Abstract
Context: It is unclear whether the pattern of GH delivery to peripheral tissues has important effects.
Objective: The aim of the study was to compare the effects of pulsatile vs. continuous administration of GH upon metabolic and IGF-I parameters in obese subjects.
Setting: The study was conducted at the General Clinical Research Center at the University of Michigan Medical Center.
Participants: Four men and five women with abdominal obesity (body mass index, 33 ± 3 kg/m2; body fat, 40 ± 3%) participated in the study.
Intervention: GH (0.5 mg/m2 · d) was given iv for 3 d as: 1) continuous infusion (C); and 2) pulsatile boluses (P) (15% of the dose at 0700, 1300, and 1800 h and 55% at 2400 h). These trials were preceded by a basal period (B) when subjects received normal saline.
Main Outcome Measures: Rate of lipolysis and hepatic glucose production were evaluated using stable isotope tracer techniques. The composite index of insulin sensitivity (Matsuda index) was assessed using oral glucose tolerance test.
Results: The increase in plasma IGF-I concentrations was greater (P < 0.05) with continuous GH infusion (211 ± 31, 423 ± 38, and 309 ± 34 μg/liter for B, C, and P, respectively). Muscle IGF-I mRNA was significantly increased (P < 0.05) only after the continuous GH infusion (1.2 ± 0.4, 4.4 ± 1.3, and 2.3 ± 0.6 arbitrary units, for B, C, and P, respectively). Only pulsatile GH augmented the rate of lipolysis (4.1 ± 0.3, 4.8 ± 0.7, and 7.1 ± 1.1 μmol/kg · min for B, C, and P, respectively). GH had no effect on hepatic glucose production, but both modes of GH administration were equally effective in impairing insulin sensitivity.
Conclusion: These findings indicate that, in obese subjects, discrete components of GH secretory pattern may differentially affect IGF-I generation and lipolytic responses.
In obese subjects, GH regulated different physiological parameters (e.g., lipolytic rate, IGF-1 production) in a tissue- and pattern-specific manner.
GH secretion is pulsatile in nature. These pulses result from acute episodic release of GH from the pituitary and typically account for the majority of the GH secreted during the day. In healthy, lean adults, 3 to 6 GH pulses are detected each day (1). Although there is usually a dominant rhythmicity to the daily GH pulses overall, the frequency and amplitude of these pulses are quite variable between individuals (2). In between these pulses, GH levels are low and relatively stable. This tonic “inter-pulse,” or “basal,” GH secretion accounts for a relatively small portion of daily GH output.
Recent studies have demonstrated sex and age dependence of hypothalamic GHRH secretion (3,4,5) and differential gender sensitivity of GH to IGF-I-negative feedback (5). Also, relative contributions of GHRH and somatostatin are important factors determining the GH secretory patterns. However, it is still largely unknown whether differences in the pattern of GH presentation to the peripheral tissues or, more specifically, the relative contributions of basal vs. pulsatile components play an important metabolic role in humans. In rats, GH pulses were suggested to be selectively responsible for the bone/muscle IGF-I mRNA generation and for somatic growth (6), hepatic epidermal growth factor receptor mRNA (7), and STAT5b activation (8) and to determine sex difference in the predisposition to thrombosis (9). In contrast, continuous GH component appears to be predominant in inducing hepatic IGF-I mRNA (6), CYP 3A4 enzymes (10,11), and GH receptors (12). In humans, GH is known to have multiple effects on peripheral tissues, such as promoting somatic growth through generation of IGF-I (6), increasing lipolysis (13), and having antiinsulin effects (14). However, whether these actions were mediated through global GH output or through a pattern-specific exposure of GH pulsatility is largely unknown. Cersosimo et al. (13) found minor (∼15%) activation of lipolysis after continuous GH infusion but far more robust lipolytic activation with pulsatile GH delivery in lean humans. However, their model of GH administration during somatostatin/insulin clamp resulted in hypoinsulinemia and grossly unphysiological GH profiles. It is not clear whether the pattern of GH exposure differentially affects lipolytic rate in obese adults, who have intrinsically elevated plasma insulin concentrations. In addition, to our knowledge the effects of the pattern of GH exposure on hepatic glucose production and insulin sensitivity have not been thoroughly explored. Therefore, the purpose of this study was to determine the effects of the pattern of GH presentation to the peripheral tissues on growth-promoting and metabolic effects of GH in obesity. In addition to the clinical relevance of improving our understanding about the regulation of key metabolic processes in obesity, it is also important to note that obese subjects serve as an informative model for understanding of GH physiology because they have impoverishment of GH secretion and are, thus, functionally GH deficient (15).
