Abstract
The mammary gland develops in a process known as branching morphogenesis, whereby a distal epithelial bud extends and bifurcates to form an extensive ductal network. Compared with other branched organs, such as the lung and kidney, little is known about the molecular basis of branching in the mammary gland. Here we report a microarray profiling strategy to identify novel genes that may regulate mammary branching. We microdissected terminal end bud (TEB) and mature duct microenvironments from β-actin–green fluorescent protein reporter mice and compared their RNA expression profiles with epithelium-free mammary stroma by means of microarray. We identified 1,074 genes enriched in the TEB microenvironment, 222 genes enriched in the mature duct microenvironment, and 385 genes enriched in both TEB and mature duct microenvironments. The microarray correctly predicted the expression of genes known to be enriched in the epithelium (Ets-5) and stroma (MMP-14) of TEBs and in the mature duct microenvironment (MMP-3). The microarray also correctly predicted the localization of previously uncharacterized genes, such as the TEB-enriched SPRR-1a, the duct-enriched casein-γ, and the general epithelial marker pleiotrophin. Analysis of genes enriched in TEBs revealed several genes in the Wnt (Wnt-2, Wnt-5a, Wnt-7b, Dsh-3, Frizzled-1, Frizzled-2), hedgehog (Dhh), ephrin (Ephrin-B1, Eph-A2), and transcription factor (Twist-1, Twist-2, Snail) families. In situ hybridization verified that these genes were enriched in the TEB epithelium (Wnt-5a, Wnt-7b, Dhh, Eph-A2) or TEB stroma (Wnt-2, Frizzled-1, Ephrin-B1). We discuss the potential roles of these genes in mammary branching morphogenesis.
Keywords: branching morphogenesis, mammary gland, microarray profiling, terminal end buds, wnt signaling, hedgehog signaling
Introduction
The branched ductal network is the most common structural motif in organisms and is found in organs such as the lung, salivary gland, kidney, and mammary gland. The development of the ductal network takes places through branching morphogenesis, a process by which a rudimentary ductal structure initiates, extends, bifurcates, and differentiates to give rise to the mature tubular organ (Affolter et al., 2003; Ghabrial et al., 2003). The iterative nature of ductal morphogenesis allows a large epithelial surface area to be generated in limited space, which is essential because these organs function in air/fluid exchange and secretion. The key structure that drives branching morphogenesis is the distal epithelial bud, which simultaneously accomplishes several cellular processes (Hinck and Silberstein, 2005). This motile structure extends into mesenchyme to elongate the ducts, interacts with the immediate mesenchyme to initiate branch points that are optimally spaced, generates a hollow lumen for ductal transport, produces and remodels the basement membrane to allow cellular adhesion to the extracellular matrix, and terminally differentiates to give rise to mature ducts (Lin et al., 2003; Sternlicht, 2006). The complexity of the process suggests that cell movement, cell adhesion, cell division, and cell death are coordinated in the distal end bud.
Branching morphogenesis in the embryonic lung end bud is mediated by interactions between the epithelium of the bud and its surrounding mesenchymal cells involving members of the fibroblast growth factor (FGF), sonic hedgehog (Shh), Wnt, and transforming growth factor-beta (TGF-β) families (Warburton et al., 2005; Cardoso and Lu, 2006). FGF-10 expressed by mesenchymal cells is a chemotactic and proliferation factor that activates FGFR-2B in the epithelium. Feedback control of this epithelial–mesenchymal crosstalk serves to regulate the size and shape of the end bud and the initiation of branch points (Lebeche et al., 1999; Hyatt et al., 2004). A key regulatory factor is Shh, which is highly expressed in the end bud epithelium (Bellusci et al., 1997). Shh is a paracrine factor that activates signaling pathways in immediate mesenchyme by means of patched/smoothened receptors and their transcriptional effectors Gli-1, Gli-2, and Gli-3 (Huangfu and Anderson, 2006). Shh signaling negatively regulates FGF-10 expression in the mesenchyme, thereby restricting FGF signaling in the end bud (Lebeche et al., 1999). This serves to control end bud size and shape by preventing widespread FGF-10 expression in the mesenchyme and FGFR-2B overactivation in the epithelium. Mice with targeted deletion of Shh or ectopic overexpression of Shh in the distal bud display severe defects in lung development, indicating an essential role for Shh in the branching process (Bellusci et al., 1997; Litingtung et al., 1998; Pepicelli et al., 1998).
