Abstract
Context: A previous report from the Study of Women Across the Nation indicated a rise in dehydroepiandrosterone sulfate (DHEAS) during the menopausal transition using data from three annual visits.
Objective: Our objective was to examine changes in DHEAS with chronological and ovarian aging, expanding the original analyses to include 10 yr of annual data.
Design: A longitudinal observational study and cross-sectional analyses of baseline data were conducted.
Outcome Measures and Subjects: DHEAS, age, menopause status, ethnicity, smoking, weight, and height were assessed in 2886 women from five ethnic groups aged 42–52 yr at entry. Hysterectomy, bilateral oophorectomy, and hormone use were excluded.
Results: Cross-sectional analysis at baseline showed a linear decline in circulating log-transformed DHEAS with increasing age for either the entire cohort (2.81% per year) or for individual ethnicities. A similar negative association with baseline age (2.44% decline per year) was seen in longitudinal linear mixed modeling including observations from premenopause through late postmenopause, an additional 0.33% decline/year. In contradistinction, a late-transition rise in DHEAS was detected when the same women were analyzed by ovarian status. The average increase in mean circulating DHEAS level between early and late menopause transition, beyond changes predicted by aging, was 3.95%, followed by an average decline of 3.96% during the late postmenopause. Approximately 84.5% of the women had an estimated within-woman increase in DHEAS from premenopause/early perimenopause to late perimenopause/early postmenopause.
Conclusion: These observations underscore differences between cross-sectional and longitudinal studies and the importance of considering ovarian status. Additional investigations regarding adrenal contribution to sex steroids in mid-aged women are warranted.
A longitudinal analysis of circulating dehydroepiandrosterone in mid-aged women reveals a positive inflection during the menopausal transition.
Dehydroepiandrosterone (DHEA) and its sulfated conjugate, DHEA sulfate (DHEAS), are the major secretory products of the human adrenal gland cortex and are produced in larger quantity than any other circulating steroid hormone (1). Whether DHEA acts as a hormone or as a prohormone is an unsettled point of contention because it lacks an identified cognate receptor (2).
Some studies suggest that DHEA has cardioprotective, antiobesity, antidiabetic, immuno-enhancing, and cancer-preventing properties (3). An antidepressant action of DHEA mediated via GABAA receptors has even been suggested (4). Observational studies have identified beneficial health associations with increased endogenous DHEAS. The Study of Women’s Health Across the Nation (SWAN) found that higher baseline DHEAS was independently associated with better perceived functional status and self-reported health in 2951 women of five race/ethnic groups (5). Higher endogenous DHEAS was also positively associated with better executive function, concentration, and working memory in 295 postmenopausal Australian women (6) and less depressed mood in another community-based study (7). Lower serum DHEAS has been associated with decreased physical functioning and more depressive symptoms in middle-aged African-American women (8) as well as increased sexual dysfunction over the menopausal transition (9).
Despite these compelling associations, short-term (1 yr or less) randomized clinical trials of exogenous DHEA have shown a negative (10) or null (11,12,13) effect on cognitive function and well-being. Thus, it has been difficult to reach a consensus conclusion from the reported associations between endogenous or exogenous DHEAS and health outcomes.
The decline in circulating DHEA/DHEAS parallels the onset of many age-related chronic diseases and disabilities, and this association has led to the hypothesis of a causal link between DHEA(S) and the aging processes (14). However, a longitudinal examination of circulating DHEAS by menopausal status in SWAN indicated that circulating DHEAS did not uniformly decline but increased before menopause (15). The current report expands that previous report from SWAN to include 9 yr of follow-up data. At this time, we hypothesized that the rise in DHEAS during the perimenopausal period is related to changes in ovarian status rather than increasing chronological age. This relationship may explain why other reports have not observed the perimenopausal inflection of DHEAS and suggest that it may be important to pursue with additional studies.
