yr | 39 | 42 | 38 | 41 | 41 | 30 | |
| Caucasian race (%) | 92 | 85 | 92 | 93 | 96 | 95 | <0.001 |
| Excellent or good health status (%) | 88 | 84 | 87 | 88 | 88 | 91 | 0.18 |
| PASE score | 152 (69) | 144 (74) | 149 (69) | 151 (61) | 155 (66) | 160 (70) | 0.08 |
| Current smoker (%) | 3 | 5 | 4 | 3 | 2 | 3 | 0.35 |
| Alcohol use (drinks/wk) | 4.7 (7.4) | 5.0 (7.9) | 3.8 (6.0) | 3.7 (5.4) | 4.9 (8.4) | 6.1 (8.7) | <0.001 |
| Total calcium intake (mg/d) | 1146 (588) | 950 (521) | 1106 (594) | 1186 (586) | 1249 (600) | 1241 (587) | <0.001 |
| Vitamin D intake (IU/d) | 390 (250) | 270 (224) | 379 (246) | 410 (252) | 427 (248) | 466 (234) | <0.001 |
| BMI (kg/m2) | 27.4 (3.8) | 28.4 (4.3) | 27.9 (3.9) | 27.3 (3.6) | 27.0 (3.5) | 26.7 (3.2) | <0.001 |
| Inability to rise from a chair (%) | 0.8 | 1.6 | 1.2 | 0.4 | 0.4 | 0.4 | 0.12 |
| Total hip BMD (g/cm2) | 0.96 (0.14) | 0.97 (0.15) | 0.94 (0.13) | 0.96 (0.13) | 0.96 (0.13) | 0.97 (0.14) | 0.33 |
Data are expressed as mean (sd) unless otherwise described.
Quintile cutpoints are 19.1, 23.7, 27.0, 31.4 ng/ml.
25(OH)D level and rate of hip bone loss
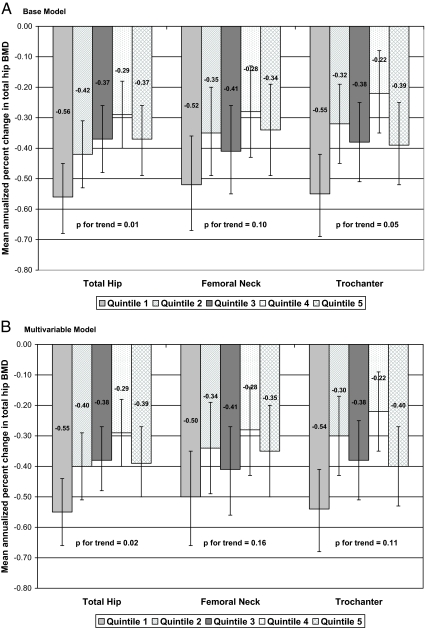
After adjustment for age, race, site, season, BMI, and baseline total hip BMD, lower 25(OH)D level was associated with higher rates of bone loss at the total hip (P for trend = 0.01) (Fig. 1A). The majority of effect was observed among men in the lowest quintile (<19.1 ng/ml) who experienced a 1.5-fold higher rate of hip bone loss (P = 0.003 for quintile 1 vs. quintiles 2–5); rates of loss were similar among men in higher quintiles and not significantly different from each other (P > 0.11 for all comparisons). A similar pattern was observed at the trochanter (P for trend = 0.05). The association was slightly attenuated in magnitude at the femoral neck, and the test for linear trend did not reach significance (P for trend = 0.10), although men in the lowest quintile had a higher rate of loss compared with men in quintiles 2–5 (P = 0.05). Results were not substantially altered after further adjustment for health status, smoking status, alcohol intake, physical activity level, and lower extremity performance, but the test for trend reached the level of significance only at the total hip (P = 0.02 at total hip, 0.16 at femoral neck, and 0.11 at trochanter) (Fig. 1B).
Figure 1.
Mean annualized rate of change in total hip BMD by quintile of total 25(OH)D level. Quintile cutpoints: 19.1, 23.7, 27.0, and 31.4 ng/ml. A, Adjusted for age, race, site, season, BMI, and baseline total hip BMD. B, Adjusted for age, race, site, season, BMI, baseline BMD, health status, smoking status, alcohol intake, physical activity level, and inability to rise from a chair.