The Wnt family of secreted proteins also plays essential roles in branching morphogenesis of the lung. The Wnt family includes at least 19 different secreted ligands that interact with 10 known frizzled receptors to regulate cell proliferation, polarity, and differentiation (Logan and Nusse, 2004). Wnt signaling takes place through a canonical pathway that involves the stabilization of β-catenin and activation of the Tcf/Lef transcription factors or a noncanonical pathway that is independent of β-catenin (Logan and Nusse, 2004). Disruption of the canonical Wnt pathway, either by targeted deletion of β-catenin or overexpression of the Wnt inhibitor dickkopf-1 (Dkk-1), leads to defects in distal epithelial differentiation and a deficiency in branching morphogenesis (Mucenski et al., 2003; De Langhe et al., 2005). The canonical pathway inhibits terminal differentiation of the distal epithelium and maintains a proliferative progenitor state in which branching morphogenesis can take place (Okubo and Hogan, 2004). The noncanonical Wnt pathway also plays an important role in branching morphogenesis. Mice lacking Wnt-5a (a noncanonical Wnt normally expressed in the epithelium and mesenchyme of the distal end bud) show overexpansion of the distal lung epithelium and increased Shh and FGF-10 expression (Li et al., 2002). The noncanonical Wnt-5a pathway thus plays a negative regulatory role in lung branching.
The mammary gland is unique among ductal epithelial organs in that its development occurs postnatally (Wiseman and Werb, 2002). Before the onset of puberty, the mammary gland is a rudimentary organ consisting of a primitive network of ductal epithelium. Shortly after the onset of puberty, specialized structures known as terminal end buds (TEBs) develop at the distal epithelial tips. The TEBs proliferate, bifurcate, and invade into a fatty stroma until 10–12 weeks of age, at which point they regress (Sternlicht, 2006). Little is known about the molecular basis of branching in the mammary gland. The hedgehog members Shh and Ihh are not necessary for mammary branching as Shh−/− and Ihh−/− rescued mammary anlagen can give rise to a normal mammary gland (Gallego et al., 2002; Michno et al., 2003). No members of the Wnt signaling pathway have been shown to be specifically expressed in the TEBs or necessary for TEB-induced (primary–secondary) branching, although Wnt-4 is necessary for side (tertiary) branching (Brisken et al., 2000). Wnt-signaling pathway components are necessary for other steps in mammary development such as placode formation and lobulo-alveolar development (Hatsell et al., 2003). Here we report an expression profiling strategy to identify novel candidates that may carry out branching morphogenesis in the mammary gland.
Results and Discussion
Expression Profiling of Mammary Microenvironments
We devised a novel microarray strategy using β-actin–green fluorescent protein (GFP) reporter mice, which display high GFP expression throughout the mammary epithelium (Hadjantonakis et al., 1998). TEBs, mature ducts, and epithelium-free stromal compartments were surgically microdissected from 5-week-old β-actin–GFP mice, and RNA was immediately harvested for analysis (Fig. 1A). The microdissection approach enabled us to determine gene expression in both the epithelium and stroma of these distinct microenvironments. The RNA expression profiles of the TEB (n = 6) and mature duct (n = 6) microenvironments were compared with the epithelium-free stroma using long-oligonucleotide spotted microarrays with 19,500 features. We identified 1,074 genes that were uniquely enriched in the TEB microenvironment, 222 genes that were uniquely enriched in the mature duct microenvironment, and 385 genes that were enriched in both TEB and mature duct microenvironments (Fig. 1B). Additionally, we identified 507 genes that were enriched in the distal stroma.
Fig. 1.
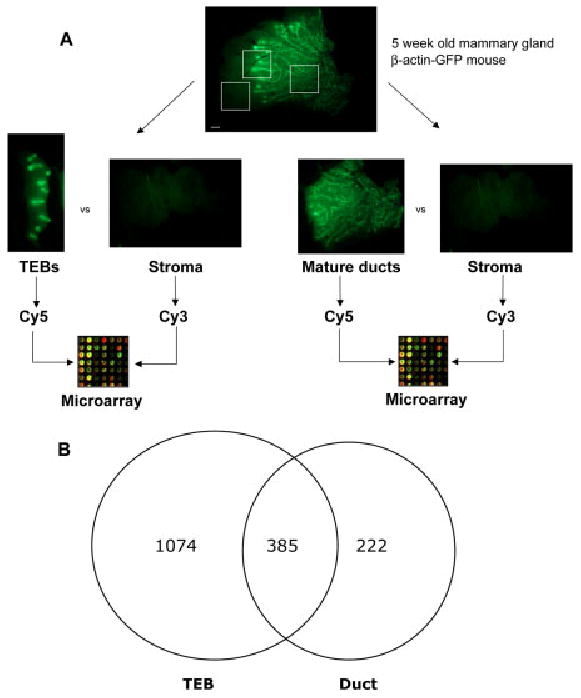
Expression profiling strategy. A: Number 4 mammary gland of 5-week-old β-actin–green fluorescent protein (GFP) mouse was microdissected into terminal end bud (TEB), mature duct, and epithelium-free distal stroma components for microarray analysis. Boxes represent the TEB, duct, and stromal microenvironments isolated for microarray analysis. B: Number of genes enriched in TEB and mature duct microenvironments (fold change > 1.5, adjusted P < 0.05). Scale bar = 1 mm.