Subjects and Methods
Participants
This report is based on data collected at baseline and nine annual follow-up evaluations of 3302 SWAN participants, who comprise a longitudinal, prospective, multiethnic, multidisciplinary, population-based study of the natural history of the menopause transition. The SWAN sampling and recruitment procedures were implemented in seven United States sites: Boston, MA; Chicago, IL; Detroit area, MI; Los Angeles, CA; Newark, NJ; Pittsburgh, PA; and Oakland, CA. The design of the study has been described elsewhere (16). Briefly, an initial screening survey was conducted between November 1995 and October 1997 to assess eligibility for enrollment and collect health, reproductive, demographic, and lifestyle data. From the 16,065 women who completed this screening, approximately 450 eligible women were recruited for the longitudinal cohort at each of the seven sites. In addition to Caucasian women, each site recruited women from one specified minority group (African-Americans in Pittsburgh, Boston, Detroit area, and Chicago; Japanese in Los Angeles; Chinese in Oakland; and Hispanic women in Newark). Eligibility criteria for the longitudinal cohort were age 42–52 yr, having an intact uterus and at least one ovary, having at least one menstrual period and not using reproductive hormones in the 3 months before the baseline interview, and self-identification with each site’s specific race/ethnic groups. The Institutional Review Boards at all participating sites approved the study protocol, and all participants provided written informed consent before enrollment.
Measures
Physical measures
Identical protocols, data collection instruments, and specimen collection were implemented across the seven clinical sites and collected annually during each of the 10 visits. Height (centimeters) and weight (kilograms) were measured annually with a stadiometer and calibrated scales, respectively. Body mass index (BMI) was calculated as weight (kilograms)/height (meters) squared. Age was calculated based on birth date and the date of the blood draw. Phlebotomy was performed in the morning after an overnight fast. Participants were scheduled for venipuncture on d 2–5 (and d 2–7 from January through June 1996) of a spontaneous menstrual cycle occurring within 60 d of recruitment or the annual anniversary of recruitment. Two attempts were made to obtain a d-2 to -5 sample. If a specimen could not be obtained within 60 d of menstruation, a fasting sample was taken within 90 d of the recruitment anniversary. Blood was refrigerated before centrifugation 1–2 h after phlebotomy, and the serum was aliquoted, frozen, and batched for shipment to the central laboratory. Samples were catalogued and assayed continuously on arrival.
Survey measures
Active and passive smoke exposure were assessed based on the American Thoracic Society questions (17) and validated questions on passive exposure (18). Although active smoking was assessed annually, few women changed smoking status; thus, analyses were based on baseline smoking status.
Menopausal status definitions
Menopause status classification was based on annual responses to questions regarding menstrual irregularity and amenorrhea. Premenopause was defined as the presence of menses within the past 3 months, with no decrease in cycle predictability. Early perimenopause was defined as the presence of menses within the past 3 months with a decrease in predictability in the previous 12 months, whereas late perimenopause was defined as 3–11 months of amenorrhea (19,20,21). Twelve consecutive months of amenorrhea with no other cause was defined as postmenopause (21). Based on data indicating that reproductive hormones continue to fluctuate after the final menstrual period but stabilize within 2 yr (17,22), this category was divided into early [≤24 months of the final menstrual period (FMP)] and late (>24 months after the FMP). Use of exogenous hormones was assessed annually by self-report and inspection of the participant’s medication containers.
Hormone assays
The DHEAS assay used a competitive immunoassay using Bayer Diagnostic’s ACS-180 (Tarrytown, NY) automated analyzer using chemiluminescent technology. The assay uses a rabbit polyclonal anti-DHEAS antibody, goat antirabbit IgG labeled with paramagnetic particle (PMP), and Dimethyl acridinium ester (DHEAS) labeled with DMAE, and 10 μl serum is required. The SWAN reporting range for the DHEAS was 1.52–450 μg/dl (actual assay range, 1.52–1020 μg/dl). The assay was standardized against DHEAS obtained from Steraloids, Inc. (Newport, RI). The intraassay coefficient of variation was 8.02% (n = 261), and interassay coefficients of variation were 11.3% (53.3 μg/dl, n = 37) and 9.74% (250.21 μg/dl, n = 37). The estradiol (E2) assay was a competitive immunoassay run on Bayer Diagnostic’s ACS-180 with an off-line incubation. E2 in the specimen competes with DMAE-labeled E2 for binding to rabbit anti-E2 antibody (antibody reagent) and monoclonal mouse antirabbit IgG, which is coupled to PMP. The SWAN reporting range for the E2 assay is 1–200 pg/ml. Inter- and intraassay coefficients of variation were 13.8 and 8.5%, respectively, over the assay range. The testosterone (T) assay was a competitive chemiluminescent immunoassay that used T labeled with DMAE, a polyclonal rabbit anti-T antibody, and a monoclonal mouse antirabbit antibody, which is coupled to PMP. The ACS T assay was standardized analytically and confirmed by gas chromatography/mass spectrometry. The SWAN reporting range for the T assay is 10–100 ng/dl (actual assay range, 2–478 ng/dl). The de novo two-site chemiluminescent assay for serum SHBG concentrations involved competitive binding of DMAE-labeled SHBG to a commercially available rabbit anti-SHBG antibody and a solid phase of goat antirabbit IgG conjugated to paramagnetic particles. Inter- and intraassay coefficients of variation for SHBG were 9.9 and 6.1%, respectively, and the lower limit of detection was 2 nm.
