Abstract
Urocortins, three paralogs of the stress-related peptide corticotropin-releasing factor (CRF) found in bony fish, amphibians, birds and mammals, have unique phylogenies, pharmacologies, and tissue distributions. As a result and despite a structural family resemblance, the natural functions of urocortins and CRF in mammalian homeostatic responses differ substantially. Endogenous urocortins are neither simply counterpoints nor mimics of endogenous CRF action. In their own right, urocortins may be clinically relevant molecules in the pathogenesis or management of many conditions, including congestive heart failure, hypertension, gastrointestinal and inflammatory disorders (irritable bowel syndrome, active gastritis, gastroparesis, rheumatoid arthritis), atopic/allergic disorders (dermatitis, urticaria, asthma), pregnancy and parturition (preeclampsia, spontaneous abortion, onset and maintenance of effective labor), major depression and obesity. Safety trials for intravenous urocortin treatment have already begun for the treatment of congestive heart failure. Further understanding the unique functions of urocortin 1, urocortin 2 and urocortin 3 action may uncover other therapeutic opportunities.
Keywords: Urocortin 1 or urocortin 2 or urocortin 3 or stresscopin or stresscopin-related peptide, corticotropin-releasing factor or CRF or corticotropin-releasing hormone or CRH or corticoliberin, stress response, peptide, anxiety or depression, pregnancy, labor or parturitition, food intake or energy balance or obesity, inflammation or immune system, salt or fluid balance, cardiovascular or hypertension or heart failure, gastrointestinal motility or irritable bowel syndrome
1. Introduction
Since the isolation of corticotropin-releasing factor (CRF) in 1981 [284], three mammalian CRF-like paralogs have been identified. The first of these – urocortin 1 (Ucn 1) – was identified in 1995 for its cross-reactivity to antisera against suckerfish urotensin I, a fish peptide structurally related to CRF and now hypothesized to be an ortholog of mammalian Ucn 1. Ucn 1 was named for its similar primary structure and bioactivity to both urotensin I and CRF [289]. Because Ucn 1 exhibited greater affinity for and activation of type 2 CRF receptors (CRF2) than did CRF, it was hypothesized to be a natural CRF2 receptor ligand [253,289]. Ucn 2 and Ucn 3 prohormones, subsequently identified in 2001 [113,165,226], were recognized for their structural relation to both CRF and Ucn 1 preproteins, but named “urocortins” due to the predominant affinity of predicted mature peptides for the CRF2 receptor. Although Ucn peptides share moderate sequence identity with one another and with CRF, each is phylogenetically distinct, with the CRF/Ucn peptide family resulting from multiple gene duplication events during evolution prior to the divergence of modern vertebrate fish from tetrapods [28,113,165]. Each peptide has a unique anatomical distribution under the control of different genes. Consequently, despite a structural family resemblance, the natural functions of Ucns and CRF in stress responses in vivo may differ significantly, as will be apparent in the current review.
1.1. Structure of Ucns, CRF and Related Peptides
The rat Ucn 1 cDNA was cloned from a library constructed from mRNA extracted from a portion of the rat midbrain that included the Edinger-Westphal (EW) nucleus in 1995 [289]. The gene codes for a 122 residue preprotein, with Ucn 1 contained in the carboxyl terminus. The Ucn 1 genes for several fish species (pufferfish, Takifugu rubripes; carp, Cyprinus carpio; goldfish, Carassius auratus; sucker, Catastomus commersoni; trout, Oncorhynchus mykiss; flounder, Platichthys flesus, sole, Hippoglossoides elassodon; and zebrafish, Danio rerio [28,36]), frog (Xenopus laevis and Xenopus tropicalis) [28], mouse [312], hamster (Mesocricetus auratus) [230], sheep (Ovis aries) [41], capuchin monkey (Cebus apella) [288], rhesus monkey (Macaca mulatta)(GenBank XM_001092536.1), dog (Canis familiaris), cow (Bos taurus)(XM_618452, XM_596525) and human [81,312] also have been cloned or predicted and found to be similar across species. The human Ucn 1 gene resides on chromosome 2 (2p23-p21) and has two exons, with the coding region residing entirely in the second exon, like the CRF gene [81]. The Several possible transcription-factor binding sites have been identified by sequence homology in the Ucn 1 promoter, but few have yet been tested for actually controlling Ucn 1 transciption. Putative regulatory elements include a TATA box, GATA-binding sites, a CCAAT enhancer binding protein (C/EBP) transcription factor-binding site, a binding site for the POU domain transcription factor Brn-2, and a cyclic adenosine monophosphate (cAMP) responsive element (CRE) [312]. Four base pairs upstream of the CRE site, the Ucn 1 promoter contains a consensus half-site for glucocorticoid response elements (GRE), consistent with the ability of glucocorticoids to upregulate Ucn 1 mRNA synthesis [130]. The CRE is involved in both constitutive and forskolin-stimulated activity [312].
The murine and human cDNAs for Ucn 2 and Ucn 3 were identified by two groups from sequence homology searches of the mouse and human genomes, with the putative Ucn 2 peptide contained in the C-terminus of a 112 amino acid residue preprotein, and the putative Ucn 3 peptide encoded in a precursor deduced to span 161 residues [113,165]. The identity and existence of endogenous peptides derived from the Ucn 2 and Ucn 3 preproteins remains predicted, as mature peptides have not been isolated or sequenced from any species (but see [235]). Reflecting this uncertainty, one group predicted sequences other than Ucn 2 and Ucn 3 to be mature peptide products of the human Ucn 2 and Ucn 3 prohormones, stresscopin-related peptide and stresscopin, respectively. These alternatively predicted peptides are N-terminally extended analogs of the predicted Ucn 2 and Ucn 3 sequences, as shown in Figure 1. To explain this ambiguity, Ucn 3 prohormone sequences from several species (fish, cow, dog, chicken, frog) contain a prototypical dibasic cleavage sequence (arginine-arginine; RR) at the N-terminus that could result in a mature 40-amino acid sequence, as exemplified in the predicted peptide stresscopin. However, the dibasic arginine residues (RR) are not conserved in humans, rhesus monkey, or rodents, which led to the alternate prediction of a mature 38-amino acid peptide, cleaved following the threonine-lysine residues (TK) of the preprotein as exemplified in rodent, dog or human Ucn 3 (see Figure 1) [28]. For the Ucn 2 prohormone, the uncertainty relates to the presence of a potential N-terminal flanking cleavage site (serine-arginine) that would predict the 38-residue peptide Ucn 2 whereas an upstream site (threonine-arginine) might predict the 43-residue stresscopin-related peptide (see Figure 1). Even further complicating the identity and existence of a human Ucn 2/stresscopin-related peptide in humans, the human Ucn 2 prohormone lacks the proteolytic cleavage site (RR) seen in Ucn 2 prohormones from other species that should follow the putative C-terminal amidation donor glycine residue.
Figure 1.
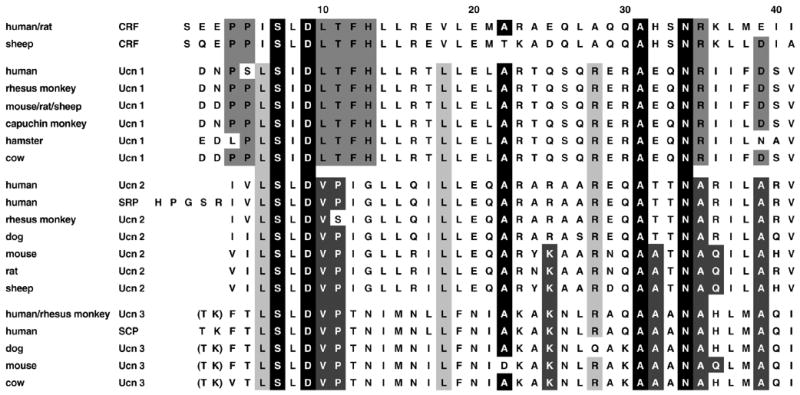
Comparison of the primary structures of urocortin/corticotropin-releasing factor (Ucn/CRF) family mammalian peptides. Selected putative amino acid sequences for CRF and Ucn 1, 2 and 3 across human (Homo sapien), mouse (Mus musculus), rat (Rattus norvegicus), rhesus monkey (Macaca mulatta), capuchin monkey (Cebus paella), hamster (Mesocricetus auratus), sheep (Ovis aries), cow (Bos taurus), and dog (Canis familiaris). Boxed regions indicate sequence identity. Black fill with white letters indicates CRF superfamily homology (only 5 residues). Dark grey fill with white letters indicates selective “type 2 Ucn” (Ucn 2, Ucn 3) lineage homology (7 residues). Grey fill with black letters indicates selective type 1 Ucn/CRF lineage (Ucn 1, CRF) homology (8 residues). Light grey fill with black letters indicates pan-Ucn homology (3 residues). The threonine-lysine (TK) residues are shown parenthetically for Ucn 3 because the flanking dibasic arginine residue (RR) cleavage site (not shown) is not conserved in humans, rhesus monkey, or rodents. This leads to the prediction of a mature 38-residue peptide cleaved after the TK residues. For species in which the RR residues are present in the preprotein, a 40-residue peptide that includes the TK residues, orthologous to the alternatively predicted peptide stresscopin (SCP), might be a mature prohormone product. Similar uncertainty regarding the N-terminal proteolytic cleavage processing has led to the alternative prediction of stresscopin-related peptide (SRP), rather than Ucn 2, as the mature product of the Ucn 2 prohormone. The definitive identity of mature peptides derived from the Ucn 2 and Ucn 3 prohormones remains uncertain.
In addition to mouse and human sequences, Ucn 2 preproteins have also been identified in rat (GenBank accession: AY044835), chicken (Gallus gallus), fish (Tetraodon nigroviridi, Takifugu rubripes), dog [28], and rhesus monkey (RefSeq accession: XM_001097967.1), and Ucn 3 preproteins likewise in rat (XM_574076), rhesus monkey (XM_001104616.1), dog (XM_845862.1), chicken, fish (Tetraodon nigroviridi, Takifugu rubripes, Danio rerio), and frog (Xenopus laevis and Xenopus tropicalis) [28]. The human genes for Ucn 2 and Ucn 3 reside on chromosomes 3 (3p21.3–4) and 10 (10p15.1), respectively [165,226]. The Ucn 2 promoter contains several GREs and is positively regulated by glucocorticoids, representing a molecular link between CRF1-mediated HPA-axis activity and Ucn 2 responses to stress [57].
In terms of primary structure (see Figure 1), Ucn 1 resembles CRF as much as, or more, than it resembles Ucn 2 and Ucn 3. In contrast, Ucn 2 and Ucn 3 resemble one another more than they do CRF, a distinction coupled with their greater selectivity for the CRF2 receptor that has led to their description as “type 2 Ucns” [318]. Thus, an ancient gene duplication event prior to the evolutionary divergence of teleost bony fishes from tetrapods is hypothesized to have resulted in separate “type 1 Ucn/CRF” (Ucn 1/CRF) vs. “type 2 Ucn” (Ucn 2/Ucn 3) lineages with subsequent gene duplication events giving rise to additional paralogs within each phylogenetic branch (see Figure 2).
Figure 2.
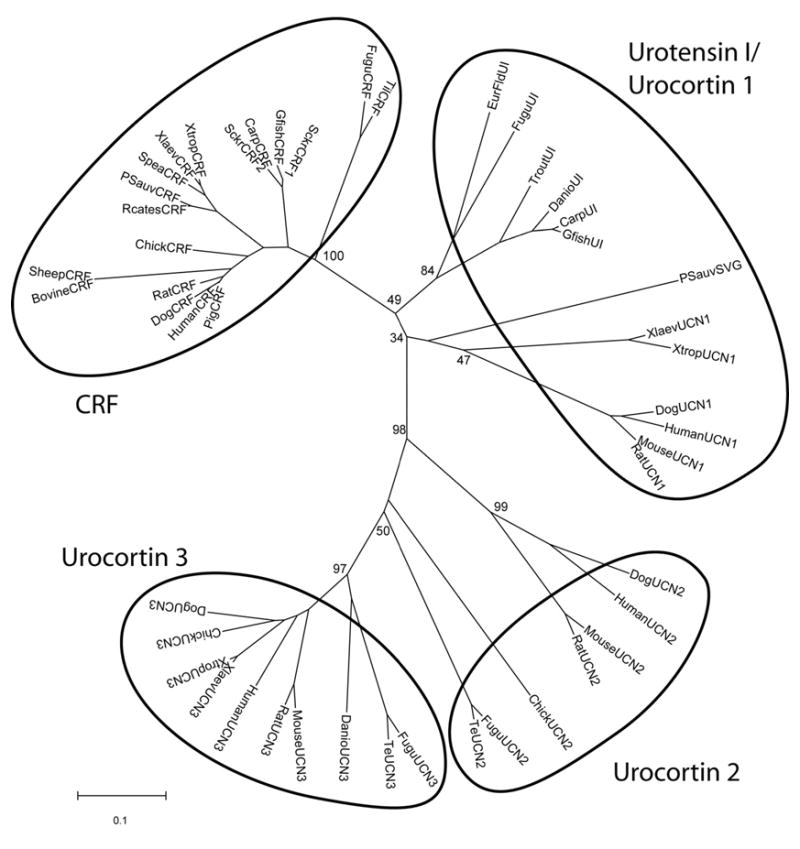
Neighbor joining phylogenetic tree using p-distance of vertebrate CRF-like prohormone amino acid sequences. Numbers at branch nodes represent the confidence level of 1000 bootstrap replications. UI, Urotensin I; SVG, sauvagine; UCN1, urocortin 1; UCN2, urocortin 2; UCN3, urocortin 3; Carp, Cyprinus carpio; Danio, Danio rerio, zebrafish; EurFld, Platichthys flesu, European flounder; Te, Tetraodon nigroviridi, pufferfish; Fugu, Takifugu rubripes, pufferfish; Gfish, Carassius auratus auratus, goldfish; Xlaev, X. laevis, South African clawed frog; Xtrop, X. tropicalis; Rcates, Rana catesbeiana, North American bullfrog; Psauv, Phyllomedusa sauvageii; Sckr, Catostomus commersoni, sucker; Spea, Spea hammondii, Western spadefoot toad; Til, Tilapia mossambicus; Trout, Oncorhynchus mykiss.
| Prohormone | GenBank Accession # / ENSEMBL ID |
|---|---|
| CRF | |
| Bovine | NM_001013400 |
| Carp | AJ317955 |
| Chick | AJ621492 |
| Dog | NM_001014278 |
| Fugu | SINFRUG00000146091 |
| Gfish | AF098629 |
| Human | NM_000756 |
| Pig | AF440229 |
| Psauv | AY596828 |
| Rat | NM_031019 |
| Rcates | AB161633 |
| Sckr1 | S65264 |
| Sckr2 | x58784 |
| Sheep | J00803 |
| Spea | AY262255 |
| Til | AJ011835 |
| Xlaev | S50096 |
| Xtrop | ENSXETG00000020294 |
| UCN2 | |
| chick | XM_425157.1 |
| dog | ENSCAFG00000012466 |
| human | NM_033199 |
| mouse | AF331517 |
| rat | NM_133385 |
| Te | AL175143 |
| UCN1 / UI / SVG | |
| carp UI | M11671 |
| Danio UI | BX510372 |
| dog UCN1 | ENSCAFG00000004852 |
| EurFld UI | AJ517171 |
| Fugu UI | SINFRUG00000137751 |
| Gfish UI | AF129115 |
| human UCN1 | NM_003353 |
| mouse UCN1 | NM_021290 |
| Psauv SVG | AY943910 |
| rat UCN1 | NM_019150 |
| Trout UI | AJ005264 |
| Xlaev UCN1 | AY596827 |
| UCN3 | |
| chick | BX930520.2 |
| Danio | BX004864.7 |
| dog | ENSCAFG00000005250 |
| Fugu | AJ251323.1 |
| human | NM_053049 |
| mouse | AF361944 |
| rat | XM_574076 |
| Te | GSTENG00027885001 |
| Xlaev | AY596826 |
| Xtrop | ENSXETG00000016289 |
1.2. Distribution of Ucns
1.2.1. Central Nervous System (see Table 1)
Table 1.
Semi-quantitative relation of prominent Ucn projection fields/expression to CRF receptor expression in rat brain
| Region | Ucn 1 | Ucn 2 | Ucn 3 | CRF1 | CRF2 |
|---|---|---|---|---|---|
| I. Forebrain | |||||
| Septum Lateral Medial/diagonal band complex |
++/+++ +/++ |
− − |
++++ −/+ |
−/+ +++ |
++++ − |
| Amygdala Central nucleus Medial nucleus Cortical nuclei Amygdalohippocampal area |
+/++ + + ++ |
− − − − |
− +++ +++ ++ |
−/+ +/++ ++ ++ |
− ++ +++ − |
| Bed nucleus of the stria terminalis
Rostral Posterior |
++ + |
− − |
+ +++ |
++ +++ |
− ++ |
| Globus pallidus | +/++ | − | − | ++ | − |
| Substantia nigra | ++ | − | − | ++/+++ | − |
| Thalamus | ++ | − | +/++ | +/++ | − |
| Hypothalamus Supraoptic nucleus Arcuate nucleus Ventromedial nucleus Lateral hypothalamus Perifornical region Lateral preoptic area Anterior hypothalamus Suprachiasmatic nucleus Dorsomedial nucleus Medial preoptic nucleus Paraventricular nucleus Mammillary nuclei |
+ −/+ −/+ ++ −/+ ++ ++/+++ +++ ++ +/++ +/++ ++ |
+++ +++ − − − − − − − − +++ − |
−/+ ++ ++++ + ++++ + + + +/++ +++ +/++ ++ |
++ ++ − + + + ++ +++ +/++ −/+ +/++ |
+ + +++ + ? + + − + +/++ −/+ −/+ |
| II. Brainstem | |||||
| Visual nuclei (sup. colliculus, anterior pretectal nucleus) | +++ | − | + | +/++ | − |
| Somatosensory nuclei (dorsal column) | ++ | − | − | ++++ | −/+ |
| Auditory nuclei (cochlear nuclei, inf. colliculus, lateral superior olive) | ++++ | − | +/++ | ++/+++ | −/+ |
| Vestibular nuclei Visceral
nuclei Solitary tract nucleus Area postrema Parabrachial nucleus |
++++
++ ++ +/++ |
− − − − |
− − − − |
++ −/+ − ++/+++ |
− ++ ++ − |
| Motor nuclei (oculomotor, facial, hypoglossal) | ++/+++ | +++ | − | +/++ | − |
| Periaqueductal gray | ++/+++ | − | +/++ | + | + |
| Tegmental nuclei | ++/+++ | − | − | ++/+++ | − |
| Red nucleus | +++ | − | − | +++ | − |
| Edinger-Westphal nucleus | ++++ | − | − | ||
| Pontine nuclei | ++ | − | − | ++/+++ | − |
| Raphe nuclei Dorsal Median |
++/+++ +/++ |
− − |
−/+ − |
+ ++ |
+++ ++ |
| Locus coeruleus | +/++ | +++ | − | −/+ | − |
| III. Cerebellum | |||||
| Deep nuclei | ++ | − | − | +++ | − |
| Cortex | +/++ | − | − | ++/+++ | − |
From [315] with permission.
