Abstract
Context: Elevated plasma free fatty acid (FFA) concentrations are observed under various clinical circumstances and are associated with impaired glucose disposal in skeletal muscle.
Objective: The aim of the study was to determine the effects of elevated plasma FFA concentrations on the response of protein synthesis and balance in muscle after essential amino acids (EAAs) ingestion.
Design: Leg protein kinetics were determined in young healthy individuals before and after the ingestion of EAAs at 10 h after the initiation of either lipid (Liposyn/heparin+EAA) or saline (saline+EAA) infusions.
Results: Plasma insulin responses where higher (P <0.05) in the Liposyn/heparin+EAA group than the saline+EAA group both before (14 ± 4 vs. 6 ± 1 μIU · ml−1) and after (1038 ± 257 vs. 280 ± 87 μIU · ml−1 · 210 min−1) the EAA ingestion. After the EAA ingestion, the rates of both leg phenylalanine disappearance (Rd; nmol · min−1 · kg lean leg mass−1) and muscle proteins fractional synthesis (FSR; % · h−1) increased (P <0.05) in both the Liposyn/heparin+EAA and saline+EAA groups, but these changes were not different between the two groups (Rd, 102 ± 32 vs. 118 ± 34; FSR, 0.014 ± 0.005 vs. 0.018 ± 0.007; P > 0.05). Although the leg phenylalanine rate of appearance (Ra; nmol · min−1 · kg lean leg mass−1) was lower (381 ± 47 vs. 518 ± 40) and the balance was greater (−109 ± 20 vs. −172 ± 17) in the Liposyn/heparin+EAA group compared to the saline+EAA group before the EAA ingestion (P <0.05), the changes in both of these parameters were not different between groups after the EAA ingestion (P > 0.05).
Conclusions: Elevated plasma FFA concentrations do not interfere with the response of muscle protein synthesis and balance to a bolus ingestion of EAAs.
Essential amino acids stimulated muscle protein turnover in the presence of elevated plasma free fatty acids.
Elevated plasma free fatty acid (FFA) concentrations are documented under various clinical circumstances such as obesity (1,2), diabetes (3), critical illness (4), and nonalcoholic steatohepatitis (5). Elevated plasma FFA concentrations are regularly observed in parallel with an increase in plasma insulin concentrations (2,3), and it has been documented that elevated plasma FFA concentrations in humans are associated with impaired insulin-mediated glucose disposal in skeletal muscle (6,7). However, very little is known with respect to the regulation of protein metabolism under these circumstances.
In the presence of physiological postabsorptive plasma FFA concentrations, increased plasma amino acid concentrations stimulate muscle protein synthesis (8). Among the plasma amino acids, the essential amino acids (EAAs) are primarily responsible for the regulation of muscle protein synthesis (8). We have shown that ingestion of a practical bolus of EAAs stimulates muscle protein synthesis without altering muscle protein breakdown, thus resulting in an overall improvement in muscle protein balance (9). For some time, animal studies have provided evidence for a decrease in protein turnover with an elevation in plasma FFA concentrations (10,11). In humans, elevated plasma FFA concentrations decrease amino acid flux (12), and have also been associated with lower rates of protein breakdown and synthesis at the muscle (12,13). Recent mechanistic evidence from animal studies indicates that the translation initiation process of muscle protein synthesis is impaired in the presence of elevated plasma FFA concentrations (14).
The anabolic response to EAA ingestion is observed simultaneously with a significant increase in the concentration of plasma insulin (9), which has been implicated in the regulation of muscle protein synthesis (15). Understanding the specific role of plasma insulin with respect to its concentration and its interaction with increased plasma amino acid availability in the regulation of muscle protein kinetics still remains an active area of research (16). It has been suggested that insulin secreted as a response to increased plasma amino acid concentrations enhances the effect of plasma amino acids on the stimulation of muscle protein synthesis (17,18). The effects of insulin on skeletal muscle are mediated through the phosphatidylinositol 3-kinase (PI3K), and insulin signaling through PI3K is the only known mechanism implicated in the regulation of not only glucose but also protein synthesis in skeletal muscle (15,19). Elevated plasma FFA concentrations, however, are known to attenuate the stimulation of PI3K by insulin in skeletal muscle (6,7) and may therefore interfere with the insulin signal required in the regulation of muscle protein synthesis.
On the basis of the evidence discussed above, we hypothesized that the response of muscle protein synthesis and balance to EAAs ingestion is impaired in the presence of elevated plasma FFA concentrations. We determined muscle protein kinetics in a physiological circumstance associated with amino acid ingestion. Muscle protein kinetics to EAAs ingestion during lipid infusion were compared with those during saline infusion.