Statistical methods
All observations from women with a hysterectomy and/or bilateral oophorectomy were excluded from analyses (n = 253). Observations from women reporting exogenous hormone therapy (HT) in the past year were omitted from analyses; subsequent observations from HT users were included if HT use had stopped at least 6 months before sample collection. Participants from the New Jersey site did not contribute observations after the fifth annual follow-up visit.
Baseline characteristics of women in the analytic sample were summarized by ethnicity using frequency distributions for categorical variables and medians and 25th and 75th percentiles for continuous variables, and distributions by ethnic groups were compared using χ2 and Kruskal-Wallis tests. Associations of DHEAS with other characteristics were estimated in the following steps. First, to assess consistency with previous studies, the cross-sectional association of baseline age with concurrent DHEAS in premenopausal women only was estimated using analysis of covariance, adjusting for ethnicity, clinical site, baseline active and passive smoking, baseline weight, and baseline height. Results presented for a specific covariate (e.g. ethnicity) represents the estimate adjusted for all other variables in the model. Second, longitudinal associations of DHEAS with concurrent menopause status were estimated from linear mixed models (23) for time-varying DHEAS, including observations concurrent with premenopausal, perimenopausal, and postmenopausal status from visits Baseline (00)-09, adjusting for baseline menopause status, baseline age, time since baseline, ethnicity, clinical site, baseline active and passive smoking, baseline weight and change in weight since baseline, baseline height, and past HT use. Results for each covariate (e.g. ethnicity) were adjusted for all other variables mentioned above. Within-woman changes in DHEAS associated with changes in menopause status were estimated from this model, with a Bonferroni correction applied to P values for multiple comparisons.
To identify women with a detectable rise in DHEAS between early and late perimenopause, the aforementioned model was reestimated in the subset of women with at least one observation during late peri- or early perimenopause and including random (i.e. woman-specific) effects for menopause status (23). The categories premenopause and early perimenopause were collapsed together as were the categories of late perimenopause and early postmenopause, because DHEAS was found not to differ between these status categories. An estimated woman-specific increase in DHEAS between premenopause/early perimenopause and late perimenopause/early postmenopause was considered as a rise in DHEAS. All analyses were conducted in SAS 9.1.
Results
Characteristics of the participants
Of the 3302 cohort participants, data from 253 with surgical menopause and 163 with missing data for DHEAS, covariates, or hormone use were excluded from analysis. This resulted in an analytic sample of 2886 participants with an average of 5.5 observations per participant from visits 00-09. Compared with the analytic sample at baseline, participants excluded from analyses were more likely to be African-American and less likely to be Caucasian or Chinese, more likely to be early perimenopausal, had higher passive smoke exposure, and were on an average younger in age and lighter in weight. At baseline (Table 1), approximately half of the subjects in the analytic sample were Caucasian (47.5%), reflecting the study recruitment design, and just over half were premenopausal. Age and SHBG did not differ significantly by ethnicity. Chinese and Japanese participants were less likely to have smoked and had lower exposure to passive smoke and had lower BMI, higher DHEAS, and lower E2. Mean levels of T were lowest among Hispanics.
Table 1.