Ucn 1
In the brain, Ucn 1 has a restricted, subcortical, predominantly caudal distribution. The major site of brain Ucn 1 synthesis is the Edinger-Westphal nucleus (E-W). The prominent synthesis and expression of Ucn 1 in the E-W is well-conserved across rats [27,158,289], sheep [41], humans [114], monkeys [288] and frogs [156]. Recent double-label immunohistochemistry studies suggest that Ucn 1-expressing neurons in the E-W, a dorsal midbrain structure originally recognized for its role in oculomotor and pupillary control, may not be the same as those which control “classic” oculomotor E-W functions, as Ucn 1-immunoreactive neurons are not preganglionic cholinergic neurons [232,298]. Perhaps reflecting this, some evidence suggests additional functions for the E-W that may be subserved by urocortinergic neurons. These proposed, but not fully substantiated, functions include the regulation of food and water intake [297], behavioral responses to stressors [127,299], temperature homeostasis [12,204,251], nociception [120,153], motor control [231], vestibular function [137], and the effects of and motivation to consume alcohol [11,13]. Certain stressful stimuli (e.g., ether, lipopolysaccharide, and restraint, but not hyperosmotic or hemorrhagic stress) activate the E-W Ucn 1 system, demonstrated by Fos expression in Ucn 1-immunoreactive neurons or increased Ucn 1 mRNA. Chronic stress results in a partial habituation of Ucn 1 mRNA, but not Fos protein, responses to stress in the E-W [93,150,154]. On the other hand, conditions that chronically increase brain CRF activity have been observed to reduce E-W Ucn 1 activity [157], whereas conditions that lower CRF levels are associated with increased E-W Ucn 1 activity [245,299], raising the possibility of a long-term, inverse relation between CRF and E-W Ucn 1 systems in homeostatic stress responses.
Descending Ucn 1-LI-positive fibers of possible E-W origin are observed in (1) midbrain: substantia nigra, periaqueductal gray, interpenduncular nucleus, and red nucleus; (2) caudal midbrain/rostral pons: dorsal raphe nucleus, ventral tegmentum, basilar pontine nuclei, and parabrachial nucleus; (3) medulla: including the facial, lateral reticular, and spinal trigeminal nuclei, inferior olive, and the dorsal column nuclei, nucleus of the solitary tract (NTS) and area postrema; (4) cerebellum: in the flocculus and paraflocculus as well as deep cerebellar and vestibular nuclei [257]; and (5) spinal cord: throughout the spinal gray and, less so, in the dorsal and ventral horns. The most prominent ascending Ucn 1-LI-positive projection from the E-W origin targets the septal/preoptic region, robustly to the lateral septum (LS) and less so to the bed nucleus of the stria terminalis (BNST), globus pallidus and medial septal/diagonal band complex. Other ascending Ucn 1-LI-positive fibers innervate the hypothalamus, the thalamus and the rostral periaqueductal gray.
Validated secondary sites of brain Ucn 1 synthesis include the lateral superior olive, the supraoptic nucleus (SON) [27], the lateral hypothalamic area, and, most caudal, several brainstem and spinal cord motoneuron nuclei [27]. Possible additional sites of Ucn 1 synthesis include the mammillary nucleus of the hypothalamus, sphenoid nucleus, substantia nigra, tegmentum, periaqueductal gray (PAG), raphe, and vestibular nucleus [27,158,310]. Ucn 1-LI generally is scarce or absent in many regions in which its paralog, CRF, is prominent, including the external layer of the median eminence, the hypophysiotropic, dorsal medial parvocellular subdivision of the paraventricular nucleus of the hypothalamus (PVN), basal ganglia, amygdala, hippocampus, locus coeruleus (LC) and cerebral cortex [27,114,158,186].
Ucn 2
Ucn 2 also exhibits a restricted, subcortical expression in rodent brain [175,226,311]. Like Ucn 1, Ucn 2 mRNA is localized in the SON and magnocellular subdivision of the PVN as well as in brainstem motoneurons and the spinal cord. Unlike Ucn 1, Ucn 2 also has marked expression in the arcuate nucleus of the hypothalamus and the LC. The projection targets of Ucn 2 neurons are unknown. Non-neuronal Ucn 2 expression is present in the meninges, but not in glial cells [226].
Ucn 3
Ucn 3 exhibits the most rostral distribution of the Ucns, again expressed subcortically [113,165,167,175,291]. The three prominent sites of forebrain Ucn 3 synthesis are (1) the median preoptic nucleus of the hypothalamus, (2) a hypothalamic region bordered laterally by the fornix and medially by the PVN that extends rostrally into the posterior BNST, and (3) the dorsal medial amygdala. Less prominent forebrain sites of Ucn 3 synthesis are the dorsomedial hypothalamus, both magnocellular and parvocellular components of the PVN, a region dorsal to the SON, and the posterior cortical and amygdalohippocampal transition areas of the amygdala. In addition to peptide expression at sites of synthesis, Ucn 3-like immunoreactive fibers of unknown origin project heavily to the VMH and arcuate nucleus, and medial amygdala Ucn 3 neurons project to the ventral premammillary nucleus [40]. Ucn 3 fibers are scarcer in the SON, PVN and anterior, dorsomedial and lateral areas of the hypothalamus and are seen in the internal, but not external, zone of the median eminence [167]. Outside the hypothalamus, forebrain Ucn 3 fibers of uncertain origin are abundant in the LS, posterior BNST and the medial amygdala and scattered in the basomedial and posterior cortical nuclei of the amygdala and the ventral hippocampus. The topographical distribution of Ucn 3 in the LS differs from that of Ucn 1 with the former innervating ventral and intermediate aspects of the structure, and the latter innervating dorsal aspects [167]. Caudally, Ucn 3 cell bodies are found in the auditory complex, notably in the superior paraolivary nucleus, and scattered Ucn 3 fibers are present in the periacqueductal gray, superior and inferior colliculi, and the ventral lateral lemniscus [167] (see Table 1 for summary).
1.2.2. Periphery
Ucn 1
Ucns also are substantially distributed in the periphery [15,27,103,130,155]. Ucn 1 expression has been observed in adipose tissue [243], heart [145,193,197] (especially ventricles), and immunological tissue [14,15,22], including thymus [130], spleen [15,130], and skin [247,248], evident at the cellular level in lymphocytes, macrophages, fibroblasts [280], and mast cells [141], as well as in synovial cells from patients with rheumatoid arthritis [149,280]. Ucn 1 also is present in the enteric nervous system of the duodenum, small intestine and colon [27,103], as well as in testis [130], kidney [130], adrenals (especially the medulla) [90], and, possibly, anterior (but not posterior) pituitary [115]. In the human pituitary, Ucn 1-like-immunoreactivity was coexpressed with growth hormone (77% of Ucn 1-positive cells) and, to a lesser degree, prolactin (22%), but negligibly with adrenocorticotropic hormone (ACTH) (<1%) [115]. Ucn1-like-immunoreactivity also has consistently been observed in parietal and oxyntic cells of the stomach [54,155], but the proportion of Ucn 1 actually synthesized by gastric tissue remains uncertain. Indeed, Ucn 1 is synthesized by lamina propria macrophages, components of the stomach’s inflammatory mucosal immune system [53,187,235,236]. Finally, Ucn 1-like-immunoreactivity is evident in human placenta and fetal membranes [21,213], produced by chorio-decidual cells [96], and is reportedly maintained at elevated levels in maternal plasma from 16 weeks of gestation through birth [64,85,87,96].
Ucn 2
In humans, Ucn 2 gene expression was observed in most peripheral tissues analyzed by polymerase chain reaction (PCR) analysis, with higher levels observed in heart, lung, muscle, stomach, adrenal and peripheral blood cells [55,113] and more recent identification in skin [246] and placenta and fetal membranes [119]. Survey of peripheral rodent tissue for Ucn 2 gene expression revealed high levels in skeletal muscle and skin, moderate levels in lung, stomach, adrenal, ovary, brown fat, spleen, thymus, and uterus, and lower or negligible levels in testes, kidney, liver, pancreas, white fat and intestine [55,311]. Unlike in humans, low Ucn 2 mRNA expression is seen in rodent heart or aorta [55,311]. Detailed studies of murine tissue confirmed that Ucn 2 is synthesized by cultured skeletal myocytes and that Ucn 2-like-immunoreactivity is present throughout epidermis and dermis regions of the skin [55]. In rat, Ucn 2-like-immunoreactivity also was seen in hypothalamus, β-endorphin-containing cells of the anterior/intermediate pituitary and adrenal medulla [311]. Expression of Ucn 2 mRNA in skin, but not skeletal muscle, is inversely related to circulating glucocorticoid levels, as manipulated by exogenous administration or adrenalectomy [55].
Ucn 3
Ucn 3 gene expression has been detected in adipose tissue [243], heart [265], and skin [165], albeit at levels considerably lower than those of Ucn 2. Ucn 3 also is present in thyroid, adrenals [90,263,265], pituitary, [265], β-cells of the pancreas [166], spleen [265], ovary [265], placenta and fetal membranes [119], kidney [113,165,265] and the muscularis mucosa of the gastrointestinal (GI) tract, notably in the stomach, small intestine, colon, and rectum but not esophagus [113,165,235].
1.3. Pharmacology of Ucns
As shown in Table 2, Ucn 1 has high affinity for every CRF binding site identified to date, including the CRF1 and CRF2 receptor families and the CRF-binding protein (CRF-BP) [25,165,289]. This promiscuity differs from that of the type 2 Ucns (as well as stresscopin-related peptide and stresscopin), each which show selective CRF2 affinity, and no or only moderate affinity for the CRF-BP in both mammalian [113,165,226,269,291] and non-mammalian vertebrates [28]. Two CRF receptor subtypes have been identified [50,62,148,169,171,207,255,293], with catfish having a duplicate genomic analog of CRF1 [7,7,49]. The subtypes are encoded by separate genes, share high sequency homology (∼70%) differing predominantly at the N-terminus, and have unique tissue distributions and pharmacological affinity profiles, implying a diversity of function [20,169,255]. CRF receptors belong to the G-protein coupled, seven transmembrane domain receptor family (GPCR). Multiple splice variant isoforms have been observed for each receptor subtype family, and include both membrane-bound and soluble variants [59,71,171,209,215].
Table 2.
Binding properties of select CRF/Ucn receptor ligands
| CRF1 (Ki) (nM) | CRF2 (Ki) (nM) | CRF-BP (Ki or IC50) (nM) | ||||
|---|---|---|---|---|---|---|
| Peptide | CRF1 | CRF2(a) | sCRF2(a) | CRF2(b) | hCRF-BP | rCRF-BP |
| r/hCRF | 0.95 | 15 (9–19) | 23 | 13.5 (10–17) | 0.21 | 0.54 |
| rUcn 1 | 0.32 (0.16–0.32) | 1.5 (0.8–2.2) | 6.6 | 0.62 (0.41–0.62) | 0.10 | 0.98 |
| hUcn 1 | --- | 0.42 (0.27–0.57) | --- | 0.44 | --- | |
| mUcn 2 | >100 | 0.58 (0.16–2.1) | 113 | 0.66 (0.25–0.66) | “No appreciable” | 4.4 |
| hUcn 2 | >100 | 0.17 (0.15–1.7) | --- | 0.50 (0.073–0.50) | “No appreciable” | --- |
| mUcn 3 | ≫100 | 9.6 (5.0–14.2) | >200 | 1.8 | “No appreciable” | >2,000 |
| hUcn 3 | ≫100 | 2.7 (1.3–21.7) | --- | 7.9 (2.3–13.5) | “No appreciable” | --- |
Values represent median (range) from mean binding affinities reported in [56,59,110,125,165,226,289]. Values were combined across receptor species (human, rat, mouse), across which large systematic differences were not discerned, but differences across species were evident for CRF-BP. For peptides, r=rat, h=human, m=mouse, CRF=corticotropin-releasing factor, and Ucn=urocortin, CRF-BP=CRF-binding protein. All values reflect inhibitory binding constants (Ki’s) except for rat CRF-BP values, which reflect half-maximal inhibitory concentrations (IC50’s).
1.3.1. CRF1 receptors
The CRF1 receptor is a class B (“secretin-like”) GPCR spanning ∼415 amino acids [62,78,207]. At least eight alternatively spliced transcripts of CRF1 have been identified in humans, three in rats, four in mice, and nine in hamsters [109,214–216]. However, only one of these splice variants is known to induce signal transduction in humans and rodents (CRF1(a)), so the following information applies to that isoform. Ucn 1 exhibits reversible, saturable, high-affinity binding to CRF1 receptors transfected in stable cell lines (Ki=0.2 nM). Indeed, Ucn 1 is ∼3 fold more potent than CRF at binding to the CRF1 receptor and 1–2.5 fold more potent than CRF in stimulating the production of cAMP from CRF1 expressing cells (see Tables 2 and 3). Ucn 1’s affinity for the CRF1 receptor is determined by regions in the first, second and third extracellular domains of the receptor [208,304]. In contrast to Ucn 1, Ucn 2 and Ucn 3 have very low potencies to bind CRF1 and thereby activate adenylate cyclase (Tables 2 and 3), and Ucn 3 also shows negligible potency to stimulate ACTH release in vitro (≫1 μM), a CRF1-mediated bioassay [165]. Unlike Ucn 3, which appears to show no functional activity whatsoever at CRF1 receptors, Ucn 2 acts as a very low potency, but full agonist at CRF1 receptors. For example, Ucn 2 showed maximum functional efficacy (100%) comparable to that of CRF (110%) and Ucn 1 (93%) to induce cAMP accumulation in AtT20 cells, but was much less potent than both peptides (EC50’s=360, 1.3 and 1.1 nM, respectively) [110] (see also Table 3). The CRF1 receptor can employ multiple signal transduction pathways when stimulated by Ucn 1 [109]. These include activation of adenylate cyclase with production of cAMP and activation of protein kinase A-dependent pathways; activation of phospholipase C with production of inositol-1,4,5-triphosphate which in turn activates protein kinase C-dependent and calcium activated pathways; MAP kinase-dependent pathways; nitric oxide production; and proximate interactions with calcium channels [32,83,109,201,289,302].
Table 3.
Functional potency of select CRF/Ucn receptor ligands
| CRF1 (EC50) (cAMP, nM) | CRF2 (EC50) cAMP, nM) | ||
|---|---|---|---|
| Peptide | CRF1 | CRF2(a) | CRF2(b) |
| r/hCRF | 1.3 (0.3–1.9) | 15 (3.1–19) | 5.8 (1.6–15) |
| rUcn 1 | 0.55 (0.1–0.9) | 0.53 (0.063–1) | 0.18 (0.087–0.84) |
| hUcn 1 | 1 | 0.42 (0.27–0.57) | 0.32 (0.19–0.44) |
| mUcn 2 | >100 (19– >100) | 0.21 (0.14–0.9) | 0.20 (0.05–0.43) |
| hUcn 2 | >100 (>100–360) | 0.17 (0.15–0.26) | 0.25 (0.027–0.42) |
| mUcn 3 | >1,500 (>1000– >1500) | 7.1 (0.073–14.2) | 0.46 (0.081–0.83) |
| hUcn 3 | >1,000 (>1,000– >1,000) | 1.3 (0.16–2.7) | 2.3 (0.12–2.7) |
Values represent median (range) from mean functional potencies reported in [56,110,125,165,226,289]. Values were combined across receptor species (human, rat, mouse), across which systematic differences were not discerned. For peptides, r=rat, h=human, m=mouse, CRF=corticotropin-releasing factor, and Ucn=urocortin.
1.3.2. CRF2 receptors
CRF2 receptors also are class B GPCRs. To date, four major CRF2 splice variants have been identified, including membrane-bound and soluble isoforms of CRF2(a), and membrane-bound CRF2(b) and CRF2(c) receptors. The CRF2(c) receptor has only been observed in human limbic neurocircuitry [151], and sub-variants of CRF2(a) and CRF2(b) also have been identified [109,199,281]. Membrane-bound CRF2 receptors are respectively ∼411, 431 and 397 residues in length, differing only in their extracellular N-terminal domains [44,151,171]. The 143 residue soluble CRF2(a) isoform was identified in mice, and results from a frame-shift deletion of exon 6 [59]. Unlike CRF, all Ucns bind with high affinity to membrane-bound CRF2 receptors transfected in stable cell lines [165,226,289] or to endogenously expressed CRF2 receptors [111]. Human Ucn 1 and Ucn 2 are each ∼1–2 orders more potent than CRF at binding to membrane CRF2 receptors, with murine and human Ucn 3 (in rank order) only slightly less potent (Ucn 1=Ucn 2>Ucn 3>CRF) (Table 2). In contrast to membrane-bound CRF2 receptors, the soluble CRF2(a) receptor unexpectedly shows high affinity for Ucn 1 and CRF (classically, CRF1 ligands), and low affinity for Ucn 2 and Ucn 3 (Table 2) [59]. Neuroanatomical and functional studies suggest that, similar to the CRF-BP (see below), the soluble CRF2(a) receptor may curb CRF1 signaling by competitively sequestering ligand.