Subjects and Methods
Subjects
Nine obese subjects (four men and five women; body weight, 99 ± 1 kg; body mass index, 33 ± 3 kg/m2; percentage body fat, 40 ± 3; fat mass, 39 ± 1 kg; fat free mass, 60 ± 2 kg; age, 33 ± 8 yr) participated in this study. Other than their excessive body weight, all subjects were in good general health and had no comorbidities (e.g. cardiovascular disease, diabetes, hypertension, or hyperlipidemia). No subjects were taking medications. All women were studied in the follicular phase of their menstrual cycle, and no subjects were pregnant as assessed using a urine pregnancy test both at screening and with each admission to the hospital. The study was approved by the Institutional Review Board of the University of Michigan. Written informed consent was obtained from all participants.
Modeling physiological GH patterns
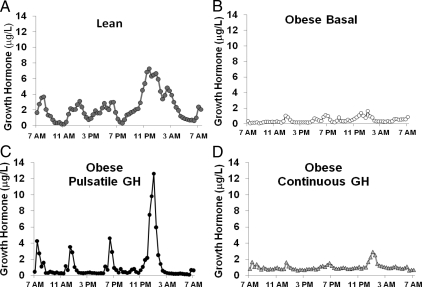
Because the object of this study was to determine the physiological/metabolic roles of GH pulses vs. the interpulse GH concentration, accurate modeling of both components was essential. To this end, we used GH concentration profiles obtained every 20 min for 24 h from healthy, lean individuals. These profiles were obtained in a total of 25 young (25 ± 1 yr), lean (body mass index, 23 ± 0.5 kg/m2), and healthy volunteers (13 men and 12 women) who were not taking any medications. The average 24-h GH profile for these lean subjects is presented in Fig. 1A. The mean 24-h GH concentration for the entire lean group was 2.3 ± 0.2 μg/liter (range, 0.5–5.3 μg/liter). Similar to our previous data (2), we discerned four dominant, average GH peaks with the maxima of 3.7 ± 1.5 μg/liter at 0820 h, 3.1 ± 1.1 μg/liter at 1320 h, 3.0 ± 1.3 μg/liter at 1820 h, and 6.7 ± 1.4 μg/liter at 0040 h. The interpeak GH concentrations were low in our lean subjects, ranging from 0.4 ± 0.1 to 2.2 ± 0.9 μg/liter at different time-points. We used these parameters from our lean subjects (i.e. mean 24-h GH concentration, number and magnitude of GH peaks, and the tonic GH concentrations) as a model to mimic “lean/young” GH profiles in our obese subjects by infusing exogenous GH in either a pulsatile or continuous manner.
Figure 1.
Mean 24-h GH profile for lean subjects under basal conditions (A), obese subjects under basal/control conditions (B), obese subjects pulsatile GH infusion (C), and obese subjects during continuous GH infusion (D). Relatively minor variability in timing of the GH pulses among the lean subjects distorts the GH profile to make the peak shapes more broad and less defined. se bars have been removed for clarity.
Experimental design
Obese subjects were admitted to the General Clinical Research Center (GCRC) at the University of Michigan Medical Center on two separate occasions. During one visit, subjects were admitted for 5 consecutive days. During the first 24 h of this 5-d admission, plasma GH concentrations were measured every 20 min. The next morning (after an overnight fast), we used stable-isotope tracer infusion techniques to assess rates of lipolysis ([d5]-glycerol rate of appearance [Ra]in plasma) and hepatic glucose production ([d2]-glucose Ra), as described previously (16,17). In addition, we obtained muscle biopsy samples (at 0800 and 1200 h) to measure IGF-I mRNA abundance in the muscle biopsy samples using quantitative real-time PCR as described previously (16). The last muscle biopsy was followed by a 2-h oral glucose tolerance test (OGTT). After these baseline metabolic measurements, we administered GH (0.5 mg/m2 · d) for the next 3 d either as: 1) a continuous infusion; or 2) pulsatile boluses (15% of the total daily dose at 0700, 1300, and 1800 h, and 55% at 2400 h). The resultant GH profiles were reexamined during the third day of GH administration. The battery of metabolic measurements (i.e. stable-isotope infusions, muscle biopsies, OGTT) were repeated in the morning after 3 d of GH treatment. During the other hospital visit, subjects were admitted for a total of 3 d, during which they received the same dose of GH (0.5 mg/m2 · d), but the pattern of GH administration (i.e. continuous or pulsatile) was opposite to that received during their other trial. The order of the pattern of GH administration was randomized, and the results were compared with those obtained during a single basal study. Throughout each hospital visit, subjects were provided with an isoenergetic diet [120% of basal metabolic rate, which was measured using indirect calorimetry (Delta Trac; SensorMedics, Yorba Linda, CA)]. The macronutrient content of the diet was 50% carbohydrate, 15% protein, and 35% fat, and the meals were divided between breakfast (0700 h), lunch (1300 h), and dinner (1900 h) with no intervening snacks. Subjects were required to stay within the GCRC throughout their entire admission, and as such physical activity was controlled and limited. The subjects were carefully observed for compliance.
