Abstract
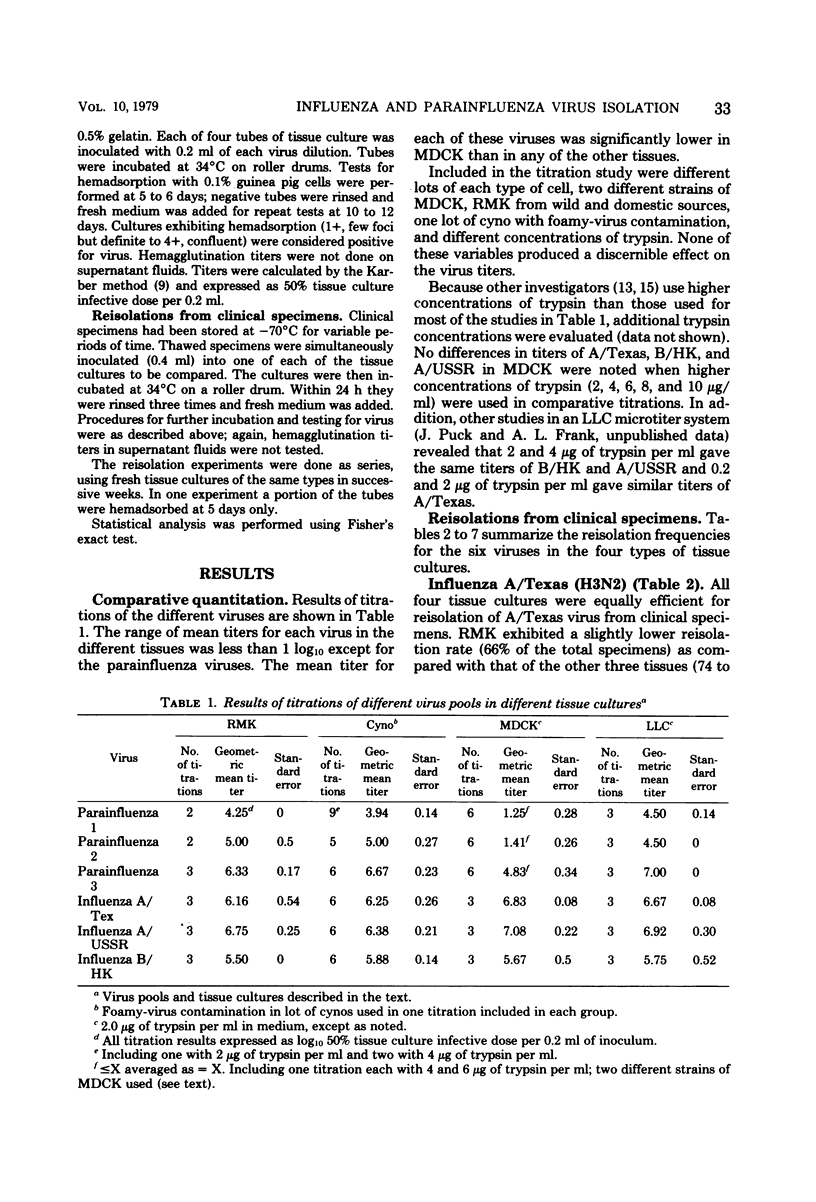
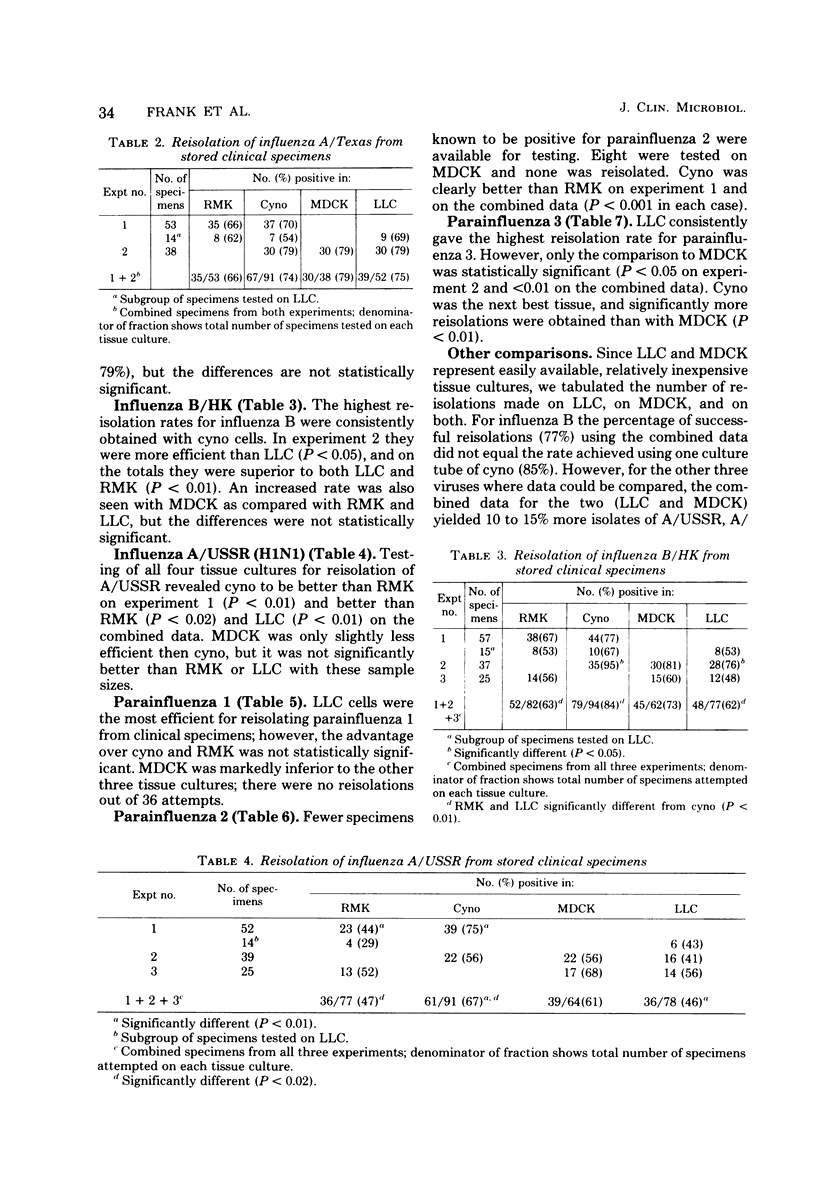
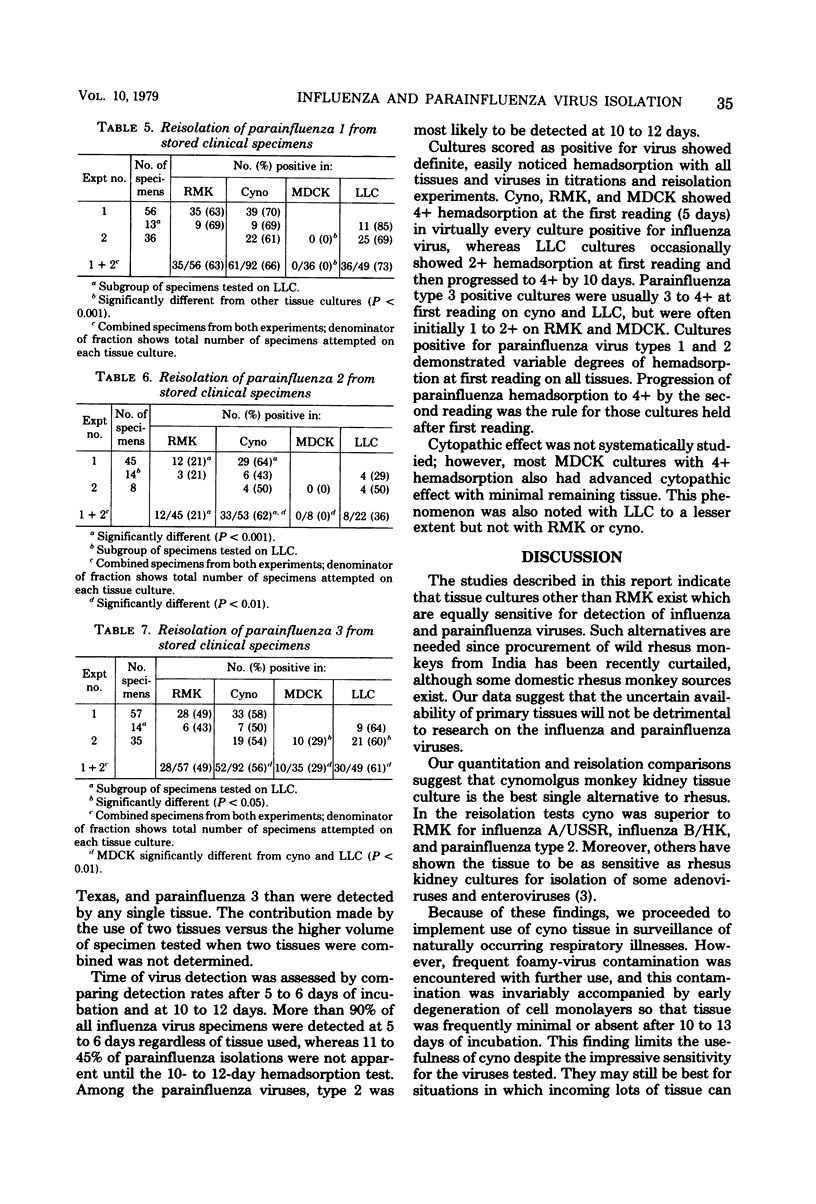
Rhesus and cynomolgus monkey kidney tissue cultures and two continuous lines, Madin-Darby canine kidney (MDCK) and LLC-MK2, were compared in titrations and isolations of influenza and parainfluenza viruses. Tube cultures were inoculated with laboratory virus strains or stored patient specimens and observed for hemadsorption. Trypsin was added to the medium of the continuous lines to increase sensitivity. All four tissue cultures gave similar titers of influenza A/USSR (H1N1), A/Texas (H3N2), and B/HK, but lower titers of parainfluenza 1, 2, and 3 were observed with MDCK. Cynomolgus kidney was the best single tissue culture for reisolation of the six viruses, but foamy-virus contamination of many lots was a serious problem. Reisolation of influenza viruses was as successful with MDCK as with primary monkey kidney. LLC-MK2 was similar to rhesus kidney but less successful than cynomolgus kidney. For reisolation of parainfluenza viruses, LLC-MK2 was superior to rhesus monkey kidney and similar to cynomolgus kidney. MDCK was less useful for parainfluenza viruses. Thus, LLC-MK2 would be an acceptable single tissue alternative