Baseline characteristics of participants in analytic sample by ethnicity
| Characteristic | % (n) or median (25–75th percentile)
|
P for ethnic differencesa | |||||
|---|---|---|---|---|---|---|---|
| All ethnicities (n = 2886) | African-American (n = 774) | Caucasian (n = 1372) | Chinese (n = 236) | Hispanic (n = 248) | Japanese (n = 256) | ||
| Menopause status | 0.0003 | ||||||
| Pre | 54.3 (1566) | 49.9 (386) | 53.2 (730) | 62.3 (147) | 57.3 (142) | 62.9 (161) | |
| Early peri | 45.7 (1320) | 50.1 (388) | 46.8 (642) | 37.7 (89) | 42.7 (106) | 37.1 (95) | |
| Age at blood draw (yr) | 46.3 (44.2–48.4) | 46.4 (44.2–48.4) | 46.1 (44.1–48.4) | 46.6 (44.4–48.2) | 46.2 (44.2–48.2) | 46.6 (44.4–48.7) | 0.5512 |
| Active smoking status | <0.0001 | ||||||
| Never | 57.3 (1653) | 53.1 (411) | 50.3 (690) | 94.1 (222) | 66.9 (166) | 64.1 (164) | |
| Past | 25.8 (744) | 22.4 (174) | 33.3 (457) | 4.7 (11) | 17.3 (43) | 23.1 (59) | |
| Current | 16.9 (489) | 24.4 (189) | 16.4 (225) | 1.3 (3) | 15.7 (39) | 12.9 (33) | |
| Passive smoking status | <0.0001 | ||||||
| None | 45.6 (1317) | 35.5 (275) | 39.7 (544) | 77.1 (182) | 63.3 (157) | 62.1 (159) | |
| 1–4 h/wk | 26.5 (765) | 25.8 (200) | 32.1 (440) | 16.5 (39) | 11.7 (29) | 22.3 (57) | |
| ≥5 h/wk | 27.9 (804) | 38.6 (299) | 28.3 (388) | 6.4 (15) | 25.0 (62) | 15.6 (40) | |
| BMI (kg/m2) | 26.5 (22.8–32.1) | 30.2 (26.0–36.1) | 26.1 (22.8–31.2) | 22.4 (20.8–24.8) | 28.3 (25.3–32.2) | 22.1 (20.4–24.5) | <0.0001 |
| DHEAS (μg/ml)b | 114.2 (75.8–169.3) | 92.1 (61.9–135.7) | 121.9 (79.4–176.7) | 154.8 (111.1–216.2) | 107.0 (63.7–146.6) | 131.6 (87.0–181.4) | <0.0001 |
| Estradiol (pg/ml)b | 55.3 (32.8–88.8) | 54.8 (32.8–90.2) | 56.9 (34.5–89.4) | 48.5 (27.7–81.3) | 61.9 (28.2–98.0) | 50.3 (30.1–81.7) | 0.0043 |
| T (ng/dl)b | 41.4 (29.7–55.8) | 41.1 (30.6–57.1) | 42.7 (30.7–57.1) | 40.3 (27.9–51.1) | 37.5 (26.8–51.8) | 38.1 (28.3–50.6) | <0.0001 |
| SHBG (nm)b | 41.3 (28.1–41.3) | 42.3 (30.5–58.2) | 41.1 (27.6–57.5) | 39.3 (26.5–54.9) | 40.1 (27.8–53.8) | 43.4 (28.0–65.1) | 0.1143 |
χ2 test for categorical variables, Kruskal-Wallis test for continuous variables.
Missing for one participant.
Associations of DHEAS with chronological aging
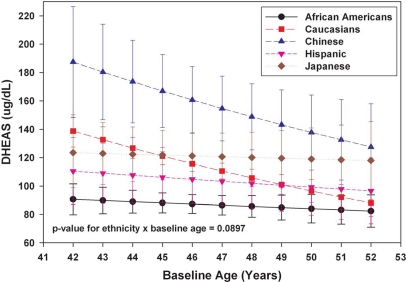
Cross-sectional analyses of baseline DHEAS for the 1565 participants who were premenopausal at baseline with an observed DHEAS level indicated a decline of 2.81% per year (P < 0.0001) with older baseline age, after adjusting for ethnicity, clinical site, weight, height, and active and passive smoking. Figure 1 presents adjusted baseline DHEAS values by age in 1-yr increments and stratified by ethnicity. A trend line shows the mean values by age for each ethnic group. A negative linear association in log DHEAS was observed for each ethnic group with different absolute and relative decreases for each group (P = 0.09 for the interaction of baseline age and ethnicity). The annual decline in DHEAS for African-Americans, Caucasians, Chinese, Hispanics, and Japanese was 0.96% (P = 0.45), 4.44% (P < 0.0001), 3.77% (P = 0.06), 1.34% (P = 0.47), and 0.46% (P = 0.82), respectively. Chinese women exhibited the highest DHEAS levels at all ages and, with Caucasians, had the greatest absolute decline with increasing age. African-American women had the lowest overall DHEAS levels and, with Japanese women, showed the lowest rate of decline with advancing age. Hispanic women had relatively low intermediate levels and decline. Caucasian women had intermediate DHEAS levels at age 42 and shared the lowest levels with African-Americans when both groups were analyzed at 53 yr of age.