The combination of high membrane CRF2 potency and low CRF1 potency makes type 2 Ucns much more selective CRF2 agonists than Ucn 1. Ucn 2 (which is a full, albeit very low affinity, CRF1 agonist) shows ∼1000-fold greater functional selectivity for the CRF2 than does Ucn 1. Ucn 3 does not show CRF1-like agonism, making it the most selective, albeit not most potent, CRF2 agonist (Tables 2 and 3). The CRF2 receptor also can activate the mitogen-activated protein (MAP) kinase pathway, with some evidence suggesting that Ucn 3 may be less efficacious than Ucn 1 or Ucn 2 at engaging this signal transduction mechanism [32,56].
Chimeric CRF2/CRF1 receptor studies show that the strong selectivity of human Ucn 2 and Ucn 3 for the CRF2 receptor is mainly determined by the extracellular domain of the CRF2 receptor with an additional contribution of the juxtamembrane domain for Ucn 2 [110]. In contrast, the (weaker) selectivity of human Ucn 1 for the CRF2 receptor is determined entirely by the juxtamembrane domain of the receptor, further underscoring different ligand CRF2 binding determinants across the Ucns [110]. The high potency binding shared by Ucns depends on a stabilizing interaction with the juxtamembrane domain of the CRF2 receptor that putatively involves the N-terminal peptide end [110]. Whereas the ability of Ucn 1 (and other agonists) to bind CRF1 receptors is highly dependent on receptor coupling to G-proteins (2–3 orders greater affinity to coupled receptors), the affinity of Ucn 2 and Ucn 3 for CRF2 is only mildly greater (<1 order) in the G-protein coupled state, and Ucn 1’s CRF2 affinity is insensitive to uncoupling [110], underscoring differential CRF1 vs. CRF2 binding determinants for Ucns.
1.3.3. CRF-Binding Protein (CRF-BP)
The CRF-BP is an evolutionarily conserved 37-kDa secreted glycoprotein that binds CRF with high affinity [26,198,300]. Cloned from human liver, rat cerebral cortex and mouse brain, CRF-BP cDNAs encode a ∼322 residue protein. The mature CRF-BP protein is not membrane-associated, lacking prototypical transmembrane domains or a phosphatidyl inositol anchor signal. The CRF-BP has been hypothesized to limit CRF receptor agonist effects by sequestering secreted ligand and facilitating subsequent enzymatic degradation, thereby limiting peptide bioavailability [23,24,24,124,211,211,241,241,317,317]. In contrast, it also has been postulated that the CRF-BP, like other binding proteins [84], may enhance the effects of bound ligand, by shielding peptide from metabolic degradation during diffusion to membrane-bound CRF receptors [139]. A more recent suggestion is that the CRF-BP may have signaling properties [45], some of which may depend on ligand/CRF-BP complexes [279]. Finally, the degree to which the Ucns themselves are sequestered by the CRF-BP under basal conditions would constitute a physiologically relevant reservoir that could be competitively “freed” by another CRF-BP ligand that otherwise has different direct pharmacological properties (such as CRF). Thus, the degree to which Ucns interact with the CRF-BP may have great biologic relevance.
Ucn 1 is approximately equipotent to CRF at binding to the CRF-BP in mammals (Table 2), less so in frogs (Ki=50.3 vs 4.1 nM for Ucn 1 vs. CRF) [28]. The region of Ucn 1 that has affinity for the CRF-BP (residues 4–28) differs from those responsible for its affinity for CRF receptors [109,123,256,300]. Ucn 1 is believed to be the predominant natural ligand for the CRF-BP in ovine brain and dissociates from the CRF-BP approximately twice as slowly as does CRF [107], potentially increasing the physiologic significance of Ucn 1/CRF-BP interactions. In contrast to CRF and Ucn 1, Ucn 3 does not appreciably bind to the human, rat or frog CRF-BP (Table 2, Ki>1 μM for frog) [28]. Again underscoring pharmacological differences between the type 2 Ucns, murine Ucn 2 does bind with moderately high affinity to the rat and frog, but not human, CRF-BP albeit slightly less potently than CRF/Ucn 1 (Table 2) [28,125,165].
1.4. Physiologic and Behavioral Effects of Ucns
Because CRF and Ucns are putative paralogs derived from a common ancestral gene [49], they are hypothesized to share complementary regulatory properties to create an integrated organism response to threats to homeostasis.
1.4.1. Hypothalamic-pituitary-adrenal axis
Unlike CRF, peripheral Ucn 2 or Ucn 3 administration does not increase corticosterone secretion [291] (Fekete, ÉM and Zorrilla, EP, unpublished observations), consistent with the relative absence of CRF2 on ACTH-secreting pituitary corticotroph cells [170]. However, intracerebroventricular (icv) or intravenous (iv) administration of Ucn 1 activates the pituitary-adrenal axis (as or more potently than CRF), via a CRF1-dependent mechanism, stimulating ACTH release and proopiomelanocortin synthesis in pituitary corticotrophs [9,73,74,223,289]. However, Ucn 1 is not a likely physiologic regulator of the HPA axis. Unlike CRF-deficient mice [290], Ucn 1-deficient mice exhibit normal basal and stress-induced HPA hormone levels [292,296]. Similarly, unlike CRF antisera, peripheral administration of specific Ucn 1 antisera does not modify basal, stress-induced or adrenalectomy-induced ACTH levels [179,278]. Finally, unlike the distribution of CRF, Ucn 1-immunoreactive fibers are scarce in the PVN and the external layer of the median eminence under basal conditions [100,101,158].
It remains possible, however, that the type 2 Ucns modulate HPA-axis activity at the hypothalamic level in paracrine or autocrine fashion. Indeed, Ucn 2 and Ucn 3 mRNA are increased in the parvocellular PVN following immobilization/restraint stress [267,291], and hypothalamic Ucn 2 expression is increased by glucocorticoids [57]. More importantly, female mice deficient for Ucn 2 or for CRF2 receptors recently were found to exhibit greater peak corticosterone (CORT) and ACTH levels at the circadian light→dark phase transition. Ucn 2 knockout mice showed greater hypothalamic magnocellular expression of AVP, which is known to augment ACTH secretion, while exhibiting normal stress endocrine responses to restraint and forced swimming. Thus, Ucn 2 via CRF2 receptors may endogenously modulate basal HPA-axis circadian amplitude via an arginine vasopressin (AVP)-dependent mechanism (see Figure 3) [58].
Figure 3.
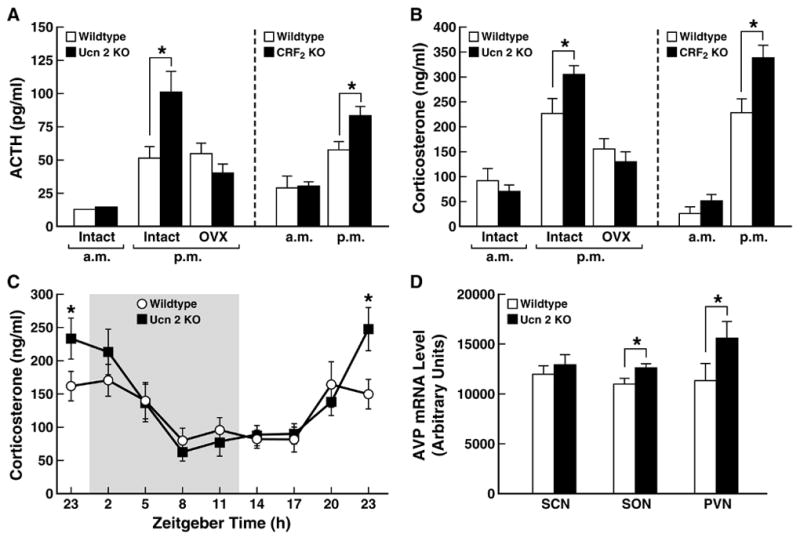
Amplified circadian peak of basal hypothalamic-pituitary-adrenal (HPA)-axis activity in urocortin 2 (Ucn 2) or corticotropin-releasing factor type 2 (CRF2) receptor-deficient female, adult mice. Panels A and B show plasma adrenocorticotropic hormone and corticosterone levels, respectively, in Ucn 2 or CRF2 null (−/−) mutant mice and their respective wildtype (+/+) littermate controls. Nocturnal peaks in ACTH and CORT levels (p.m., -1hr dark onset) were greater in both mutant mice models, which did not differ in circulating nadir levels (a.m., -1 hr light onset). Effects of Ucn 2 deficiency were abrogated by ovariectomy (OVX). The amplified circadian amplitude of adrenocortical activity was confirmed by time course analysis in Ucn 2-deficient female mice (Panel C, shading indicates the 12-hr dark cycle), and may be related to their increased expression of arginine vasopressin in the supraoptic (SON) and paraventricular (PVN), but not suprachiasmatic nuclei (SCN) of the hypothalamus (Panel D). Data reflect M±SEM. *p<0.05 vs. wildtype controls. Adapted from [58] with permission.
Ucns also might directly modulate other adrenal functions (if not adrenocorticoal activity). Ucn 1 and Ucn 3, as well as CRF receptors, are observed in non-pathological adrenal glands, but at reduced levels in tumor cells of pheochromocytomas, adrenocortical adenomas and carcinomas [90,266], suggesting physiologic relevance or regulation of their expression. Ucn 2 may regulate catecholamine synthesis and release in the adrenal medulla, as, in PC12 cells, it induces noradrenaline release and phosporylation of tyrosine hydroxylase through protein kinase A and protein kinase A-Erk1/2 pathways, respectively [190].
1.4.2. Osmoregulation
Because of the presence of Ucn 1 and Ucn 2 in magnocellular neurons of the SON and the existence of Ucn 1 projections to the posterior pituitary, an osmoregulatory role for Ucns is hypothesized. Such a function would correspond well to the ancestral phylogenetic relation of the CRF/Ucn lineages to orthologous diuretic hormones in teleost fish, insects and other invertebrates [49]. Accordingly, salt loading, dehydration and hypophysectomy increase Ucn-like-immunoreactivity in magnocellular SON and PVN neurons, whereas food deprivation decreases Ucn- like-immunoreactivity in the SON (studies in which the antibody specificity for Ucn 1 vs. Ucn 2 is uncertain) [100–102]. Specific increases in SON Ucn 1 mRNA expression also have been observed following salt loading [118]. Chronic osmotic stimulation (by salt loading or water deprivation) increases CRF2 mRNA levels in the SON and magnocellular PVN, potentially increasing sensitivity to resident Ucns [8]. Finally, female Ucn 2 KO mice recently were found to exhibit increased hypothalamic magnocellular AVP expression (Figure 3) and a blunted circadian regulation of water intake across the day, failing to show the typical difference in food/water ratios between light and dark phases which reflects a greater sensitivity to osmotic stress during the nocturnal/feeding phase [58]. Collectively, the findings support an endogenous role for Ucns 1 and 2 in the regulation of salt/water balance.
Peripheral Ucns also may control body fluid homeostasis as a result of their potency to stimulate atrial natriuretic peptide release from cardiomyocytes [116], through a putative CRF2 mechanism. Atrial natriuretic peptide, present in cardiac atrial tissue, has profound effects on salt and water homeostasis [105], as it reduces blood volume, acutely by sequestering plasma and longer term by promoting renal salt and water excretion. Atrial natriuretic peptide antagonizes the renin-angiotensin-aldosterone system at many levels [6]. Interestingly, this property of Ucns would reduce the preload or after-load on a compromised heart, to complement their direct vasodilatory effects, modulation of cardiac function and cardioprotection (see below) [74].
1.4.3. Cardiovascular function
Ucns (and CRF) are vasodilatory via arteriole and cardiac CRF2 receptors when given intravenously in animal models [1,2,51,60,66,92,131,203,223,268,289]. Conversely, CRF2-deficient mice exhibit elevated mean arterial pressure suggesting an endogenous relaxant function of Ucn/CRF2 interactions on vasculature in vivo [18]. The vasodilatory effects of Ucns are orthologous to the properties of urotensin I and other fish and invertebrate peptides of the CRF/Ucn superfamily that evolved to subserve osmoregulatory functions [49]. Vasodilatory effects of Ucn 2 in rat thoracic aorta are mediated by protein kinase A and MAP kinase signaling pathways [131], and relaxation of pulmonary arteries involves inhibition of a protein kinase C-dependent contractile mechanism [46]. Vasodepressor effects of Ucn 1 in rodents did not appear to involve activation of the nitric oxide/L-arginine pathway, prostanoid production, or K+ channels and were not counteracted by compensatory vasoconstrictive mechanisms (e.g., angiotensin, endothelin) [2,92].
The human cardiovascular system also expresses high numbers of CRF2 receptors [63,303]. Accordingly, both Ucn 2 and Ucn 3 produced potent, sustained, direct, endothelium-independent vasodilating effects in an in vitro human internal mammary artery model of endothelin-1 induced constrictions [303]. Ucn 1 also produced endothelium-dependent vasodilating effects in this model, putatively mediated (unlike in vivo rodent studies) by nitric oxide and, downstream, cyclic guanine 3′,5′ monophosphate-dependent stimulation of calcium-activated K+ channels in vascular smooth muscle [63]. However, in humans, in vivo hemodynamic effects of Ucn 1 were not observed following acute bolus doses (i.v. 50 μg) that were sufficient to activate the HPA-axis [73,74]. Perhaps higher doses or CRF2 selective ligands would produce such effects.
Complementing their hemodynamic effects, Ucns have cardioprotective and cardiovascular function-enhancing effects on compromised heart [37,196,220,237]. Systemic or in vitro Ucn 1 infusion has prolonged cardiac inotropic actions [202,268], increasing cardiac contractility, heart rate and aortic blood flow independent of changes in peripheral vascular resistance [19,51]. Ucns also are cardioprotective when added to post-ischemic/hypoxic cardiomyocytes or to isolated intact heart during reperfusion after regional ischemia [33,35,47,126,161,163,164,239,285]. For example, Ucn 1 promoted hemodynamic and bioenergetic recovery of isolated, paced rat hearts following post-ischemia reperfusion, effects associated with improved ventricular performance [239]. Ucn 2 and Ucn 3 reduced infarct size in isolated rat hearts after post-ischemia reperfusion [33] and also protected human heart from reperfusion injury [51]. In ventricular pacing large animal models of congestive heart failure, Ucn 1 and Ucn 2 similarly had palliative effects, the latter reducing left atrial pressure, brain natriuretic peptide and vascular resistance (see Figure 4). These changes were accompanied by normalizing decreases in circulating vasopressin, aldosterone, endothelin-1, and epinephrine levels, with corresponding increases in sodium and water excretion, indicating effective “unloading” of compromised cardiovascular/renal systems [37,220,221,223] (see also Figure 4).
Figure 4.
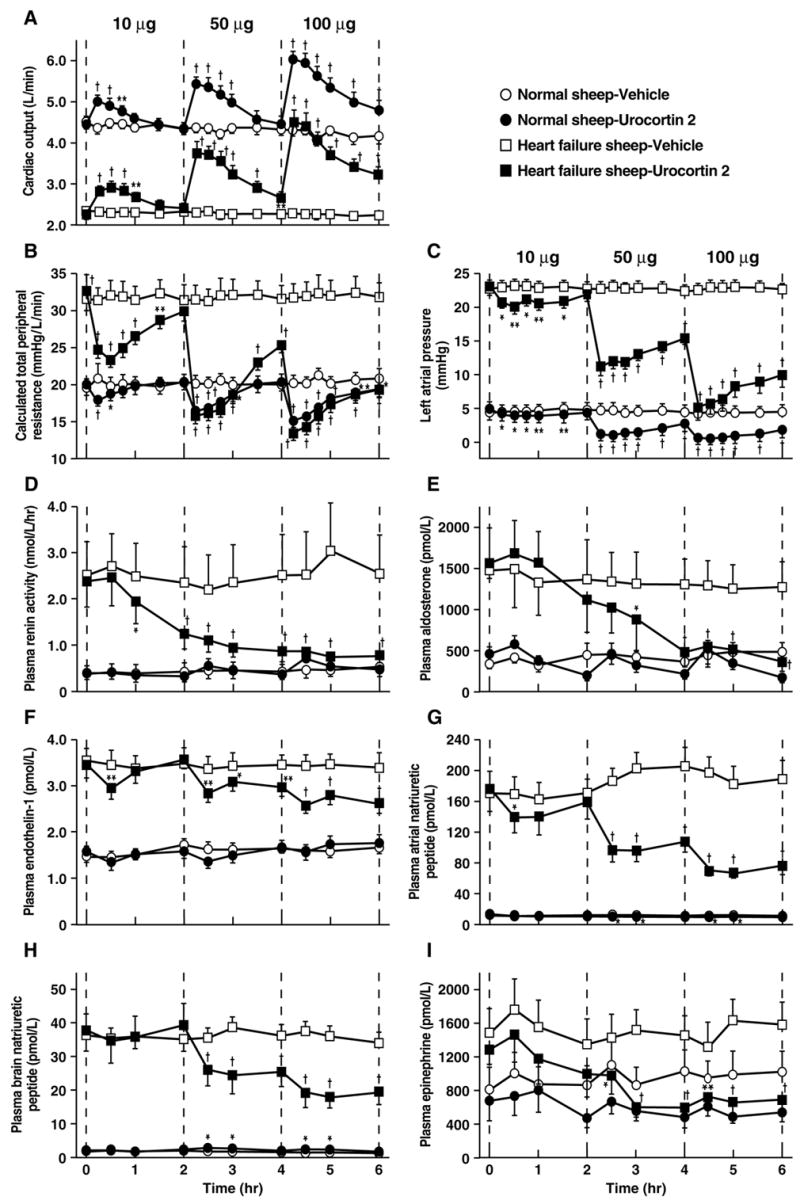
Effects of bolus intravenous mouse urocortin 2 (Ucn 2) infusion on hemodynamic (Panels A–C) and cardiovascular-relevant hormonal responses (D–I) in sheep before or after induction of heart failure by 7 days of rapid (225 beats/minutes) left ventricular pacing, as compared to vehicle-infused (10 ml isotonic saline) controls. Ucn 2 tended to normalize all parameters in the heart failure model, while having lesser direct effects in healthy sheep. Data reflect M±SEM. *P<0.05, **P<0.01, P<0.001 vs. respective vehicle-treated controls. Adapted from [220] with permission.