Analytical methods
Plasma GH, IGF-I, and insulin concentrations were measured by immunoluminometric assay (ILMA; Quest Diagnostics, Madison, NJ) and RIA (Diagnostics Systems Laboratories Inc., Webster, TX), respectively. Tracer enrichment analysis was performed via gas chromatography/mass spectrometry as described by Wolfe (18) with slight modifications.
Calculations
Whole-body plasma glycerol Ra and glucose Ra were determined from the steady-state isotope dilution equation (Steele equation): Ra = I/TTRA (19), where I is the tracer infusion rate (μmol · kg−1 · min−1) and TTRA is the tracer-to-tracee ratio (TTR) of [d5]-glycerol or [d2]-glucose in the arterial sample. Because these measurements are performed under basal conditions at metabolic steady state, Ra = Rd.
Total body insulin sensitivity was assessed with Matsuda’s Composite Index (20), using plasma glucose and insulin concentrations measured during the OGTT.
Statistical analysis
Discrete parameters of GH concentration profiles were established by Cluster (1). A one-way ANOVA for repeated measures with Tukey post hoc analysis was used to assess statistical differences in the major endpoints among the different GH treatments (i.e. baseline, continuous GH administration, and pulsatile GH administration). The same statistical analysis was used to assess sex-related differences between our male and female participants. Statistical analysis was performed using Sigmastat for Windows (version 3.1; Systat Software Inc., Point Richmond, CA) and Excel 2000 (Microsoft Corp., Redmond, WA). Data are shown as mean ± sem. Statistical significance was defined as P < 0.05.
Results
Plasma GH profiles
Mean spontaneous 24-h GH concentrations in obese subjects were lower than in lean controls (0.4 ± 0.1 vs. 2.3 ± 0.3 μg/liter; P < 0.001; Fig. 1, A and B). In the obese subjects, only the nocturnal endogenous GH pulse could be discerned with certainty (Fig. 1B). Otherwise, marked GH pulses were not evident, and most of the plasma GH concentrations remained very low (0.1–0.3 μg/liter). Pulsatile administration of GH to obese subjects more than doubled their 24-h mean plasma GH concentration (1.3 ± 0.3 μg/liter) to within the lower part of the range of GH concentrations observed in the control group of young, lean, and healthy individuals, and four distinct GH peaks were observed (Fig. 1C). These artificial GH pulses closely resembled the pattern of the major naturally occurring dominant (average) GH peaks seen in lean subjects in terms of timing and magnitude. The interpulse GH concentrations during the pulsatile GH infusion trials were 0.7 ± 0.2 μg/liter, similar to lean controls, and higher than the tonic GH levels in untreated obese subjects. Continuous administration of GH to obese subjects also more than doubled their 24-h mean plasma GH concentration (1.0 ± 0.1 μg/liter) and quadrupled their interpulse GH levels (Fig. 1D). Therefore, the modes of GH administration employed in this study were able to selectively recapitulate the average tonic and the pulsatile GH secretory components observed in lean individuals while keeping the mean daily GH milieu within the normal physiological range. Additional data comparing the GH profiles for obese and lean subjects is presented in Table 1.
Table 1.
Cluster analysis of GH profiles for obese vs. lean subjects
| Obese basal (no GH) | Obese + continuous GH | Obese + pulsatile GH | Lean | |
|---|---|---|---|---|
| Mean 24-h GH concentration (μg/liter) | 0.4 ± 0.1 | 1.0 ± 0.1a | 1.3 ± 0.3a | 2.3 ± 0.3a |
| Mean daytime GH peak amplitude (μg/liter) | 0.8 ± 0.2 | 2.0 ± 0.9 | 5.3 ± 0.5a | 5.9 ± 0.9a,b |
| Mean nocturnal GH peak amplitude (μg/liter) | 1.9 ± 0.4 | 1.8 ± 1.2 | 9.4 ± 2.1a | 13.3 ± 2.2a,b |
| Mean interpulse GH concentration (μg/liter) | 0.2 ± 0.1 | 0.9 ± 0.1a | 0.7 ± 0.2a | 0.7 ± 0.1a |
Values are means ± se. Daytime = 0700–2100 h; nocturnal = 2100–0700 h.
