Abstract
Purpose of review
Antenatally detected renal abnormalities are frequently encountered. Recommended postnatal evaluation of these infants has evolved to minimize invasive testing while maximizing detection of significant abnormalities.
Recent findings
There is a low rate of detectable renal abnormalities in infants with a normal postnatal sonogram at 4–6 weeks of age. Routine prophylactic antibiotics are not indicated in infants with isolated antenatal hydronephrosis. Infants with a multicystic dysplastic kidney and a normal contralateral kidney on renal ultrasound do not require further evaluation. Parents of these children should be counseled on symptoms of urinary tract infections to allow prompt diagnosis.
Summary
All infants with abnormalities on antenatal sonogram should undergo postnatal evaluation with a sonogram after birth and at 4–6 weeks of age. Further evaluation can be safely limited when the postnatal sonogram is normal at 6 weeks of age.
Keywords: antenatal hydronephrosis, neonates, obstructive uropathy, vesicoureteral reflux
Introduction
Abnormalities of the kidneys and urinary tract are the most common abnormalities detected during routine antenatal ultrasound imaging [1,2]. Over the last decade, recommendations for postnatal evaluation of these abnormalities have been under intense scrutiny. Large cohort studies have resulted in significant changes to the current standard of care. This review will focus on the most commonly detected antenatal renal abnormalities and the current recommendations for postnatal evaluation.
Antenatal renal abnormalities
There is a variety of renal abnormalities that may be detected on antenatal sonogram. Commonly found renal abnormalities are listed in Table 1, and common pathologic diagnoses are listed in Table 2. The most common renal abnormality is hydronephrosis (also known as renal pelvic dilatation) with an incidence estimated between 0.5 and 1% [3,4]. Two grading systems exist for fetal hydronephrosis – the anterior posterior diameter (APD) and the Society for Fetal Urology (SFU) grade. The majority of studies use the APD, but the normal values for APD are not universally agreed upon. Many studies use an APD greater than or equal to 5 mm at any gestational age as abnormal [5–7,8•,9,10], necessitating postnatal evaluation.
Table 1.
Abnormalities of the kidneys and urinary tract detectable on antenatal sonogram
| Hydronephrosis (unilateral or bilateral) |
| Hydroureter (unilateral or bilateral) |
| Thickened or rugated bladder |
| Cystic kidney |
| Small or dysplastic kidney |
| Absent kidney (renal agenesis) |
Table 2.
Common renal abnormalities detected in neonates
| Ureteropelvic junction (UPJ) obstruction |
| Vesicoureteral reflux (VUR) |
| Posterior urethral valves (PUV) |
| Ureterovesical junction (UVJ) obstruction |
| Multicystic dysplastic kidney (MCDK) |
| Prune belly syndrome (PBS) |
| Duplicated collecting system with ureterocele |
| Solitary kidney |
| Urethral atresia or stricture |
| Autosomal recessive polycystic kidney disease (ARPKD) |
The likelihood of significant renal abnormality correlates with the severity of APD dilatation [5,11,12]. A meta-analysis of 17 studies reported the risk of renal abnormality for three classifications of antenatal hydronephrosis: mild, moderate, and severe [12]. Mild hydronephrosis (APD ≤7 mm in the second or ≤9 mm in the third trimester) had an 11.9% risk of postnatal abnormality. The risk of postnatal abnormality increased to 45.1% in the moderate hydronephrosis group (APD 7–10 mm in the second or 9–15 mm in the third trimester), and the risk further increased to 88.3% in the severe group (APD ≥10 mm in the second or ≥15 mm in the third trimester) [12]. The probability of ureteropelvic junction (UPJ) obstruction increased with increasing APD, and there was no association of vesicoureteral reflux (VUR) with APD measurement.
Postnatal evaluation
The postnatal evaluation of an infant with antenatally detected renal abnormalities should always begin with a physical examination. A palpable abdominal mass may be detected in an infant with a multicystic dysplastic kidney (MCDK), UPJ obstruction, or autosomal recessive polycystic kidney disease (ARPKD). Absent abdominal wall musculature with bilateral undescended testicles suggests a diagnosis of prune belly syndrome (PBS). Infants with a palpable bladder may have posterior urethral valves (PUV) or urethral atresia or stricture. Features of Potter sequence (Table 3) secondary to low amniotic fluid volume may be present. Infants with severe bilateral hydronephrosis or severe unilateral hydronephrosis in a single functioning kidney warrant immediate postnatal evaluation with a renal sonogram and voiding cystourethrogram (VCUG).
Table 3.
Characteristics of Potter sequence
| Potter’s facies |
| Low-set ears |
| Posteriorly rotated ears |
| Hypertelorism |
| Prominent epicanthal folds |
| Flattened nasal bridge |
| Micrognathia |
| Short neck |
| Redundant skin |
| Pulmonary hypoplasia |
| Orthopedic complications |
| Vertebral anomalies |
| Talipes equinovarus |
| Bowing of legs |
| Limb contractures |
| Wide, broad hands |
| Hip dislocation |
| Abnormal genitalia |
Radiologic evaluation of all infants with antenatal renal abnormalities begins with a postnatal renal ultrasound. Results of the postnatal sonogram will dictate subsequent evaluations. Many studies recommend that postnatal renal sonograms be performed soon after birth and again at 4–6 weeks of age [9,10,13••,14,15]. This allows early detection of severe abnormalities while not missing significant abnormality. Neonates have relative oliguria immediately after birth which could result in an inappropriately normal sonogram.