to primary monkey kidney. The combination of MDCK and LLC-MK2 would provide optimal sensitivity for isolation of all six viruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GREEN I. J. Serial propagation of influenza B (Lee) virus in a transmissible line of canine kidney cells. Science. 1962 Oct 5;138(3536):42–43. doi: 10.1126/science.138.3536.42. [DOI] [PubMed] [Google Scholar]
- HAMBLING M. H., DAVIS P. M. SUSCEPTIBILITY OF THE LLC-MK2 LINE OF MONKEY KIDNEY CELLS TO HUMAN ENTEROVIRUSES. J Hyg (Lond) 1965 Jun;63:169–174. doi: 10.1017/s0022172400045071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULL R. N., CHERRY W. R., TRITCH O. J. Growth characteristics of monkey kidney cell strains LLC-MK1, LLC-MK2, and LLC-MK2(NCTC-3196) and their utility in virus research. J Exp Med. 1962 May 1;115:903–918. doi: 10.1084/jem.115.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollick G. E., Reichrath L., Smith T. F. Comparison of primary rhesus and cynomolgus monkey kidney cell cultures for viral isolation from clinical specimens. Am J Clin Pathol. 1977 Aug;68(2):276–278. doi: 10.1093/ajcp/68.2.276. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Morimoto Y., Iwase I., Doi Y., Sanpe T. Effect of trypsin on viral susceptibility of Vero cell cultures--cercopithecus kidney line. Jpn J Med Sci Biol. 1970 Aug;23(4):227–235. doi: 10.7883/yoken1952.23.227. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Meguro H., Bryant J. D., Torrence A. E., Wright P. F. Canine kidney cell line for isolation of respiratory viruses. J Clin Microbiol. 1979 Feb;9(2):175–179. doi: 10.1128/jcm.9.2.175-179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. M., Scheid A., Choppin P. W. Loss on serial passage of rhesus monkey kidney cells of proteolytic activity required for Sendai virus activation. Infect Immun. 1978 Apr;20(1):235–241. doi: 10.1128/iai.20.1.235-241.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita K., Maru M., Sato K. A sensitive plaque assay for Sendai virus in an established line of monkey kidney cells. Jpn J Microbiol. 1974 May;18(3):262–264. doi: 10.1111/j.1348-0421.1974.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Tobita K. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B viruses. Med Microbiol Immunol. 1975 Dec 30;162(1):23–27. doi: 10.1007/BF02123574. [DOI] [PubMed] [Google Scholar]
- Tobita K., Sugiura A., Enomote C., Furuyama M. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol. 1975 Dec 30;162(1):9–14. doi: 10.1007/BF02123572. [DOI] [PubMed] [Google Scholar]