Further analysis of these genes revealed that that the most highly enriched genes families in mature ducts were milk proteins, such as casein-α, Apod, and lactalbumin, and myoepithelial-specific genes, such as Myh11, Cnn1, and Mylk (Table 1). This finding is consistent with the differentiated status of the mature ducts relative to TEBs. In contrast, the most highly enriched gene families in TEBs were cell cycle genes, such as Cdc20, Cyclin B1, and Mcm3, and actin remodeling genes, such as Myh9, Fscn1, and Capg (Table 1). These data are consistent with the highly proliferative and motile nature of TEBs compared with the quiescent mature ducts.
Table 1. Most Highly Enriched Gene Families in Terminal End Buds and Ducts.
| Gene family (no. of genes) | Accession no. | TEB ratioa | Gene family (no. of genes) | Accession no. | Duct ratiob |
|---|---|---|---|---|---|
| Cell cycle (33) | 2.3 | Milk proteins (12) | 4.0 | ||
| Cdc20 | NM_023223 | 4.4 | Csn-γ | NM_007785 | 15.6 |
| Cyclin B1 | NM_007629 | 2.6 | Apod | NM_007470 | 12.1 |
| Mcm3 | X62154 | 2.5 | Csn-α | NM_007784 | 6.9 |
| Rrm2 | NM_009104 | 2.6 | Lalba | NM_010679 | 4.3 |
| Actin remodeling (20) | 2.3 | Myoepithelial (6) | 2.3 | ||
| Myh9 | AJ312390 | 2.8 | Myh11 | NM_013607 | 4.6 |
| Fscn1 | NM_007984 | 2.1 | Cnn1 | NM_009922 | 3.4 |
| Capg | NM_007599 | 1.9 | Mylk | AF314149 | 2.0 |
| Nucleotide synth. (5) | 2.2 | ECM/Structural (15) | 2.0 | ||
| Dutp | NM_023595 | 2.4 | Lmnb1 | BC006696 | 8.0 |
| Folr4 | AK003087 | 2.3 | Fgg | AK013887 | 3.5 |
| Protease (8) | 2.2 | Anti-microbial (9) | 2.0 | ||
| imp1 | NM_011593 | 4.8 | Lao1 | BC017599 | 3.7 |
| MMP-14 | NM_008608 | 2.6 | Alox12e | X99252 | 1.9 |
| Transporter (10) | 2.1 | Protease (12) | 1.7 | ||
| Kcnn4 | NM_008433 | 2.3 | MMP-3 | NM_010809 | 3.1 |
| Slc7a5 | NM_011404 | 2.3 | Cst3 | NM_009976 | 2.1 |
| Apoptosis (3) | 2.1 | Transporter (20) | 1.7 | ||
| Expi | NM_007969 | 2.6 | Ttr | NM_013697 | 4.9 |
| Transcrip. Factor (18) | 2.0 | Cell cycle control(6) | 1.6 | ||
| Klf-5 | AK015853 | 3.1 | Osmr | NM_011019 | 2.4 |
| Tcf-7 | NM_009331 | 2.8 | Gas5 | NM_013525 | 2.1 |
| ECM/Structural (12) | 2.0 | Signal trans. (6) | 1.6 | ||
| Upk3a | NM_023478 | 2.9 | Erbb2ip | NM_021563 | 2.7 |
| Lad1 | BC016257 | 2.3 | Gna14 | NM_008137 | 1.6 |
| Cell adhesion (8) | 1.9 | Cytoskeletal (5) | 1.6 | ||
| Cldn3 | NM_009902 | 2.0 | Ktn | NM_008477 | 2.6 |
| Patterning/Ep-Mes Crosstalk (13) | 1.8 | Cell adhesion (5) | 1.5 | ||
| Wnt-7b | NM_009528 | 2.1 | Ceacam10 | NM_007675 | 1.8 |
| Wnt-5a | NM_009524 | 2.0 | Pcdh21 | AF426393 | 1.6 |
| Ribosomal (8) | 1.8 | Transcrip. Factor(13) | 1.5 | ||
| Rpl13a | NM_009438 | 2.0 | FoxI1 | BC007475 | 2.7 |
TEB ratio = M(TEB) − M(duct), expressed as fold change.
Duct ratio = M(duct) − M(TEB), expressed as fold change.
Proof-of-Concept Analysis
We determined how well the microarray predicted the expression of genes known to be enriched in the TEBs or mature ducts. The matrix metalloproteinases MMP-14 (MT1-MMP) and MMP-3 (stromelysin-1) are enriched in the stroma of the TEBs and mature ducts, respectively (Wiseman et al., 2003). The transcription factor Ets-5 is enriched in the TEB epithelium, whereas cytokeratins 8 and 18 are expressed throughout the epithelium of TEBs and the luminal epithelium of mature ducts (Chotteau-Lelievre et al., 2003). The microarray data correctly recapitulated the expression localization of these genes (Fig. 2A; Supplementary Material Table S1, which can be viewed at http://www.interscience.wiley.com/jpages/1058-8388/suppmat). MMP-14 and Ets-5 were enriched in the TEB microenvironment, while MMP-3 was enriched in the mature duct microenvironment. Cytokeratins 8 and 18 were highly enriched in both the TEBs and mature duct microenvironments. The housekeeping genes HPGRT and GAPDH were not enriched in any compartment. Thus the microarray strategy correctly predicted the expression of known genes and was also capable of detecting genes expressed in either the stroma or epithelium of the TEBs and mature ducts.