Traditionally, all infants with antenatal hydronephrosis (APD ≥5 mm) received prophylactic antibiotics and underwent postnatal radiologic evaluation with a renal ultrasound and VCUG, even in the setting of a normal postnatal renal ultrasound [16–19]. The VCUG was included because VUR can be present in the setting of a normal ultrasound [10,12,16–19]. Recent studies suggest that VUR is typically mild and self-limited in infants with normal postnatal ultrasounds [11,13••,14]. Because routine prophylactic antibiotics are no longer recommended [20••], `missing' the diagnosis of mild VUR may have no clinical significance. Instead of routine prophylactic antibiotics, families should be counseled on signs and symptoms of urinary tract infections (UTIs) in infants to allow prompt diagnosis and treatment should UTI occur. Prophylactic antibiotics are still recommended in infants with severe obstruction [21••].
Infants with persistent hydronephrosis on postnatal ultrasound (APD >7 mm) should undergo evaluation with a VCUG [9,13••,14,22]. Fluoroscopic VCUG is recommended as it provides visualization of the anatomy of the bladder and urinary system, allowing the diagnosis of PUV, ureteroceles, and other anatomic abnormalities that would be missed on a nuclear VCUG. If the VCUG is normal and the ultrasound shows a pelvic dilatation >10 mm, diuretic renography should be done to assess for UPJ or ureterovesical (UVJ) obstruction [5,7,16]. Figure 1 depicts an algorithm for the work-up of antenatal hydronephrosis.
Figure 1.
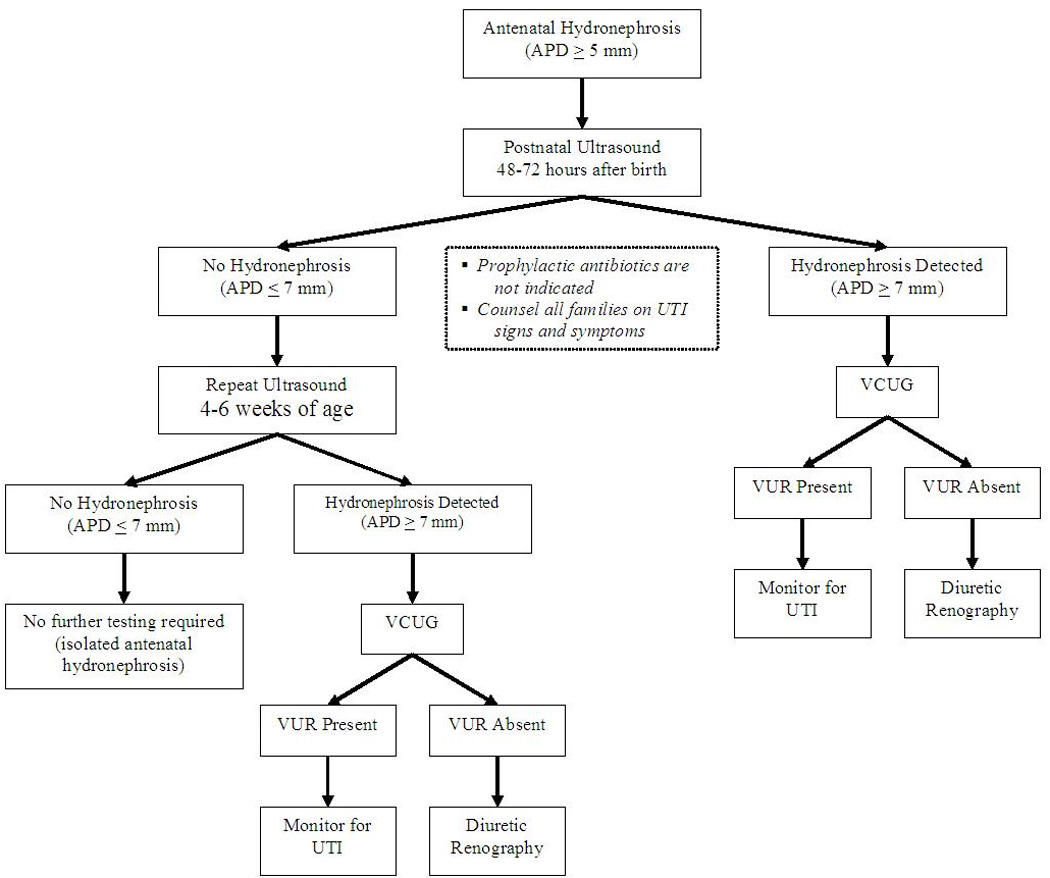
Algorithm for postnatal evaluation of antenatal hydronephrosis gr1 APD, anterior–posterior diameter, VCUG, voiding cystourethrogram, VUR, vesicoureteral reflux.