Subjects and Methods
Subjects
Young healthy subjects were randomly assigned to two groups; one group ingested a bolus of EAAs while receiving lipid infusion (Liposyn/heparin+EAA), whereas the other group ingested the same bolus of EAAs while receiving saline infusion (saline+EAA). Subjects included in the study were determined to be healthy based on a medical history report, physical examination, resting electrocardiogram, and routine blood and urine tests. Body composition was determined using dual-energy x-ray absorptiometry. Subject characteristics for the two groups are presented in Table 1. The study protocol was approved by the Institutional Review Board and the General Clinical Research Center (GCRC) of the University of Texas Medical Branch at Galveston.
Table 1.
Subject characteristics
| Saline+EAA | Liposyn/heparin +EAA | P value | |
|---|---|---|---|
| Gender (males/females) | 5/3 | 5/2 | |
| Age (yr) | 31.1 ± 6.0 | 28.4 ± 6.0 | 0.40 |
| Body weight (kg) | 71.7 ± 12.7 | 83.3 ± 14.1 | 0.12 |
| Height (cm) | 172.4 ± 4.7 | 172.9 ± 8.2 | 0.89 |
| BMI (kg/m2) | 24.1 ± 4.0 | 27.9 ± 4.6 | 0.12 |
| Body fat (%) | 22.9 ± 10.3 | 25.0 ± 8.7 | 0.67 |
| Lean body mass (kg) | 51.9 ± 10.0 | 60.2 ± 10.7 | 0.15 |
| Leg lean mass (kg) | 8.5 ± 1.7 | 10.3 ± 2.6 | 0.13 |
Values are expressed as means ± sd. Saline+EAA, EAA ingestion (control); Liposyn/heparin+EAA, EAA ingestion combined with lipid infusion to increase plasma FFA concentrations; BMI, body mass index.
Experimental protocol and samples analysis
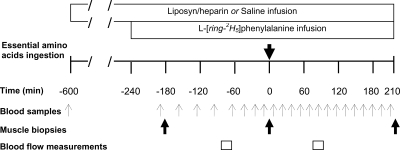
Subjects reported to the GCRC in the afternoon of the day before the experiment and were not allowed to consume any food after 2200 h. At approximately 0100 h, a combined 20% Liposyn (90 ml · h−1) and heparin (0.28 IU · kg−1 · min−1) infusion was initiated into an antecubital vein of an arm of subjects in the Liposyn/heparin+EAA group. The saline+EAA group received normal saline without heparin. The Liposyn infusion protocol employed in the present study is well-documented to elevate plasma FFA concentrations and result in impaired insulin action in skeletal muscle (6,20,21,22,23). Another catheter was inserted into the antecubital vein of the opposite arm for blood sampling. At approximately 0600 h, 3-French, 8-cm polyethylene catheters were inserted in the femoral artery and vein of a leg. A primed (2.0 μmol · kg−1) constant (0.05 μmol · kg−1 · min−1) infusion of L-[ring-2H5]phenylalanine was started at approximately 0700 h. Blood samples were drawn simultaneously from the femoral artery and vein catheters (Fig. 1). Indocyanine green dye was infused at a constant rate (0.5 mg · min−1) into the femoral artery for the determination of the leg blood flow (Fig. 1), using procedures that have previously been described (24). Subjects ingested a bolus of 250 ml of noncaloric/noncaffeinated soft drink at time 0 (Fig. 1) containing the following EAAs (in grams): histidine (0.30), isoleucine (0.78), leucine (1.72), lysine (1.36), methionine (0.36), phenylalanine (0.51), threonine (0.95), and valine (0.74). The relative contribution of individual amino acids in the mixture was based on the distribution of these amino acids in whey protein, and we have previously shown that ingestion of these amounts of EAAs in a mixture effectively stimulates muscle protein synthesis in healthy subjects (9). At the same time, the overall amount of amino acids chosen in this mixture was not large enough to overcome a potentially impaired response of muscle protein synthesis to plasma amino acids, as we have previously evidenced in physiological circumstances associated with aging (9,25). L-[ring-2H5]phenylalanine (0.04 g) was included in the amino acids mixture to maintain the steady-state isotopic enrichment of blood phenylalanine after the amino acids ingestion. Muscle biopsy samples (∼50 mg) were taken from the lateral portion of vastus lateralis. The muscle was cleaned from any visible fat and connective tissue, rinsed with ice-cold saline to remove any blood, and blotted dry. The muscle was immediately frozen in liquid nitrogen and then stored at −80 C.
Figure 1.