Figure 1.
Adjusted mean DHEAS (95% confidence interval) by baseline age within ethnicity from SWAN visit 00 (1565 premenopausal women).
Associations of DHEAS with time on study: longitudinal analysis
In longitudinal analyses, the menopause status-adjusted difference by baseline age was similar to the estimate from cross-sectional premenopausal observations, at 2.44% decline per year (P < 0.0001). The adjusted within-woman decline with each additional year on study was 0.33% (P = 0.06).
Associations of DHEAS with menopausal status
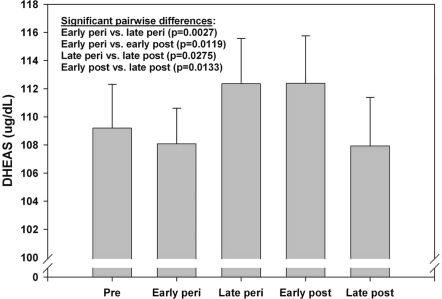
DHEAS differed significantly by menopause status (Fig. 2) using the longitudinal model described above adjusting for chronological age and other characteristics also listed in the preceding section. Consistent with the earlier SWAN analyses through follow-up visit 02 (16), there was a significant (P = 0.003) within-woman increase in DHEAS from early to late perimenopause of 4.27 μg/ml (3.95%) on average, beyond changes associated with chronological age/aging and other characteristics. Of the 1423 women with at least one observation during late perimenopause or early perimenopause, 1202 (84.5%) displayed an early-to-late perimenopause increase. Mean DHEAS levels were similar during late perimenopause and early postmenopause (within 24 months of FMP) but declined significantly between premenopause and late postmenopause by 3.96% on average, beyond changes associated with age and other characteristics.
Figure 2.
Adjusted mean DHEAS (95% confidence interval) by menopause status from SWAN visits 00-09 (15,930 observations from 2,886 women).
The minority of women that did not exhibit a detectable rise in DHEAS differed from those who did only in that they had lower circulating DHEAS at the beginning of their menopausal transition and were more likely to be early perimenopausal rather than premenopausal. An overall higher DHEAS level before the menopausal transition in women who subsequently show a rise is consistent with an intrinsic predisposition to produce more rather than less weak androgen. Sociodemographic variables such as self-assessed health, perceived stress, smoking history, and BMI in the early transition were not predictive of the inflection in circulating DHEAS.
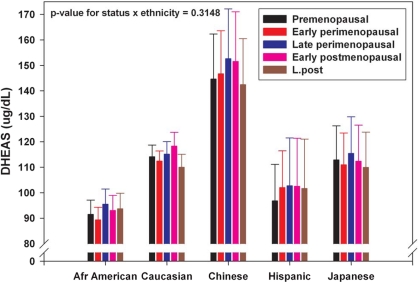
Circulating DHEAS concentrations across the menopausal transition differed significantly by ethnicity even after adjustment for other characteristics (P < 0.0001), with the highest levels in Chinese women and lowest levels in African-American women. Despite the ethnic difference in absolute circulating DHEAS levels, similar mean profiles were observed for each ethnic group when stratified by menopausal status (Fig. 3), and there was no significant interaction between ethnicity and menopausal status (P = 0.31).
Figure 3.
Adjusted mean DHEAS (95% confidence interval) by menopause status within ethnicity from SWAN visits 00-09 (15,930 observations from 2,886 women).