Isolated antenatal hydronephrosis
Infants with antenatal hydronephrosis but no other detectable abnormalities are classified as having isolated antenatal hydronephrosis. Several cohort studies have provided information on the prevalence of renal abnormalities in infants with isolated antenatal hydronephrosis. A meta-analysis by Sidhu et al. [23] reported that 98% of all mild hydronephrosis (defined at an APD <12 mm) resolved, stabilized, or improved on follow-up. In a study of 143 infants with antenatal hydronephrosis, 50 infants had postnatal sonograms without hydronephrosis. In this group, only three had VUR (by routine VCUG screening), and all were low-grade reflux [14]. Another study of 103 infants with antenatal hydronephrosis had 53 infants with normal postnatal renal ultrasounds. Of those 53, only 3 infants had VUR on routine VCUG screening, and all 3 were grade I VUR [13••]. This group of infants did not receive prophylactic antibiotics and none of them developed a UTI. Also in this cohort, 34 infants were identified with mildly dilated APD postnatally (APD >7 mm but <15 mm) but no other abnormalities. This group of infants did not receive antibiotics, and only one infant developed a UTI during the 2-year follow-up period [13••]. The infants in this study underwent routine dimercaptosuccinic acid (DMSA) scanning at 2 years of age to detect renal scars. Forty-nine of the 53 infants with normal postnatal ultrasounds completed the DMSA scan, and all were normal, including the infants with a previous UTI. Of the 34 infants with mild postnatal hydronephrosis and no other abnormalities, only one had an abnormal DMSA scan at 2 years [24•]. An additional cohort study of 125 infants with isolated antenatal hydronephrosis had 106 infants with an APD 5–14 mm. This subgroup of children had a low rate of VUR, and only two infants developed a UTI in the follow-up period [8•].
Outcome data on a set of infants who underwent limited postnatal screening are available. Mallik and Watson [21••] divide their cohort of infants with isolated antenatal hydronephrosis into two groups. The first group, designated NSD1 (NSD = nonspecific dilatation), consists of infants with antenatal hydronephrosis but normal postnatal ultrasounds (APD <10 mm and no ureteral, renal cortical, or bladder abnormalities). The second group, NSD2, contains infants with a postnatal APD >10 mm but normal VCUG and diuretic renography scans. These infants underwent repeat ultrasound evaluation during the first year of life to confirm a decrease in the APD to <10 mm. Infants in the NSD1 and NSD2 groups did not receive prophylactic antibiotics. Out of the 170 infants in the two groups combined, only one child developed a UTI [21••]. This study highlights the safety in using a limited postnatal evaluation of a select group of infants with antenatal hydronephrosis. Therefore, routine VCUG is not indicated in infants with normal postnatal ultrasounds after birth and again at 4–6 weeks of age. A `normal' ultrasound includes no hydronephrosis, no ureteral dilatation, normal-sized kidneys, no renal cortical abnormalities, and no bladder abnormalities.
Vesicoureteral reflux
Vesicoureteral reflux (VUR) is the upward flow of urine out of the bladder and into the ureters during voiding. A common cause of antenatal hydronephrosis, VUR is found in up to 38% of infants [25], even in the setting of a normal postnatal ultrasound [5]. VUR is graded by the International Reflux Study in Children classification system, detailed in Table 4[26]. Recurrent UTIs from VUR can cause parenchymal scarring resulting in hypertension and/or renal insufficiency [27]. Spontaneous resolution of VUR occurs in 78–90% of grades I–III VUR that is diagnosed during evaluation of antenatal hydronephrosis [22,28]. Grades IV and V may spontaneously resolve but usually require surgical intervention. Infants with VUR of grade III or higher should be referred to a pediatric urologist. Historically, all infants with VUR received antibiotic prophylaxis to prevent UTIs. Pennesi et al. [20••] recently reported on an open-label, randomized, control trial of prophylactic antibiotics versus no prophylaxis in infants with grades II–IV VUR. During the 4-year follow-up, there was no difference between the two groups in the number of UTIs or renal scars [20••]. Current recommendations for treatment of VUR do not include routine prophylactic antibiotics. Instead, parents should be counseled on signs and symptoms of UTIs such as fever, irritability, and foul-smelling urine.
Table 4.
Classification of vesicoureteral reflux (VUR)
| VUR grade | Description |
|---|---|
| I | Reflux of urine into a nondilated ureter |
| II | Reflux of urine into a nondilated renal pelvis |
| III | Reflux of urine into a dilated ureter up to the renal pelvis with possible blunting of the calyceal fornices |
| IV | Reflux of urine into a grossly dilated ureter with moderate blunting of the calyces |
| V | Reflux of urine into a massively dilated and tortuous ureter with loss of the papillary impression |
Multicystic dysplastic kidney
Multicystic dysplastic kidney is a commonly encountered renal abnormality, seen in 1 in 4300 live births [29]. If the antenatal sonogram suggests a MCDK, a postnatal sonogram should be done to confirm the diagnosis. Noncommunicating cysts of various sizes, lack of identifiable renal parenchyma, and atretic proximal ureters are classic ultrasound findings of MCDK. Historically, verification of a nonfunctioning kidney with a DMSA scan was necessary to confirm the diagnosis of MCDK. Recent studies have shown that the DMSA scan is not necessary in the setting of well done ultrasound with features diagnostic of MCDK [30,31]. If there is a question of possible functional renal parenchyma, a DMSA scan should be performed.