Experimental design of the infusion protocol. Leg arterio-venous blood samples were collected for the determination of leg phenylalanine kinetics, before and after ingestion of approximately 7 g of EAAs at time 0. Muscle biopsies were collected for the determination of FSR of mixed-muscle proteins. Leg blood flow was determined by the infusion of indocyanine green.
Blood phenylalanine concentration was determined using the internal standard approach with the addition of L-[U-13C9-15N]phenylalanine. Free phenylalanine concentration in muscle was determined in a similar way as for blood, using the chloride method (26). Blood and muscle samples were analyzed for phenylalanine enrichment, including muscle protein-bound phenylalanine enrichment, as previously described (9,24). Serum from the femoral and peripheral veins was used for the determination of the leg blood flow by measuring the indocyanine green dye absorbance at 805 nm and as previously described (24). Arterial concentrations of plasma glucose (YSI; YSI Inc., Yellow Springs, OH), FFAs (Wako Chemicals, Richmond, VA), and insulin (ALPCO Diagnostics, Windham, NH) were determined using commercially available procedures.
Calculations
The phenylalanine balance (PB) across the leg at each sampling time point was calculated based on the blood phenylalanine concentrations in the artery (Ca) and vein (Cv) and the measured leg blood flow (BF): PB = (Ca − Cv) × BF. At any given period that the intracellular free phenylalanine concentration remains constant, the rates of phenylalanine disappearance (Rd) from the artery and appearance (Ra) to the vein reflect the rates of incorporation of blood phenylalanine into muscle proteins and release from muscle proteins breakdown, respectively, because phenylalanine is not metabolized in muscle. Rd and Ra were calculated as follows:
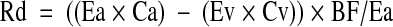 |
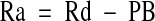 |
where Ea and Ev are the blood phenylalanine enrichments in the femoral artery and vein, respectively, and Ca, Cv, BF, and PB are as defined above. Calculated PB, Rd, and Ra were averaged for the periods before and after the amino acid ingestion to calculate an average response for PB, Rd, and Ra during each of these periods. Fractional synthesis rate (FSR; % · h−1) of mixed-muscle proteins was calculated as previously described (24):
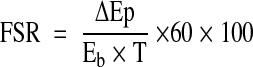 |
where ΔEp defines the increment in the muscle protein-bound phenylalanine tracer-to-tracee ratio (t/T) between two biopsies and Eb is the average arterial blood phenylalanine t/T during isotopic steady-state between two biopsies. Using a weighted average from several blood enrichments to calculate the precursor enrichment provides a more reliable approach to calculate the FSR than using a precursor enrichment based on only two muscle samples. T is the time interval (minutes) between biopsies, and the factors 60 and 100 are used to express the FSR values in % · h−1.
Statistical analyses
Experimental data are summarized as means ± se unless otherwise stated. Changes in the main parameters of interest as a result of the amino acid ingestion were compared between groups using unpaired t tests. Paired t tests were used to compare differences within a group (e.g. average phenylalanine Rd values before vs. average phenylalanine Rd values after the amino acids ingestion). Data from the two groups were also analyzed using two-factor (group × time) ANOVA. Values in the parameters of interest after the amino acid ingestion were compared with that in the postabsorptive period within a group using one-way ANOVA followed by Dunnett’s post hoc test. A P value less than 0.05 was considered statistically significant.
Results
Leg blood flow
There were no significant main effects for either group or time. Leg blood flow before the amino acid ingestion was 3.6 ± 0.4 and 3.3 ± 0.4 ml · min−1 · 100 ml leg volume−1 for the Liposyn/heparin+EAA and saline+EAA groups, respectively (P > 0.05). Blood flow did not change significantly (P > 0.05) in either group after the amino acids ingestion.
Plasma concentrations of FFAs, insulin, and glucose
As expected, the average plasma FFAs concentration in the period before the amino acids ingestion was higher in the Liposyn/heparin+EAA group (3.0 ± 0.5 mmol · liter−1) than the saline+EAA group (0.4 ± 0.1 mmol · liter−1; P < 0.05). This difference remained significant also in the period after the amino acids ingestion (Liposyn/heparin+EAA group, 2.6 ± 0.5 mmol · liter−1; saline+EAA group, 0.5 ± 0.1 mmol · liter−1; P < 0.05).