The cardioprotective effects of Ucns are mediated by a CRF2-dependent mechanism [19,33] and involve multiple signal transduction pathways. Ucn 1 increased synthesis, expression and translocation/activation of the protein kinase C epsilon isozyme in primary rat cardiomyocytes and in Langendorff perfused ex vivo heart. Ucn 1 no longer reduced apoptosis resulting from ischemia-reperfusion in isolated cardiomyocytes when peptide inhibitors of the isozyme were present, and the cardioprotective effects of Ucn 1 on whole heart were absent in protein kinase C epsilon-deficient mice [162,164]. Similarly, chelerythrine, a specific PKC inhibitor, eliminated Ucn 1-induced cardioprotection of isolated rat heart following ischemia-reperfusion [91]. Conversely, Ucn 1 reduced ischemia-induced increases in mRNA and protein expression of a calcium-insensitive phospholipase A2 enzyme (iPLA2) as well as levels of its toxic metabolite lysophosphatidylcholine in isolated cardiac myocytes [163,164]. Ucn 1, Ucn 2 and Ucn 3 cardioprotection also involves MAP kinase signaling, as each peptide phosphorylated Erk1/2-p42, 44 in neonatal cardiomyocytes and reduced post-reperfusion infarct size by a CRF2 dependent mechanism, effects abolished by inhibitors of MEK1, Ras, or Raf-1 [32,34,47,160,240]. Ucn 1 also increased synthesis of the mitochondrial KATP potassium channel subunit Kir 6.1. in cardiomyocytes, with general and mitochondrial-specific KATP channel blockers blocking the cardioprotective effects of Ucn 1 both in isolated cardiac cells and in intact heart [161,164]. Finally, both cardioprotective and (undesired) hypertrophic effects of Ucns also involve activation of a phosphatidylinositol-3 (PI-3) kinase/Akt-dependent pathway [47,48,126], which ultimately reduces Beclin-1 expression and resulting autophagic cell death in cardiomyocytes [285]. Importantly, some of Ucns’ cardioprotective effects are dissociable from their hypertrophic effects, opening potential therapeutic avenues [48,72,225].
Supporting an endogenous compensatory response for Ucns in cardioprotection, Ucn 1, 2 and 3 are present in the heart [55,145,193,197,264], with Ucn 2 and Ucn 3 expression abundant in myocardium. Plasma Ucn 1-like-immunoreactivity is increased in human systolic heart failure [191] as well as in an experimental model of heart failure [52], and Ucn 1 expression is increased in diseased human heart [117,193]. Ucn 1 expression also increased more than 9-fold in viable, apoptotic-resistant human myocytes after surgical cardioplegic arrest-reperfusion for coronary bypass surgery [237,238]. Finally, Ucn 1 mRNA is increased in post-hypoxic cardiomyocytes, and isolated cardiomyocytes treated with CRF receptor antagonists or from CRF2-deficient mice are more susceptible to ischemia-perfusion injury [19,35]. Endogenous Ucn/CRF-related peptides also may be involved in hemodynamic adaptations to heart failure, because intravenous infusion of a preferential CRF2 receptor antagonist produced greater acute effects on arterial pressure, peripheral resistance and circulating renin and endothelin-1 levels in a pacing-induced, ovine model of heart failure than in the non-diseased state [222]. Finally, human venous endothelial cells synthesize and secrete Ucn 1 in response to inflammatory cytokines, such as tumor necrosis factor-α and interferon-γ. This response has been hypothesized to oppose damaging effects of oxidative stress because incubation with Ucn 1 suppressed angiotensin II-induced accumulation of reactive oxygen species in human umbilical vein endothelial cells [112].
1.4.4. Energy balance
Exogenous administration of Ucns promotes negative energy balance, by both increasing energy expenditure and decreasing food intake. The reviewed distributions of the Ucns support the hypothesis that they may regulate metabolism or food intake by interacting with CRF2 receptors, including concordant hypothalamic expression in the VMH (Ucn 3), arcuate nucleus (Ucn 2, Ucn 3) and PVN (Ucn 1, Ucn 2) of the hypothalamus, lateral septum (Ucn 1, Ucn 3), and in the NTS of the caudal hindbrain (Ucn 1) [27,43,165,167,170,226,287]. In addition, VMH CRF2 mRNA levels vary directly with circulating leptin levels [104,174,194,227,272], potentially modulating sensitivity to catabolic Ucn action, and circulating leptin facilitates the entry of peripheral Ucn 1 into the central compartment [134,135]. The presence of Ucns in the GI tract, glucoregulating tissue (e.g., pancreas, VMH) and adipose tissue also is consistent with a hypothesized peripheral role for Ucns in the short- or long-term regulation of energy balance.
Energy expenditure
With respect to energy expenditure, Ucn 1 (i.c.v.) increases whole body oxygen consumption as measured by indirect calorimetry, and increases signs of sympathetic nervous system activity, including mean arterial pressure and colonic body temperature [76,253]. The hypothalamus is a candidate site of metabolic action for Ucns, because intra-PVN Ucn 1 increases plasma leptin levels, induces BAT uncoupling protein-1 (UCP1) mRNA synthesis, and increases relative utilization of fat as an energy substrate [152]. Similarly, intra-PVN Ucn 1 blocked the ability of NPY to promote food intake or the preferential utilization of carbohydrates as an energy substrate [68]. Whether the energy expenditure-increasing effects of Ucn 1 are shared by the type 2 Ucns and mediated by CRF2 receptors is unclear. Supporting a role for CRF2 receptors in the body weight-reducing effects of Ucns, Ucn 1 promoted greater, more sustained reductions in body weight in CRF1 knockout mice relative to their wild-type littermates despite achieving similar anorexia across genotypes [30]. In addition to these central actions of Ucns, peripheral activation of CRF2 receptors in oxidative skeletal muscle also directly promotes thermogenesis via substrate cycling between de novo lipogenesis and lipid oxidation [252].
Food intake
With respect to food intake, peripheral administration of Ucn 1 or stresscopin reduces food intake in rodents (see Figure 5A for effects in fasted mice), possibly in part by slowing gastric emptying [10,113,294], and Ucn 1 suppresses operant responding for food reward [146]. Sustained intravenous infusion of Ucn 1 also reduced food intake in an experimental ovine heart failure model for 2 days, but with subsequent tolerance observed [223]. The receptor subtype mediating these actions remains uncertain.
Figure 5.
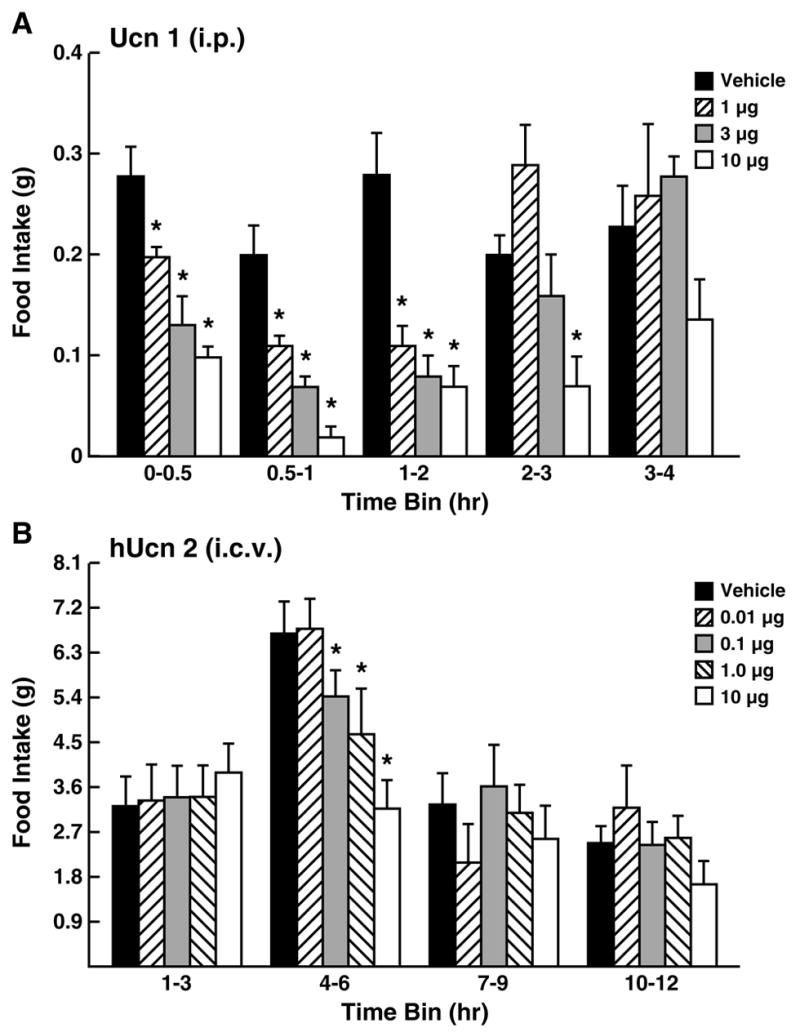
Anorectic effects of urocortins on incremental food intake. Panel A shows acute-onset, dose-dependent anorectic effects of intraperitoneal (i.p.) rat urocortin 1 (Ucn 1) in previously fasted (18–20 hr) adult male mice, with refeeding intake monitored by intermittent (30, 1 hr) weighing. Panel B shows delayed-onset, dose-dependent anorectic effects of intracerebroventricular human urocortin 2 (hUcn 2) in non-food deprived adult male rats, with spontaneous nocturnal intake monitored by an automated precision-pellet system. Data reflect M±SEM. *p<0.05 vs. vehicle-condition. Adapted from [294] and [121], respectively, with permission.
Central infusion of Ucns also potently suppresses feeding in mammalian and non-mammalian vertebrates, effects shown to be at least partly CRF2-mediated in studies that used selective agonists (see Figure 5B for effects in non-fasted rats) or antagonists, antisense knockdown of receptor expression, and knockout mice [28,67,77,195,206,253,316,318]. CRF2 KO mice are constitutively hyperphagic on high-fat diets [17] or on 15% corn syrup-sweetened chow pellets (Consoli D., Diaz-Chaves Y., Monseingeon M., Corcuff J., Drago F., Vale W., Bale T., Koob G., Contarino A., Zorrilla E., and Tabarin A., unpublished observations). Unlike CRF1 agonists, type 2 Ucns do not produce malaise, arousal or anxiety-like effects at the minimum central doses needed to reduce food intake in rats [77,121,195,206,226,282,316,318]. Also unlike ligands with CRF1 affinity, the anorectic effects of i.c.v. type 2 Ucns are delayed approximately 2-6 hr in onset, again perhaps reflecting slowed gastric emptying (see below). The degree to which endogenous brain Ucns produce these effects under physiologic conditions remains unclear, because Ucn 1 and Ucn 2 deficient mice exhibited normal spontaneous food intake [58,292]. On the other hand Ucn 2 deficiency did blunt the anorectic effects of fenfluramine, suggesting a downstream role in serotonin’s satiating effects [58].
In addition to the hypothesized anorectic role of central Ucns via the CRF2 receptor, brain Ucn 1 also may reduce food intake via additional acute onset CRF1-dependent mechanisms [318]. Brain CRF1 stimulation suppresses feeding through a different behavioral mechanism than CRF2 receptor activation, as evidenced by different time courses, effects on meal patterning and dietary self-selection [316,318]. Possible loci for endogenous Ucn 1-CRF1 mediated anorexia include the dorsomedial nucleus of the hypothalamus, the parabrachial nucleus and other caudal hindbrain glucoregulatory sites. Ucn 1 infused into the fourth ventricle reduced intraoral sucrose solution intake even in chronically maintained decerebrate rats, supporting a hindbrain mechanism of anorectic action for brainstem Ucn 1 [70].
Ucn 3 is present in pancreatic islet β-cells, and secretion of Ucn 3-like immunoreactivity by MIN6 cells is increased by extracellular glucose [166]. In vivo and in vitro studies demonstrated that exogenous Ucn 3 increased glucagon and insulin levels, resulting in a net increase in blood glucose levels. Effects were abolished by pretreatment with a selective CRF2 antagonist, and suggest an autocrine or paracrine regulation of glucose homeostasis by pancreatic Ucn 3 [166].
Human genetic studies also suggest a relation of CRF2 receptors and, by association, Ucns to energy balance. Four genome-wide linkage analyses have revealed an association between energy balance-related endpoints, including body mass index (BMI) [3,306], type 2 diabetes [305], and lean body mass [42], with the portion of chromosome 7 that includes the CRF2 gene (7p15–7p21) [180]. In addition, a study of early-onset (<10 years of age) obesity identified a single nucleotide mutation substitution (Val411Met) in the CRF2 gene of a hyperphagic, severely obese 5-year old girl that was not evident in 140 alleles from control subjects. The heterozygous substitution also was observed in the hyperphagic proband’s mother and maternal grandfather, both of whom also were obese.
1.4.5. Gastrointestinal motility and function
Stressors release CRF-related peptides which inhibit gastric emptying through brain-gut CRF2 receptor systems [177,318]. For example, stress-induced gastric stasis is reversed by central or peripheral pretreatment with nonselective or selective CRF2 receptor antagonists [106,143,177,183,262]. Similarly, nonselective or selective CRF2 receptor antagonists, but not CRF1 antagonists, block the ability of i.c.v., i.v., or i.p. administered Ucns/CRF to slow gastric emptying [61,142,177,189,229,294]. Through vagal efferents, central infusion of CRF or Ucn 1 reduces antral gastric motility, inhibits high amplitude gastric contractions, and shifts duodenal activity from fasted to fed motor patterns [142]. Intracisternal infusion of Ucn 2 also suppresses gastric emptying, but unlike Ucn 1 and CRF, its effects are mediated by a non-vagal, central CRF2-dependent alpha-adrenergic1 receptor mechanism [69].
Parallel to the central pathways for stress-induced gastric stasis, peripheral CRF2 receptor activation delays gastric emptying, with peripheral (i.v. or i.p.) administration of agonists with high (i.e., Ucn 1) or selective (i.e., Ucn 2) CRF2 affinity delaying gastric emptying more potently than CRF (see Figure 6) [177,178]. Similar to central administration, peripheral Ucn 1 reduces antral gastric motility in fed rats and shifts gastric motor patterns from a fasted to fed state [142]. Ucn 1 also hyperpolarizes stomach smooth muscle [210]. Candidate substrates that mediate CRF receptor-induced gastric stasis include, centrally, the PVN and dorsal vagal complex, via an undefined subset of its descending autonomic efferents [294,295], and, peripherally, myenteric fibers of the enteric nervous system [54,142] and the gastric antrum, each which expresses CRF, Ucn 1, Ucn 2 and both CRF receptor subtypes [219]. Outside the stomach, CRF-like peptides also inhibit phasic contractions of the CRF2-expressing ileum of the small intestine, an effect that, similar to stomach, was blocked by CRF2, but not CRF1, antagonists [218]. An endogenous role for Ucns in stress-induced changes in gastrointestinal motor function is also supported by the presence of Ucns in the PVN, NTS of the caudal hindbrain, GI tract and enteric nervous system. Consistent with this hypothesis, site-specific RNA interference knockdown of Ucn 2 (or CRF) expression in the terminal ileum increased diurnal fecal output under basal conditions, suggesting a physiologic role for Ucn 2 in the regulation of ileal motility [159].
Figure 6.
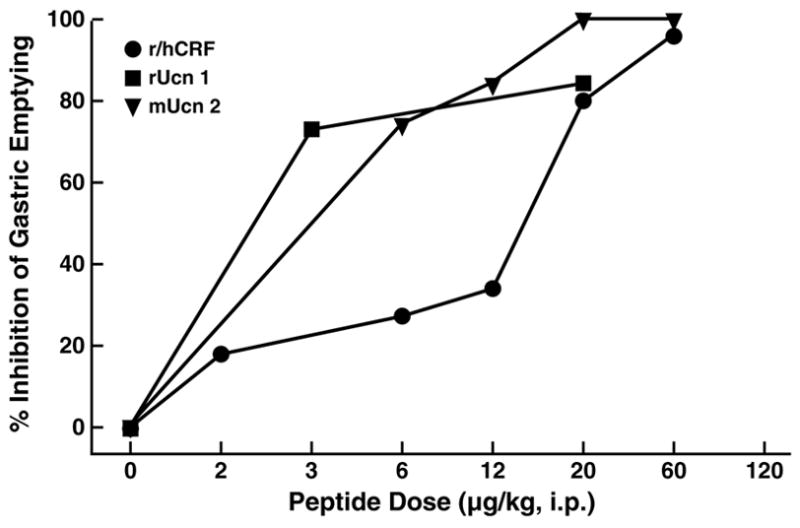
Relative potency of intraperitoneal administration of urocortins (Ucn 1, Ucn 2) or rat/human corticotropin-releasing factor (r/hCRF) to inhibit gastric emptying in conscious mice. Graphs represent the % mean inhibition of gastric emptying of a solid nutrient meal in the 2 hr (mice) after peptide administration; r=rat, m=murine, h=human. Adapted from [177] with permission.