Multicystic dysplastic kidneys are associated with a high rate of contralateral renal abnormalities. As many as 50% of infants will have a contralateral renal abnormality, with VUR and UPJ obstruction the most common [32]. Because of the potential for additional abnormalities, previous recommendations for postnatal evaluation of MCDK included routine VCUG and diuretic renography [32,33]. Recent studies suggest a more conservative approach is indicated in this patient population. In a study of 76 infants with unilateral MCDK, 61 infants had a normal renal ultrasound in the newborn period (after 3 days of age) and at 1 month of age [34]. Within this group, four infants had VUR verified by VCUG. All had low-grade VUR (grades I or II), and all had spontaneously resolution of VUR by 2 years of age. The authors conclude that infants with unilateral MCDK who have two consecutive normal ultrasounds of the contralateral kidney do not require routine screening with VCUG [34]. Another study of 90 infants with unilateral MCDK identified 7 infants with VUR [35•]. Four of these infants had a normal ultrasound of the contralateral kidney, but all four had low-grade VUR (grade II). One infant had a documented urinary tract infection, but no scarring was identified in follow-up. Multiple other studies have reported a low rate of VUR in infants with MCDK and a normal contralateral kidney on ultrasound. The VUR was low grade in all of these infants, resolved spontaneously, and likely inconsequential [31,36–38]. Therefore, infants with unilateral MCDK and a normal contralateral kidney on postnatal ultrasounds can be conservatively managed without routine VCUG screening. Families should be counseled on the signs and symptoms of UTI to allow prompt diagnosis and treatment [39••]. Abnormalities of the contralateral kidney (hydronephrosis, small size, lack of corticomedullary differentiation, dilated ureter) should be investigated with a VCUG. If the VCUG is normal, further work-up should include diuretic renography.
Solitary kidney
Solitary kidney may result from complete renal agenesis or from involution of dysplastic kidney tissue [40]. Abnormalities of the solitary kidney are often present and include vesicoureteral reflux, UPJ obstruction, and UVJ obstruction [41,42]. Detection and treatment of abnormalities of a solitary kidney are important in maintaining renal function. In order to achieve this goal, the standard of care has required routine screening with in all infants with a solitary kidney [41–43]. Recent studies, however, have suggested a more conservative approach may be acceptable. In a study of 45 infants with a solitary kidney and normal postnatal ultrasound, only 3 infants had VUR [44••]. None of these infants appeared to require surgical intervention. Therefore, infants with a normal appearing solitary kidney on postnatal ultrasound do not require further imaging studies. If any abnormality is detected on postnatal ultrasound, investigation with a VCUG and diuretic renography should be performed. These children should have close monitoring as they age for the development of hypertension and/or proteinuria as these conditions cause renal damage and may result in renal impairment [45•,46,47].
Ureteropelvic junction obstruction
Ureteropelvic junction obstruction has an incidence of 1 in 1000–1500 newborns and is the most common cause of antenatal hydronephrosis [48,49]. Bilateral obstruction is present in 20–25% of cases, and it is three times more common in males [5]. Many potential causes have been proposed for UPJ obstruction including intrinsic stenosis, insertion anomaly of the ureters, peripelvic fibrosis, peristaltic abnormalities, and blood vessels crossing the ureter [48]. Dilatation of the renal pelvis with normal ureters and bladder are characteristic findings on renal ultrasound. Diuretic renography should be performed to confirm the diagnosis and provide an estimation of differential renal function. Some controversy exists regarding the indications for surgical intervention in UPJ obstruction. However, most experts recommend a conservative approach of observation when obstruction is unilateral with a well functioning kidney (differential function >35%) [7]. Referral to a pediatric urologist is indicated for patients with bilateral UPJ obstruction or unilateral UPJ obstruction with decreased differential function.
Posterior urethral valves
Posterior urethral valves are the most common cause of lower urinary tract obstruction in male infants and occur with an incidence of 1 in 5000–8000 live births. The obstruction results from a thin membrane of tissue in the posterior urethra which blocks urinary flow out of the bladder [50]. A thorough evaluation including renal ultrasound, VCUG, urinalysis, urine culture, and serum electrolytes, creatinine, and blood urea nitrogen should be completed on all infants suspected of having PUV. Ultrasound findings of PUV include bilateral hydronephrosis, hydroureter, posterior urethral dilatation, and a distended, thick-walled bladder. Signs of renal dysplasia may also be seen. Fluoroscopic VCUG confirms the diagnosis with a linear filling defect in the column of radiocontrast in a markedly dilated posterior urethra [51]. VUR is seen in 25–50% of infants with PUV and will be detected on the VCUG [52]. Prenatal diagnosis of PUV allows the physician to begin prompt treatment of the infant, prior to the development of sepsis or metabolic complications. Unfortunately, the outcome of patients with PUV has not been significantly improved with prenatal diagnosis [53]. Infants with PUV require referral to a pediatric urologist for valve ablation and further management.
