Abstract
Context: In postmenopausal women, bone mineral density (BMD) declines after teriparatide therapy is stopped. The pattern of BMD loss after teriparatide therapy is stopped in men is less clear.
Objective: The aim of the study was to determine whether the pattern of teriparatide-induced bone accrual and post-teriparatide bone loss differs between postmenopausal women and eugonadal men.
Design: We conducted a prospective cohort substudy.
Patients: The study included 14 postmenopausal women and 17 eugonadal men, ages 46–85 yr, with lumbar spine or femoral neck BMD T-scores below −2.
Intervention: Teriparatide (37 μg sc daily) was administered for 24 months, followed by 12 months off therapy.
Main Outcome Measures: We measured BMD at various anatomic sites by dual-energy x-ray absorptiometry, trabecular spine BMD by quantitative computed tomography, and bone turnover markers during the treatment and observation periods. The response to teriparatide administration and discontinuation was compared between females and males.
Results: BMD of the spine, femoral neck, total hip, and trabecular spine increased similarly during the treatment period in men and women, whereas BMD at the radius was stable in men but decreased by 8.1 ± 3.3% in women (P < 0.0001). After teriparatide was stopped, BMD at the posterior-anterior spine decreased by 7.1 ± 3.8% in women and by 4.1 ± 3.5% in men (P = 0.036). BMD at the total hip and femoral neck decreased by 3.8 ± 3.9 and 3.1 ± 4.3%, respectively, in women but remained stable in men (P < 0.05 for both sites). BMD at the distal radius remained stable in men but increased in women by 1.6 ± 3.1% (P = 0.069).
Conclusions: Teriparatide appears to increase BMD similarly in postmenopausal women and eugonadal men with osteoporosis. After teriparatide is stopped, the decline in BMD is greater in women than in men. If confirmed in larger cohorts, these findings would suggest that the indication for immediate antiresorptive therapy after teriparatide may not be as urgent in men as in women.
This study demonstrates that the response to teriparatide discontinuation differs between osteoporotic men and women.
Osteoporosis affects more than 10 million Americans over the age of 50, with 1.5 million U.S. men and women experiencing an osteoporotic-related fracture each year (1). Although the past decade has seen the approval of several new drugs for the treatment of osteoporosis, teriparatide [human PTH 1–34, hPTH (1–34)] is the only Food and Drug Administration (FDA)-approved therapy that stimulates new bone formation. It potently increases bone mineral density (BMD) in both men and women, particularly at sites of predominantly trabecular bone, and reduces spine and nonspine fracture incidence in postmenopausal women (2). Unlike other osteoporosis therapies, however, teriparatide therapy is limited to a maximum of 24 months due to safety concerns related to the development of osteogenic sarcomas in rodents (3). Also, whereas bisphosphonates accumulate in the skeleton and have persistent effects on bone resorption that can be observed for many months after drug exposure (4,5), the biological activity of teriparatide does not endure in the absence of continued administration. In fact, previous studies have demonstrated that bone loss occurs within 6–12 months after withdrawal of teriparatide or the related compound PTH (1–84) in postmenopausal women (6). Although controlled studies of teriparatide discontinuation in men are not available, uncontrolled data suggest that rapid bone loss may occur in men as well (7). In this study, we sought to compare bone loss and changes in biochemical markers of bone turnover after the maximal approved period of teriparatide therapy in men and women. Because the men were eugonadal and the women were estrogen deficient, we hypothesized that post-teriparatide bone loss would be greater in women than in men.
Subjects and Methods
Study subjects
The subjects described in this report are limited to the men and women who received teriparatide alone as part of our larger study comparing the effect of alendronate, teriparatide, or both on BMD and bone turnover. The details of this protocol, as it relates to male subjects, have been described previously (8). An identical protocol was performed in postmenopausal women. In total, we mailed 120,000 recruitment letters to subjects in the local area, and 575 men and 293 women were interested and eligible after screening with a questionnaire alone. Of these 868 people, 201 subjects remained eligible by BMD and screening laboratory criteria. Forty-nine subjects subsequently decided not to participate and an additional 24 were recruited from our clinic or bone density center, leaving a final cohort of 176 subjects (93 women and 83 men). Seventeen of the 31 women and 17 of the 27 men assigned to the teriparatide-alone group successfully completed the first 30 months of the study. Of the subjects who completed the protocol to 30 months, three women declined to continue to month 42, leaving 31 subjects (17 men and 14 women) who form the basis of this report. The gender-specific baseline characteristics did not differ between the subjects described in this report and those subjects assigned to the teriparatide-alone grouping in the original cohort.