Findings were similar in analyses expressing 25-hydroxy levels by category of vitamin D status (Table 2). For example, the mean annualized rate of change in total hip BMD was −0.59%/yr among men with a 25(OH)D below 15.0 ng/ml, −0.54%/yr among men with a 25(OH)D level of 15.0–19.9 ng/ml, −0.35%/yr among men with a 25(OH)D level of 20.0–29.9 ng/ml, and −0.37%/yr among men with a 25(OH)D level of at least 30 ng/ml (P for trend = 0.008 for multivariable model). Results were consistent at hip subregions (P for trend multivariable model 0.06 at femoral neck and 0.02 at trochanter).
Table 2.
Mean annualized rate of change in hip BMD by category of 25(OH)D level
| Hip subregion | Mean annualized % change in BMD (95% CI)
|
||||
|---|---|---|---|---|---|
| <15.0 ng/ml (n = 110) | 15.0–19.9 ng/ml (n = 184) | 20.0–29.9 ng/ml (n = 655) | ≥30.0 ng/ml (n = 330) | P trend | |
| Total hip | |||||
| Base modela | −0.61 (−0.78, −0.43) | −0.56 (−0.70, −0.43) | −0.35 (−0.42, −0.28) | −0.36 (−0.46, −0.26) | 0.002 |
| Multivariable modelb | −0.59 (−0.76, −0.42) | −0.54 (−0.67, −0.41) | −0.35 (−0.42, −0.28) | −0.37 (−0.47, −0.27) | 0.008 |
| Femoral neck | |||||
| Base modela | −0.56 (−0.79, −0.33) | −0.48 (−0.66, −0.31) | −0.36 (−0.45, −0.26) | −0.30 (−0.44, −0.17) | 0.04 |
| Multivariable modelb | −0.54 (−0.78, −0.31) | −0.48 (−0.65, −0.30) | −0.36 (−0.45, −0.26) | −0.32 (−0.45, −0.18) | 0.06 |
| Trochanter | |||||
| Base modela | −0.63 (−0.84, −0.43) | −0.52 (−0.68, −0.37) | −0.30 (−0.38, −0.21) | −0.35 (−0.47, −0.23) | 0.01 |
| Multivariable modelb | −0.62 (−0.82, −0.41) | −0.50 (−0.65, −0.34) | −0.29 (−0.37, −0.21) | −0.36 (−0.48, −0.25) | 0.02 |
CI, Confidence interval.
Adjusted for age, race, site, season, BMI, and baseline BMD.
Adjusted for age, race, site, season, BMI, baseline BMD, health status, smoking status, alcohol intake, physical activity level, and inability to rise from a chair.
Association between 25(OH)D level and rate of change in hip BMD according to risk subgroup
There was no evidence that baseline total hip BMD or physical activity level modified the association between 25(OH)D level and rate of change in hip BMD [P for tests of interaction between 25(OH)D and BMD >0.10 and between 25(OH)D and PASE score >0.96]. Among men with BMD above and below the median value and among men with PASE score above and below the median value, lower 25(OH)D levels were similarly associated with higher rates of bone loss (Table 3). However, there was evidence to support an interaction between 25(OH)D level (expressed in quintiles) and age for the prediction of rate of change in total hip BMD [P = 0.08 for interaction term with age expressed as a dichotomous variable (<75 yr vs. ≥75 yr) and 0.07 with age expressed as a continuous variable]. Among men at least 75 yr old, lower levels of 25(OH)D were associated with higher rates of hip bone loss (P for trend <0.001), whereas there was no evidence of an association between 25(OH)D level and rate of hip bone loss among men younger than 75 yr (P for trend = 0.43). Results were similar when 25(OH)D was expressed as categories (P for trend <0.001 among men ≥75 yr and 0.55 among men <75 yr). Further adjustment for renal function (eGFR) did not alter the association between 25(OH)D level and rate of bone loss at the total hip (P for trend = 0.005), and there was no evidence of an interaction between 25(OH)D levels and eGFR (P for interaction terms >0.12). Finally, excluding the 102 nonwhite men or excluding the 27 men taking bone-active agents from the analysis did not alter findings concerning the association between 25(OH)D levels and rate of change in hip BMD (P for trend at the total hip = 0.003 and 0.01, respectively) (results not shown).
Table 3.