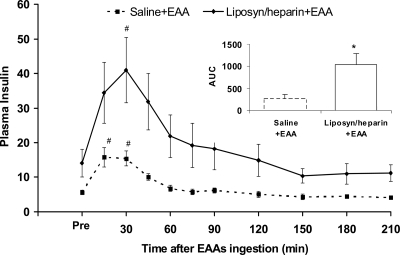
In the postabsorptive period, plasma insulin concentration was higher in the lipid infusion group (Liposyn/heparin+EAA, 14.1 ± 3.9 μIU · ml−1; saline+EAA, 5.6 ± 0.6 μIU · ml−1; P < 0.05). The response of plasma insulin to amino acids ingestion in both groups is shown in Fig. 2. When assessed by the area under the curve (AUC), this response of plasma insulin was more than 3.5 times higher in the lipid infusion group (Liposyn/heparin+EAA, 1038 ± 257 μIU · ml−1 · 210 min−1; saline+EAA, 280 ± 87 μIU · ml−1 · 210 min−1; P < 0.05).
Figure 2.
Plasma insulin concentration (μIU · ml−1) in the period before (Pre; average value) and the period after the ingestion of EAAs, in the control group (saline+EAA) and the group receiving lipid infusion (Liposyn/heparin+EAA). The inset depicts the AUC for the plasma insulin concentration (μIU · ml−1 · 210 min−1) in the period after the EAAs ingestion. #, Significantly different from Pre within-group (P < 0.05); *, significant difference between groups (P < 0.05).
With respect to plasma glucose concentration, there were no differences between groups in the period before the amino acid ingestion (Liposyn/heparin+EAA, 102 ± 3 mg · dl−1; saline+EAA, 96 ± 3 mg · dl−1; P > 0.05). After the amino acid ingestion, the average plasma glucose concentration was significantly different between groups (Liposyn/heparin+EAA, 97 ± 3 mg · dl−1; saline+EAA, 88 ± 2 mg · dl−1; P < 0.05).
The Quantitative Insulin-Sensitivity Check Index (QUICKI), as proposed to incorporate the plasma FFA concentrations (27) [QUICKI = 1/(Log insulin + Log glucose + Log FFA)], immediately before the amino acids ingestion was lower in the lipid infusion group (Liposyn/heparin+EAA, 0.29 ± 0.01; saline+EAA, 0.44 ± 0.02; P < 0.05), whereas it was not different between the two groups before the initiation of any infusions (P > 0.05). The same index calculated from the average plasma insulin, glucose, and FFA responses after the amino acid ingestion was lower in the lipid infusion group (Liposyn/heparin+EAA, 0.28 ± 0.01; saline+EAA, 0.40 ± 0.02; P < 0.05).
Arterial blood phenylalanine concentration and enrichment
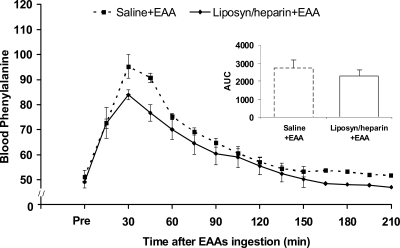
The arterial blood phenylalanine concentration was not significantly different between groups in the period before the amino acids ingestion (Liposyn/heparin+EAA, 49 ± 2 nmol · ml−1; saline+EAA, 51 ± 3 nmol · ml−1; P > 0.05). In the period after the amino acids ingestion, the average blood phenylalanine concentration was higher (P < 0.05) compared with the period before the amino acids ingestion in both groups, with no differences between groups in the blood phenylalanine concentration AUC (P > 0.05; Fig. 3). Average enrichments for arterial blood phenylalanine (L-[ring-2H5] phenylalanine/phenylalanine ratio) in the Liposyn/heparin+EAA group were 0.076 ± 0.003 and 0.086 ± 0.003 before and after the amino acids ingestion, respectively. The corresponding values in the saline+EAA group were 0.072 ± 0.003 and 0.080 ± 0.003 before and after the amino acids ingestion, respectively.
Figure 3.
Blood phenylalanine concentration (nmol · ml−1) in the period before (Pre; average value) and the period after the ingestion of EAAs, in the control group (saline+EAA) and the group receiving lipid infusion (Liposyn/heparin+EAA). The inset depicts the AUC for the blood phenylalanine concentration (nmol · ml−1 · 210 min−1) in the period after the EAAs ingestion.
Muscle free phenylalanine concentration
Average muscle free phenylalanine concentration before the amino acids ingestion was 61 ± 7 and 63 ± 3 nmol · ml−1 in the Liposyn/heparin+EAA and saline+EAA groups, respectively (P > 0.05). The concentration of muscle free phenylalanine was not different (P > 0.05) in either group at the end of the experimental period compared with the concentration in the period before the amino acids ingestion (Liposyn/heparin+EAA, 62 ± 4 nmol · ml−1; saline+EAA, 64 ± 7 nmol · ml−1).