Fig. 2.
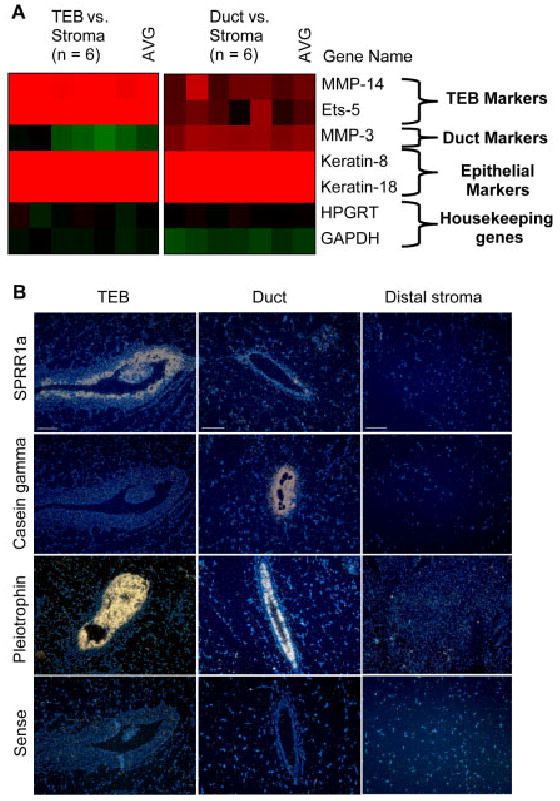
Proof-of-concept analysis. A: Clustered image map of microarray data comparing Cy5-labeled terminal end buds (TEBs; red, left) or mature ducts (red, right) and Cy3-labeled distal stroma (green). Color intensity represents M = log2(Cy5 fluorescence/Cy3 fluorescence). B: In situ hybridization of TEBs, mature ducts, and distal stroma in 5-week-old mammary gland. 4′,6-diamidine-2-phenylidole-dihydrochloride (DAPI; blue) and darkfield signal (white) were merged in the images. Scale bar = 50 μm.
We then used in situ hybridization to determined how well the microarray predicted the expression of genes that had not been previously characterized in the mammary gland. The small proline-rich protein SPRR-1a (TEB ratio, 7.4-fold change) and casein-γ (duct ratio, 15.6-fold change) were predicted to be localized to the TEBs and mature ducts, respectively. The growth factor pleiotrophin was predicted to be highly expressed in both TEBs (M(TEB), 4.5-fold change) and mature ducts (M(duct), 5.9-fold change). In situ hybridization of 5-week mammary tissue verified these expression profiles (Fig. 2B). SPRR-1a was highly expressed in the TEB epithelium and not in mature ducts, casein-γ was highly expressed in mature ducts and not in TEBs, and pleiotrophin was highly expressed throughout the TEB and mature duct epithelium (Fig. 2B). These data provided a proof-of-concept that our microarray strategy could correctly predict gene expression in the developing mammary gland. We then analyzed the genes that were exclusively enriched in the TEBs to identify candidate regulators of mammary branching morphogenesis.
Wnt Expression
Several Wnt family components showed restricted localization to the TEBs. Analysis of 18 Wnt ligands revealed that six Wnt ligands were enriched in the mammary epithelium. Three Wnt genes (Wnt-2, Wnt-5a, Wnt-7b) were exclusively enriched in the TEB microenvironment (Fig. 3B; Supplementary Table S1). Three other Wnt genes (Wnt-4, Wnt-5b, Wnt-6) were enriched in both the TEBs and mature duct microenvironments relative to distal stroma. In situ hybridization verified the spatial expression patterns for these Wnt ligands. Wnt-5a and Wnt-7b signal were detected in the epithelium of TEBs while Wnt-2 was detected in the stroma surrounding the TEBs (Fig. 4). We did not detect the expression of Wnt-2, Wnt-5a, or Wnt-7b in the mature ducts (Fig. 4). Wnt-5b was detected in the epithelium of both TEBs and mature ducts (Fig. 4). Other Wnt pathway components that showed restricted localization to the TEBs were Frizzled-1, Frizzled-2, and Dsh-3 (Fig. 3A). Of interest, in situ hybridization showed that Frizzled-1 was localized to the stroma surrounding TEBs, a pattern that mirrored Wnt-2 expression (Fig. 4). These data implicate specific members of the Wnt pathway (Wnt-2, Wnt-5a, Wnt-7b, Frizzled-1, Frizzled-2, Dsh-3) as potential regulators of mammary branching development.
Fig. 3.