Significantly different than ″obese basal″; P ≤ 0.01.
Significantly different than ″obese + continuous GH″; P ≤ 0.015.
IGF-I in plasma and skeletal muscle
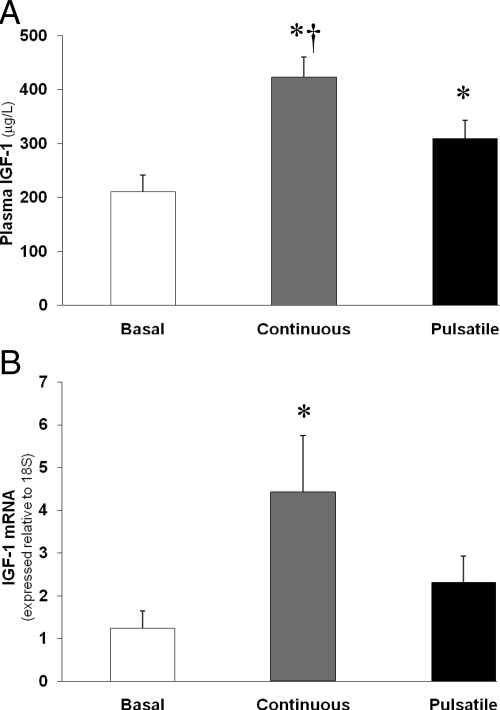
Pulsatile administration of exogenous GH in our obese subjects significantly increased their plasma IGF-I concentrations (Fig. 2A; P < 0.05) as well as their IGF-I mRNA abundance in skeletal muscle (Fig. 2B; P < 0.05). Importantly, continuous GH administration was about twice as effective at increasing both plasma IGF-I concentration and IGF-I mRNA in muscle compared with pulsatile GH infusion (both P < 0.05).
Figure 2.
Plasma IGF-I concentration (A) and IGF-I mRNA abundance (B) in skeletal muscle during basal/control conditions, continuous GH infusion, and pulsatile GH infusion. *, Significantly different than basal conditions, P < 0.05. †, Significantly different from pulsatile, P < 0.05.
Substrate metabolism
Whole-body lipolytic rate
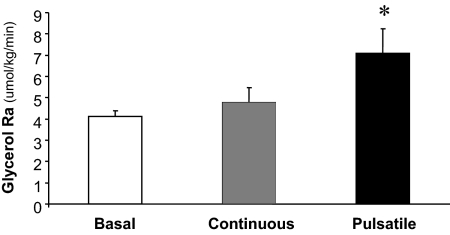
Without exogenous GH administration, the rate of whole-body lipolysis (i.e. glycerol Ra) after an overnight fast was 4.1 ± 0.3 μmol/kg · min. Continuous GH administration was ineffective at increasing lipolytic rate (4.8 ± 0.7 μmol/kg · min), but pulsatile GH administration nearly doubled the rate of lipolysis (7.1 ± 1.1 μmol/kg · min; Fig. 3; P < 0.05).
Figure 3.
Glycerol rate of appearance in plasma (Ra), an index of whole-body lipolytic rate, during basal/control conditions, continuous GH infusion, and pulsatile GH infusion. *, Significantly different than basal conditions, P < 0.05.
Glucose metabolism
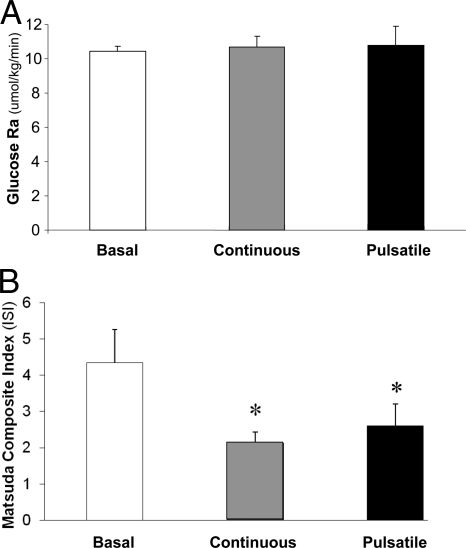
Neither continuous nor pulsatile GH administration altered hepatic glucose production (i.e. glucose Ra) (Fig. 4A), and both modes of GH administration equally decreased insulin sensitivity compared with basal measures (P < 0.05 for both), as assessed using the Matsuda’s Composite Index (Fig. 4B).
Figure 4.
Glucose Ra, an index of hepatic glucose production (A) and Matsuda Composite index, as a marker for insulin sensitivity (B) during basal/control conditions, continuous GH infusion, and pulsatile GH infusion. *, Significantly different than basal conditions, P < 0.05.