At entry, participants were required to be 46 to 85 yr old and have BMD of the lumbar spine in the posterior-anterior (PA) or lateral projection or the femoral neck at least 2 sd values below the mean of gender-matched young adults (T-score <−2), serum calcium level below 10.6 mg/dl, serum creatinine below 2 mg/dl, serum alkaline phosphatase below 150 U/liter, and serum 25-hydroxyvitamin D above 15 ng/ml. Subjects were also required to have normal serum PTH and TSH levels. In men, the serum testosterone level was required to be at least 270 ng/dl, the lower limit of normal in our assay. Women were required to be at least 5 yr removed from surgical or natural menopause. Subjects with disorders or taking medications known to affect bone metabolism were excluded, as were subjects with nephrolithiasis, peptic ulcer disease, severe reflux esophagitis, significant cardiac disease, or malignancy (with the exception of basal cell skin cancer).
Study protocol
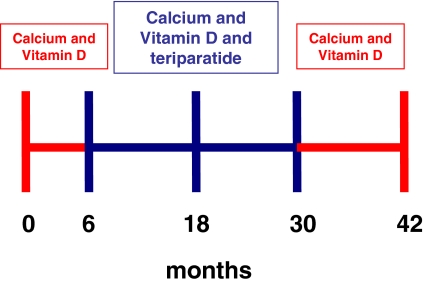
This report describes changes in BMD and bone turnover in the men and women who were assigned to receive teriparatide alone. The protocol timeline is shown in Fig. 1. Calcium intake was estimated by a research dietitian at baseline and periodically during months 0–42 and was maintained at 1000 to 1200 mg daily through diet and/or supplements. Subjects also received 400 IU of vitamin D daily. At the 6-month visit, subjects began self-administration of teriparatide (37 μg sc daily) and continued this therapy for 24 months (to month 30). For the next 12 months (months 30 to 42), subjects received no specific osteoporosis therapy other than calcium and vitamin D supplementation. Blood was collected at baseline and months 1, 2, 3, 6, 7, 8, 9, 12, 18, 24, 30, 36, and 42 to assess serum N-telopeptide (NTX), osteocalcin (OC), and N-terminal propeptide of type 1 collagen (P1NP). Serum calcium was measured before and 4 to 6 h after teriparatide injection. Twenty-four-hour urinary calcium excretion was measured at baseline and every 6 months, as well as 1 month after starting teriparatide (month 7). BMD at the PA spine, femoral neck, total hip, and one third distal radius was measured by dual-energy x-ray absorptiometry (DXA) every 6 months. Trabecular bone density of the lumbar spine was measured by quantitative computed tomography (QCT) at baseline, month 30, and month 42. Compliance with study medications was assessed by diaries and medication counts. The study was approved by the Institutional Review Board of Partners Healthcare and Massachusetts General Hospital. All subjects provided written informed consent.
Figure 1.
Timeline of the treatments administered during the 42-month study period.
Teriparatide preparation and dose adjustments
Good manufacturing practices grade synthetic hPTH (1–34) (Bachem, Inc., Torrence, CA) was vialed as a sterile lyophilized powder (with mannitol, U.S.P.) under good manufacturing practices conditions by Ben-Venue Laboratories (Bedford, OH). Amino acid and high-pressure liquid chromatography analysis of these preparations confirmed their peptide content and stability. The teriparatide dose was reduced by 25% if serum calcium was above 11.5 mg/dl or if the investigators felt that the subject was experiencing a side effect of therapy. If hypercalcemia or symptoms persisted, the teriparatide dose was then reduced by another 25%. If hypercalcemia or symptoms persisted after two dose reductions, teriparatide was discontinued. If the 24-h urinary calcium excretion exceeded 400 mg/d, dietary calcium and/or sodium intake was reduced by 25 to 50%. If hypercalciuria persisted after this dietary intervention, the teriparatide dose was reduced by 25 to 50% as described above. If hypercalciuria persisted after a 50% dose reduction, teriparatide was discontinued.