Prune belly syndrome
Prune belly syndrome is believed to result from early urethral obstruction leading to massive bladder distention, abdominal wall musculature degeneration, and prevention of testicular descent [51]. It has an incidence of 1 in 29 000–40 000 male births [54]. Evaluation of an infant suspected of having PBS should begin with a physical examination, renal ultrasound, VCUG, urinalysis, urine culture, and serum electrolytes, creatinine, and blood urea nitrogen. Typical ultrasound findings include increased bladder wall thickness and dilated, tortuous ureters with thickened walls. Other potential findings include hydronephrosis, renal dysplasia, and a patent urachus. VCUG will detect concomitant VUR, seen in approximately 85% of infants with PBS [55]. PBS is associated with anomalies of many systems including gastrointestinal (malrotation, volvulus, gastroschisis, omphalocele, Hirschsprung’s, imperforate anus), pulmonary (hypoplasia secondary to oligohydramnios), cardiac (atrial septal defect, ventricular septal defect, tetralogy of Fallot), and orthopedic (developmental dislocation of the hip, scoliosis, pectus excavatum, talipes equinovarus, torticollis) [51]. Infants with PBS should be referred promptly to a pediatric urologist.
Ureterovesical obstruction
Ureterovesical obstruction occurs more frequently on the left and four times more frequently in males. Twenty-five percentage of cases are bilateral. Ultrasound findings include ureteral dilatation (megaureter) and dilated renal pelvis. UVJ obstruction results from an aperistaltic segment of the distal ureter, near the UVJ. Many urologists promote conservative management [56,57], but surgical repair may be necessary. Referral to a pediatric urologist is recommended to determine surgical need which typically includes excision of the distal ureteric segment, tapering, and reimplantation of the ureter [58].
Ureterocele
Ureteroceles are formed from the cystic dilatation of the intravesical ureter. They are commonly associated with duplicated collecting systems or ectopic ureteral insertions. The incidence of ureterocele is 1 in 5000 infants, occurring 2.5–3 times more often in females and bilaterally 20–50% of the time [59]. On ultrasound examination, the ureterocele may be detected as a thin membrane within the bladder. Ureteroceles associated with duplicated collecting systems typically will manifest upper pole hydroureteronephrosis on ultrasound. Postnatal work-up should include a VCUG to confirm the diagnosis of ureterocele and assess for concomitant VUR. On a VCUG, the ureterocele will appear as a round filling defect within the bladder. If a duplicated collecting system is suspected, nuclear renography should be performed to determine the function of the upper renal pole. Treatment includes referral to a pediatric urologist for endoscopic incision of the ureterocele [60]. If the upper pole moiety of a duplicated collecting system is nonfunctioning, upper pole heminephro-ureterectomy may be performed.
Conclusion
Routine ultrasound screening of pregnant women has led to an increase in the number of detected renal abnormalities. The most commonly detected renal abnormality is hydronephrosis. Evaluation of all infants with an abnormal antenatal sonogram should begin with postnatal renal ultrasounds after birth and again at 4–6 weeks of age. If abnormalities on postnatal sonogram are detected, further work-up should continue with VCUG followed by diuretic renography if indicated. Routine VCUG screening of all infants with antenatal hydronephrosis is not indicated if the postnatal ultrasounds are normal. Additionally, routine antibiotic prophylaxis for antenatal hydronephrosis is not recommended. Families should receive education on signs and symptoms of UTIs to allow prompt diagnosis and treatment should a UTI occur.
Acknowledgement
A.B. is supported in part by KL2RR024983.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
•of special interest
••of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Carvalho MH, Brizot ML, Lopes LM, et al. Detection of fetal structural abnormalities at the 11–14 week ultrasound scan. Prenat Diagn. 2002;22:1–4. doi: 10.1002/pd.200. [DOI] [PubMed] [Google Scholar]
- 2.Dillon E, Walton SM. The antenatal diagnosis of fetal abnormalities: a 10 year audit of influencing factors. Br J Radiol. 1997;70:341–346. doi: 10.1259/bjr.70.832.9166068. [DOI] [PubMed] [Google Scholar]
- 3.Dudley JA, Haworth JM, McGraw ME, et al. Clinical relevance and implications of antenatal hydronephrosis. Arch Dis Child Fetal Neonatal Ed. 1997;76:F31–F34. doi: 10.1136/fn.76.1.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth JA, Diamond DA. Prenatal hydronephrosis. Curr Opin Pediatr. 2001;13:138–141. doi: 10.1097/00008480-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Woodward M, Frank D. Postnatal management of antenatal hydronephrosis. BJU Int. 2002;89:149–156. doi: 10.1046/j.1464-4096.2001.woodward.2578.x. [DOI] [PubMed] [Google Scholar]
- 6.Podevin G, Mandelbrot L, Vuillard E, et al. Outcome of urological abnormalities prenatally diagnosed by ultrasound. Fetal Diagn Ther. 1996;11:181–190. doi: 10.1159/000264300. [DOI] [PubMed] [Google Scholar]
- 7.Shokeir AA, Nijman RJ. Antenatal hydronephrosis: changing concepts in diagnosis and subsequent management. BJU Int. 2000;85:987–994. doi: 10.1046/j.1464-410x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 8. de Kort EH, Bambang OS, Zegers SH. The long-term outcome of antenatal hydronephrosis up to 15 millimetres justifies a noninvasive postnatal follow-up. Acta Paediatr. 2008;97:708–713. doi: 10.1111/j.1651-2227.2008.00749.x. This study of infants with antenatal hydronephrosis demonstrated a good outcome for infants with an anterior–posterior diameter less than 15 mm on postnatal ultrasound.