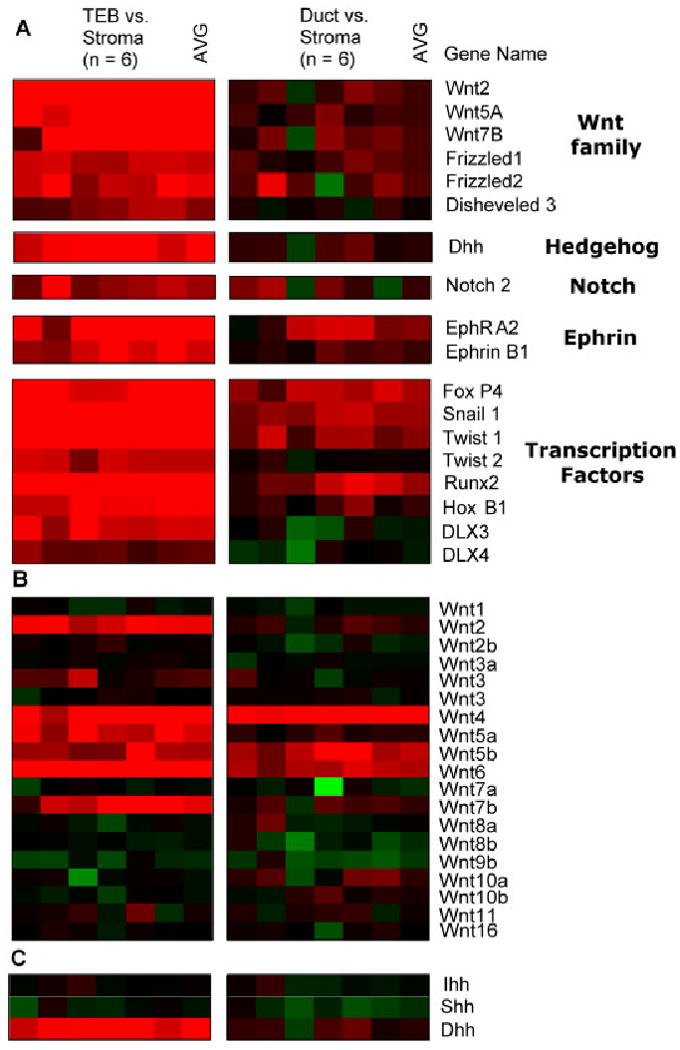
Genes enriched in terminal end buds. A: Clustered image maps of genes in the Wnt, hedgehog, notch, ephrin, and transcription factor families. Red denotes Cy5 signal in TEBs (left) and mature ducts (right), while green denotes Cy3 signal in distal stroma. Color intensity represents M = log2(Cy5 fluorescence/Cy3 fluorescence). B,C: Expression analysis of 18 Wnt ligands (B) and 3 hedgehog ligands (C).
Fig. 4.
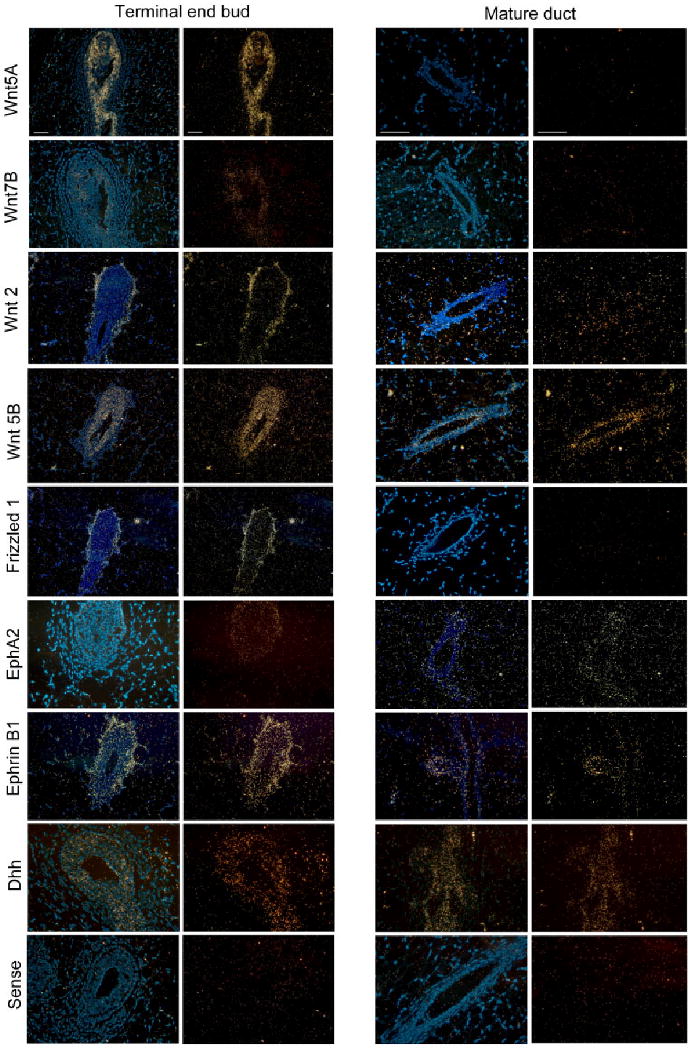
In situ hybridization of TEB-enriched genes. Darkfield views (right) and merged 4′,6-diamidine-2-phenylidole-dihydrochloride (DAPI)/darkfield views (left) of TEBs and mature ducts in a 5-week-old mammary gland. Probes used include Wnt-5a, Wnt-7b, Wnt-2, Wnt-5b, Frizzled-1, Dhh, ephrin-B1, Eph-A2, and sense control. Scale bar = 50 μm.