Gastric Ucn 1 also may participate in the control of gastric acid secretion [54]. Ucn 1 colocalizes with tyrosine hydroxylase in parietal cells of the stomach, in proximity to CRF2, but not CRF1 receptors [54,155]. CRF2 receptors, in turn, co-localize with H+/K+-ATPase the enzyme gastric proton pump, and somatostatin, which inhibits parietal cell activity and secretion of gastrin and histamine [54]. Perhaps accordingly, peripheral administration of nonselective CRF2 receptor agonists inhibits gastric acid secretion and increases gastric mucosal blood flow.
In contrast to their inhibitory effects on gastric and ileal motility, diverse stressors stimulate colonic motor function, seen as increased colonic motility, decreased colonic transit time, defecation and watery diarrhea [259,261,318]. Central and peripheral administration of CRF1, but not CRF2, agonists also stimulates colonic motility, and selective CRF1, but not CRF2, antagonists attenuate stress- or CRF/Ucn 1-induced colonic hypermotility [177,318]. An apparent exception to this pattern of CRF1 activation selectively stimulating colonic motility is that central murine Ucn 2 infusion also increased colonic motility. However, central administration of Ucn 3 (an even more selective CRF2 agonist) did not similarly stimulate colonic motility, and actions of Ucn 2 could be reversed by a selective CRF1 receptor antagonist. Thus, CRF1 activation was still an essential (if not proximate) mediator of the colonic motor stimulating effects of i.c.v. Ucn 2 [177]. Candidate substrates for CRF1-mediated stimulation of colonic motility include, centrally, the PVN and LC/Barrington nuclei that activate sacral parasympathetic nervous system activity, and, peripherally, the colonic myenteric nervous system, tissue in which Ucn 1 is expressed. Ucn 1 also was recently shown to increase duodenal contractile activity in vitro, an effect reversed by CRF1, but nor CRF2, antagonists [218]. Thus, endogenous Ucn 1 may partly mediate the colonic and duodenal motor stimulating effects of stress.
Peripheral CRF receptor signaling, perhaps initiated by Ucns, also modifies visceral nociception, in particular that related to painful gastrointestinal stimuli. For example, peripheral (i.p. or i.v.) or intrathecal administration of Ucn 2 or stresscopin, selective CRF2 agonists, reduced behavioral and visceromotor responses to duodenal or colorectal distension [176,192]. Similarly, peripheral sauvagine (an amphibian CRF/Ucn-related peptide with high CRF1/CRF2 affinity) and Ucn 2, but not CRF, prevented experience-induced increases (i.e., sensitization) in visceromotor responses to colorectal distension. These palliative effects were reversed by CRF2 antagonist pretreatment [182,184]. Peripheral antinociceptive effects of Ucns and Ucn-related peptides were not CRF1-mediated, as i.p. CRF produced pronociceptive effects in the duodenal distension pain model via a CRF1-dependent mechanism [192,259]. In fact, selective CRF1 receptor antagonists reversed stress and experience-induced increases in visceromotor hyperalgesic-like responses to colorectal distension [181,182]. The actions of exogenous CRF and Ucns on GI function as well as the ability of selective CRF antagonists to modify similar effects of stress have led to a proposed involvement of endogenous Ucns (and CRF) in stress-related functional GI disorders, such as irritable bowel syndrome [258].
1.4.6. Immune function
As reviewed previously, Ucns are expressed in immunological tissue, including thymus, spleen, and/or skin, with Ucn 1 seen at the cellular level in lymphocytes, macrophages, fibroblasts, and mast cells. Exogenous Ucn 1 administration has palliative effects in experimental models of autoimmune encephalomyelitis [217], thermal injury-induced edema [277], and Crohn’s disease [95], though these actions may reflect glucocorticoid, vasodilatory or cytoprotective (rather than direct immune) mediated mechanisms [4]. Still, converging lines of evidence suggest that immune-derived Ucns locally and directly modulate proinflammatory responses to perceived environmental insults, with the direction of this effect depending on the tissue or CRF receptor subtype [270].
For example, Ucn 1 mRNA is expressed in lamina propria macrophages, and Ucn 1-like-immunoreactivity is detected throughout the entire lamina propria layer of the intestine [187,236]. Although intestinal lamina propria inflammatory cells are evident as early as 12–18 weeks of gestation, Ucn 1-like-immunoreactivity is not detectable until after birth [187]. This suggests that intestinal Ucn 1 activity may be regulated by changes in the intestinal milieu, such as passage of dietary factors or food-associated bacterial antigens. Ucn 1-like-immunoreactivity is further elevated in intestinal lamina propria macrophages of patients with ulcerative colitis, where it is hypothesized to have a proinflammatory effect via CRF1 receptors [236]. Intraperitoneal administration of CRF1 agonists, such as Ucn 1, also increases intestinal mucosal permeability to macromolecules [258,260]. Interestingly, a history of early trauma potentiates the effects of acute stress on intestinal mucosal dysfunction in adult rats, a response blocked by peripheral injection of a CRF receptor antagonist [258,260]. Chronic psychosocial stress also reduces intestinal host defense and initiates intestinal inflammation through a mast cell, putatively CRF1 receptor dependent, mechanism [258,260]. In contrast to Ucn 1, Ucn 3 was hardly detected in lamina propria inflammatory cells in colonic mucosa, although it was detected in other gastrointestinal tissue, underscoring peptide specificity in the physiological roles of Ucns [235].
Other stress-related inflammatory conditions are also accompanied by locally increased Ucn 1 expression. In rheumatoid arthritis, Ucn 1-like-immunoreactivity and Ucn 1 mRNA are substantially elevated in synovium, and the number of Ucn 1-positive cells in synovia [280], including leukocytes and marcophages, is higher and significantly correlates with inflammation severity [149,280]. The same degree of synovial Ucn 1 activation is not observed in osteoarthritis [149]. Supporting a proinflammatory action of secreted Ucn 1, Ucn 1 stimulates IL-1β and IL-6 secretion by peripheral blood mononuclear cells in vitro, presumably via a CRF1-mediated mechanism [149].
Some stress-related dermatological inflammatory conditions also have altered Ucn/CRF crosstalk between mast cells, neurons and keratinocytes [246–250,302]. Mast cells, which play a role in allergy and inflammation by releasing histamine, proteases (tryptase, chymase), proteoglycans, prostaglandin D2, leukotriene C4, and several multifunctional cytokines, are distributed widely in the skin. Mast cells recently were recognized to synthesize and secrete both CRF and Ucn 1 in response to psychosocial stress or immunoglobulin E receptor crosslinking [141,172,244,270,271]. Mast cells also express CRF receptors, activation of which leads to the release of cytokines and other pro-inflammatory mediators (e.g., vascular endothelial growth factor) and increased skin vascular permeability [38,39,82,270]. Finally hair follicles are both sources and targets of CRF/Ucn 1 lineage peptides with resulting effects on pigmentation [122,138]. Thus, it has been suggested that disorders such as atopic dermatitis, psoriasis [270], alopecia areata [31,136], and chronic urticaria [200] involve a stress-related precipitation or exacerbation of skin mast cell activation (and possibly other resident skin cells) via local CRF/Ucn 1–CRF receptor signaling.
The endometrium, myometrium, and outer decidua of the reproductive tract also contain mast cells [140,173]. Ucn 1-like-immunoreactivity is increased more than 10-fold in spontaneous abortion products (which include myometrium, fetal membranes and chorionic villi) from women with a history of multiple non-elective abortions relative to products from elective or non-habitual abortions [86,173]. Supporting the hypothesis that the increase in Ucn 1-like-immunoreactivity is related to mast cell activation, levels of tryptase, which constitutes 20% of total protein in mast cells, and IL-8, an abortogenic mast cell-derived cytokine, also are robustly elevated in habitual spontaneous abortions. Endometriosis also is associated with an increased number of activated mast cells in association with strong positive Ucn 1 immunostaining [140]. Normal endometrium, in contrast, shows low tryptase and Ucn 1 immunoreactivity [140]. Thus, Ucn 1 expression and mast cell activation, increased in inflammatory conditions of the reproductive tract, may correlate with increased risk for spontaneous abortion.
Increased Ucn 1-like-immunoreactivity and mRNA also were observed in lung airway epithelial cells of rats with experimental allergic asthma, where Ucn 1 increases pulmonary vascular permeability via a mast-cell dependent mechanism [307,308]. Ucn 1 elevations were reversed by effective glucocorticoid treatment. Finally, Ucn 1-IR was elevated in stomach biopsies from patients with active gastritis [53]. The stomach does not contain the rich CRF1 distribution of the colon, but rather is rich in CRF2 receptors [54]. Correspondingly, in contrast to the CRF1-mediated proinflammatory effects of intestinal Ucn 1, stomach Ucn 1 has been hypothesized, via CRF2 receptors, to protect or repair gastric mucosa from injury following noxious stimuli [53]. Consistent with this possibility, immunoreactive Ucn 1 increases further in treatment-responding, but not treatment-resistant, patients during disease regression. Perhaps underlying this relation, both Ucn 1 and Ucn 2 reportedly have short-onset pro-apoptotic effects on macrophages via CRF2 receptors [276], and Ucn 1 suppressed lipopolysaccharide-induced TNF-α production by rat Kupffer cells in vitro ([4], but see [275]).
1.4.7. Reproductive function
In addition to resident and infiltrating mast cells, the human reproductive system itself expresses Ucn 1, CRF receptors and CRF-BP [89,196,242,301]. In the ovary, Ucn 1 may suppress ovarian steroidogenesis [188], and Ucn 1 and Ucn 2 levels change dynamically in relation to the luteal phase [309]. In addition, as reviewed previously, Ucn 1-like-immunoreactivity is produced by choriodecidual and placental tissue and is elevated in maternal plasma from mid-gestation through birth [85,87,274]. Ucn 2 and Ucn 3 gene expression also were recently observed in trophoblast and maternal decidua and, especially in late pregnancy, fetal membranes [119].
Placental Ucn 1 appears to act as a relaxant on uteroplacental vasculature via local action at CRF receptors. Supporting the physiological relevance of this function, pregnant women with impaired uterine artery blood flow during mid-gestation exhibit significantly reduced circulating Ucn 1 levels in proportion to the degree of increased arterial resistance [85]. Similarly, placental explants from women with preeclampsia were deficient in their cGMP response to Ucn perfusion, a mechanism through which Ucns are hypothesized to produce vasodilative effects on fetoplacental circulation [133].
Reproductive Ucns also may stimulate uterine contractility or augment contractility from other stimuli (e.g., prostaglandins) [108,132,212]. Myometrium expresses both CRF1 and CRF2 receptors [88,242]. Ucns increase contractility via autocrine and paracrine CRF2-mediated actions [132] and may facilitate degradation of extracellular matrices (and thereby rupture of fetal membranes) via their ability to increase production of matrix metalloproteinase-9 [168]. Consistent with this hypothesis higher Ucn 1 levels at the time of medical induction of post-term labor strongly predicts a shorter time to delivery (see Figure 7) [274]. Thus, Ucns may regulate placental vessel resistance to blood flow and augment uterine contractility, suggesting an important role in the physiology of pregnancy and parturition.
Figure 7.
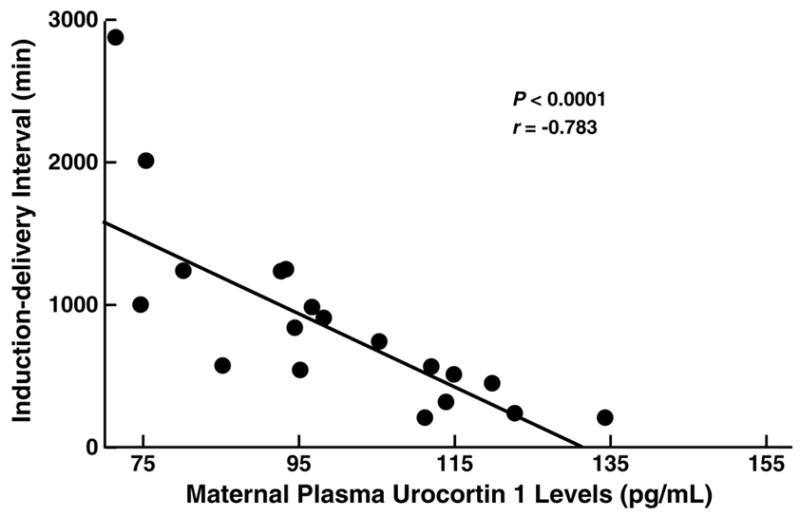
Higher preinduction maternal plasma urocortin 1 levels correlate significantly with a shorter time to delivery following intravaginal prostaglandin labor induction in post-term pregnancies. Figure depicts scatterplot of individual observation with fit regression line. Adapted from [274] with permission.
1.4.8. Anxiety- and depressive-related behavior
Central Ucn 1 administration shares many neurochemical and behavioral properties of i.c.v. CRF treatment, reflecting their pharmacological similarity. These include behavioral arousing properties in familiar environments and proconvulsant and anxiogenic-like effects [315]. The anxiogenic-like properties of central Ucn 1 infusion, mediated at least partly by CRF1 receptors, have been shown in several paradigms, including the open field [185,317], elevated plus-maze [128,185,254], light-dark box [185], defensive withdrawal [254] and social interaction tests [94,233,234]. Ucn 1’s effects on acoustic startle responding appear to differ from those of CRF, however, as exogenous Ucn 1 dampens rather than potentiates the acoustic startle response [128].
Quite unlike CRF1 receptor agonists, i.c.v. administration of type 2 Ucns does not consistently have anxiogenic-like effects in rats [77,273,282,283,291]. For example, Ucn 3 did not increase anxiety-like behavior in the open field [291], elevated plus-maze [283,291], light/dark box [291], social interaction [313], or defensive burying tests [313], under conditions in which CRF1 agonists produced anxiogenic-like changes. In fact, i.c.v. Ucn 3 acutely produced anxiolytic-like changes in the elevated plus-maze and light/dark box tests [283,291]. Ucn 2 (i.c.v.) also lacked anxiogenic-like effects in the rat open field and elevated plus-maze tests [282]. Rather, Ucn 2 had delayed, anxiolytic-like effects under high baseline anxiety conditions in the plus maze [282] and opposed the anxiogenic-like effects of CRF in the open field [291]. Finally, whereas CRF1 agonists increased activity in familiar environments, type 2 Ucns had mild motor suppressing effects and opposed the activating effects of CRF [195,282,283,316].
On the other hand, some studies have been interpreted as showing pro-stress-like effects of CRF2 receptor activation in the lateral septum or dorsal raphe of rats [16,99,224], findings apparently incongruous with the reviewed effects of i.c.v. type 2 Ucn administration. Furthermore, exogenous i.c.v. administration of high doses of Ucn 2 (but not Ucn 3) to mice increased anxiety-like behavior in the plus-maze [205,206] and acoustic startle responses [228]. However, because Ucn 2 (unlike Ucn 3) can activate CRF1 receptors at high doses [110] and displaces CRF1 agonists from the CRF-BP [125], the role of CRF1 vs. CRF2 receptors in mediating these effects remains unclear. Overall, whereas CRF1 receptor activation has known anxiogenic-like effects [314], conclusions regarding anxiety-related effects of central type 2 Ucn administration are not yet possible and may be brain site-specific.
Moreover, the endogenous anxiety-related roles of Ucns remain similarly unclear as one Ucn 1-deficient mouse model exhibited normal anxiety-like behavior and autonomic responses to stress [296], whereas another Ucn 1-deficient mouse model showed increased anxiety-like behavior on the plus maze and open field tests [292]. Recently generated Ucn 2 deficient mice also did not exhibit altered anxiety-like behavior in the plus maze, light/dark box or conditioned fear tests [58].
While the role of endogenous Ucns in anxiety-related behavior is unclear, accumulating evidence suggests a role for Ucn 2 in the regulation of depressive-related behavior, perhaps via modulation of serotonergic signaling by the dorsal raphe nucleus (DRN). The DRN densely contains CRF2 and, less so, CRF1 receptors [75,287]. Several studies indicate that CRF1 activation inhibits 5-HT release, whereas CRF2 activation has excitatory effects [97–99,147,286]. Ucn 2, which is highly expressed in the locus coeruleus, may innervate the DRN via a known reciprocal connection between these two brain regions [144]. Supporting a role for Ucn 2-CRF2 signaling in the DRN, intra-DRN administration of Ucn-2 dose-dependently increased 5-HT efflux in the basolateral amygdala [5] and led to “learned helplessness”-like behavioral changes at doses 100-fold lower than those required for CRF, a less potent CRF2 agonist [99]. Most recently, Ucn 2 deficient female (but not male) mice were shown to exhibit greater antidepressant-like behavior in the forced swim and tail suspension tests (see Figure 8 [58]). The resistance of Ucn 2 null females to acquire forced swim immobility resulted from a persistence of swimming, rather than climbing [58], a behavioral profile linked to serotonergic acting antidepressants in rodents [79,80]. Interestingly, female, but not male, 5-HT1B-deficient mice also selectively show reduced immobility in the forced-swim and tail-suspension tests [129], and other studies have seen a differential sensitivity of females to genetic deletion or polymorphisms of serotonergic modulators [29,65,129]. These findings support the hypothesis that Ucn 2-CRF2 signaling modulates 5-HT function..
Figure 8.