- 9.Ek S, Lidefeldt KJ, Varricio L. Fetal hydronephrosis; prevalence, natural history and postnatal consequences in an unselected population. Acta Obstet Gynecol Scand. 2007;86:1463–1466. doi: 10.1080/00016340701714802. [DOI] [PubMed] [Google Scholar]
- 10.Aksu N, Yavascan O, Kangin M, et al. Postnatal management of infants with antenatally detected hydronephrosis. Pediatr Nephrol. 2005;20:1253–1259. doi: 10.1007/s00467-005-1989-3. [DOI] [PubMed] [Google Scholar]
- 11.Coelho GM, Bouzada MC, Pereira AK, et al. Outcome of isolated antenatal hydronephrosis: a prospective cohort study. Pediatr Nephrol. 2007;22:1727–1734. doi: 10.1007/s00467-007-0539-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee RS, Cendron M, Kinnamon DD, Nguyen HT. Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics. 2006;118:586–593. doi: 10.1542/peds.2006-0120. [DOI] [PubMed] [Google Scholar]
- 13. Lidefelt KJ, Herthelius M. Antenatal hydronephrosis: infants with minor postnatal dilatation do not need prophylaxis. Pediatr Nephrol. 2008;23:2021–2024. doi: 10.1007/s00467-008-0893-z. This study describes the incidence of VUR in infants with antenatal hydronephrosis and normal postnatal ultrasounds. VUR is infrequent in this group, and the use of routine antibiotic prophylaxis is not supported.
- 14.Merlini L, Parvex P, nooshiravani-Dumont M, et al. Postnatal management of isolated mild pelvic dilatation detected in antenatal period. Acta Paediatr. 2007;96:1131–1134. doi: 10.1111/j.1651-2227.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 15.Ismaili K, Hall M, Piepsz A, et al. Insights into the pathogenesis and natural history of fetuses with renal pelvis dilatation. Eur Urol. 2005;48:207–214. doi: 10.1016/j.eururo.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Jaswon MS, Dibble L, Puri S, et al. Prospective study of outcome in antenatally diagnosed renal pelvis dilatation. Arch Dis Child Fetal Neonatal Ed. 1999;80:F135–F138. doi: 10.1136/fn.80.2.f135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan V, Traubici J, Hershenfield B, et al. Vesicoureteral reflux in infants with isolated antenatal hydronephrosis. Pediatr Nephrol. 2003;18:1224–1228. doi: 10.1007/s00467-003-1287-x. [DOI] [PubMed] [Google Scholar]
- 18.Alconcher L, Tombesi M. Mild antenatal hydronephrosis: management controversies. Pediatr Nephrol. 2004;19:819–820. doi: 10.1007/s00467-004-1476-2. [DOI] [PubMed] [Google Scholar]
- 19.Tibballs JM, De BR. Primary vesicoureteric reflux: how useful is postnatal ultrasound? Arch Dis Child. 1996;75:444–447. doi: 10.1136/adc.75.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pennesi M, Travan L, Peratoner L, et al. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics. 2008;121:e1489–e1494. doi: 10.1542/peds.2007-2652. This is a randomized, controlled trial investigating the effectiveness of prophylactic antibiotics in preventing UTIs in children with VUR. Rates of UTIs and renal scars were the same in both groups. Thus, prophylactic antibiotics do not offer any benefit in the treatment of VUR.
- 21. Mallik M, Watson AR. Antenatally detected urinary tract abnormalities: more detection but less action. Pediatr Nephrol. 2008;23:897–904. doi: 10.1007/s00467-008-0746-9. This longitudinal study provides outcome data on a cohort of infants who underwent limited postnatal evaluation for isolated antenatal hydronephrosis. Infants with normal postnatal ultrasounds did not have voiding cystourethrograms or receive prophylactic antibiotics. Only one infant developed a UTI.
- 22.Ismaili K, Hall M, Piepsz A, et al. Primary vesicoureteral reflux detected in neonates with a history of fetal renal pelvis dilatation: a prospective clinical and imaging study. J Pediatr. 2006;148:222–227. doi: 10.1016/j.jpeds.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Sidhu G, Beyene J, Rosenblum ND. Outcome of isolated antenatal hydronephrosis: a systematic review and meta-analysis. Pediatr Nephrol. 2006;21:218–224. doi: 10.1007/s00467-005-2100-9. [DOI] [PubMed] [Google Scholar]
- 24. Lidefelt KJ, Herthelius M, Soeria-Atmadja S. Antenatal renal pelvis dilatation: 2-year follow-up with DMSA scintigraphy. Pediatr Nephrol. 2008 doi: 10.1007/s00467-008-1043-3. [Epub ahead of print] This study provides follow-up on a cohort of infants with antenatal hydronephrosis. None of the infants with normal postnatal ultrasounds developed renal scars.