Measurements of BMD
BMD of the lumbar spine in the PA projection, total hip, femoral neck, and distal one third radius shaft was measured by DXA (QDR 4500A; Hologic, Waltham, MA). For the radius shaft, two measurements were made at each visit, and their mean was used in all analyses. Our short-term in vivo measurement sd values are 0.005, 0.007, and 0.006 g/cm2 for PA spine, femoral neck, and total hip, respectively. Individual vertebrae with obvious deformities or areas of focal sclerosis were excluded from analyses. Trabecular BMD of the lumbar spine was determined by QCT (QCT Lightspeed Plus, GE Medical Systems, Milwaukee, WI). Axial scans of L1–L4 were obtained, and density was determined by comparison to an internal hydroxyapatite standard. The precision error for this technique is 3–5 mg/cm3 (9). All bone density scans were analyzed by individuals blinded to study treatment.
Measurements of bone turnover
Serum NTX was measured using an enzyme-linked immunoassay kit (Wampole Laboratories, Princeton, NJ). Serum P1NP was measured using an RIA kit (Orion Diagnostica, Espoo, Finland). Serum OC was measured using an enzyme-linked immunoassay kit (ALPCO Diagnostics, Windham, NH). For each subject, all samples from months 0 to 30 were assayed together as were all samples from months 30 to 42. Because the kit used to measure OC in samples from months 0 to 30 was modified before the month 30 to 42 samples were assayed, month 30 samples were measured with both kits, and a correction factor was applied to all OC measurements after month 30.
Statistical analysis
The predetermined primary end point for this analysis was the comparison of the difference in the percentage loss in PA spine BMD from month 30 to month 42 in male vs. female subjects. Secondary endpoints included the same analysis for other BMD sites and for biochemical markers of bone turnover. These analyses, as well as analyses of baseline characteristics, were assessed by two-sample t tests. Additionally, gender-specific month 30–42 changes in BMD and biochemical markers of bone turnover were examined using paired t tests. The same statistical methods used to analyze 30- to 42-month changes were then used to analyze the responses to initial therapy (month 0–30). We also performed an adjusted analysis for the significant gender differences initially detected by the above univariate analyses by analysis of covariance. Baseline age, body weight, and serum 25-hydroxyvitamin D level were included in this analysis.
All P values are two-sided. Values less than or equal to 0.05 are considered statistically significant. Unless otherwise noted, data are presented as the mean ± sd in the text and mean ± se in figures. Statistical analyses were performed using SAS version 9.1 for Windows (SAS Institute, Cary, NC).
Results
Characteristics of the subjects
The baseline characteristics of the 31 subjects are shown in Table 1. Male subjects were significantly younger and heavier and ingested less calcium than female subjects. Mean PTH doses and weight-adjusted doses were similar between men and women, as were serum 25-hydroxyvitamin D levels and serum PTH levels. As expected, BMD was higher in males than in females at all measured sites although the difference at the PA spine was not statistically significant. Baseline levels of biochemical markers of bone turnover were similar between males and females with the exception of a trend toward higher serum NTX in female subjects.
Table 1.
Baseline characteristics of the male and female study subjects
| Characteristic | Females | Males | P value |
|---|---|---|---|
| n | 14 | 17 | |
| Age (yr) | 65 ± 7 | 57 ± 9 | 0.008 |
| Weight (kg) | 67 ± 10 | 78 ± 13 | 0.021 |
| Dose (mg) | 30 ± 7 | 31 ± 6 | 0.701 |
| Dose/weight (mg/kg) | 0.41 ± 0.08 | 0.45 ± 0.09 | 0.243 |
| Calcium intake (mg) | 1376 ± 693 | 1023 ± 545 | 0.013 |
| 25-Hydroxyvitamin D (ng/ml) | 27 ± 11 | 22 ± 9 | 0.170 |
| PTH (pg/ml) | 46 ± 15 | 46 ± 21 | 0.970 |
| PA spine BMD (g/cm2) | 0.81 ± .10 | 0.87 ± .12 | 0.104 |
| Femoral neck BMD (g/cm2) | 0.60 ± 0.05 | 0.70 ± 0.09 | 0.001 |
| Total hip BMD (g/cm2) | 0.76 ± .11 | 0.88 ± 0.09 | 0.003 |
| Radius shaft BMD (g/cm2) | 0.58 ± 0.09 | 0.74 ± 0.04 | <0.001 |
| NTX (nmol BCE) | 17 ± 5 | 14 ± 3 | 0.098 |
| P1NP (ng/ml) | 46 ± 10 | 45 ± 12 | 0.931 |
| OC (ng/ml) | 29 ± 12 | 27 ± 6 | 0.627 |
Values shown are mean± sd. To convert values for 25-hydroxyvitamin D to nanomoles per liter, multiply by 2.496; to convert values for PTH to picomoles per liter, divide by 9.5.