Leg phenylalanine kinetics
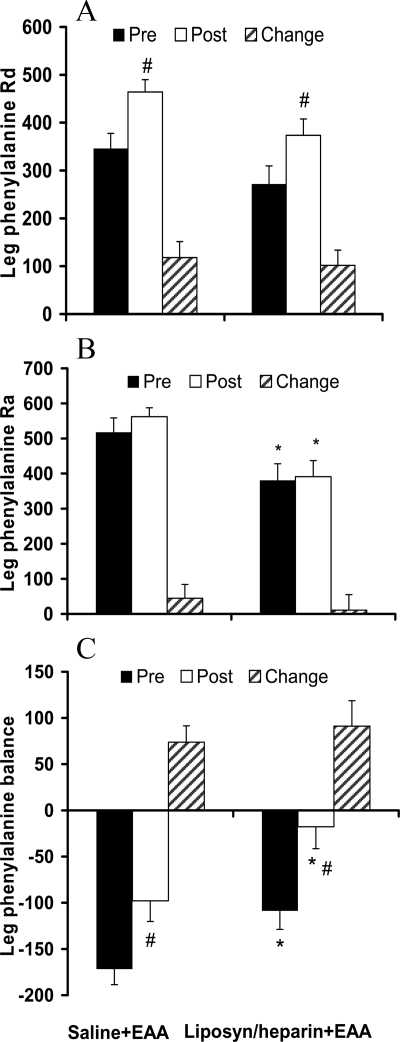
The leg phenylalanine Rd in the period before the amino acids ingestion was not different between the saline+EAA and Liposyn/heparin+EAA groups (346 ± 31 vs. 272 ± 38 nmol · min−1 · kg lean leg mass−1; P > 0.05). As shown in Fig. 4A, the leg phenylalanine Rd improved significantly (P < 0.05) in both groups in the period after the amino acids ingestion, and this change in leg phenylalanine Rd was not different between groups (P > 0.05). As shown in Fig. 4B, the leg phenylalanine Ra in the period before the amino acids ingestion was lower in the Liposyn/heparin+EAA group than the saline+EAA group (381 ± 47 vs. 518 ± 40 nmol · min−1 · kg lean leg mass−1; P < 0.05). The leg phenylalanine Ra in the period after the amino acids ingestion remained lower (P < 0.05) in the Liposyn/heparin+EAA group compared with that in the saline+EAA group, but it was not different compared with the period before the amino acids ingestion in either group (P > 0.05). Data for the leg PB are presented in Fig. 4C. The leg PB in the period before the amino acids ingestion was greater in the Liposyn/heparin+EAA group than the saline+EAA group (−109 ± 20 vs. −172 ± 17 nmol · min−1 · kg lean leg mass−1; P < 0.05). The leg PB improved (P < 0.05) in both groups after the amino acid ingestion, and during this period it remained greater in the Liposyn/heparin+EAA group when compared with that in the saline+EAA group (P < 0.05). However, there was no difference between groups with respect to the change in leg PB as a result of the amino acids ingestion (P > 0.05).
Figure 4.
Average values for the leg phenylalanine Rd (A), Ra (B), and balance (C) (nmol · min−1 · kg lean leg mass−1) in the period before (Pre) and the period after (Post) the ingestion of EAAs, as well as the change (Change; Post − Pre) in these parameters as a result of the EAAs ingestion. Saline+EAA, Control group; Liposyn/heparin+EAA, group receiving lipid infusion. #, Significantly different from Pre within-group (P < 0.05); *, significant difference between groups (P < 0.05).
Muscle protein FSR
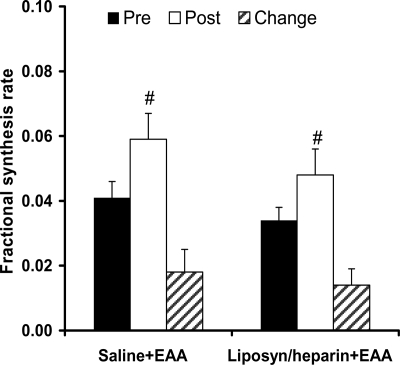
There were no significant differences (P > 0.05) between groups in mixed-muscle proteins FSR either before or after the amino acids ingestion. As shown in Fig. 5, the FSR increased significantly (P < 0.05) in both groups as a result of the amino acids ingestion, but this change was not different between groups (P > 0.05).
Figure 5.
FSR (% · h−1) of mixed-muscle proteins in the period before (Pre) and the period after (Post) the ingestion of EAAs, as well as its change (Change; Post − Pre) as a result of the EAAs ingestion. Saline+EAA, Control group; Liposyn/heparin+EAA, group receiving lipid infusion. #, Significantly different from Pre within-group (P < 0.05).