Mean annualized rate of change in total hip BMD by quintile of 25(OH)D level according to risk subgroup
| Mean annualized % change in BMD (95% CI)
|
||||||
|---|---|---|---|---|---|---|
| Q1 (n = 131) | Q2 (n = 161) | Q3 (n = 193) | Q4 (n = 222) | Q5 (n = 229) | P trend | |
| Total hip BMD (g/cm2)a,b | ||||||
| <0.954 (n = 640) | −0.65 (−0.83, −0.47) | −0.31 (−0.47, −0.14) | −0.42 (−0.59, −0.24) | −0.26 (−0.43, −0.09) | −0.40 (−0.57, −0.22) | 0.08 |
| ≥0.954 (n = 639) | −0.47 (−0.61, −0.32) | −0.54 (−0.69, −0.39) | −0.32 (−0.45, −0.18) | −0.34 (−0.48, −0.20) | −0.37 (−0.51, −0.23) | 0.10 |
| Physical activitya,c | ||||||
| PASE score <147 (n = 637) | −0.58 (−0.73, −0.42) | −0.42 (−0.59, −0.26) | −0.37 (−0.52, −0.21) | −0.32 (−0.48, −0.16) | −0.42 (−0.60, −0.25) | 0.10 |
| PASE score ≥147 (n = 640) | −0.50 (−0.67, −0.34) | −0.43 (−0.58, −0.28) | −0.39 (−0.54, −0.24) | −0.28 (−0.43, −0.13) | −0.34 (−0.48, −0.19) | 0.07 |
| Age group (yr)d | ||||||
| <75 (n = 786) | −0.29 (−0.43, −0.16) | −0.30 (−0.43, −0.17) | −0.34 (−0.47, −0.21) | −0.15 (−0.28, −0.02) | −0.28 (−0.40, −0.15) | 0.43 |
| ≥75 (n = 493) | −0.98 (−1.18, −0.78) | −0.61 (−0.82, −0.41) | −0.46 (−0.65, −0.26) | −0.51 (−0.71, −0.32) | −0.46 (−0.69, −0.23) | <0.001 |
| Renal function (ml/min/1.73 m2)c | ||||||
| ≤60 (n = 179) | −0.84 (−1.17, −0.50) | −0.73 (−1.05, −0.41) | −0.75 (−1.07, −0.42) | −0.56 (−0.88, −0.23) | −0.50 (−0.79, −0.22) | 0.09 |
| >60 (n = 1021) | −0.49 (−0.62, −0.37) | −0.39 (−0.51, −0.26) | −0.33 (−0.45, −0.21) | −0.22 (−0.33, −0.10) | −0.36 (−0.49, −0.23) | 0.03 |
Quintile cutpoints are 19.1, 23.7, 27.0, 31.4 ng/ml. CI, Confidence interval.
Stratified by median value.
Adjusted for age, site, season, and BMI.
Adjusted for age, baseline total hip BMD, site, season, and BMI.
Adjusted for baseline total hip BMD, site, season, and BMI.
25(OH)D3 level and rate of hip bone loss
Among the 1279 men in the analytical cohort, the mean 25(OH)D3 level was 23.8 ng/ml. Only 343 men (27%) had detectable levels of 25(OH)D2, and the mean 25(OH)D2 level was 8.4 ng/ml in this group. In the overall cohort, levels of 25(OH)D3 were not related to the rate of change in total hip BMD (P for trend = 0.23) (results not shown). However, there was some evidence to suggest that this association depended on whether 25(OH)D2 was detectable or not. Among the 936 men without detectable 25(OH)D2, lower levels of 25(OH)D3 were associated with higher rates of loss (P for trend = 0.02), whereas there was no association between 25(OH)D3 level and rate of BMD change among the 343 men with detectable 25(OH)D2 (P for trend = 0.59). However, the test for an interaction between 25(OH)D3 and 25(OH)D2 (detectable or not) for the prediction of rate of change in total hip BMD did not reach the level of significance (P for interaction term = 0.15).
Discussion
In this prospective cohort study, we found that community-dwelling older men with total 25(OH)D level below 20 ng/ml experienced greater rates of hip bone loss, whereas rates of loss were similar among men with higher levels. This association was most apparent among men aged 75 yr and older. These findings suggest that low 25(OH)D levels are detrimental to BMD in older men.