The pattern of Wnt expression in the mammary gland closely mirrored expression in the lung, suggesting that these genes may have overlapping functions in both systems. In the developing lung, Wnt-7b and Wnt-2 are localized to the epithelium and mesenchyme, respectively, of the distal bud (Mucenski et al., 2003). Wnt-5a is expressed in both the epithelium and mesenchyme of the distal lung bud (Li et al., 2002). Targeted deletion analyses have shown that Wnt-7b and Wnt-5a regulate branching morphogenesis of the lung. Wnt-7b participates in epithelial–mesenchymal crosstalk to regulate mesenchymal proliferation in the distal end bud, which may in turn affect epithelial differentiation. As a result, Wnt7b−/− mice exhibit severe defects in lung development due to deficiencies in mesenchymal proliferation, epithelial maturation, and vascular development (Shu et al., 2002). Wnt-5a also regulates lung branching, as described earlier. Of interest, Wnt-5a is localized to the distal compartments in several developmental systems that extend from the primary body axis, including the limb, genitals, face, and outer ear. Mice lacking Wnt-5a show defects in these caudal structures, including loss of tail and digits as well as shortening of the body axis, mandible, head, and tongue (Yamaguchi et al., 1999). The defects are attributed to the conserved role of Wnt-5a in maintaining the proliferative state of progenitor cells, which suggests that Wnt-5a may have a similar role in mammary TEBs. Taken together, these data further implicate Wnt-5a, Wnt-7b, and Wnt-2 as putative regulators of branching morphogenesis in the mammary gland.
Hedgehog Expression
Analysis of the hedgehog family members revealed that desert hedgehog (Dhh) was enriched in the TEB microenvironment, whereas Ihh and Shh did not show enrichment in either the TEBs or mature ducts (Fig. 3C; Supplementary Table S1). In situ hybridization verified that Dhh was highly expressed in the epithelium of TEBs, although we did detect low expression in side branches of mature ducts (Fig. 4). This pattern of Dhh expression suggests that it may regulate mammary branching. Previous work showed that hedgehog effector molecules Gli-2 and Patched-1 function in the stroma to regulate mammary development (Lewis et al., 1999, 2001). However, hedgehog ligands Shh and Ihh do not regulate mammary development, as described earlier (Gallego et al., 2002; Michno et al., 2003). Our data suggest that it may be epithelial-derived Dhh that activates stromal hedgehog effector molecules to regulate mammary branching morphogenesis. Further work will be necessary to analyze this potential role for Dhh in the mammary gland.
Ephrin Expression
The ephrin (Eph) receptors represent the largest family of receptor tyrosine kinases, with nine Eph-A type receptors and six Eph-B receptors identified to date. These cell-surface receptors interact with six GPI-anchored ephrin-A ligands and three transmembrane ephrin-B ligands. Direct cell-to-cell contact between ephrins and their receptors leads to intracellular signaling events in both the receptor-expressing (forward signaling) and ligand-expressing (reverse signaling) cells, a process known as bidirectional signaling (Pasquale, 2005). Ephrin signaling is necessary for a broad range of developmental processes, including vascular development, axon guidance, and tissue-border formation (Zhang and Hughes, 2006). The roles of ephrins in cell adhesion, cell migration, and tissue-border maintenance makes them prime candidates for mammary branching morphogenesis.
Analysis of ephrin expression in the mammary gland revealed that a single ephrin ligand, ephrin-B1, is enriched in the TEB microenvironment relative to mature ducts (Fig. 3C; Supplementary Table S1). In situ hybridization verified that ephrin-B1 is highly expressed in the TEB stroma, although a low-level of expression was detected in the TEB epithelium (Fig. 4). Low-level ephrin-B1 was also detected in mature ducts, particularly in sites of lateral budding (Fig. 4). It was shown previously that ephrin-B1−/− mice are perinatally lethal and that ephrin-B1 normally controls neural crest cell migration to target tissues (Davy et al., 2004). Given its expression pattern and its developmental functions, it is possible that ephrin-B1 in mammary gland may regulate TEB motility and/or establish the TEB border at the stromal interface.
Microarray analysis of the ephrin family also revealed that Eph-A2 was the only ephrin receptor to be enriched in TEBs relative to ducts (Fig. 3C; Supplementary Table S1). In situ hybridization showed restricted Eph-A2 expression in the TEB epithelium (Fig. 4). Although it is classically believed that EphA and EphB receptors interact solely with ephrin-A and ephrin-B ligands, respectively, recent work has demonstrated crosstalk between these ephrin subfamilies (Himanen et al., 2004). Future work will be necessary to determine the roles of ephrin-B1 and Eph-A2 in mammary development and to evaluate potential crosstalk between these cell-surface proteins.