Gender-specific analysis
Despite the small number of men (n = 4) and women (n = 5) in our study, we attempted to see whether there might be gender-related differences in hormonal and metabolic responses to either pulsatile or continuous GH administration. No gender differences were found in responses of IGF-I, IGF-I mRNA, hepatic glucose production, or insulin sensitivity as measured by Matsuda’s Index. However, we found the lipolytic rate during the pulsatile GH administration to be greater in men compared with women (9.9 ± 0.8 vs. 4.8 ± 0.7 μmol/kg · min; P < 0.05).
Discussion
In this study, we investigated the effects of pulsatile vs. continuous administration of GH in a model of human obesity, a well-documented GH-impoverished state (15). The dose of exogenous GH used in this study raised mean 24-h GH concentrations in our obese subjects into the lower part of the normal range for lean adults, and the pulsatile mode of administration mimicked the dominant pattern of GH concentrations observed in young, healthy, lean individuals. Similarly, the continuous infusion of GH resulted in steady plasma GH concentrations closely resembling tonic component of GH pulsatility in young and lean controls. Therefore, in contrast to other studies assessing GH effects after once-daily injections (21,22,23,24), our model closely approached normal physiology. The major overall findings from our study indicate that GH differentially affects various physiological processes in a tissue- and pattern-specific mode.
The best-studied effect of GH is its ability to increase production of IGF-I in different organs and tissues and thereby to promote somatic growth (6). Most of the circulating IGF-I is of hepatic origin (25), but somatic growth is largely due to the autocrine/paracrine generation of IGF-I because selective abrogation of IGF-I production by the liver did not affect growth rate in transgenic animals (25). These observations were made in rodents and were further supported by the finding that the rate of growth was parallel to the expression of IGF-I mRNA in the muscle and bone (6). Importantly, only pulsatile administration of GH increased muscle and bone tissue IGF-I mRNA abundance in previous studies performed in rodents (6). In contrast, studies performed in hypopituitary humans suggested that a continuous GH administration might be more effective than pulsatile GH injections at increasing circulating (i.e. hepatic) IGF-I concentrations (26). In contrast, in an identical model employed by Jørgensen et al. (27), both continuous and frequent pulsatile modes of GH administration were equally effective in increasing plasma IGF-I concentrations, whereas two large GH boluses were less effective.
We show that continuous GH administration recapitulating the basal, tonic GH levels in lean healthy subjects was more effective than pulsatile injections of the same daily dose. In contrast to the data in rodents (6), we found that the continuous GH administration was also more effective than the pulsatile GH pattern at increasing muscle IGF-I mRNA. Therefore, our data point to a major difference between the rodent and human models and may have major implications for the development of therapeutic strategies of GH administration to promote somatic growth: the formulations that maintain elevated steady-state GH levels over long periods of time may have improved growth-promoting efficacy, without requiring supraphysiological GH doses. Our findings may also help explain the clinical syndrome of acromegaly in patients with apparently normal mean daily GH concentrations because their tonic component of GH secretion is markedly and selectively augmented, in association with excessively high plasma IGF-I concentrations and exaggerated somatic growth (28).
To date, the metabolic effects of GH in humans have been primarily studied in normal and hypopituitary adults during either chronic once-daily sc GH administration or in short-term studies during continuous iv GH infusions (29,30). In these previous studies, GH was shown to be important for augmenting lipolysis, and the resultant increase in fatty acid availability has been suggested to be a key contributor to protein conservation and induction of insulin resistance during fasting (30,31). However, the doses of GH used in these earlier studies were excessively high, resulting in mean GH concentrations two to four times the physiological levels, and the mix of GH bolus injections superimposed on continuous GH infusion in these studies prevented the ability to assess separate contributions of discrete components of GH secretion profiles (i.e. GH pulses vs. interpulse GH levels) on these metabolic processes. A study by Cersosimo et al. (13) attempted to address this issue by administering GH in a pulsatile vs. a continuous pattern in healthy lean subjects during a short-term “pituitary-pancreatic clamp” (i.e. continuous infusion of somatostatin and insulin). However, interpretation of their findings is complicated by the suppression of insulin secretion during the clamp and by a nonphysiological replication of GH pulsatility (13).
Our study employed a pulsatile mode of GH delivery that resulted in circulating GH profiles that closely reproduced those found in healthy, lean individuals. We show here that only pulsatile GH delivery stimulated lipolysis in humans, whereas the continuous mode was virtually ineffective. These data also fully support the results of our earlier study (32) in which inhibition of GH pulsatility by GHRH receptor antagonist abolished the fasting-induced increase in the rate of lipolysis despite exceptionally low plasma insulin concentrations. Interestingly, the higher lipolytic rate in response to the pulsatile GH infusion in our male subjects compared with our female subjects disagrees with previous work from Cersosimo et al. (13) in which they reported no apparent difference in palmitate flux between their male and female participants in response to pulsatile GH infusion. The reason for this discrepancy is unclear, but the small numbers of male and female subjects in our present study and in the study by Cersosimo et al. (13) warrant further investigation to explore potential sex-related differences in the metabolic responses to pulsatile GH exposure.