Discussion
A rise in circulating DHEAS coincident with the latter part of the menopausal transition is common to a majority of women in all five ethnic groups examined. The biological significance of this temporary increase in endogenous DHEAS may reflect an adaptive endocrine response to other hormone changes. A positive relationship has been observed between circulating levels of DHEA/DHEAS and physical (5) and cognitive (6) function in mid-aged women but not men (24,25), suggesting that adrenal androgens may play a more important role in women compared with men. It is possible that in women, the increased circulating DHEA and DHEAS during the perimenopause are taken up by granulosa cells, resulting in sustained ovarian follicle function by providing androgen precursors ordinarily supplied by the theca. In the face of a failing theca, an increased supply of exogenous DHEA in nonrandomized studies has been hypothesized to maintain hormone homeostasis in some individuals (26,27) because DHEA can be a substrate for both T (28) and E2 production (29). However, the conversion of DHEA to bioactive androgens and/or estrogens occurs at the tissue level with potentially different consequences for different individuals. Thus, circulating levels of DHEA/DHEAS may provide only limited information.
An increase in circulating DHEAS, which could contribute to peripheral conversion to bioactive sex steroids at the time of declining E2 production and increasing circulating FSH may attenuate the loss of estrogens before the last menstrual period (17,30) and explain some differences in menopausal symptoms. Although the mean circulating DHEAS increase observed in this study is small, this approximate 4% increase may be important in terms of downstream conversion of DHEA(S) to bioactive products given the several orders of magnitude higher concentration of the precursor compared with the normal circulating levels of the bioactive products. Furthermore, such an increase in peripheral P450 17c substrate may also partially explain the persistence of androgen levels through most of the menopausal transition (31). Perhaps most intriguing is the possibility that increased peripheral conversion of DHEA/DHEAS to bioactive androgens may be responsible for lower SHBG and a shift in the androgen/estrogen ratio (32). Such mechanisms are supported by a recent report that indicates supplementation of DHEA at 50 mg/d results in an increase in T, the free T index, E2, estrone, and the free estrogen index as well as a decrease in SHBG in 58 postmenopausal women (33).
The positive transitional trend in DHEAS that is confirmed here for most women raises several questions. The first question is whether this rise in DHEAS is a result of increased production or decreased clearance because both would have the same impact on circulating levels. The more likely of the two possibilities is an increase in DHEAS production because increased DHEAS production occurs at least two other times during a human’s life history, once in utero and again at puberty (adrenarche). There is no example of a change in DHEAS clearance that leads to an increase in circulating concentrations. Therefore, our findings are more likely to be due to increased production of DHEAS. If so, then the next question is what is (are) the mechanism(s) that drive(s) this increase. Although there are several theoretical explanations for the fetal and pubertal increase in adrenal weak androgens (1), none of these theories have been proven. In both earlier cases of a rise in circulating DHEAS, there is a concomitant shift in human chorionic gonadotropin or LH drive, and in this case, the rise coincides with an overt increase in circulating FSH. This may indicate that a shift in gonadotropin drive subsequent to decreased ovarian feedback in the menopausal transition may operate in the majority of mid-aged women to increase DHEAS production. Because serum LH was not measured in SWAN, we were unable to examine the hypothesis that the DHEAS rise was associated with increased LH. In addition, the higher baseline levels of DHEAS in women exhibiting a later overt rise in circulating DHEAS may indicate an inherent difference in the sensitivity to factors that control DHEAS production.
The present cross-sectional and longitudinal data confirm a continuous decline of mean DHEAS with chronological age and agree with the previously reported age-related decline in circulating DHEAS. The present findings show that a significant number of women exhibit a rise in circulating DHEAS during the menopausal transition and confirms our earlier report (15). This rise in DHEAS was not detected in other studies because either menopausal status was not considered in the analysis or if menopausal status was included, the results were not presented by menopausal status after adjusting for chronological age. The ethnic differences we had previously observed in our 3-yr longitudinal study were preserved in this subsequent analysis with Caucasian and Chinese women demonstrating the most rapid declines in DHEAS over time and Japanese women demonstrating the least rapid decline. Overall, African-American women had the lowest circulating DHEAS concentrations. Despite these seemingly large ethnic differences in baseline concentrations and rate of decline, the direction of change in DHEAS during the menopausal transition was similar for all ethnic groups, and there was no overall effect of ethnicity in our final model. These findings underscore the value of longitudinal analysis and the importance of considering ovarian status in assessing the endocrine aspects of the menopausal transition.