BMD response to initial therapy (months 0–30)
Figure 2 shows the mean percentage changes in BMD during the 24-month teriparatide treatment phase of the study. BMD of the PA spine, femoral neck, total hip, and trabecular spine (QCT) increased significantly during the treatment period in both males and females (P < 0.001 for all within-group comparisons from baseline). The magnitude of these increases, whether expressed as percentage increase from baseline or as absolute change from baseline, did not differ between men and women. The mean female-male differences in the change in BMD [95% confidence interval (CI)] were 0.3 (−6.0, 6.6), 0.1 (−4.9, 5.0), 0.4 (−4.5, 5.2) and 13.4 (−8.0, 34.7) for PA spine, femoral neck, total hip, and trabecular spine, respectively.
Figure 2.
Mean (± se) percentage change in BMD during the initial treatment phase of the study (months 0 to 30). *, P < 0.001 for the comparison between male and female subjects.
Conversely, BMD at the radius was stable in men, whereas it decreased by 8.1 ± 3.3% in women (P < 0.0001 vs. baseline and vs. men), corresponding to a mean female-male difference in BMD change of −7.2 (95% CI, −9.4, −4.9).
BMD response to teriparatide discontinuation (months 30–42)
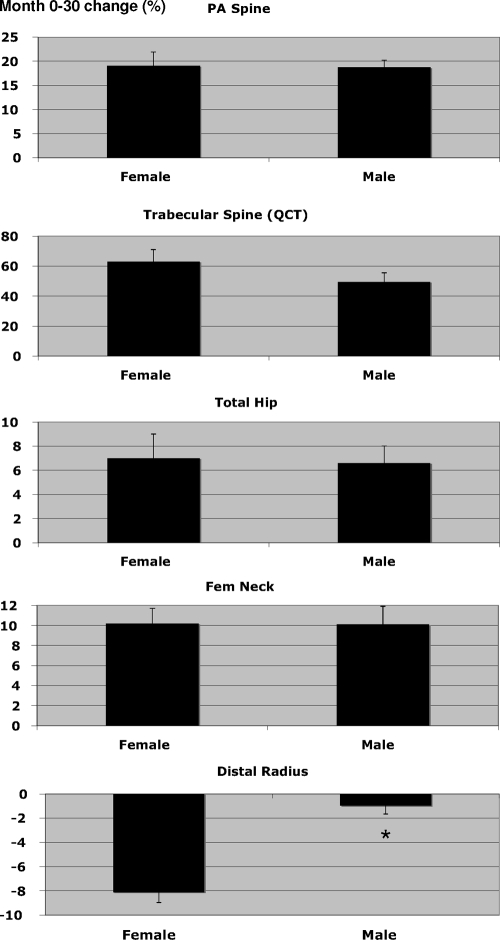
Figure 3 shows the mean percentage change in BMD during the 12-month off-treatment phase of the study (months 30–42). Figure 4 shows the cumulative effects of treatment followed by observation on absolute BMD of the PA spine and femoral neck. In the spine, DXA BMD decreased by 7.1 ± 3.8% (absolute change, 0.07 ± 0.04 g/cm2) in women and by 4.1 ± 3.5% (absolute change, 0.04 ± 0.04 g/cm2) in men (P < 0.001 vs. baseline in both men and women). The percentage loss of BMD was 3.0% greater in women than in men (P = 0.036; 95% CI, −5.8, −0.2). The absolute loss of BMD was also greater in women than in men, but the difference was of borderline statistical significance (P = 0.075). Trabecular BMD of the lumbar spine by QCT also decreased between months 30 and 42 in both men and women (P < 0.001 vs. baseline for both). Trabecular BMD decreased more in women than in men when teriparatide was stopped; 17.0 ± 8.9% (absolute change, 21.6 ± 14.3 mg/cm3) in women vs. 11.1 ± 12.2% (absolute change, 15.4 ± 13.0 mg/cm3) in men. The 5.9% female-male difference in trabecular BMD loss was statistically significant (P = 0.037; 95% CI, −11.2, −0.4), but the difference in absolute trabecular BMD loss was not (P = 0.181).
Figure 3.
Mean (± se) percentage change in BMD after teriparatide discontinuation (months 30 to 42). *, P < 0.05 for the comparison between male and female subjects.