Discussion
The primary end-points of this study were the response of muscle protein synthesis and balance to amino acids ingestion in the presence of elevated plasma FFA concentrations. The present data demonstrate that elevation in plasma FFA concentrations does not interfere with the postprandial response of muscle protein synthesis and balance to a bolus ingestion of amino acids.
Previous studies performed in a postabsorptive state have reported that the rates of forearm phenylalanine uptake and release were significantly lower (13) or tended to be lower (12) without a significant difference in the overall forearm phenylalanine balance with lipid infusion. These results appear, at least in part, to be different from the finding of the present study where an improved muscle protein balance was observed with elevated plasma FFA concentrations. In addition to possible differences related to the arteriovenous model used (leg vs. forearm) (28), this discrepancy in the results between studies most likely reflects differences in study design. It should be indicated that, in both of the previous studies (12,13), forearm protein kinetics were evaluated in the presence of basal insulin concentrations, whereas the lipid infusion in the present study resulted in an increase in plasma insulin concentration, similar to that observed in other studies (29,30). These differences in plasma insulin concentrations between studies may relate to differences in the plasma FFA concentrations and a decrease in the rate of plasma insulin clearance as a result of the prolonged presence of elevated plasma FFA concentrations (31). Concurrent elevations in the concentration of plasma FFAs and insulin are frequent observations under various clinical circumstances (2,3,32,33,34).
It has been shown that small increases in plasma insulin concentrations increase muscle protein anabolism by inhibiting muscle protein breakdown (35). This is in line with the lower leg phenylalanine Ra and improved muscle protein balance in the presence of higher plasma insulin concentrations before the amino acids ingestion in the Liposyn/heparin+EAA group when compared with the saline+EAA group in the present study. However, the lower leg phenylalanine Ra in the present study can also be a result of a direct effect of plasma FFAs on muscle protein breakdown and according to what has been reported previously in the presence of unchanged plasma insulin concentrations (10,11,36). After the amino acids ingestion, and similar to what we have previously shown (9), muscle protein breakdown did not change relative to the muscle protein breakdown in the postabsorptive state in the saline+EAA group. The current study extends these findings by showing that muscle protein breakdown is also unaffected by amino acids ingestion in the presence of elevated plasma FFA concentrations.
With respect to the muscle protein synthesis in the period before the amino acids ingestion, we found this not to be significantly affected by lipid infusion. In line with our results, others have found no difference in muscle protein synthesis with lipid infusion resulting in plasma FFA concentrations close to those observed in the present study (12). Contrary to these observations in humans, Lang (14) has shown a decrease in muscle protein synthesis during lipid infusion in rats. In that study (14), as well as in other studies in rats (37), plasma insulin concentrations appear unaffected by the lipid infusion, whereas they increased in the present study, and similarly to what has been reported in other studies in humans (29,30). Therefore, determination of muscle protein kinetics in the presence of increased plasma insulin concentrations, secondary to increased plasma FFA concentrations, may have played a role in the present findings and given the role of insulin in regulating muscle protein turnover (16). The results of the present study with respect to muscle protein synthesis extend findings in the postabsorptive state to a physiological state associated with amino acids ingestion and in the presence of elevated plasma FFA concentrations. We have previously documented in healthy young subjects that ingestion of amino acids results in an increase in plasma amino acid concentrations and stimulation of muscle protein synthesis (9), and the present findings show a similar response in the presence of elevated plasma FFA concentrations.
It is known that plasma EAAs are insulin secretagogues, and this response is clearly observed in the saline+EAA group after the amino acids ingestion. Both insulin and amino acids have been implicated in the stimulation of skeletal muscle protein synthesis (38), and increased plasma insulin response to amino acids ingestion has been suggested to enhance the effect of the circulating amino acids on stimulating muscle protein synthesis (17,18). However, despite the greater plasma insulin response after the amino acids ingestion in the Liposyn/heparin+EAA group in the present study, the increase in muscle protein synthesis was not different between the two groups. It is possible that after the amino acids ingestion the higher plasma insulin response in the Liposyn/heparin+EAA group overcompensated for an impaired insulin action in the skeletal muscle. However, the calculated insulin sensitivity index was still lower in the Liposyn/heparin+EAA group when compared with that in the saline+EAA group in the period after the amino acid ingestion, suggestive of resistance to insulin action. Furthermore, recent evidence shows that stimulation of muscle protein synthesis at a given concentration of plasma amino acids is not enhanced further by increasing the concentration of plasma insulin beyond that observed in the postabsorptive state (39).