The principal limitation of this study was the incomplete data for the Hispanic women because the New Jersey site contributed data only for the first 5 yr. Despite these missing data, the circulating DHEAS trends were similar to the other ethnic groups in showing a slight decline by age and a late perimenopausal rise when analyzed by menopausal status. It is possible that these missing data led to a lower overall BMI in Hispanics because, as a group, these women were younger and possibly healthier than women represented though the entire study. However, there was no indication that BMI was related to circulating DHEAS levels.
In summary, the present report clarifies the relationships between DHEAS, chronological aging, and menopausal status. Specifically, this report confirms a rise in circulating DHEAS that occurs in a majority of women and relates this rise to ovarian stage during the menopausal transition rather than chronological age. These data suggest the late menopausal transition is associated with a potentially compensatory increase in adrenal androgen secretion, but an ovarian contribution cannot be completely ruled out. Additional studies that take differences in circulating DHEAS levels during the menopausal transition into account are warranted.
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN.
Clinical centers included the following: University of Michigan, Ann Arbor (MaryFran Sowers, PI); Massachusetts General Hospital, Boston, MA (Robert Neer, PI 1994–1999; Joel Finkelstein, PI 1999 to present); Rush University, Rush University Medical Center, Chicago, IL (Lynda Powell, PI 1994–2009; Howard Kravitz, PI 2009); University of California, Davis/Kaiser (Ellen Gold, PI); University of California, Los Angeles, Los Angeles, CA (Gail Greendale, PI); University of Medicine and Dentistry-New Jersey Medical School, Newark, NJ (Gerson Weiss, PI 1994–2004; Nanette Santoro, PI 2004 to present); and the University of Pittsburgh, Pittsburgh, PA (Karen Matthews, PI). National Institutes of Health (NIH) Program Office included National Institute on Aging (NIA), Bethesda, MD (Marcia Ory 1994–2001; Sherry Sherman 1994 to present) and National Institute of Nursing Research, Bethesda, MD (Program Officers). The central laboratory was University of Michigan, Ann Arbor, MI (Daniel McConnell) (Central Ligand Assay Satellite Services). The coordinating centers were University of Pittsburgh, Pittsburgh, PA (Kim Sutton-Tyrrell, PI 2001 to present) and New England Research Institutes, Watertown, MA (Sonja McKinlay, PI 1995 to 2001). Steering committee chairs were Chris Gallagher and Susan Johnson.
Footnotes
The SWAN has grant support from the NIH, Department of Health and Human Services, through the NIA, the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Disclosure Summary: The authors have nothing to disclose.
First Published Online May 26, 2009
Abbreviations: BMI, Body mass index; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; DMAE, Dimethyl acridiniumester; E2, estradiol; FMP, final menstrual period; HT, hormone therapy; PMP, paramagnetic particle; SWAN, Study of Women’s Health Across the Nation; T, testosterone.
References
- Nguyen AD, Conley AJ 2008 Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev 13:33–54 [DOI] [PubMed] [Google Scholar]
- Widstrom RL, Dillon JS 2004 Is there a receptor for dehydroepiandrosterone or dehydroepiandrosterone sulfate? Semin Reprod Med 22:289–298 [DOI] [PubMed] [Google Scholar]
- Yen SS, Morales AJ, Khorram O 1995 Replacement of DHEA in aging men and women. Potential remedial effects. Ann NY Acad Sci 774:128–142 [DOI] [PubMed] [Google Scholar]
- Genud R, Merenlender A, Gispan-Herman I, Maayan R, Weizman A, Yadid G 2008 DHEA lessens depressive-like behavior via GABA-ergic modulation of the mesolimbic system. Neuropsychopharmacology 10:1038–1046 [DOI] [PubMed] [Google Scholar]
- Santoro N, Torrens J, Crawford S, Allsworth JE, Finkelstein JS, Gold EB, Korenman S, Lasley WL, Luborsky JL, McConnell D, Sowers MF, Weiss G 2005 Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J Clin Endocrinol Metab 90:4836-4845 [DOI] [PubMed] [Google Scholar]
- Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ 2008 Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab 93:801–808 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, von Mühlen D, Laughlin GA, Kripke A 1999 Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc 47:685–691 [DOI] [PubMed] [Google Scholar]