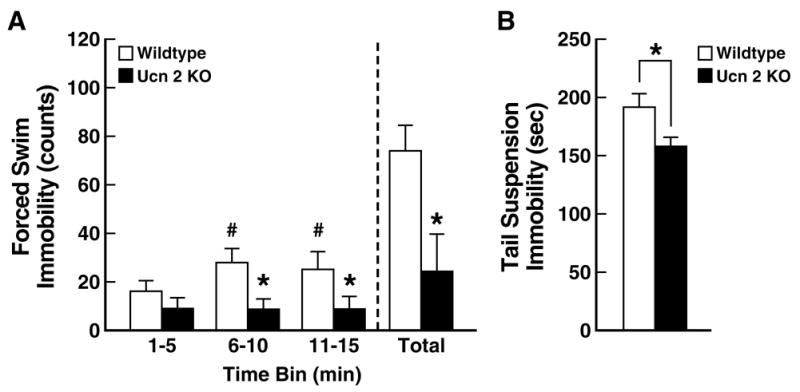
Increased antidepressant-like behavior in female urocortin 2 (Ucn 2) null mutant mice. Panel A shows that female Ucn 2 null mice did not become increasingly immobile during a modified forced-swim test, unlike female wildtype (WT) mice. Behavioral analysis indicated that this resulted mainly from persistent swimming, a behavior linked to serotonergic acting antidepressants. Panel B shows that female Ucn 2 null mutant mice also were significantly less immobile in the tail-suspension test. Data reflect M±SEM. *p<0.05 versus WT mice; #p<0.05 versus 1–5 min time bin. Adapted from [58] with permission.
1.4.9. Hearing
Ucn 1 may be required for the development and maintenance of normal hearing. The distribution of Ucn 1-like-immunoreactive neurons within the margin of the lateral superior olive of the brainstem as well as the presence of Ucn 1 fibers and terminals in the inner spiral bundle of the organ of Corti suggests a role for Ucn 1 in audition [292]. Ucn-like-immunoreactivity appears in the rat organ of Corti at 8 days of age, present only in the inner hair-cell region, and not within the tunnel or in the outer hair cell region [292]. CRF1 and CRF2 receptors also localize to the lateral portions of the organ of Corti, and include other hair cells, Deiters cells, Henson cells and Claudius cells [292]. Supporting a functional role for Ucn 1 in hearing, Ucn 1-null mutant mice have shorter hair-cells and a higher response threshold in the auditory brainstem response examination than wild-type littermates [292]. Perhaps reflecting impaired hearing, a different murine model of Ucn 1 deficiency had a reduced acoustic startle response [296].
1.5. Concluding remarks
In sum, understanding of Ucns has emerged from the shadow cast by their paralog CRF, with each peptide having a unique phylogenetic history, gene, pharmacology, tissue distribution, and functions. In several organ systems, type 2 Ucns exert pharmacological effects superficially opposite to those induced by CRF1 ligands, including possible anti-inflammatory properties, visceral antinociception, dearousal, possible anxiolytic-like activity, stasis of the gastrointestinal tract, and hypotension. However, it appears to be an oversimplification that Ucns are simply the yang to CRF’s yin, as Ucns, especially but not exclusively Ucn 1, share several general properties with CRF (e.g., anorexia, increased energy expenditure/thermogenesis, CRF1-mediated proinflammatory effects, roles in parturition and reproduction, as reviewed elsewhere [14,20,78,106,108,260,314,318]) and have distinct ones of their own (hearing, cardioprotection, hemodynamic and osmoregulatory actions). Even where apparently “opposite” pharmacological effects exist, it is not clear whether the actions reflect counter-regulatory actions of endogenous Ucns as opposed to self-regulating, negative feedback actions of CRF at CRF2 receptors (e.g., anxiolytic-like behavior, dearousal) or indeed whether they even are physiologically opposing actions (e.g., gastric stasis vs. colonic hypermotility, peripherally-induced hypotension vs. centrally-induced hypertension). Also, because CRF has previously been implicated as playing roles in many of the domains reviewed for Ucns, a critical area of future research will be to clarify how each endogenous ligand singly, in combination or in opposition subserves particular physiological functions. For example, the role of CRF, but not Ucn 1, in endogenous HPA-stress responses was partly clarified by distinguishing the degree to which each ligand did (CRF) or did not (Ucn 1) have stress-regulated access (PVN->median eminence-> portal blood) to the anatomical targets that govern ACTH release (anterior pituitary corticotrophs) and which accordingly express the principal identified regulatory receptor (CRF1 subtype). Analogously, further understanding of CRF/Ucn physiology and pathophysiology in the functional domains reviewed above will involve even closer study of how each natural ligand is differentially expressed and secreted in tissue-specific fashion in concordance with its putative cognate receptor(s).
Thus, in their own right, Ucns may be clinically relevant molecules in the pathogenesis, treatment or management of many conditions, including congestive heart failure, hypertension, inflammatory disorders (irritable bowel syndrome, active gastritis, rheumatoid arthritis), atopic/allergic disorders (dermatitis, urticaria, asthma), gastroparesis, pregnancy and parturition (preeclampsia, spontaneous abortion, onset and maintenance of effective labor), major depression and obesity. Safety trials for intravenous Ucn treatment have already begun for the treatment of congestive heart failure. Further understanding the unique functions of Ucn 1, Ucn 2 and Ucn 3 action may uncover other therapeutic opportunities.
Acknowledgments
The authors thank Mike Arends for editorial assistance. This is publication number 18347 from The Scripps Research Institute. Supported by DK70118, DK64871, and DK26741 from the National Institute of Diabetes and Digestive and Kidney Diseases. ÉMF was supported by the Hungarian Eötvös Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdelrahman AM, Lin LS, Pang CC. Influence of urocortin and corticotropin releasing factor on venous tone in conscious rats. Eur J Pharmacol. 2005;510:107–111. doi: 10.1016/j.ejphar.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Abdelrahman AM, Syyong HT, Tjahjadi AA, Pang CC. Analysis of the mechanism of the vasodepressor effect of urocortin in anesthetized rats. Pharmacology. 2005;73:175–179. doi: 10.1159/000082749. [DOI] [PubMed] [Google Scholar]
- 3.Adeyemo A, Luke A, Cooper R, Wu X, Tayo B, Zhu X, Rotimi C, Bouzekri N, Ward R. A genome-wide scan for body mass index among Nigerian families. ObesRes. 2003;11:266–273. doi: 10.1038/oby.2003.40. [DOI] [PubMed] [Google Scholar]
- 4.Agnello D, Bertini R, Sacco S, Meazza C, Villa P, Ghezzi P. Corticosteroid-independent inhibition of tumor necrosis factor production by the neuropeptide urocortin. Am J Physiol. 1998;275:E757–E762. doi: 10.1152/ajpendo.1998.275.5.E757. [DOI] [PubMed] [Google Scholar]
- 5.Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84:169–208. doi: 10.1152/physrev.00017.2003. [DOI] [PubMed] [Google Scholar]
- 7.Arai M, Assil IQ, Abou-Samra AB. Characterization of three corticotropin-releasing factor receptors in catfish: a novel third receptor is predominantly expressed in pituitary and urophysis. Endocrinology. 2001;142:446–454. doi: 10.1210/endo.142.1.7879. [DOI] [PubMed] [Google Scholar]
- 8.Arima H, Aguilera G. Vasopressin and oxytocin neurones of hypothalamic supraoptic and paraventricular nuclei co-express mRNA for Type-1 and Type-2 corticotropin-releasing hormone receptors. J Neuroendocrinol. 2000;12:833–842. doi: 10.1046/j.1365-2826.2000.00528.x. [DOI] [PubMed] [Google Scholar]
- 9.Asaba K, Makino S, Hashimoto K. Effect of urocortin on ACTH secretion from rat anterior pituitary in vitro and in vivo: comparison with corticotropin-releasing hormone. Brain Res. 1998;806:95–103. doi: 10.1016/s0006-8993(98)00747-1. [DOI] [PubMed] [Google Scholar]
- 10.Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology. 1999;116:1287–1292. doi: 10.1016/s0016-5085(99)70491-9. [DOI] [PubMed] [Google Scholar]
- 11.Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Strain differences in urocortin expression in the Edinger-Westphal nucleus and its relation to alcohol-induced hypothermia. Neuroscience. 2002;113:421–434. doi: 10.1016/s0306-4522(02)00174-4. [DOI] [PubMed] [Google Scholar]
- 12.Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Identification of temperature-sensitive neural circuits in mice using c-Fos expression mapping. Brain Res. 2003;960:157–164. doi: 10.1016/s0006-8993(02)03807-6. [DOI] [PubMed] [Google Scholar]
- 13.Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baigent SM. Peripheral corticotropin-releasing hormone and urocortin in the control of the immune response. Peptides. 2001;22:809–820. doi: 10.1016/s0196-9781(01)00395-3. [DOI] [PubMed] [Google Scholar]
- 15.Baigent SM, Lowry PJ. Urocortin is the principal ligand for the corticotrophin-releasing factor binding protein in the ovine brain with no evidence for a sauvagine-like peptide. J Mol Endocrinol. 2000;24:53–63. doi: 10.1677/jme.0.0240053. [DOI] [PubMed] [Google Scholar]
- 16.Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144:2580–2587. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- 18.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 19.Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee KF, Rivier J, Chien KR, Vale WW, Peterson KL. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proc Natl Acad Sci USA. 2004;101:3697–3702. doi: 10.1073/pnas.0307324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 21.Bamberger CM, Minas V, Bamberger AM, Charalampopoulos I, Fragouli Y, Schulte HM, Makrigiannakis A. Expression of urocortin in the extravillous human trophoblast at the implantation site. Placenta. doi: 10.1016/j.placenta.2006.03.013. in press. [DOI] [PubMed] [Google Scholar]
- 22.Bamberger CM, Wald M, Bamberger AM, Ergun S, Beil FU, Schulte HM. Human lymphocytes produce urocortin, but not corticotropin-releasing hormone. J Clin Endocrinol Metab. 1998;83:708–711. doi: 10.1210/jcem.83.2.4693. [DOI] [PubMed] [Google Scholar]
- 23.Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- 24.Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 25.Behan DP, Khongsaly O, Ling N, De Souza EB. Urocortin interaction with corticotropin-releasing factor (CRF) binding protein (CRF-BP): a novel mechanism for elevating “free’ CRF levels in human brain. Brain Res. 1996;725:263–267. doi: 10.1016/0006-8993(96)00347-2. [DOI] [PubMed] [Google Scholar]
- 26.Behan DP, Linton EA, Lowry PJ. Isolation of the human plasma corticotrophin-releasing factor-binding protein. J Endocrinol. 1989;122:23–31. doi: 10.1677/joe.0.1220023. [DOI] [PubMed] [Google Scholar]
- 27.Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- 28.Boorse GC, Crespi EJ, Dautzenberg FM, Denver RJ. Urocortins of the South African clawed frog, Xenopus laevis: conservation of structure and function in tetrapod evolution. Endocrinology. 2005;146:4851–4860. doi: 10.1210/en.2005-0497. [DOI] [PubMed] [Google Scholar]
- 29.Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–2212. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- 30.Bradbury MJ, McBurnie MI, Denton DA, Lee KF, Vale WW. Modulation of urocortin-induced hypophagia and weight loss by corticotropin-releasing factor receptor 1 deficiency in mice. Endocrinology. 2000;141:2715–2724. doi: 10.1210/endo.141.8.7606. [DOI] [PubMed] [Google Scholar]
- 31.Brajac I, Tkalcic M, Dragojevic DM, Gruber F. Roles of stress, stress perception and trait-anxiety in the onset and course of alopecia areata. J Dermatol. 2003;30:871–878. doi: 10.1111/j.1346-8138.2003.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 32.Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology. 2004;145:1718–1729. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- 33.Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjos OD, Latchman DS, Lee KF, Vale W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24–35. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]
- 34.Brar BK, Jonassen AK, Stephanou A, Santilli G, Railson J, Knight RA, Yellon DM, Latchman DS. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- 35.Brar BK, Stephanou A, Okosi A, Lawrence KM, Knight RA, Marber MS, Latchman DS. CRH-like peptides protect cardiac myocytes from lethal ischaemic injury. Mol Cell Endocrinol. 1999;158:55–63. doi: 10.1016/s0303-7207(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 36.Brunner B, Grutzner F, Yaspo ML, Ropers HH, Haaf T, Kalscheue VM. Molecular cloning and characterization of the Fugu rubripes MEST/COPG2 imprinting cluster and chromosomal localization in Fugu and Tetraodon nigroviridis. Chromosome Research. 2000;8:465–476. doi: 10.1023/a:1009263504671. [DOI] [PubMed] [Google Scholar]
- 37.Burnett JC., Jr Urocortin: advancing the neurohumoral hypothesis of heart failure. Circulation. 2005;112:3544–3546. doi: 10.1161/CIRCULATIONAHA.105.584441. [DOI] [PubMed] [Google Scholar]
- 38.Cao J, Cetrulo CL, Theoharides TC. Corticotropin-releasing hormone induces vascular endothelial growth factor release from human mast cells via the cAMP/protein kinase A/p38 mitogen-activated protein kinase pathway. Mol Pharmacol. 2006;69:998–1006. doi: 10.1124/mol.105.019539. [DOI] [PubMed] [Google Scholar]
- 39.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 40.Cavalcante JC, Sita LV, Mascaro MB, Bittencourt JC, Elias CF. Distribution of urocortin 3 neurons innervating the ventral premammillary nucleus in the rat brain. Brain Res. 2006;1089:116–125. doi: 10.1016/j.brainres.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 41.Cepoi D, Sutton S, Arias C, Sawchenko P, Vale WW. Ovine genomic urocortin: cloning, pharmacologic characterization, and distribution of central mRNA. Brain Res Mol Brain Res. 1999;68:109–118. doi: 10.1016/s0169-328x(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 42.Chagnon YC, Borecki IB, Perusse L, Roy S, Lacaille M, Chagnon M, Ho-Kim MA, Rice T, Province MA, Rao DC, Bouchard C. Genome-wide search for genes related to the fat-free body mass in the Quebec family study. Metabolism. 2000;49:203–207. doi: 10.1016/s0026-0495(00)91299-x. [DOI] [PubMed] [Google Scholar]
- 43.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- 45.Chan RK, Vale WW, Sawchenko PE. Paradoxical activational effects of a corticotropin-releasing factor-binding protein “ligand inhibitor” in rat brain. Neuroscience. 2000;101:115–129. doi: 10.1016/s0306-4522(00)00322-5. [DOI] [PubMed] [Google Scholar]
- 46.Chan YC, Yao XQ, Lau CW, Chan FL, He GW, Bourreau JP, Huang Y. The relaxant effect of urocortin in rat pulmonary arteries. Regul Pept. 2004;121:11–18. doi: 10.1016/j.regpep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Chanalaris A, Lawrence KM, Stephanou A, Knight RD, Hsu SY, Hsueh AJ, Latchman DS. Protective effects of the urocortin homologues stresscopin (SCP) and stresscopin-related peptide (SRP) against hypoxia/reoxygenation injury in rat neonatal cardiomyocytes. J Mol Cell Cardiol. 2003;35:1295–1305. doi: 10.1016/s0022-2828(03)00244-x. [DOI] [PubMed] [Google Scholar]
- 48.Chanalaris A, Lawrence KM, Townsend PA, Davidson S, Jamshidi Y, Stephanou A, Knight RD, Hsu SY, Hsueh AJ, Latchman DS. Hypertrophic effects of urocortin homologous peptides are mediated via activation of the Akt pathway. Biochem Biophys Res Commun. 2005;328:442–448. doi: 10.1016/j.bbrc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Chang CL, Hsu SY. Ancient evolution of stress-regulating peptides in vertebrates. Peptides. 2004;25:1681–1688. doi: 10.1016/j.peptides.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Chang CP, Pearse RV, 2nd, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- 51.Charles CJ, Rademaker MT, Richards AM. Urocortins: putative role in cardiovascular disease. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:43–47. doi: 10.2174/1568016043477341. [DOI] [PubMed] [Google Scholar]
- 52.Charles CJ, Rademaker MT, Richards AM, Yandle TG. Plasma urocortin 1 in sheep: Regional sampling and effects of experimental heart failure. Peptides. 2006;27:1801–1805. doi: 10.1016/j.peptides.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Chatzaki E, Charalampopoulos I, Leontidis C, Mouzas IA, Tzardi M, Tsatsanis C, Margioris AN, Gravanis A. Urocortin in human gastric mucosa: relationship to inflammatory activity. J Clin Endocrinol Metab. 2003;88:478–483. doi: 10.1210/jc.2002-020853. [DOI] [PubMed] [Google Scholar]
- 54.Chatzaki E, Murphy BJ, Wang L, Million M, Ohning GV, Crowe PD, Petroski R, Tache Y, Grigoriadis DE. Differential profile of CRF receptor distribution in the rat stomach and duodenum assessed by newly developed CRF receptor antibodies. J Neurochem. 2004;88:1–11. doi: 10.1046/j.1471-4159.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- 55.Chen A, Blount A, Vaughan J, Brar B, Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145:2445–2457. doi: 10.1210/en.2003-1570. [DOI] [PubMed] [Google Scholar]