Leucine is a branched-chain amino acid that is considered to play, among other plasma amino acids, a major role in the up-regulation of cell signaling mechanisms involved in protein synthesis (40). Lang (14) has shown that muscle protein synthesis remains responsive to the stimulatory effect of plasma leucine despite the existence of impaired cell signaling and lower rates of muscle protein synthesis with elevated plasma FFA concentrations in the postabsorptive state. Elevated plasma FFA concentrations in the postabsorptive state have been associated with lower plasma leucine concentrations in some (10,30), but not all studies (11,14). In the present study, leucine concentration was not determined, but an increase in plasma leucine concentration is expected after the amino acid ingestion as a result of the leucine-containing amino acid mixture. Relative to that, the findings by Lang (14) could suggest that leucine may have played a role in maintaining the up-regulation of muscle protein synthesis in the period after the amino acid ingestion in the Liposyn/heparin+EAA group in the present study.
In conclusion, elevated concentrations in plasma FFAs in humans decrease muscle protein breakdown and improve the protein balance at postabsorptive plasma amino acid concentrations. More importantly, elevated concentrations in plasma FFAs per se do not interfere with the response of muscle protein synthesis and balance to a bolus ingestion of EAAs. Therefore, our results suggest that in clinical circumstances associated with elevated plasma FFA concentrations such as obesity, diabetes, critical illness, and nonalcoholic steatohepatitis, muscle protein synthesis can be stimulated by amino acids ingestion.
Acknowledgments
The authors thank the nurses and the staff at the General Clinical Research Center at the University of Texas Medical Branch in Galveston, Texas, as well as Dan Creson, R.N. We gratefully acknowledge Stephaine J. Blasé, Melissa Bailey, Christopher Danesi, Gaurang K. Jariwala, and Ming-Qian Zheng for skillful technical assistance.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants AR 49038 and M01 RR 00073 and Shriners Hospital Grant 8490. Studies were conducted on the General Clinical Research Center (GCRC) at the University of Texas Medical Branch at Galveston, funded by Grant M01 RR 00073 from the National Center for Research Resources, NIH, and the U.S. Public Health Service.
Disclosure Summary: C.S.K., A.A., and M.G.C. have nothing to declare. R.R.W. has financial interest in HealthSpan, LLC.
First Published Online May 19, 2009
For editorial see page 2725
Abbreviations: AUC, Area under the curve; EAA, essential amino acid; FFA, free fatty acid; FSR, fractional synthesis rate; PB, phenylalanine balance; PI3K, phosphatidylinositol 3-kinase; Ra, rate of appearance; Rd, rate of disappearance.
References
- Boden G 2008 Obesity and free fatty acids. Endocrinol Metab Clin North Am 37:635–646, viii-ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod JP, Brotman DJ 2003 The metabolic syndrome as a vicious cycle: does obesity beget obesity? Med Hypotheses 60:584–589 [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD 1988 Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37:1020–1024 [DOI] [PubMed] [Google Scholar]
- Wolfe RR 1997 Substrate utilization/insulin resistance in sepsis/trauma. Baillieres Clin Endocrinol Metab 11:645–657 [DOI] [PubMed] [Google Scholar]
- de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME 2002 Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr 21:219–223 [DOI] [PubMed] [Google Scholar]
- Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI 1999 Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap SR, Belfort R, Berria R, Suraamornkul S, Pratipranawatr T, Finlayson J, Barrentine A, Bajaj M, Mandarino L, DeFronzo R, Cusi K 2004 Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab 287:E537–E546 [DOI] [PubMed] [Google Scholar]
- Wolfe RR 2002 Regulation of muscle protein by amino acids. J Nutr 132:3219S–3224S [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR 2005 Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82:1065–1073 [DOI] [PubMed] [Google Scholar]
- Beaufrere B, Tessari P, Cattalini M, Miles J, Haymond MW 1985 Apparent decreased oxidation and turnover of leucine during infusion of medium-chain triglycerides. Am J Physiol 249:E175–E182 [DOI] [PubMed] [Google Scholar]
- Tessari P, Nissen SL, Miles JM, Haymond MW 1986 Inverse relationship of leucine flux and oxidation to free fatty acid availability in vivo. J Clin Invest 77:575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormsen LC, Gjedsted J, Gjedde S, Norrelund H, Christiansen JS, Schmitz O, Jorgensen JO, Moller N 2008 Dose-response effects of free fatty acids on amino acid metabolism and ureagenesis. Acta Physiol (Oxf) 192:369–379 [DOI] [PubMed] [Google Scholar]