- Haren MT, Malmstrom TK, Banks WA, Patrick P, Miller DK, Morley JE 2007 Lower serum DHEAS levels are associated with a higher degree of physical disability and depressive symptoms in middle-aged to older African American women. Maturitas 57:347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia CR, Freeman EW, Sammel MD, Lin H, Mogul M 2007 Hormones and sexuality during transition to menopause. Obstet Gynecol 109:831–840 [DOI] [PubMed] [Google Scholar]
- Parsons TD, Kratz KM, Thompson E, Stanczyk FZ, Buckwalter JG 2006 DHEA supplementation and cognition in postmenopausal women. Int J Neurosci 116:141–155 [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Freeman E, Grisso JA, Rader DJ, Sammel M, Kapoor S, Nestler JE 1999 The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab 84:3896–3902 [DOI] [PubMed] [Google Scholar]
- Dayal M, Sammel MD, Zhao J, Hummel AC, Vandenbourne K, Barnhart KT 2005 Supplementation with DHEA: effect on muscle size, strength, quality of life, and lipids. J Womens Health (Larchmt) 14:391–400 [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, von Mühlen D, Laughlin GA, Bettencourt R 2008 Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and Well-Ness (DAWN) Trial. J Am Geriatr Soc 56:1292–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE 1996 Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocrinol Metab 81:3147–3151 [DOI] [PubMed] [Google Scholar]
- Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF 2002 The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab 87:3760–3767 [DOI] [PubMed] [Google Scholar]
- Sowers M 2000 SWAN: A multicenter, multiethnic, community-based cohort study of women and the menoausal transition. In: Lobo R, Kelsey J, Marcus R, eds. Menopause: biology and pathobiology. New York: Academic Press; 175–188 [Google Scholar]
- Ferris BG 1978 Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 118:1–120 [PubMed] [Google Scholar]
- Coghlin J, Hammond SK, Gann PH 1989 Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol 130:696–704 [DOI] [PubMed] [Google Scholar]
- Brambilla DJ, McKinlay SM, Johannes CB 1994 Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol 140:1091–1095 [DOI] [PubMed] [Google Scholar]
- Dudley EC, Hopper JL, Taffe J, Guthrie JR, Burger HG, Dennerstein L 1998 Using longitudinal data to define the perimenopause by menstrual cycle characteristics. Climacteric 1:18–25 [DOI] [PubMed] [Google Scholar]
- World Health Organization 1996 Research on the menopause in the 1990s. WHO Technical Services Report. Geneva: World Health Organization [Google Scholar]
- Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L 1999 Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab 84:4025–4030 [DOI] [PubMed] [Google Scholar]
- Fitzmaurice G, Laird N, Ware J 2004 Applied longitudinal analysis. Hoboken, NJ: Wiley Interscience [Google Scholar]
- Fonda SJ, Bertrand R, O'Donnell A, Longcope C, McKinlay JB 2005 Age, hormones, and cognitive functioning among middle-aged and elderly men: cross-sectional evidence from the Massachusetts Male Aging Study. J Gerontol A Biol Sci Med Sci 60:385–390 [DOI] [PubMed] [Google Scholar]
- Moffat S, Zonderman A, Harman S, Blackman MR, Kawas C, Resnick SM 2002 The relationship between longitudinal decline in dehydroepiandrosterone sulfate concentrations and cognitive performance in older men. Arch Intern Med 160:2193–2198 [DOI] [PubMed] [Google Scholar]
- Barad D, Brill H, Gleicher N 2007 Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet 24:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Barad D 2007 DHEA and testosterone in the elderly. N Engl J Med 356:636–637; author reply 637 [PubMed] [Google Scholar]
- Arlt W, Callies F, Allolio B 2000 DHEA replacement in women with adrenal insufficiency: pharmacokinetics, bioconversion and clinical effects on well-being, sexuality and cognition. Endocr Res 26:505–511 [DOI] [PubMed] [Google Scholar]
- Labrie F, Cusan L, Gomez JL, Martel C, Bérubé R, Bélanger P, Chaussade V, Deloche C, Leclaire J 2008 Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol 110:1–9 [DOI] [PubMed] [Google Scholar]
- Randolph Jr JF, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL 2003 Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 88:1516–1522 [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL 2000 A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab 85:2832–2838 [DOI] [PubMed] [Google Scholar]
- Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K 2008 Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med 168:1568–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM 2008 Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab 93:4767–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]