- 56.Chen A, Perrin M, Brar B, Li C, Jamieson P, DiGruccio M, Lewis K, Vale W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol. 2005;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- 57.Chen A, Vaughan J, Vale WW. Glucocorticoids regulate the expression of the mouse urocortin II gene: a putative connection between the corticotropin-releasing factor receptor pathways. Mol Endocrinol. 2003;17:1622–1639. doi: 10.1210/me.2003-0054. [DOI] [PubMed] [Google Scholar]
- 58.Chen A, Zorrilla E, Smith S, Rousso D, Levy C, Vaughan J, Donaldson C, Roberts A, Lee KF, Vale W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. A soluble mouse brain splice variant of type 2{alpha} corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci USA. 2005;102:2620–2625. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen CY, Doong ML, Rivier JE, Tache Y. Intravenous urocortin II decreases blood pressure through CRF2 receptor in rats. Regul Pept. 2003;113:125–130. doi: 10.1016/s0167-0115(03)00003-x. [DOI] [PubMed] [Google Scholar]
- 61.Chen CY, Million M, Adelson DW, Martinez V, Rivier J, Taché Y. Intracisternal urocortin inhibits vagally stimulated gastric motility in rats: role of CRF(2) Br J Pharmacol. 2002;136:237–247. doi: 10.1038/sj.bjp.0704713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen ZW, Huang Y, Yang Q, Li X, Wei W, He GW. Urocortin-induced relaxation in the human internal mammary artery. Cardiovasc Res. 2005;65:913–920. doi: 10.1016/j.cardiores.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 64.Clifton VL, Gu Q, Murphy VE, Schwartz J, Madsen G, Smith R. Localization and characterization of urocortin during human pregnancy. Placenta. 2000;21:782–788. doi: 10.1053/plac.2000.0570. [DOI] [PubMed] [Google Scholar]
- 65.Cornelissen LL, Brooks DP, Wibberley A. Female, but not male, serotonin reuptake transporter (5-HTT) knockout mice exhibit bladder instability. Auton Neurosci. 2005;122:107–110. doi: 10.1016/j.autneu.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Coste SC, Quintos RF, Stenzel-Poore MP. Corticotropin-releasing hormone-related peptides and receptors: emergent regulators of cardiovascular adaptations to stress. Trends Cardiovasc Med. 2002;12:176–182. doi: 10.1016/s1050-1738(02)00157-3. [DOI] [PubMed] [Google Scholar]
- 67.Cullen MJ, Ling N, Foster AC, Pelleymounter MA. Urocortin, corticotropin releasing factor-2 receptors and energy balance. Endocrinology. 2001;142:992–999. doi: 10.1210/endo.142.3.7989. [DOI] [PubMed] [Google Scholar]
- 68.Currie PJ, Coscina DV, Bishop C, Coiro CD, Koob GF, Rivier J, Vale W. Hypothalamic paraventricular nucleus injections of urocortin alter food intake and respiratory quotient. Brain Res. 2001;916:222–228. doi: 10.1016/s0006-8993(01)02851-7. [DOI] [PubMed] [Google Scholar]
- 69.Czimmer J, Million M, Tache Y. Urocortin 2 acts centrally to delay gastric emptying through sympathetic pathways while CRF and urocortin 1 inhibitory actions are vagal dependent in rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G511–G518. doi: 10.1152/ajpgi.00289.2005. [DOI] [PubMed] [Google Scholar]
- 70.Daniels D, Markison S, Grill HJ, Kaplan JM. Central structures necessary and sufficient for ingestive and glycemic responses to Urocortin I administration. J Neurosci. 2004;24:11457–11462. doi: 10.1523/JNEUROSCI.2702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dautzenberg FM, Higelin J, Wille S, Brauns O. Molecular cloning and functional expression of the mouse CRF2(a) receptor splice variant. Regul Pept. 2004;121:89–97. doi: 10.1016/j.regpep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 72.Davidson SM, Townsend PA, Carroll C, Yurek-George A, Balasubramanyam K, Kundu TK, Stephanou A, Packham G, Ganesan A, Latchman DS. The transcriptional coactivator p300 plays a critical role in the hypertrophic and protective pathways induced by phenylephrine in cardiac cells but is specific to the hypertrophic effect of urocortin. Chembiochem. 2005;6:162–170. doi: 10.1002/cbic.200400246. [DOI] [PubMed] [Google Scholar]
- 73.Davis ME, Pemberton CJ, Yandle TG, Lainchbury JG, Rademaker MT, Nicholls MG, Frampton CM, Richards AM. Urocortin-1 infusion in normal humans. J Clin Endocrinol Metab. 2004;89:1402–1409. doi: 10.1210/jc.2003-031231. [DOI] [PubMed] [Google Scholar]
- 74.Davis ME, Pemberton CJ, Yandle TG, Lainchbury JG, Rademaker MT, Nicholls MG, Frampton CM, Richards AM. Effect of urocortin 1 infusion in humans with stable congestive cardiac failure. Clin Sci (Lond) 2005;109:381–388. doi: 10.1042/CS20050079. [DOI] [PubMed] [Google Scholar]
- 75.Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Fanti BA, Martinez JA. Central urocortin activation of sympathetic-regulated energy metabolism in Wistar rats. Brain Res. 2002;930:37–41. doi: 10.1016/s0006-8993(01)03401-1. [DOI] [PubMed] [Google Scholar]
- 77.de Groote L, Penalva RG, Flachskamm C, Reul JM, Linthorst AC. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor type 1 and type 2 agonists. J Neurochem. 2005;94:45–56. doi: 10.1111/j.1471-4159.2005.03164.x. [DOI] [PubMed] [Google Scholar]
- 78.De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20:789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 79.Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 80.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 81.Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, Vaughan JM, Vale WW. Cloning and characterization of human urocortin. Endocrinology. 1996;137:2167–2170. doi: 10.1210/endo.137.5.8612563. [DOI] [PubMed] [Google Scholar]
- 82.Donelan J, Boucher W, Papadopoulou N, Lytinas M, Papaliodis D, Dobner P, Theoharides TC. Corticotropin-releasing hormone induces skin vascular permeability through a neurotensin-dependent process. Proc Natl Acad Sci USA. 2006;103:7759–7764. doi: 10.1073/pnas.0602210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fazal N, Slominski A, Choudhry MA, Wei ET, Sayeed MM. Effect of CRF and related peptides on calcium signaling in human and rodent melanoma cells. FEBS Lett. 1998;435:187–190. doi: 10.1016/s0014-5793(98)01067-9. [DOI] [PubMed] [Google Scholar]
- 84.Ferry RJ, Jr, Cerri RW, Cohen P. Insulin-like growth factor binding proteins: new proteins, new functions. Horm Res. 1999;51:53–67. doi: 10.1159/000023315. [DOI] [PubMed] [Google Scholar]
- 85.Florio P, Calonaci G, Severi FM, Torricelli M, Bocchi C, Fiore G, Linton EA, Petraglia F. Reduced maternal plasma urocortin concentrations and impaired uterine artery blood flow at human mid pregnancy. J Soc Gynecol Investig. 2005;12:191–194. doi: 10.1016/j.jsgi.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Florio P, Ciarmela P, Arcuri F, Petraglia F. High levels of intrauterine corticotrophin-releasing hormone, urocortin, tryptase, and interleukin-8 in spontaneous abortions. J Clin Endocrinol Metab. 2003;88:5580–5581. doi: 10.1210/jc.2003-031250. [DOI] [PubMed] [Google Scholar]
- 87.Florio P, Cobellis L, Woodman J, Severi FM, Linton EA, Petraglia F. Levels of maternal plasma corticotropin-releasing factor and urocortin during labor. J Soc Gynecol Investig. 2002;9:233–237. [PubMed] [Google Scholar]
- 88.Florio P, Franchini A, Reis FM, Pezzani I, Ottaviani E, Petraglia F. Human placenta, chorion, amnion and decidua express different variants of corticotropin-releasing factor receptor messenger RNA. Placenta. 2000;21:32–37. doi: 10.1053/plac.1999.0461. [DOI] [PubMed] [Google Scholar]
- 89.Florio P, Vale W, Petraglia F. Urocortins in human reproduction. Peptides. 2004;25:1751–1757. doi: 10.1016/j.peptides.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 90.Fukuda T, Takahashi K, Suzuki T, Saruta M, Watanabe M, Nakata T, Sasano H. Urocortin 1, urocortin 3/stresscopin, and corticotropin-releasing factor receptors in human adrenal and its disorders. J Clin Endocrinol Metab. 2005;90:4671–4678. doi: 10.1210/jc.2005-0090. [DOI] [PubMed] [Google Scholar]
- 91.Garcia-Villalon AL, Amezquita YM, Monge L, Fernandez N, Climent B, Sanchez A, Dieguez G. Mechanisms of the protective effects of urocortin on coronary endothelial function during ischemia-reperfusion in rat isolated hearts. Br J Pharmacol. 2005;145:490–494. doi: 10.1038/sj.bjp.0706208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gardiner SM, March JE, Kemp PA, Davenport AP, Wiley KE, Bennett T. Regional hemodynamic actions of selective corticotropin-releasing factor type 2 receptor ligands in conscious rats. J Pharmacol Exp Ther. 2005;312:53–60. doi: 10.1124/jpet.104.075259. [DOI] [PubMed] [Google Scholar]
- 93.Gaszner B, Csernus V, Kozicz T. Urocortinergic neurons respond in a differentiated manner to various acute stressors in the Edinger-Westphal nucleus in the rat. J Comp Neurol. 2004;480:170–179. doi: 10.1002/cne.20343. [DOI] [PubMed] [Google Scholar]
- 94.Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez-Rey E, Chorny A, Varela N, Robledo G, Delgado M. Urocortin and adrenomedullin prevent lethal endotoxemia by down-regulating the inflammatory response. Am J Pathol. 2006;168:1921–1930. doi: 10.2353/ajpath.2006.051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gu Q, Clifton VL, Schwartz J, Madsen G, Sha J, Smith R. Characterization of urocortin in human pregnancy. Chin Med J (Engl) 2001;114:618–622. [PubMed] [Google Scholar]
- 97.Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 98.Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hara Y, Ueta Y, Isse T, Kabashima N, Shibuya I, Hattori Y, Yamashita H. Increase of urocortin-like immunoreactivity in the rat hypothalamo-neurohypophysial system after salt loading and hypophysectomy. Neurosci Lett. 1997;227:127–130. doi: 10.1016/s0304-3940(97)00327-3. [DOI] [PubMed] [Google Scholar]
- 101.Hara Y, Ueta Y, Isse T, Kabashima N, Shibuya I, Hattori Y, Yamashita H. Increase of urocortin-like immunoreactivity in the rat supraoptic nucleus after dehydration but not food deprivation. Neurosci Lett. 1997;229:65–68. doi: 10.1016/s0304-3940(97)00419-9. [DOI] [PubMed] [Google Scholar]
- 102.Hara Y, Ueta Y, Isse T, Serino R, Shibuya I, Hattori Y, Yamashita H. Increase of urocortin-like immunoreactivity in the supraoptic nucleus of Dahl rats given a high salt diet. Neurosci Lett. 2000;279:17–20. doi: 10.1016/s0304-3940(99)00957-x. [DOI] [PubMed] [Google Scholar]
- 103.Harada S, Imaki T, Naruse M, Chikada N, Nakajima K, Demura H. Urocortin mRNA is expressed in the enteric nervous system of the rat. Neurosci Lett. 1999;267:125–128. doi: 10.1016/s0304-3940(99)00349-3. [DOI] [PubMed] [Google Scholar]
- 104.Hashimoto K, Nishiyama M, Tanaka Y, Noguchi T, Asaba K, Hossein PN, Nishioka T, Makino S. Urocortins and corticotropin releasing factor type 2 receptors in the hypothalamus and the cardiovascular system. Peptides. 2004;25:1711–1721. doi: 10.1016/j.peptides.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 105.Hedner T, Hedner J, Andersson O, Persson B, Pettersson A. ANP--a cardiac hormone and a putative central neurotransmitter. Eur Heart J. 1987;8(Suppl B):87–98. doi: 10.1093/eurheartj/8.suppl_b.87. [DOI] [PubMed] [Google Scholar]
- 106.Heinrichs SC, Tache Y. Therapeutic potential of CRF receptor antagonists: a gut-brain perspective. Expert Opin Investig Drugs. 2001;10:647–659. doi: 10.1517/13543784.10.4.647. [DOI] [PubMed] [Google Scholar]
- 107.Henriot S, Dautzenberg FM, Kilpatrick GJ. Urocortin: slower dissociation than corticotropin releasing factor from the CRF binding protein. Eur J Pharmacol. 1999;376:321–324. doi: 10.1016/s0014-2999(99)00415-x. [DOI] [PubMed] [Google Scholar]
- 108.Hillhouse EW, Grammatopoulos DK. Role of stress peptides during human pregnancy and labour. Reproduction. 2002;124:323–329. doi: 10.1530/rep.0.1240323. [DOI] [PubMed] [Google Scholar]
- 109.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- 110.Hoare SR, Sullivan SK, Fan J, Khongsaly K, Grigoriadis DE. Peptide ligand binding properties of the corticotropin-releasing factor (CRF) type 2 receptor: pharmacology of endogenously expressed receptors, G-protein-coupling sensitivity and determinants of CRF2 receptor selectivity. Peptides. 2005;26:457–470. doi: 10.1016/j.peptides.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 111.Hoare SR, Sullivan SK, Ling N, Crowe PD, Grigoriadis DE. Mechanism of corticotropin-releasing factor type I receptor regulation by nonpeptide antagonists. Mol Pharmacol. 2003;63:751–765. doi: 10.1124/mol.63.3.751. [DOI] [PubMed] [Google Scholar]
- 112.Honjo T, Inoue N, Shiraki R, Kobayashi S, Otsui K, Takahashi M, Hirata K, Kawashima S, Yokozaki H, Yokoyama M. Endothelial urocortin has potent antioxidative properties and is upregulated by inflammatory cytokines and pitavastatin. J Vasc Res. 2006;43:131–138. doi: 10.1159/000090132. [DOI] [PubMed] [Google Scholar]
- 113.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 114.Iino K, Sasano H, Oki Y, Andoh N, Shin RW, Kitamoto T, Takahashi K, Suzuki H, Tezuka F, Yoshimi T, Nagura H. Urocortin expression in the human central nervous system. Clin Endocrinol (Oxf) 1999;50:107–114. doi: 10.1046/j.1365-2265.1999.00624.x. [DOI] [PubMed] [Google Scholar]
- 115.Iino K, Sasano H, Oki Y, Andoh N, Shin RW, Kitamoto T, Totsune K, Takahashi K, Suzuki H, Nagura H, Yoshimi T. Urocortin expression in human pituitary gland and pituitary adenoma. J Clin Endocrinol Metab. 1997;82:3842–3850. doi: 10.1210/jcem.82.11.4371. [DOI] [PubMed] [Google Scholar]
- 116.Ikeda K, Tojo K, Sato S, Ebisawa T, Tokudome G, Hosoya T, Harada M, Nakagawa O, Nakao K. Urocortin, a newly identified corticotropin-releasing factor-related mammalian peptide, stimulates atrial natriuretic peptide and brain natriuretic peptide secretions from neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 1998;250:298–304. doi: 10.1006/bbrc.1998.9297. [DOI] [PubMed] [Google Scholar]
- 117.Ikeda K, Tojo K, Tokudome G, Ohta M, Sugimoto K, Tamura T, Tajima N, Mochizuki S, Kawakami M, Hosoya T. Cardiac expression of urocortin (Ucn) in diseased heart; preliminary results on possible involvement of Ucn in pathophysiology of cardiac diseases. Mol Cell Biochem. 2003;252:25–32. doi: 10.1023/a:1025551305777. [DOI] [PubMed] [Google Scholar]
- 118.Imaki T, Katsumata H, Miyata M, Naruse M, Imaki J, Minami S. Expression of corticotropin releasing factor (CRF), urocortin and CRF type 1 receptors in hypothalamic-hypophyseal systems under osmotic stimulation. J Neuroendocrinol. 2001;13:328–338. doi: 10.1046/j.1365-2826.2001.00629.x. [DOI] [PubMed] [Google Scholar]
- 119.Imperatore A, Florio P, Torres PB, Torricelli M, Galleri L, Toti P, Occhini R, Picciolini E, Vale W, Petraglia F. Urocortin 2 and urocortin 3 are expressed by the human placenta, deciduas, and fetal membranes. Am J Obstet Gynecol. 2006;195:288–295. doi: 10.1016/j.ajog.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 120.Innis RB, Aghajanian GK. Cholecystokinin-containing and nociceptive neurons in rat Edinger-Westphal nucleus. Brain Res. 1986;363:230–238. doi: 10.1016/0006-8993(86)91008-5. [DOI] [PubMed] [Google Scholar]
- 121.Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther. 2003;305:385–393. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- 122.Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. J Invest Dermatol. 2004;122:235–237. doi: 10.1046/j.1523-1747.2003.22145.x. [DOI] [PubMed] [Google Scholar]
- 123.Jahn O, Eckart K, Sydow S, Hofmann BA, Spiess J. Pharmacological characterization of recombinant rat corticotropin releasing factor binding protein using different sauvagine analogs. Peptides. 2001;22:47–56. doi: 10.1016/s0196-9781(00)00356-9. [DOI] [PubMed] [Google Scholar]
- 124.Jahn O, Radulovic J, Stiedl O, Tezval H, Eckart K, Spiess J. Corticotropin-releasing factor binding protein--a ligand trap? Mini Rev Med Chem. 2005;5:953–960. doi: 10.2174/138955705774329500. [DOI] [PubMed] [Google Scholar]
- 125.Jahn O, Tezval H, van Werven L, Eckart K, Spiess J. Three-amino acid motifs of urocortin II and III determine their CRF receptor subtype selectivity. Neuropharmacology. 2004;47:233–242. doi: 10.1016/j.neuropharm.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 126.Janjua S, Lawrence KM, Ng LL, Latchman DS. The cardioprotective agent urocortin induces expression of CT-1. Cardiovasc Toxicol. 2003;3:255–262. doi: 10.1385/ct:3:3:255. [DOI] [PubMed] [Google Scholar]
- 127.Jansen AS, Farkas E, Sams JM, Loewy AD. Local connections between the columns of the periaqueductal gray matter: a case for intrinsic neuromodulation. Brain Res. 1998;784:329–336. doi: 10.1016/s0006-8993(98)00473-9. [DOI] [PubMed] [Google Scholar]
- 128.Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology. 1998;138:124–132. doi: 10.1007/s002130050654. [DOI] [PubMed] [Google Scholar]