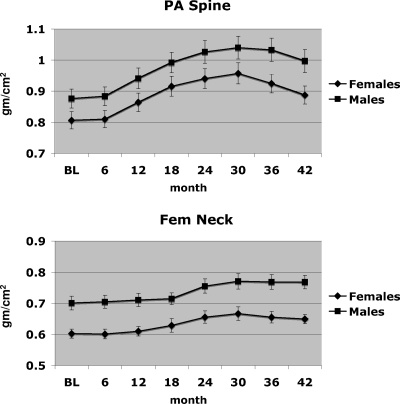
Figure 4.
Mean (± se) BMD of the PA spine and femoral neck during months 0 to 42.
In women, total hip and femoral neck BMD decreased by 3.8 ± 3.9 and 3.1 ± 4.3%, respectively, between months 30 and 42 (P < 0.05 vs. baseline for both sites), whereas in men BMD remained stable at both sites. The percentage change in total hip (P = 0.005) and femoral neck (P = 0.047) BMD was greater in women than in men after teriparatide was stopped, as was the absolute change in total hip BMD (P = 0.021). Specifically, the mean female-male differences in the change in BMD (95% CI) were −3.1% (−6.1, −0.1), and −3.5% (−5.8, −1.1) for femoral neck and total hip, respectively. BMD at the one third distal radius (which was stable in male subjects but decreased in female subjects during teriparatide treatment) remained stable in men but increased in women by 1.6 ± 3.1%. The 1.5% female-male difference in radial BMD during months 30–42 was not statistically significant (P = 0.069; 95% CI, −0.5, 3.5).
Multivariate analysis
To assess the influence of the baseline differences between female and male subjects on subsequent changes in BMD both during and after teriparatide administration, we repeated the analyses after multivariate adjustment for subject age, body weight, and serum 25-hydroxyvitamin D level. Multivariate adjustment had no impact on the comparison of the female-male response to initial teriparatide administration (mean percentage decrease in radius BMD remained greater in women than men, P = 0.0002). Multivariate adjustment did, however, affect the 30- to 42-month analysis. Although the gender differences in the mean percentage change in spine BMD by both DXA and QCT remained significant (P = 0.010 and P = 0.045, respectively), gender differences in the amount of BMD lost in the femoral neck in total hip no longer were.
Biochemical markers of bone turnover
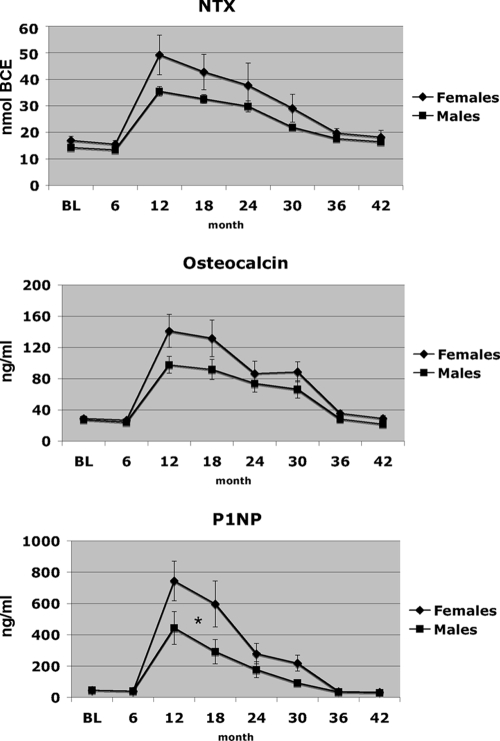
Figure 5 shows the changes in biochemical markers of bone turnover during both the teriparatide treatment phase and the observation phase. Bone turnover markers increased in both female and male subjects during the period of teriparatide administration and decreased during the period of observation (months 30–42) (P < 0.05 for the within-group comparisons of month 30 vs. month 0 and month 42 vs. month 30). The magnitude of the changes in serum NTX and serum OC did not differ between female and male subjects, but the increases in serum P1NP between months 0 and 30 as well as the decreases between months 30 and 42 were greater in women than in men (P < 0.05 for the between-group comparison during both periods of interest). Mean serum NTX remained slightly higher at month 42 (12 months after teriparatide treatment) than before initiating teriparatide therapy (month 6) in subjects overall [14.3 ± 4.1 nmol bone collagen equivalents (BCE) at month 6 and 17.2 ± 6.8 nmol BCE at month 42; P = 0.008]. Although this pattern was observed in both female and male subjects, it was significant only in males (P = 0.002 in men and P = 0.217 in women). Mean serum P1NP also remained slightly higher at month 42 vs. month 6 (P = 0.002 overall, P = 0.008 in men, P = 0.131 in women), but mean serum OC levels had returned to baseline values by month 42 in both men and women.