The diabetogenic effect of GH in humans is a well-established metabolic phenomenon. This is primarily manifest as an induction of insulin resistance (33). Indeed, in our experiments, hepatic glucose production was not affected by GH, similar to our earlier data in fed humans (32). Some evidence of the stimulatory GH effects on hepatic glucose production was found in starvation (32), after abolition of gross GH excess in acromegaly (34,35), or after supraphysiological, long-term GH exposure (36), but the relevance of these models to normal physiology is uncertain. However, both continuous and pulsatile modes of GH administration were equipotent in lowering insulin sensitivity as measured by the Matsuda Composite Index, which was shown to correlate extremely well with the rate of whole-body glucose disposal as measured by the euglycemic insulin clamp (21). Holck et al. (37) have previously shown that muscle biopsy by itself can diminish insulin sensitivity. In our study, estimation of insulin sensitivity by OGTT on all occasions was done at the same time of day, after identical exposure to two consecutive muscle biopsies and to either naturally occurring major GH increase or artificial GH pulse of the same amplitude. Therefore, it is the total GH output, rather than the manner of GH administration to humans that is responsible for the worsening of glucose homeostasis. It follows that the diminished GH pulsatility in obesity is not an inherently deleterious phenomenon, but may be rather an adaptive or protective mechanism. Indeed, an increase in the mean GH concentrations even to the low-normal “lean” values was accompanied by worsened insulin resistance, a prelude to the development of diabetes mellitus. This is in complete agreement with the development of insulin resistance or frank diabetes in GH-deficient humans treated with exogenous GH (38).
In conclusion, we have shown that the manner of GH presentations to the peripheral tissues is an important parameter of GH action and is independent of the average daily GH milieu within the physiological range. The continuous GH administration (and by inference, the tonic component of GH secretion) is largely responsible for increasing plasma IGF-I concentrations, likely by augmenting hepatic IGF-I production (25). Similarly, it is also predominant in increasing muscle IGF-I mRNA concentrations. In contrast, only pulsatile GH administration (and by inference, the pulsatile component of GH secretion) augments adipose tissue lipolysis. Both components are equally effective at worsening insulin resistance, but neither affects hepatic glucose production, at least in the nonfasting state. Our results suggest that the decline in GH pulsatility that occurs in obesity (as well as with aging) is not an inherently undesirable effect, but rather may be an adaptive mechanism protecting against excessive lipolytic rates to avoid insulin resistance and the development of type 2 diabetes. The largely preserved tonic component of GH secretory patterns in these conditions (3,15) helps in maintaining hepatic and skeletal tissue production of IGF-I, which at least partially counteracts the global decline of GH milieu. Most importantly, our data demonstrate that the biological effects of GH in obese humans are not determined merely by the overall magnitude of GH output. Rather, the complex pulsatile pattern of GH presentation to the peripheral tissues may encode within it a set of messages that determine growth-promoting and metabolic effects in a pattern- and tissue-specific manner. It is intriguing to speculate that this may help explain the evolutionary basis for the regulation of GH secretion by two separate hypothalamic hormones, somatostatin and GHRH, each preferentially regulating basal and pulsatile components of GH release.
Acknowledgments
We are thankful to Dr. Alexander Hinko and Amy Kaufman for their technical assistance and expertise. We are also very grateful to the nursing and dietary staff of the General Clinical Research Center at The University of Michigan Hospital for the excellent assistance.
Footnotes
This study was supported by National Institutes of Health Grant R01 DK071955 (to J.F.H. and A.L.B.), the DVA Merit Review Program (to A.L.B.), the University of Michigan General Clinical Research Center (UL1RR024986), and training Fellowships funded by Pfizer (to S.S.) and Genentech (N.G.).
Disclosure Summary: The authors have nothing to declare.
First Published Online May 26, 2009
Abbreviations: OGTT, Oral glucose tolerance test; Ra, rate of appearance.