Although several prior studies (16,17,18,19,20,21,22) have examined the association between 25(OH)D level and BMD in older adults, most were cross-sectional investigations or case-control studies in select populations, and they excluded or did not describe the association among men or inadequately controlled for confounders. A cross-sectional study of 881 men aged 19 to 85 yr (23) that included 595 men aged 55 to 85 yr reported that 25(OH)D3 levels (measured by RIA) were weakly correlated with BMD at the total hip (r = 0.12) and whole body (r = 0.11) in older men after adjustment for age, body weight, and season. The largest reported cross-sectional study was the third National Health and Nutritional Examination Survey (NHANES III) of a representative sample of U.S. adults, which included 13,432 participants. This study (24) reported that age group (25 to 49 yr vs. ≥50 yr) and race/ethnicity (Whites, Mexican-Americans, Blacks), but not sex, modified the association between 25(OH)D3 level (measured by RIA) and hip BMD. After adjustment for multiple potential confounders, mean hip BMD was 2 to 5% higher among adults in the highest quintile compared with those in the lowest quintile among younger and older whites, younger and older Mexican-Americans, and older Blacks (24). Race/ethnicity-specific quintile cutpoints in this study ranged from 12.6 to 21.2 ng/ml for quintile 1 and from 25.9 to 39.3 ng/ml for quintile 5.
The few prospective studies limited to older adults have reported conflicting results. One population-based study (6) of 316 Caucasian adults aged 60–75 yr (173 men and 143 women) living in the community found no evidence of an association between 25(OH)D level (measured with a competitive binding protein assay) and subsequent rate of change in BMD at the hip or spine. Another study of 669 Caucasian postmenopausal women (mean age, 62 yr) in a population-based cohort (7) reported no association between 25(OH)D level (measured with a competitive binding protein assay) and subsequent rates of bone loss at the radius. However, a third prospective study of approximately 200 community-dwelling Caucasian women aged 65 yr and older (10) found that lower 25(OH)D3 levels (measured by RIA) were associated with higher rates of bone loss at the hip, but not calcaneus.
Although low levels of 25(OH)D have been reported to be a marker of conditions such as darker skin (1,25) and greater adiposity (6), we found that the effect of 25(OH)D level on rate of change in hip BMD remained essentially unchanged despite adjustment for several factors including age, race, site, season of blood draw, BMI, baseline BMD, health status, physical activity level, smoking status, alcohol intake, and lower extremity strength. The association between low 25(OH)D level and higher rates of hip bone loss was also consistent across risk subgroups defined by bone density, physical activity level, and renal function in our cohort. However, our results suggest that the adverse effect of low 25(OH)D levels on rates of hip bone loss varies according to age; we found strong evidence to support an association among men aged 75 yr and older, but no evidence to support an association among younger men.
Our finding of an association between low 25(OH)D level and higher rates of hip bone loss in older men is supported by the results of some (26,27,28,29), but not all (7,30,31) prospective studies of 25(OH)D levels and risk of fracture. Of the four studies reporting an association, three investigations suggested a similar optimal level of 25(OH)D as the one identified in our analysis (at or above 20 ng/ml) for the prevention of hip fractures in postmenopausal women (26) and non-Hispanic older white adults (28), and for the prevention of clinical fractures in older women (27). The fourth study (29) observed no evidence of an association between lower 25(OH)D levels and an increased risk of fracture among older people aged 65 yr and older, except among those aged 65–75 yr with a level of 12 ng/ml or less. None of these prospective studies reported that lower 25(OH)D levels were associated in a graded manner with an increased risk of fracture. Similarly, we found no evidence to suggest that 25(OH)D levels between 20.0 and 29.9 ng/ml had adverse effects on the rate of change in hip BMD.
Levels of 25(OH)D2 and 25(OH)D3 were individually quantified in this study and summed for total 25(OH)D level. Only 27% of the men in this study had detectable levels of 25(OH)D2, and the primary difference between men with and without detectable 25(OH)D2 levels was a much greater prevalence of vitamin D supplementation use among the former group (32). Although lower total 25(OH)D levels were associated with higher rates of hip bone loss in the overall cohort, analyses examining the effect of 25(OH)D3 levels on rate of change in BMD found no evidence that men with lower 25(OH)D3 levels had higher rates of hip bone loss, with the exception of a possible association among the subgroup of men without detectable 25(OH)D2 levels. These results provide some evidence that low 25(OH)D3 levels do not have adverse effects on hip bone loss as long as 25(OH)D2 is available. However, the effect of vitamin D supplementation (D2 or D3) on rates of bone loss in older people, including among a target population defined by 25(OH)D status, can only be definitively addressed in a study using a randomized trial design.