Transcription Factors
Several transcription factors were enriched in the TEB microenvironments, including Fox-P4, Snail-1, Twist-1, Twist-2, Runx-2, Hox-B1, Dlx-3, and Dlx-4 (Fig. 3; Supplementary Table S1). Of interest, the Snail and Twist transcription factors have been characterized for their roles in epithelial–mesenchymal transition (EMT), a developmental process by which a polarized epithelium converts to a motile fibroblastoid or mesenchymal tissue. EMT occurs in several developmental process that require epithelial remodeling and multilayering, such as gastrulation in vertebrates (Thiery, 2002; Huber et al., 2005). Snail and Twist function as transcriptional repressors to inhibit expression of epithelial genes, such as E-cadherin. The restricted expression of these transcription factors in TEBs suggests that they may potentially repress epithelial differentiation in TEBs and activate a motile phenotype. Further work will be necessary to characterize the potential roles of these transcription factors and EMT in branching morphogenesis.
In conclusion, our microarray data have implicated several genes as potential players in mammary ductal morphogenesis. These genes include members of the Wnt (Wnt-2, Wnt-5a, Wnt-7b, Dsh-3, Frizzled-1 Frizzled-2), hedgehog (Dhh), ephrin (ephrin-B1, Eph-A2), and transcription factor families. These genes play essential roles in other developmental systems, making it challenging for study in a postpubertal developing organ such as the mammary gland. However, the mammary gland offers several experimental strategies for study of these genes. First, targeted inducible deletion of these genes, such as with the Cre/lox recombination system, can be used to specifically inhibit gene expression in the mammary gland (Utomo et al., 1999). Second, primary mammary glands can be cultured, infected with lentiviral or retroviral expression vectors, and transplanted back into wild-type mammary glands (Welm et al., 2005). Lastly, primary mammary organoids cultured in Matrigel can develop into highly branched structures in the presence of growth factors (Sternlicht et al., 2005). The organoids can be cultured with specific inhibitors or viral vectors for analysis of mammary branching. These experimental procedures offer a rapid and reproducible method for analyzing the function of these genes in mammary development and for dissecting their precise cellular functions in the branching process.
Experimental Procedures
Expression Profiling
All animal experiments were performed with protocols approved by the UCSF IACUC. TEB microenvironments (n = 6), mature duct microenvironments (n = 6), and distal stroma regions (n = 12) of mammary glands 2–5 were microdissected from anesthetized 5-week-old β-actin–GFP reporter mice (FVB/n, Jackson laboratory) using a Leica MZFLIII fluorescence microscope (Hadjantonakis et al., 1998). RNA was extracted with Trizol Reagent (Tel-Test), reverse transcribed in the presence of aminoallyl-dUTP, and coupled to CyScribe dyes (Amersham). Unamplified Cy5-labeled TEB or mature duct cDNAs and Cy3-labeled stromal cDNAs were hybridized onto 70-mer oligonucleotide microarrays with 19,500 features (Operon, mouse version 2.0), as described elsewhere (Barczak et al., 2003). Lowess print-tip normalization of microarray data was performed using the Acuity software program. For each gene, we calculated M = log2(Cy5/Cy3) where Cy5 labeled TEB or mature duct RNA and Cy3 labeled distal stroma RNA. Genes with M > 0.6 (1.5-fold change) and adjusted P value < 0.05 (Benjamini–Hochberg correction) were considered differentially expressed in TEB or mature ducts relative to distal stroma (Benjamini, 1995). TEB ratio = M(TEB) − M(duct), converted to fold change. Duct ratio = M(duct) − M(TEB), converted to fold change.
In Situ Hybridization
Number 4 (inguinal) mammary glands of 5-week-old female mice (FVB/n) were fixed in 4% paraformaldehye–phosphate buffered saline (PBS) overnight at 4°C and paraffin embedded. Five-micrometer sections were cut for hybridization with 35S-labeled RNA probes. First-strand cDNA from 5-week-old mammary glands were used as templates for PCR with the following primers: SPRR-1a (F-CATGAGTTCCCACCAGCAG, R-GGATAGACAGCAGCCTCAGC), casein-γ (F-CCAATGTTGCACACCTCTTC, R-TGCAGTTAATACGGCTCCAC), pleiotrophin (F-GTGAAGGCAGGATCAGGTTC, R-TTCAAGGCGGTATTGAGGTC), Dhh (F-AGATGCCCAATTGACAGGAG, R-ACCGCCAGTGAGTTATCAGC), Wnt-5a (F-CTGGCAGGACTTTCTCAAGG, R-TGGGGTCGATGTAGACCAG), Wnt-5b (F-TGGAGACAACGTGGAGTACG, R-CTCTTGAAGCGGTCATAGCC), Wnt-2 (F-AATTCCACTGGTGCTGTGC, R-TTGGTGTCAGCTCATTCTGC), Wnt-7b (F-CCCACATGACAGATGGACAG, R-GAGGCTTGGAACATCCTGAC), ephrin-B1 (F-ACCCTCATGAGACAATGCTG, R-ACAAAAATCCCTTCCGACTG), Eph-A2 (F-GAGGACCCACTGTCCATGTC, R-GTATCCGGAGCAAGGTCATC), Frizzled-1 (F-AACAAGTTCGGCTTCCAGTG, R-CAAACTTGTCGTTGCACACC). Primers included T7 (reverse primers) and SP6 (forward primers) promoter sites. The PCR products were used as templates for in vitro transcription (Maxiscript) to generate 35S-labeled antisense probes, which were purified on G-50 spin columns (Roche). Hybridization of probes onto mammary sections was performed as described (Diaz et al., 2003).