References
- Ho PJ, Friberg RD, Barkan AL 1992 Regulation of pulsatile growth hormone secretion by fasting in normal subjects and patients with acromegaly. J Clin Endocrinol Metab 75:812–819 [DOI] [PubMed] [Google Scholar]
- Surya S, Symons K, Rothman E, Barkan AL 2006 Complex rhythmicity of growth hormone secretion in humans. Pituitary 9:121–125 [DOI] [PubMed] [Google Scholar]
- Russell-Aulet M, Dimaraki EV, Jaffe CA, DeMott-Friberg R, Barkan AL 2001 Aging-related growth hormone (GH) decrease is a selective hypothalamic GH-releasing hormone pulse amplitude mediated phenomenon. J Gerontol A Biol Sci Med Sci 56:M124–M129 [DOI] [PubMed] [Google Scholar]
- Russell-Aulet M, Jaffe CA, Demott-Friberg R, Barkan AL 1999 In vivo semiquantification of hypothalamic growth hormone-releasing hormone (GHRH) output in humans: evidence for relative GHRH deficiency in aging. J Clin Endocrinol Metab 84:3490–3497 [DOI] [PubMed] [Google Scholar]
- Jaffe CA, Ocampo-Lim B, Guo W, Krueger K, Sugahara I, DeMott-Friberg R, Bermann M, Barkan AL 1998 Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest 102:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgaard J, Carlsson L, Isaksson OG, Jansson JO 1988 Pulsatile intravenous growth hormone (GH) infusion to hypophysectomized rats increases insulin-like growth factor I messenger ribonucleic acid in skeletal tissues more effectively than continuous GH infusion. Endocrinology 123:2605–2610 [DOI] [PubMed] [Google Scholar]
- Ekberg S, Carlsson L, Carlsson B, Billig H, Jansson JO 1989 Plasma growth hormone pattern regulates epidermal growth factor (EGF) receptor messenger ribonucleic acid levels and EGF binding in the rat liver. Endocrinology 125:2158–2166 [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Miles GD, Wauthier V, Holloway MG, Zhang X, Hodor P, Ray WJ, Waxman DJ 2007 Role of STAT5a in regulation of sex-specific gene expression in female but not male mouse liver revealed by microarray analysis. Physiol Genomics 31:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JH, Dukes J, Levy RE, Sos B, Mason SE, Fong TS, Weiss EJ 2008 Sex differences in thrombosis in mice are mediated by sex-specific growth hormone secretion patterns. J Clin Invest 118:2969–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C 2006 Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20:2613–2629 [DOI] [PubMed] [Google Scholar]
- Choi HK, Waxman DJ 2000 Pulsatility of growth hormone (GH) signalling in liver cells: role of the JAK-STAT5b pathway in GH action. Growth Horm IGF Res 10(Suppl B):S1–S8 [DOI] [PubMed] [Google Scholar]
- Bick T, Hochberg Z, Amit T, Isaksson OG, Jansson JO 1992 Roles of pulsatility and continuity of growth hormone (GH) administration in the regulation of hepatic GH-receptors, and circulating GH-binding protein and insulin-like growth factor-I. Endocrinology 131:423–429 [DOI] [PubMed] [Google Scholar]
- Cersosimo E, Danou F, Persson M, Miles JM 1996 Effects of pulsatile delivery of basal growth hormone on lipolysis in humans. Am J Physiol 271:E123–E126 [DOI] [PubMed] [Google Scholar]
- Glenn KC, Rose KS, Krivi GG 1988 Somatotropin antagonism of insulin-stimulated glucose utilization in 3T3–L1 adipocytes. J Cell Biochem 37:371–383 [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Lizarralde G, Veldhuis JD 1991 Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab 73:1081–1088 [DOI] [PubMed] [Google Scholar]
- Harber MP, Schenk S, Barkan AL, Horowitz JF 2005 Effects of dietary carbohydrate restriction with high protein intake on protein metabolism and the somatotropic axis. J Clin Endocrinol Metab 90:5175–5181 [DOI] [PubMed] [Google Scholar]
- Harber MP, Schenk S, Barkan AL, Horowitz JF 2005 Alterations in carbohydrate metabolism in response to short-term dietary carbohydrate restriction. Am J Physiol Endocrinol Metab 289:E306–E312 [DOI] [PubMed] [Google Scholar]
- Wolfe RR 1992 Radioactive and stable isotope tracers in biomedicine: principles and practice of kinetic analysis. 1st ed. New York: Wiley-Liss [Google Scholar]
- Steele R 1959 Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82:420–430 [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- Trepp R, Flück M, Stettler C, Boesch C, Ith M, Kreis R, Hoppeler H, Howald H, Schmid JP, Diem P, Christ ER 2008 Effect of GH on human skeletal muscle lipid metabolism in GH deficiency. Am J Physiol Endocrinol Metab 294:E1127–E1134 [DOI] [PubMed] [Google Scholar]