Strengths of our study include its prospective design; study population comprised of a large cohort of older men not selected on the basis of BMD status; measurement of total 25(OH) vitamin D, D2, and D3; and adjustment for several potential confounders. However, our study had several limitations. Participants were older community-dwelling men, and our results may not apply to other populations. Other than Caucasian men, we had insufficient power to examine the association between 25(OH)D level and rate of bone loss within specific race/ethnic groups. In addition, our power was inadequate to examine the association between severe vitamin D deficiency (e.g. 25(OH)D level <10 ng/ml) and rates of hip bone loss. Our analyses were adjusted for several factors, but given the observational design of this study, the possibility of confounding cannot be eliminated. Other than renal function, we did not evaluate potential biological mechanisms underlying the association; future studies should examine whether other pathways (such as PTH, sex steroid hormones, and bone turnover markers) mediate the relationship. Of the 1606 men with baseline 25(OH)D measurements, 327 men were excluded from the analytical cohort because they did not return for a repeat BMD measurement. Because these men had lower 25(OH)D level and lower hip BMD at baseline, our findings may underestimate the magnitude of the true association between 25(OH)D level and rates of bone loss. Finally, comparison of the findings of this study with those of prior investigations are limited in part due to differences in 25(OH)D assay methods between studies.
We conclude that community-dwelling older men with 25(OH)D levels below 20 ng/ml (including men with levels <15.0 ng/ml and those with levels 15.0–19.9 ng/ml) had higher subsequent rates of hip bone loss, whereas rates of loss were similar among men with levels between 20.0 and 29.9 ng/ml and those with levels of at least 30 ng/ml. The association between lower 25(OH)D levels and higher rates of hip bone loss was most evident among men aged 75 yr and older. These findings provide support to the view that low 25(OH)D levels are detrimental to BMD in older men.
Footnotes
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health (NIH) funding. The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research provide support under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
Disclosure Summary: The authors have nothing to declare.
First Published Online May 19, 2009
Abbreviations: BMD, Bone mineral density; BMI, body mass index; CV, coefficient of variation; DXA, dual-energy x-ray absorptiometry; eGFR, estimated glomerular filtration rate; 25(OH)D, 25-hydroxyvitamin D; PASE, Physical Activity Scale for the Elderly.
References
- Zadshir A, Tareen N, Pan D, Norris K, Martins D 2005 The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis 15(4 Suppl 5):S5–S101 [PubMed] [Google Scholar]
- Lips P 2001 Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Aloia JF, Talwar SA, Pollack S, Yeh J 2005 A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med 165:1618–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Puente A, Esposito A, Savastano S, Carpinelli A, Postiglione L, Oriente P 2002 Dietary calcium intake and serum vitamin D are major determinants of bone mass variations in women. A longitudinal study. Aging Clin Exp Res 14:382–388 [DOI] [PubMed] [Google Scholar]
- Dennison E, Eastell R, Fall CH, Kellingray S, Wood PJ, Cooper C 1999 Determinants of bone loss in elderly men and women: a prospective population-based study. Osteoporos Int 10:384–391 [DOI] [PubMed] [Google Scholar]
- Garnero P, Munoz F, Sornay-Rendu E, Delmas PD 2007 Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone 40:716–722 [DOI] [PubMed] [Google Scholar]
- Melin A, Wilske J, Ringertz H, Sääf M 2001 Seasonal variations in serum levels of 25-hydroxyvitamin D and parathyroid hormone but no detectable change in femoral neck bone density in an older population with regular outdoor exposure. J Am Geriatr Soc 49:1190–1196 [DOI] [PubMed] [Google Scholar]
- Storm D, Eslin R, Porter ES, Musgrave K, Vereault D, Patton C, Kessenich C, Mohan S, Chen T, Holick MF, Rosen CJ 1998 Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo-controlled trial. J Clin Endocrinol Metab 83:3817–3825 [DOI] [PubMed] [Google Scholar]