Supplementary Material
Acknowledgments
We thank Andrea Barczak, Agnes Paquet, and David Erle of the Sandler/UCSF Genomics Core Facility for their assistance with microarray profiling and data analysis. We thank Kristin Butcher and Richard Schneider for assistance with in situ hybridization. H.K.M. was supported by the UCSF Medical Scientist Training Program (MSTP), a California Breast Cancer Research Program (CBCRP) dissertation award, and the Paul and Daisy Soros Fellowship for New Americans.
Grant sponsor: National Cancer Institute; Grant number: CA057621; Grant sponsor: National Institute of Environmental Health Sciences; Grant number: ES012801.
Footnotes
Accession Numbers: Microarray data were submitted to the NCBI Gene Expression Omnibus with the accession number GSE2988.
References
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Barczak A, Rodriguez MW, Hanspers K, Koth LL, Tai YC, Bolstad BM, Speed TP, Erle DJ. Spotted long oligonucleotide arrays for human gene expression analysis. Genome Res. 2003;13:1775–1785. doi: 10.1101/gr.1048803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124:53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- Benjamini Y. Controlling the false discover rate: a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, factsandcontroversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Chotteau-Lelievre A, Montesano R, Soriano J, Soulie P, Desbiens X, de Launoit Y. PEA3 transcription factors are expressed in tissues undergoing branching morphogenesis and promote formation of duct-like structures by mammary epithelial cells in vitro. Dev Biol. 2003;259:241–257. doi: 10.1016/s0012-1606(03)00182-9. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol. 2005;277:316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Diaz E, Yang YH, Ferreira T, Loh KC, Okazaki Y, Hayashizaki Y, Tessier-Lavigne M, Speed TP, Ngai J. Analysis of gene expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2003;100:5491–5496. doi: 10.1073/pnas.0831080100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego MI, Beachy PA, Hennighausen L, Robinson GW. Differential requirements for shh in mammary tissue and hair follicle morphogenesis. Dev Biol. 2002;249:131–139. doi: 10.1006/dbio.2002.0761. [DOI] [PubMed] [Google Scholar]
- Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA. Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol. 2003;19:623–647. doi: 10.1146/annurev.cellbio.19.031403.160043. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2003;8:145–158. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Hinck L, Silberstein GB. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Shangguan X, Shannon JM. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1116–1126. doi: 10.1152/ajplung.00033.2004. [DOI] [PubMed] [Google Scholar]
- Lebeche D, Malpel S, Cardoso WV. Fibroblast growth factor interactions in the developing lung. Mech Dev. 1999;86:125–136. doi: 10.1016/s0925-4773(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Scott MP, Daniel CW. Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development. 1999;126:5181–5193. doi: 10.1242/dev.126.22.5181. [DOI] [PubMed] [Google Scholar]
- Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Hui C, Daniel CW. The Gli2 transcription factor is required for normal mouse mammary gland development. Dev Biol. 2001;238:133–144. doi: 10.1006/dbio.2001.0410. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhang S, Tuukkanen J, Peltoketo H, Pihlajaniemi T, Vainio S. Patterning parameters associated with the branching of the ureteric bud regulated by epithelial-mesenchymal interactions. Int J Dev Biol. 2003;47:3–13. [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Michno K, Boras-Granic K, Mill P, Hui CC, Hamel PA. Shh expression is required for embryonic hair follicle but not mammary gland development. Dev Biol. 2003;264:153–165. doi: 10.1016/s0012-1606(03)00401-9. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Utomo AR, Nikitin AY, Lee WH. Temporal, spatial, and cell type-specific control of Cre-mediated DNA recombination in transgenic mice. Nat Biotechnol. 1999;17:1091–1096. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, Mailleux A, Tefft D, Unbekandt M, Wang K, Shi W. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res. 2005;57:26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- Welm AL, Kim S, Welm BE, Bishop JM. MET and MYC cooperate in mammary tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:4324–4329. doi: 10.1073/pnas.0500470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hughes S. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol. 2006;208:453–461. doi: 10.1002/path.1937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