- 25.Zerin JM, Ritchey ML, Chang AC. Incidental vesicoureteral reflux in neonates with antenatally detected hydronephrosis and other renal abnormalities. Radiology. 1993;187:157–160. doi: 10.1148/radiology.187.1.8451404. [DOI] [PubMed] [Google Scholar]
- 26.Lebowitz RL, Olbing H, Parkkulainen KV, et al. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr Radiol. 1985;15:105–109. doi: 10.1007/BF02388714. [DOI] [PubMed] [Google Scholar]
- 27.Vanderheyden T, Kumar S, Fisk NM. Fetal renal impairment. Semin Neonatol. 2003;8:279–289. doi: 10.1016/S1084-2756(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 28.Steele BT, Robitaille P, DeMaria J, Grignon A. Follow-up evaluation of prenatally recognized vesicoureteric reflux. J Pediatr. 1989;115:95–96. doi: 10.1016/s0022-3476(89)80337-3. [DOI] [PubMed] [Google Scholar]
- 29.Hartman GE, Shochat SJ. Abdominal mass lesions in the newborn: diagnosis and treatment. Clin Perinatol. 1989;16:123–135. [PubMed] [Google Scholar]
- 30.Feldenberg LR, Siegel NJ. Clinical course and outcome for children with multicystic dysplastic kidneys. Pediatr Nephrol. 2000;14:1098–1101. doi: 10.1007/s004670000391. [DOI] [PubMed] [Google Scholar]
- 31.Alconcher L, Tombesi M. Multicystic dysplastic kidney detected by prenatal ultrasonography: conservative management. Pediatr Nephrol. 2005;20:1024–1025. doi: 10.1007/s00467-005-1866-0. [DOI] [PubMed] [Google Scholar]
- 32.Atiyeh B, Husmann D, Baum M. Contralateral renal abnormalities in multicystic-dysplastic kidney disease. J Pediatr. 1992;121:65–67. doi: 10.1016/s0022-3476(05)82543-0. [DOI] [PubMed] [Google Scholar]
- 33.Okada T, Yoshida H, Matsunaga T, et al. Multicystic dysplastic kidney detected by prenatal ultrasonography: natural history and conservative management. Pediatr Surg Int. 2003;19:207–210. doi: 10.1007/s00383-002-0920-2. [DOI] [PubMed] [Google Scholar]
- 34.Ismaili K, Avni FE, Alexander M, et al. Routine voiding cystourethrography is of no value in neonates with unilateral multicystic dysplastic kidney. J Pediatr. 2005;146:759–763. doi: 10.1016/j.jpeds.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 35. Kiyak A, Yilmaz A, Turhan P, Sander S, Aydin G, Aydogan G. Unilateral multicystic dysplastic kidney: single-center experience. Pediatr Nephrol. 2008 doi: 10.1007/s00467-008-0942-7. [Epub ahead of print] This study details the outcomes of children with multicystic dysplastic kidneys from a single center over a period of 17 years.
- 36.Rahman RC, Amoreo O. Multicystic dysplastic kidney: diagnosis and evolution. Pediatr Nephrol. 2005;20:1023. doi: 10.1007/s00467-005-1865-1. [DOI] [PubMed] [Google Scholar]
- 37.Kuwertz-Broeking E, Brinkmann OA, Von Lengerke HJ, et al. Unilateral multicystic dysplastic kidney: experience in children. BJU Int. 2004;93:388–392. doi: 10.1111/j.1464-410x.2003.04623.x. [DOI] [PubMed] [Google Scholar]
- 38.Aslam M, Watson AR. Unilateral multicystic dysplastic kidney: long term outcomes. Arch Dis Child. 2006;91:820–823. doi: 10.1136/adc.2006.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hains DS, Bates CM, Ingraham S, Schwaderer AL. Management and etiology of the unilateral multicystic dysplastic kidney: a review. Pediatr Nephrol. 2008;24:233–241. doi: 10.1007/s00467-008-0828-8. This is a comprehensive review of MCDKs. It includes pathogenesis, associated abnormalities, natural history, and recommended diagnostic studies.