- Stone K, Bauer DC, Black DM, Sklarin P, Ensrud KE, Cummings SR 1998 Hormonal predictors of bone loss in elderly women: a prospective study. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 13:1167–1174 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K 2005 Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR 2005 Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568 [DOI] [PubMed] [Google Scholar]
- Singh RJ, Taylor RL, Reddy GS, Grebe SK 2006 C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 91:3055–3061 [DOI] [PubMed] [Google Scholar]
- Washburn RA, Ficker JL 1999 Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness 39:336–340 [PubMed] [Google Scholar]
- Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F 2007 Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53:766–772 [DOI] [PubMed] [Google Scholar]
- Al-oanzi ZH, Tuck SP, Raj N, Harrop JS, Summers GD, Cook DB, Francis RM, Datta HK 2006 Assessment of vitamin D status in male osteoporosis. Clin Chem 52:248–254 [DOI] [PubMed] [Google Scholar]
- Boonen S, Mohan S, Dequeker J, Aerssens J, Vanderschueren D, Verbeke G, Broos P, Bouillon R, Baylink DJ 1999 Down-regulation of the serum stimulatory components of the insulin-like growth factor (IGF) system (IGF-I, IGF-II, IGF binding protein [BP]-3, and IGFBP-5) in age-related (type II) femoral neck osteoporosis. J Bone Miner Res 14:2150–2158 [DOI] [PubMed] [Google Scholar]
- Khaw KT, Sneyd MJ, Compston J 1992 Bone density parathyroid hormone and 25-hydroxyvitamin D concentrations in middle aged women. BMJ 305:273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landin-Wilhelmsen K, Wilhelmsen L, Bengtsson BA 1999 Postmenopausal osteoporosis is more related to hormonal aberrations than to lifestyle factors. Clin Endocrinol (Oxf) 51:387–394 [DOI] [PubMed] [Google Scholar]
- Thiébaud D, Burckhardt P, Costanza M, Sloutskis D, Gilliard D, Quinodoz F, Jacquet AF, Burnand B 1997 Importance of albumin, 25(OH)-vitamin D and IGFBP-3 as risk factors in elderly women and men with hip fracture. Osteoporos Int 7:457–462 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Civitelli R, Chines A, Avioli LV 1991 Subclinical vitamin D deficiency in postmenopausal women with low vertebral bone mass. J Clin Endocrinol Metab 72:628–634 [DOI] [PubMed] [Google Scholar]
- Yan L, Zhou B, Wang X, D'Ath S, Laidlaw A, Laskey MA, Prentice A 2003 Older people in China and the United Kingdom differ in the relationships among parathyroid hormone, vitamin D, and bone mineral status. Bone 33:620–627 [DOI] [PubMed] [Google Scholar]
- Szulc P, Munoz F, Marchand F, Chapuy MC, Delmas PD 2003 Role of vitamin D and parathyroid hormone in the regulation of bone turnover and bone mass in men: the MINOS study. Calcif Tissue Int 73:520–530 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B 2004 Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634–639 [DOI] [PubMed] [Google Scholar]
- Pal BR, Marshall T, James C, Shaw NJ 2003 Distribution analysis of vitamin D highlights differences in population subgroups: preliminary observations from a pilot study in UK adults. J Endocrinol 179:119–129 [DOI] [PubMed] [Google Scholar]
- Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR 2008 Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 149:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K 2005 Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int 16:1425–1431 [DOI] [PubMed] [Google Scholar]
- Looker AC, Mussolino ME 2008 Serum 25-hydroxyvitamin D and hip fracture risk in older U.S. white adults. J Bone Miner Res 23:143–150 [DOI] [PubMed] [Google Scholar]
- van Schoor NM, Visser M, Pluijm SM, Kuchuk N, Smit JH, Lips P 2008 Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 42:260–266 [DOI] [PubMed] [Google Scholar]
- Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B 1998 Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med 339:733–738 [DOI] [PubMed] [Google Scholar]
- Roddam AW, Neale R, Appleby P, Allen NE, Tipper S, Key TJ 2007 Association between plasma 25-hydroxyvitamin D levels and fracture risk: the EPIC-Oxford study. Am J Epidemiol 166:1327–1336 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Nielson CM, Marshall LM, Lambert L, Holton KF, Hoffman AR, Barrett-Connor E, Shikany JM, Dam T, Cauley JA 2009 Vitamin D deficiency in older men. J Clin Endocrinol Metab 94:1214–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]