Figure 5.
Mean (± se) levels of serum NTX, OC, and P1NP during months 0 to 42. *, P < 0.05 refers to the comparison between male and female subjects for both the increase between months 0 and 30 and the subsequent decrease between months 30 and 42.
In addition to the parametric tests performed, we also performed the Mann-Whitney test to compare the female-male response in bone turnover, and the results were unchanged.
Discussion
To our knowledge, this is the first study to assess the effect of discontinuing teriparatide therapy on BMD prospectively in men as well as the first to compare the response to teriparatide administration and discontinuation in postmenopausal women and eugonadal men in the same protocol. Additionally, this is the first study to investigate the effects of teriparatide discontinuation after the maximal allowable duration of teriparatide use (24 months), the duration that is most commonly recommended by physicians. Aside from gender and gonadal status, all study participants satisfied identical entry criteria, and all were prescribed the same daily teriparatide dose. During initial treatment, the increases in BMD of the lumbar spine (by DXA and QCT), total hip, and femoral neck were similar in male and female subjects, whereas the amount of teriparatide-induced bone mineral loss at the radius shaft was greater in women. The similarity in the anabolic effect of teriparatide in our men and women is consistent with results reported in postmenopausal women and in men with idiopathic osteoporosis, albeit those studies had differing lengths of teriparatide therapy (10,11). When teriparatide was discontinued in the present study, the degree of bone loss at all anatomic sites was greater in women than in men. Specifically, BMD at the spine decreased less in men than in women, and BMD at the femoral neck and total hip remained stable in men but decreased in women. Of note, however, when these comparisons were adjusted for weight, age, and vitamin D level, gender differences in the total hip and femoral neck were no longer statistically significant, whereas differences in the spine (by DXA or QCT) remained.
There are several clinical ramifications of our findings. First, these data confirm the importance of considering antiresorptive therapy after completing 24 months of teriparatide therapy in postmenopausal women. Although BMD in women at the end of the 42-month study period remained higher than at baseline, the loss of BMD observed during months 30–42 was large enough to be clinically significant. Indeed, the magnitude of bone loss was generally similar to that observed in controlled and uncontrolled studies that also demonstrated that this bone loss was preventable with antiresorptive drugs (6,12,13,14). In men, however, the stability of BMD in the femoral neck and total hip, along with the smaller amount of bone loss at the spine suggests that some patients may be suitable for observation as opposed to automatic antiresorptive therapy, particularly if BMD at the spine is relatively normal after 24 months of teriparatide therapy. In fact, it has been reported that there is a persistent antivertebral fracture effect as long as 30 months after teriparatide discontinuation in men, despite only 29% of these subjects transitioning to antiresorptive therapy (7). Furthermore, it is not yet clear that spine and hip BMD will increase in men, as has been shown in women, if a bisphosphonate is started after completion of teriparatide or hPTH (1–84) therapy.
In the present study, BMD of the radius shaft responded to 37 μg daily teriparatide differently in men and women. This is consistent with earlier studies in which 40 μg of teriparatide was given to women only or men only (10,11). The etiology of the differential response in distal radius BMD to teriparatide therapy between men and women is unclear. It is possible that the distal radius in male subjects, which is comprised predominantly of cortical bone, is relatively protected from PTH-induced bone loss by the presence of circulating gonadal steroids. Indeed, the influence of gonadal steroids on cortical bone loss in diverse clinical settings has been well documented (15,16). Additionally, studies have clearly shown that the bone-resorbing properties of PTH are enhanced in hypogonadal vs. eugonadal men and women (16,17,18,19,20,21,22). It is also tempting to attribute this differential effect of teriparatide discontinuation in men vs. women to the presence or absence of gonadal steroid production in our male and female subjects, respectively. Indeed, this explanation is supported by long-standing clinical observations and clinical studies, as well as newer in vitro and animal models elucidating the molecular effects of gonadal steroids on osteoblast and osteoclast function through genomic and nongenomic mechanisms (23). Additionally, some studies have suggested that FSH, which would be expected to be much higher in our female cohort, also directly stimulates osteoclastic bone resorption leading to greater bone loss (24). Finally, because IGF-I is a central mediator of the osteoblastic response to teriparatide (25,26) and the women in our study would be expected to have lower levels of circulating IGF-I than the men based on both their gender and older age (27,28), differences in IGF-I may also be influencing the differential skeletal responses between sexes.