- 40.Zaffanello M, Brugnara M, Zuffante M, Franchini M, Fanos V. Are children with congenital solitary kidney at risk for lifelong complications? A lack of prediction demands caution. Int Urol Nephrol. 2008 doi: 10.1007/s11255-008-9437-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Cascio S, Paran S, Puri P. Associated urological anomalies in children with unilateral renal agenesis. J Urol. 1999;162:1081–1083. doi: 10.1016/S0022-5347(01)68074-1. [DOI] [PubMed] [Google Scholar]
- 42.Kaneyama K, Yamataka A, Satake S, et al. Associated urologic anomalies in children with solitary kidney. J Pediatr Surg. 2004;39:85–87. doi: 10.1016/j.jpedsurg.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Song JT, Ritchey ML, Zerin JM, Bloom DA. Incidence of vesicoureteral reflux in children with unilateral renal agenesis. J Urol. 1995;153:1249–1251. [PubMed] [Google Scholar]
- 44. Calisti A, Perrotta ML, Oriolo L, et al. The risk of associated urological abnormalities in children with pre and postnatal occasional diagnosis of solitary, small or ectopic kidney: is a complete urological screening always necessary? World J Urol. 2008;26:281–284. doi: 10.1007/s00345-008-0249-0. This is a study of solitary kidneys and associated urologic abnormalities. Children with a normal contralateral kidney on ultrasound had a low likelihood of significant abnormalities. The authors recommend discriminate screening with voiding cystourethrogram in patients with abnormal ultrasounds of the contralateral kidney.
- 45. Vu KH, Van DM, Daniels H, Proesmans W. Renal outcome of children with one functioning kidney from birth. A study of 99 patients and a review of the literature. Eur J Pediatr. 2008;167:885–890. doi: 10.1007/s00431-007-0612-y. This is a study describing the long-term outcome of a cohort of patients with a solitary functioning kidney from birth. Outcomes measured include glomerular filtration rate, hypertension, and proteinuria.
- 46.Hellerstein S, Chambers L. Solitary kidney. Clin Pediatr (Phila) 2008;47:652–658. doi: 10.1177/0009922808315661. [DOI] [PubMed] [Google Scholar]
- 47.Hegde S, Coulthard MG. Renal agenesis and unilateral nephrectomy: what are the risks of living with a single kidney? Pediatr Nephrol. 2008 doi: 10.1007/s00467-008-0924-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Williams B, Tareen B, Resnick MI. Pathophysiology and treatment of ureteropelvic junction obstruction. Curr Urol Rep. 2007;8:111–117. doi: 10.1007/s11934-007-0059-8. [DOI] [PubMed] [Google Scholar]
- 49.Chevalier RL. Perinatal obstructive nephropathy. Semin Perinatol. 2004;28:124–131. doi: 10.1053/j.semperi.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Imaji R, Moon DA, Dewan PA. Congenital posterior urethral membrane: variable morphological expression. J Urol. 2001;165:1240–1242. [PubMed] [Google Scholar]
- 51.Strand WR. Initial management of complex pediatric disorders: prunebelly syndrome, posterior urethral valves. Urol Clin North Am. 2004;31:399–415. doi: 10.1016/j.ucl.2004.04.010. vii. [DOI] [PubMed] [Google Scholar]
- 52.Agarwal S. Urethral valves. BJU Int. 1999;84:570–578. doi: 10.1046/j.1464-410x.1999.00307.x. [DOI] [PubMed] [Google Scholar]
- 53.El-Ghoneimi A, Desgrippes A, Luton D, et al. Outcome of posterior urethral valves: to what extent is it improved by prenatal diagnosis? J Urol. 1999;162:849–853. doi: 10.1097/00005392-199909010-00076. [DOI] [PubMed] [Google Scholar]
- 54.Wheatley JM, Stephens FD, Hutson JM. Prune-belly syndrome: ongoing controversies regarding pathogenesis and management. Semin Pediatr Surg. 1996;5:95–106. [PubMed] [Google Scholar]
- 55.Walker J, Prokurat AI, Irving IM. Prune belly syndrome associated with exomphalos and anorectal agenesis. J Pediatr Surg. 1987;22:215–217. doi: 10.1016/s0022-3468(87)80331-7. [DOI] [PubMed] [Google Scholar]
- 56.Oliveira EA, Diniz JS, Rabelo EA, et al. Primary megaureter detected by prenatal ultrasonography: conservative management and prolonged follow-up. Int Urol Nephrol. 2000;32:13–18. doi: 10.1023/a:1007101227302. [DOI] [PubMed] [Google Scholar]
- 57.Keating MA, Escala J, Snyder HM, III, et al. Changing concepts in management of primary obstructive megaureter. J Urol. 1989;142:636–640. doi: 10.1016/s0022-5347(17)38841-9. [DOI] [PubMed] [Google Scholar]
- 58.Shenoy MU, Rance CH. Is there a place for the insertion of a JJ stent as a temporizing procedure for symptomatic partial congenital vesico-ureteric junction obstruction in infancy? BJU Int. 1999;84:524–525. doi: 10.1046/j.1464-410x.1999.00256.x. [DOI] [PubMed] [Google Scholar]
- 59.Pohl HG, Joyce GF, Wise M, Cilento BG., Jr Vesicoureteral reflux and ureteroceles. J Urol. 2007;177:1659–1666. doi: 10.1016/j.juro.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 60.Vergani P, Ceruti P, Locatelli A, et al. Accuracy of prenatal ultrasonographic diagnosis of duplex renal system. J Ultrasound Med. 1999;18:463–467. doi: 10.7863/jum.1999.18.7.463. [DOI] [PubMed] [Google Scholar]