- Walker M, Shmueli E, Daley SE, Cooper BG, Alberti KG 1993 Do nonesterified fatty acids regulate skeletal muscle protein turnover in humans? Am J Physiol 265:E357–E361 [DOI] [PubMed] [Google Scholar]
- Lang CH 2006 Elevated plasma free fatty acids decrease basal protein synthesis, but not the anabolic effect of leucine, in skeletal muscle. Am J Physiol Endocrinol Metab 291:E666–E674 [DOI] [PubMed] [Google Scholar]
- Proud CG 2006 Regulation of protein synthesis by insulin. Biochem Soc Trans 34:213–216 [DOI] [PubMed] [Google Scholar]
- Phillips SM 2008 Insulin and muscle protein turnover in humans: stimulatory, permissive, inhibitory, or all of the above? Am J Physiol Endocrinol Metab 295:E731 [DOI] [PubMed] [Google Scholar]
- Garlick PJ 2005 The role of leucine in the regulation of protein metabolism. J Nutr 135:1553S–1556S [DOI] [PubMed] [Google Scholar]
- Suryawan A, O'Connor PM, Bush JA, Nguyen HV, Davis TA 2009 Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids 37:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS 2002 Invited review: role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol 93:1168–1180 [DOI] [PubMed] [Google Scholar]
- Bachmann OP, Dahl DB, Brechtel K, Machann J, Haap M, Maier T, Loviscach M, Stumvoll M, Claussen CD, Schick F, Häring HU, Jacob S 2001 Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 50:2579–2584 [DOI] [PubMed] [Google Scholar]
- Boden G, Lebed B, Schatz M, Homko C, Lemieux S 2001 Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 50:1612–1617 [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G 2002 Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51:2005–2011 [DOI] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI 1996 Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR 2006 A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291:E381–E387 [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR 2004 Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286:E321–E328 [DOI] [PubMed] [Google Scholar]
- Bergström J, Fürst P, Norée LO, Vinnars E 1974 Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol 36:693–697 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Caumo A, Caloni M, Testolin G, Luzi L 2001 Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J Clin Endocrinol Metab 86:4776–4781 [DOI] [PubMed] [Google Scholar]
- Möller-Loswick AC, Bennegård K, Lundholm K 1991 The forearm and leg perfusion techniques in man do not give the same metabolic information. Clin Physiol 11:385–395 [DOI] [PubMed] [Google Scholar]
- Magnan C, Cruciani C, Clément L, Adnot P, Vincent M, Kergoat M, Girard A, Elghozi JL, Velho G, Beressi N, Bresson JL, Ktorza A 2001 Glucose-induced insulin hypersecretion in lipid-infused healthy subjects is associated with a decrease in plasma norepinephrine concentration and urinary excretion. J Clin Endocrinol Metab 86:4901–4907 [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Barrett EJ, Bevilacqua S, Jacob R, Walesky M, Sherwin RS, DeFronzo RA 1986 Effect of free fatty acids on blood amino acid levels in human. Am J Physiol 250:E686–E694 [DOI] [PubMed] [Google Scholar]
- Balent B, Goswami G, Goodloe G, Rogatsky E, Rauta O, Nezami R, Mints L, Angeletti RH, Stein DT 2002 Acute elevation of NEFA causes hyperinsulinemia without effect on insulin secretion rate in healthy human subjects. Ann NY Acad Sci 967:535–543 [DOI] [PubMed] [Google Scholar]
- El-Atat FA, Stas SN, McFarlane SI, Sowers JR 2004 The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol 15:2816–2827 [DOI] [PubMed] [Google Scholar]
- Hsu IR, Kim SP, Kabir M, Bergman RN 2007 Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr 86:s867–871 [DOI] [PubMed] [Google Scholar]
- Kaplan NM 1989 The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med 149:1514–1520 [DOI] [PubMed] [Google Scholar]
- Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS 2006 Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab 291:E729–E736 [DOI] [PubMed] [Google Scholar]
- Haymond MW, Tessari P, Beaufrere B, Rodriguez N, Bailey J, Miles JM 1988 Effects of parenteral lipid on leucine metabolism: dependence of fatty acid chain length. JPEN J Parenter Enteral Nutr 12(Suppl 6):94S–97S [DOI] [PubMed] [Google Scholar]
- Magnan C, Collins S, Berthault MF, Kassis N, Vincent M, Gilbert M, Pénicaud L, Ktorza A, Assimacopoulos-Jeannet F 1999 Lipid infusion lowers sympathetic nervous activity and leads to increased β-cell responsiveness to glucose. J Clin Invest 103:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'homme M, Balage M, Debras E, Farges MC, Kimball S, Jefferson L, Grizard J 2005 Differential effects of insulin and dietary amino acids on muscle protein synthesis in adult and old rats. J Physiol 563:235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ 2008 Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295:E595–E604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Shantz LM, Horetsky RL, Jefferson LS 1999 Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem 274:11647–11652 [DOI] [PubMed] [Google Scholar]