The centrality of the underlying estrogen deficiency (or other hormonal differences) in our female subjects in explaining observed differences in bone loss after teriparatide therapy is challenged, however, in that the mean age of our female subjects puts them beyond the period of accelerated menopausal bone loss. In support of this, the pretreatment levels of biochemical markers of bone turnover were generally comparable in our male and female subjects (NTX being slightly but not significantly higher in women), and these markers dropped similarly in men and women after teriparatide administration. Together, these observations suggest that the intrinsic rates of bone metabolism were similar in our two groups of subjects despite differences in gonadal steroid production or gonadotropin levels.
Other possible explanations for the greater bone loss in female subjects are the effects of age and weight. Female subjects in this study were older and lighter than male subjects. Notably, after multivariate adjustment, the male/female differences in BMD loss after teriparatide discontinuation remained significant in the spine (as measured by both DXA and QCT), and the magnitude of the gender differences was not reduced. Conversely, differences at the femoral neck and total hip were reduced slightly and were no longer statistically significant after multivariate adjustment, implying an influence of these variables on the rate of bone loss at these sites. There are conflicting data regarding the age at which bone loss begins and accelerates in men and women (29,30), but it is conceivable that by virtue of their younger age (as well as their increased weight), our male subjects would have been less likely to be actively losing bone in the absence of intervention. Notably, however, in the first 6 months of observation (during which only calcium and vitamin D were administered) neither group experienced measurable bone loss, suggesting that any differences in baseline levels of bone loss were minor. Finally, although the men and women in our study were enrolled based on the same entry criteria, baseline BMD was significantly lower in our female subjects as measured by both absolute BMD (all sites) and T-score (all sites except the PA spine, data not shown). Thus, it is possible that female subjects had a higher predisposition to bone loss due to unmeasured factors. While this explanation may account for some of the differences, however, it important to note that the male and female responses to teriparatide therapy were similar at all sites but the radius.
Several limitations of our study deserve mention. First, the limited sample size included in these observations is not large enough to make definitive determinations regarding gender differences, and the multiple comparison tests raise the possibility that some of the significant findings may have been found by chance. Conversely, it is important to note that given the limited sample size, it is also possible that some negative findings may have been due to insufficient power to detect small between-group differences (the study was powered to detect differences approximately equal to 1 within-group sd for each variable). Finally, the dose of teriparatide used in this study was larger than the FDA-approved dose of teriparatide (20 μg daily) that is commonly used in clinical practice. It is thus possible that the gender-specific responses to teriparatide administration and/or discontinuation may be different when patients receive 20 μg daily.
In summary, teriparatide increases BMD similarly in men and women, but the loss of bone density after discontinuing teriparatide treatment appears to be greater in women than in men. The mechanisms underlying this potential relative resistance to post-teriparatide bone loss in men are unclear. This observation requires confirmation in larger studies and in men and women receiving the FDA-approved teriparatide dose of 20 μg daily.
Acknowledgments
We thank Annmarie Hayes, Kate Gibson, Melissa Davis, and Carol Shea for their meticulous administration of the study protocol; Robbin Cleary, Sarah Zhang, and Annmarie Swarcz for performing the bone density measurements; and the nursing and dietary staff of the Mallinckrodt General Clinical Research Center for their dedicated care of the patients.
Footnotes
This work was supported by National Institutes of Health Grants P50 AR44855, RR-1066 and K24DK02759 (to J.S.F.).
Clinical trial registration at www.clinicaltrials.gov: NCT00000400.
Disclosure Summary: B.Z.L., H.W.L., J.J.W., S.A.B., and R.M.N. have nothing to declare. J.S.F. has been a consultant for Merck.
First Published Online May 12, 2009
Abbreviations: BCE, Bone collagen equivalents; BMD, bone mineral density; CI, confidence interval; DXA, dual-energy x-ray absorptiometry; hPTH (1–34), human PTH 1–34; NTX, N-telopeptide of type I collagen; OC, osteocalcin; PA, posterior-anterior; P1NP, N-terminal propeptide of type 1 collagen; QCT, quantitative computer tomography.