- Yuen KC, Dunger DB 2007 Therapeutic aspects of growth hormone and insulin-like growth factor-I treatment on visceral fat and insulin sensitivity in adults. Diabetes Obes Metab 9:11–22 [DOI] [PubMed] [Google Scholar]
- Laursen T, Jørgensen JO, Jakobsen G, Hansen BL, Christiansen JS 1995 Continuous infusion versus daily injections of growth hormone (GH) for 4 weeks in GH-deficient patients. J Clin Endocrinol Metab 80:2410–2418 [DOI] [PubMed] [Google Scholar]
- Laursen T, Gravholt CH, Heickendorff L, Drustrup J, Kappelgaard AM, Jørgensen JO, Christiansen JS 2001 Long-term effects of continuous subcutaneous infusion versus daily subcutaneous injections of growth hormone (GH) on the insulin-like growth factor system, insulin sensitivity, body composition, and bone and lipoprotein metabolism in GH-deficient adults. J Clin Endocrinol Metab 86:1222–1228 [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D 1999 Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96:7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins PB 2002 Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab 283:E1008–E1015 [DOI] [PubMed] [Google Scholar]
- Jørgensen JO, Møller N, Lauritzen T, Christiansen JS 1990 Pulsatile versus continuous intravenous administration of growth hormone (GH) in GH-deficient patients: effects on circulating insulin-like growth factor-I and metabolic indices. J Clin Endocrinol Metab 70:1616–1623 [DOI] [PubMed] [Google Scholar]
- Dimaraki EV, Jaffe CA, DeMott-Friberg R, Chandler WF, Barkan AL 2002 Acromegaly with apparently normal GH secretion: implications for diagnosis and follow-up. J Clin Endocrinol Metab 87:3537–3542 [DOI] [PubMed] [Google Scholar]
- Møller N, Jørgensen JOL 2009 Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Jørgensen JO, Hartmund T, Nørrelund H, Nair KS, Christiansen JS, Møller N 2002 Effects of lowering circulating free fatty acid levels on protein metabolism in adult growth hormone deficient patients. Growth Horm IGF Res 12:425–433 [DOI] [PubMed] [Google Scholar]
- Nørrelund H, Nair KS, Nielsen S, Frystyk J, Ivarsen P, Jørgensen JO, Christiansen JS, Møller N 2003 The decisive role of free fatty acids for protein conservation during fasting in humans with and without growth hormone. J Clin Endocrinol Metab 88:4371–4378 [DOI] [PubMed] [Google Scholar]
- Sakharova AA, Horowitz JF, Surya S, Goldenberg N, Harber MP, Symons K, Barkan A 2008 Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J Clin Endocrinol Metab 93:2755–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C, Gormsen LC, Jessen N, Pedersen SB, Møller N, Lund S, Jørgensen JO 2008 Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab 93:2842–2850 [DOI] [PubMed] [Google Scholar]
- Lindberg-Larsen R, Møller N, Schmitz O, Nielsen S, Andersen M, Orskov H, Jørgensen JO 2007 The impact of pegvisomant treatment on substrate metabolism and insulin sensitivity in patients with acromegaly. J Clin Endocrinol Metab 92:1724–1728 [DOI] [PubMed] [Google Scholar]
- Höybye C, Chandramouli V, Efendic S, Hulting AL, Landau BR, Schumann WC, Wajngot A 2008 Contribution of gluconeogenesis and glycogenolysis to hepatic glucose production in acromegaly before and after pituitary microsurgery. Horm Metab Res 40:498–501 [DOI] [PubMed] [Google Scholar]
- Hannon TS, Danadian K, Suprasongsin C, Arslanian SA 2007 Growth hormone treatment in adolescent males with idiopathic short stature: changes in body composition, protein, fat, and glucose metabolism. J Clin Endocrinol Metab 92:3033–3039 [DOI] [PubMed] [Google Scholar]
- Holck P, Pørksen N, Nielsen MF, Nyholm B, Bak JF, Andreasen F, Møller N, Schmitz O 1994 Effect of needle biopsy from the vastus lateralis muscle on insulin-stimulated glucose metabolism in humans. Am J Physiol 267:E544–E548 [DOI] [PubMed] [Google Scholar]
- Rosenfalck AM, Maghsoudi S, Fisker S, Jørgensen JO, Christiansen JS, Hilsted J, Vølund AA, Madsbad S 2000 The effect of 30 months of low-dose replacement therapy with recombinant human growth hormone (rhGH) on insulin and C-peptide kinetics, insulin secretion, insulin sensitivity, glucose effectiveness, and body composition in GH-deficient adults. J Clin Endocrinol Metab 85:4173–4181 [DOI] [PubMed] [Google Scholar]