References
- 2004 Bone health and osteoporosis: a report of the Surgeon General. Washington, DC: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General [PubMed] [Google Scholar]
- Cosman F 2008 Parathyroid hormone treatment for osteoporosis. Curr Opin Endocrinol Diabetes Obes 15:495–501 [DOI] [PubMed] [Google Scholar]
- Miller PD 2008 Safety of parathyroid hormone for the treatment of osteoporosis. Curr Osteoporos Rep 6:12–16 [DOI] [PubMed] [Google Scholar]
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR 2006 Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM 2004 Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res 19:1259–1269 [DOI] [PubMed] [Google Scholar]
- Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ 2005 One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353:555–565 [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, Lindsay R, Mitlak BH 2005 Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 16:510–516 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM 2003 The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226 [DOI] [PubMed] [Google Scholar]
- Rosenthal DI, Ganott MA, Wyshak G, Slovik DM, Doppelt SH, Neer RM 1985 Quantitative computed tomography for spinal density measurement. Factors affecting precision. Invest Radiol 20:306–310 [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH 2001 Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441 [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA 2003 The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17 [DOI] [PubMed] [Google Scholar]
- Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, Reginster JY, Stepan JJ, Myers SL, Mitlak BH 2004 Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med 164:2024–2030 [DOI] [PubMed] [Google Scholar]
- Adami S, San Martin J, Muñoz-Torres M, Econs MJ, Xie L, Dalsky GP, McClung M, Felsenberg D, Brown JP, Brandi ML, Sipos A 2008 Effect of raloxifene after recombinant teriparatide [hPTH(1–34)] treatment in postmenopausal women with osteoporosis. Osteoporos Int 19:87–94 [DOI] [PubMed] [Google Scholar]
- Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, Halse J, Lindsay R, Dalsky GP, Mitlak BH 2005 Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 20:1507–1513 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Atkinson EJ, O'Fallon WM 2001 Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab 86:3555–3561 [DOI] [PubMed] [Google Scholar]
- Khosla S, Amin S, Orwoll E 2008 Osteoporosis in men. Endocr Rev 29:441–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman F, Shen V, Xie F, Seibel M, Ratcliffe A, Lindsay R 1993 Estrogen protection against bone resorbing effects of parathyroid hormone infusion. Assessment by use of biochemical markers. Ann Intern Med [Erratum (1994) 120:698] 118:337–343 [DOI] [PubMed] [Google Scholar]
- Joborn C, Ljunghall S, Larsson K, Lindh E, Naessen T, Wide L, Akerstrom G, Rastad J 1991 Skeletal responsiveness to parathyroid hormone in healthy females: relationship to menopause and oestrogen replacement. Clin Endocrinol (Oxf) 34:335–339 [DOI] [PubMed] [Google Scholar]
- Leder BZ, Smith MR, Fallon MA, Lee ML, Finkelstein JS 2001 Effects of gonadal steroid suppression on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab 86:511–516 [DOI] [PubMed] [Google Scholar]
- Lee H, Finkelstein JS, Miller M, Comeaux SJ, Cohen RI, Leder BZ 2006 Effects of selective testosterone and estradiol withdrawal on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab 91:1069–1075 [DOI] [PubMed] [Google Scholar]
- Tsai KS, Ebeling PR, Riggs BL 1989 Bone responsiveness to parathyroid hormone in normal and osteoporotic postmenopausal women. J Clin Endocrinol Metab 69:1024–1027 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Schoenfeld DA 1999 Effects of gonadal suppression on the regulation of parathyroid hormone and 1,25-dihydroxyvitamin D secretion in women. J Clin Endocrinol Metab 84:2151–2156 [DOI] [PubMed] [Google Scholar]
- Venken K, Callewaert F, Boonen S, Vanderschueren D 2008 Sex hormones, their receptors and bone health. Osteoporos Int 19:1517–1525 [DOI] [PubMed] [Google Scholar]
- Zaidi M, Blair HC, Iqbal J, Zhu LL, Kumar TR, Zallone A, Sun L 2007 Proresorptive actions of FSH and bone loss. Ann NY Acad Sci 1116:376–382 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP 2002 Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res 17:1570–1578 [DOI] [PubMed] [Google Scholar]
- Canalis E, Centrella M, Burch W, McCarthy TL 1989 Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest 83:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant G, Wallaschofski H 2007 Normal levels of serum IGF-I: determinants and validity of current reference ranges. Pituitary 10:129–133 [DOI] [PubMed] [Google Scholar]
- O'Connor KG, Tobin JD, Harman SM, Plato CC, Roy TA, Sherman SS, Blackman MR 1998 Serum levels of insulin-like growth factor-I are related to age and not to body composition in healthy women and men. J Gerontol A Biol Sci Med Sci 53:M176–M182 [DOI] [PubMed] [Google Scholar]
- Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, Guralnik JM, Ferrucci L 2008 Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res 23:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Melton Iii 3rd LJ, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S 2004 Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19:1945–1954 [DOI] [PubMed] [Google Scholar]