- 129.Jones MD, Lucki I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology. 2005;30:1039–1047. doi: 10.1038/sj.npp.1300664. [DOI] [PubMed] [Google Scholar]
- 130.Kageyama K, Bradbury MJ, Zhao L, Blount AL, Vale WW. Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology. 1999;140:5651–5658. doi: 10.1210/endo.140.12.7223. [DOI] [PubMed] [Google Scholar]
- 131.Kageyama K, Furukawa K, Miki I, Terui K, Motomura S, Suda T. Vasodilative effects of urocortin II via protein kinase A and a mitogen-activated protein kinase in rat thoracic aorta. J Cardiovasc Pharmacol. 2003;42:561–565. doi: 10.1097/00005344-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 132.Karteris E, Hillhouse EW, Grammatopoulos D. Urocortin II is expressed in human pregnant myometrial cells and regulates myosin light chain phosphorylation: potential role of the type-2 corticotropin-releasing hormone receptor in the control of myometrial contractility. Endocrinology. 2004;145:890–900. doi: 10.1210/en.2003-1210. [DOI] [PubMed] [Google Scholar]
- 133.Karteris E, Vatish M, Hillhouse EW, Grammatopoulos DK. Preeclampsia is associated with impaired regulation of the placental nitric oxide-cyclic guanosine monophosphate pathway by corticotropin-releasing hormone (CRH) and CRH-related peptides. J Clin Endocrinol Metab. 2005;90:3680–3687. doi: 10.1210/jc.2004-2210. [DOI] [PubMed] [Google Scholar]
- 134.Kastin AJ, Akerstrom V, Pan W. Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier: meaningful controls. Peptides. 2001;22:2127–2136. doi: 10.1016/s0196-9781(01)00569-1. [DOI] [PubMed] [Google Scholar]
- 135.Kastin AJ, Pan W, Akerstrom V, Hackler L, Wang C, Kotz CM. Novel peptide-peptide cooperation may transform feeding behavior. Peptides. 2002;23:2189–2196. doi: 10.1016/s0196-9781(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 136.Katsarou-Katsari A, Singh LK, Theoharides TC. Alopecia areata and affected skin CRH receptor upregulation induced by acute emotional stress. Dermatology. 2001;203:157–161. doi: 10.1159/000051732. [DOI] [PubMed] [Google Scholar]
- 137.Kaufman GD, Anderson JH, Beitz AJ. Fos-defined activity in rat brainstem following centripetal acceleration. J Neurosci. 1992;12:4489–4500. doi: 10.1523/JNEUROSCI.12-11-04489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kauser S, Slominski A, Wei ET, Tobin DJ. Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides. FASEB J. 2006;20:882–895. doi: 10.1096/fj.05-5257com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kemp CF, Woods RJ, Lowry PJ. The corticotrophin-releasing factor-binding protein: an act of several parts. Peptides. 1998;19:1119–1128. doi: 10.1016/s0196-9781(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 140.Kempuraj D, Papadopoulou N, Stanford EJ, Christodoulou S, Madhappan B, Sant GR, Solage K, Adams T, Theoharides TC. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. 2004;52:267–275. doi: 10.1111/j.1600-0897.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- 141.Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, Boucher W, Christodoulou S, Athanassiou A, Theoharides TC. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 142.Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G406–G419. doi: 10.1152/ajpgi.2001.280.3.G406. [DOI] [PubMed] [Google Scholar]
- 143.Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am J Physiol GastrointestLiver Physiol. 2001;280:G406–G419. doi: 10.1152/ajpgi.2001.280.3.G406. [DOI] [PubMed] [Google Scholar]
- 144.Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 145.Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab. 2002;87:340–346. doi: 10.1210/jcem.87.1.8160. [DOI] [PubMed] [Google Scholar]
- 146.Kinney JW, Scruggs B, Avery DD. Peripheral administration of urocortin suppresses operant responding for food reward. Peptides. 2001;22:583–587. doi: 10.1016/s0196-9781(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 147.Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 148.Kishimoto T, Pearse RV, 2nd, Lin CR, Rosenfeld MG. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci USA. 1995;92:1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kohno M, Kawahito Y, Tsubouchi Y, Hashiramoto A, Yamada R, Inoue KI, Kusaka Y, Kubo T, Elenkov IJ, Chrousos GP, Kondo M, Sano H. Urocortin expression in synovium of patients with rheumatoid arthritis and osteoarthritis: relation to inflammatory activity. J Clin Endocrinol Metab. 2001;86:4344–4352. doi: 10.1210/jcem.86.9.7827. [DOI] [PubMed] [Google Scholar]
- 150.Korosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res. 2005;1046:172–179. doi: 10.1016/j.brainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 151.Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. MolEndocrinol. 1998;12:1077–1085. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- 152.Kotz CM, Wang C, Levine AS, Billington CJ. Urocortin in the hypothalamic PVN increases leptin and affects uncoupling proteins-1 and -3 in rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R546–R551. doi: 10.1152/ajpregu.00436.2001. [DOI] [PubMed] [Google Scholar]
- 153.Koyama N, Nishio T, Yokota T. Non-serotonergic midbrain neurons are involved in picrotoxin-induced analgesia. An immunohistochemical study in the rat. Neurosci Lett. 2000;291:147–150. doi: 10.1016/s0304-3940(00)01387-2. [DOI] [PubMed] [Google Scholar]
- 154.Kozicz T. Neurons colocalizing urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neuroscience. 2003;116:315–320. doi: 10.1016/s0306-4522(02)00772-8. [DOI] [PubMed] [Google Scholar]
- 155.Kozicz T, Arimura A. Distribution of urocortin in the rat’s gastrointestinal tract and its colocalization with tyrosine hydroxylase. Peptides. 2002;23:515–521. doi: 10.1016/s0196-9781(01)00639-8. [DOI] [PubMed] [Google Scholar]
- 156.Kozicz T, Arimura A, Maderdrut JL, Lazar G. Distribution of urocortin-like immunoreactivity in the central nervous system of the frog Rana esculenta. J Comp Neurol. 2002;453:185–198. doi: 10.1002/cne.10403. [DOI] [PubMed] [Google Scholar]
- 157.Kozicz T, Korosi A, Korsman C, Tilburg-Ouwens D, Groenink L, Veening J, van der Gugten J, Roubos E, Olivier B. Urocortin expression in the Edinger-Westphal nucleus is down-regulated in transgenic mice over-expressing neuronal corticotropin-releasing factor. Neuroscience. 2004;123:589–594. doi: 10.1016/j.neuroscience.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 158.Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 159.La Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc NatlAcadSci USA. 2005;102:7647–7652. doi: 10.1073/pnas.0408531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Latchman DS. Urocortin protects against ischemic injury via a MAPK-dependent pathway. Trends Cardiovasc Med. 2001;11:167–169. doi: 10.1016/s1050-1738(01)00093-7. [DOI] [PubMed] [Google Scholar]
- 161.Lawrence KM, Chanalaris A, Scarabelli T, Hubank M, Pasini E, Townsend PA, Comini L, Ferrari R, Tinker A, Stephanou A, Knight RA, Latchman DS. K(ATP) channel gene expression is induced by urocortin and mediates its cardioprotective effect. Circulation. 2002;106:1556–1562. doi: 10.1161/01.cir.0000028424.02525.ae. [DOI] [PubMed] [Google Scholar]
- 162.Lawrence KM, Kabir AM, Bellahcene M, Davidson S, Cao XB, McCormick J, Mesquita RA, Carroll CJ, Chanalaris A, Townsend PA, Hubank M, Stephanou A, Knight RA, Marber MS, Latchman DS. Cardioprotection mediated by urocortin is dependent on PKCepsilon activation. FASEB J. 2005;19:831–833. doi: 10.1096/fj.04-2506fje. [DOI] [PubMed] [Google Scholar]
- 163.Lawrence KM, Scarabelli TM, Turtle L, Chanalaris A, Townsend PA, Carroll CJ, Hubank M, Stephanou A, Knight RA, Latchman DS. Urocortin protects cardiac myocytes from ischemia/reperfusion injury by attenuating calcium-insensitive phospholipase A2 gene expression. FASEB J. 2003;17:2313–2315. doi: 10.1096/fj.02-0832fje. [DOI] [PubMed] [Google Scholar]
- 164.Lawrence KM, Townsend PA, Davidson SM, Carroll CJ, Eaton S, Hubank M, Knight RA, Stephanou A, Latchman DS. The cardioprotective effect of urocortin during ischaemia/reperfusion involves the prevention of mitochondrial damage. Biochem BiophysRes Commun. 2004;321:479–486. doi: 10.1016/j.bbrc.2004.06.170. [DOI] [PubMed] [Google Scholar]
- 165.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- 167.Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li W, Challis JR. Corticotropin-releasing hormone and urocortin induce secretion of matrix metalloproteinase-9 (MMP-9) without change in tissue inhibitors of MMP-1 by cultured cells from human placenta and fetal membranes. J Clin Endocrinol Metab. 2005;90:6569–6574. doi: 10.1210/jc.2005-1445. [DOI] [PubMed] [Google Scholar]
- 169.Liaw CW, Lovenberg TW, Barry G, Oltersdorf T, Grigoriadis DE, De Souza EB. Cloning and characterization of the human corticotropin-releasing factor-2 receptor complementary deoxyribonucleic acid. Endocrinology. 1996;137:72–77. doi: 10.1210/endo.137.1.8536644. [DOI] [PubMed] [Google Scholar]
- 170.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 171.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin- releasing factor receptor subtype from rat brain. Proc Natl Acad SciUSA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lytinas M, Kempuraj D, Huang M, Boucher W, Esposito P, Theoharides TC. Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int Arch Allergy Immunol. 2003;130:224–231. doi: 10.1159/000069516. [DOI] [PubMed] [Google Scholar]
- 173.Madhappan B, Kempuraj D, Christodoulou S, Tsapikidis S, Boucher W, Karagiannis V, Athanassiou A, Theoharides TC. High levels of intrauterine corticotropin-releasing hormone, urocortin, tryptase, and interleukin-8 in spontaneous abortions. Endocrinology. 2003;144:2285–2290. doi: 10.1210/en.2003-0063. [DOI] [PubMed] [Google Scholar]
- 174.Makino S, Nishiyama M, Asaba K, Gold PW, Hashimoto K. Altered expression of type 2 CRH receptor mRNA in the VMH by glucocorticoids and starvation. Am J Physiol. 1998;275:R1138–R1145. doi: 10.1152/ajpregu.1998.275.4.R1138. [DOI] [PubMed] [Google Scholar]
- 175.Mano-Otagiri A, Shibasaki T. Distribution of urocortin 2 and urocortin 3 in rat brain. J Nippon Med Sch. 2004;71:358–359. doi: 10.1272/jnms.71.358. [DOI] [PubMed] [Google Scholar]
- 176.Martinez V, Wang L, Million M, Rivier J, Tache Y. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733–1744. doi: 10.1016/j.peptides.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 177.Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Martinez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 179.Masuzawa M, Oki Y, Ozawa M, Watanabe F, Yoshimi T. Corticotropin-releasing factor but not urocortin is involved in adrenalectomy-induced adrenocorticotropin release. J Neuroendocrinol. 1999;11:71–74. doi: 10.1046/j.1365-2826.1999.00302.x. [DOI] [PubMed] [Google Scholar]
- 180.Meyer AH, Ullmer C, Schmuck K, Morel C, Wishart W, Lubbert H, Engels P. Localization of the human CRF2 receptor to 7p21-p15 by radiation hybrid mapping and FISH analysis. Genomics. 1997;40:189–190. doi: 10.1006/geno.1996.4521. [DOI] [PubMed] [Google Scholar]
- 181.Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, Zhou H, Saunders PR, Maillot C, Mayer EA, Tache Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res. 2003;985:32–42. doi: 10.1016/s0006-8993(03)03027-0. [DOI] [PubMed] [Google Scholar]
- 182.Million M, Maillot C, Adelson DA, Nozu T, Gauthier A, Rivier J, Chrousos GP, Bayati A, Mattsson H, Tache Y. Peripheral injection of sauvagine prevents repeated colorectal distension-induced visceral pain in female rats. Peptides. 2005;26:1188–1195. doi: 10.1016/j.peptides.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 183.Million M, Maillot C, Saunders P, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF-related peptide, displays selective CRF2-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G34–G40. doi: 10.1152/ajpgi.00283.2001. [DOI] [PubMed] [Google Scholar]
- 184.Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu V, Wei JY, Rivier J, Vale W, Mayer EA, Tache Y. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moreau JL, Kilpatrick G, Jenck F. Urocortin, a novel neuropeptide with anxiogenic-like properties. Neuroreport. 1997;8:1697–1701. doi: 10.1097/00001756-199705060-00027. [DOI] [PubMed] [Google Scholar]
- 186.Morin SM, Ling N, Liu XJ, Kahl SD, Gehlert DR. Differential distribution of urocortin- and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience. 1999;92:281–291. doi: 10.1016/s0306-4522(98)00732-5. [DOI] [PubMed] [Google Scholar]
- 187.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- 188.Muramatsu Y, Sugino N, Suzuki T, Totsune K, Takahashi K, Tashiro A, Hongo M, Oki Y, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in normal cycling human ovaries. J Clin Endocrinol Metab. 2001;86:1362–1369. doi: 10.1210/jcem.86.3.7299. [DOI] [PubMed] [Google Scholar]
- 189.Nagata T, Uemoto M, Yuzuriha H, Asakawa A, Inui A, Fujimiya M, Sakamaki R, Kasuga M, Shinfuku N. Intracerebroventricularly administered urocortin inhibits gastric emptying in mice. Int J Mol Med. 2005;15:1041–1043. [PubMed] [Google Scholar]
- 190.Nemoto T, Mano-Otagiri A, Shibasaki T. Urocortin 2 induces tyrosine hydroxylase phosphorylation in PC12 cells. Biochem Biophys Res Commun. 2005;330:821–831. doi: 10.1016/j.bbrc.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 191.Ng LL, Loke IW, O’Brien RJ, Squire IB, Davies JE. Plasma urocortin in human systolic heart failure. Clin Sci (Lond) 2004;106:383–388. doi: 10.1042/CS20030311. [DOI] [PubMed] [Google Scholar]
- 192.Nijsen M, Ongenae N, Meulemans A, Coulie B. Divergent role for CRF1 and CRF2 receptors in the modulation of visceral pain. Neurogastroenterol Motil. 2005;17:423–432. doi: 10.1111/j.1365-2982.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 193.Nishikimi T, Miyata A, Horio T, Yoshihara F, Nagaya N, Takishita S, Yutani C, Matsuo H, Matsuoka H, Kangawa K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am J Physiol Heart Circ Physiol. 2000;279:H3031–H3039. doi: 10.1152/ajpheart.2000.279.6.H3031. [DOI] [PubMed] [Google Scholar]
- 194.Nishiyama M, Makino S, Asaba K, Hashimoto K. Leptin effects on the expression of type-2 CRH receptor mRNA in the ventromedial hypothalamus in the rat. J Neuroendocrinol. 1999;11:307–314. doi: 10.1046/j.1365-2826.1999.00331.x. [DOI] [PubMed] [Google Scholar]
- 195.Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides. 2004;25:1703–1709. doi: 10.1016/j.peptides.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 196.Oki Y, Sasano H. Localization and physiological roles of urocortin. Peptides. 2004;25:1745–1749. doi: 10.1016/j.peptides.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 197.Okosi A, Brar BK, Chan M, D’Souza L, Smith E, Stephanou A, Latchman DS, Chowdrey HS, Knight RA. Expression and protective effects of urocortin in cardiac myocytes. Neuropeptides. 1998;32:167–171. doi: 10.1016/s0143-4179(98)90033-6. [DOI] [PubMed] [Google Scholar]
- 198.Orth DN, Mount CD. Specific high-affinity binding protein for human corticotropin-releasing hormone in normal human plasma. Biochem Biophys Res Commun. 1987;143:411–417. doi: 10.1016/0006-291x(87)91369-6. [DOI] [PubMed] [Google Scholar]
- 199.Palchaudhuri MR, Hauger RL, Wille S, Fuchs E, Dautzenberg FM. Isolation and pharmacological characterization of two functional splice variants of corticotropin-releasing factor type 2 receptor from Tupaia belangeri. J Neuroendocrinol. 1999;11:419–428. doi: 10.1046/j.1365-2826.1999.00348.x. [DOI] [PubMed] [Google Scholar]
- 200.Papadopoulou N, Kalogeromitros D, Staurianeas NG, Tiblalexi D, Theoharides TC. Corticotropin-releasing hormone receptor-1 and histidine decarboxylase expression in chronic urticaria. J Invest Dermatol. 2005;125:952–955. doi: 10.1111/j.0022-202X.2005.23913.x. [DOI] [PubMed] [Google Scholar]
- 201.Parham KL, Zervou S, Karteris E, Catalano RD, Old RW, Hillhouse EW. Promoter analysis of human corticotropin-releasing factor (CRF) type 1 receptor and regulation by CRF and urocortin. Endocrinology. 2004;145:3971–3983. doi: 10.1210/en.2004-0194. [DOI] [PubMed] [Google Scholar]
- 202.Parkes DG, Vaughan J, Rivier J, Vale W, May CN. Cardiac inotropic actions of urocortin in conscious sheep. Am J Physiol. 1997;272:H2115–H2122. doi: 10.1152/ajpheart.1997.272.5.H2115. [DOI] [PubMed] [Google Scholar]
- 203.Parkes DG, Weisinger RS, May CN. Cardiovascular actions of CRH and urocortin: an update. Peptides. 2001;22:821–827. doi: 10.1016/s0196-9781(01)00396-5. [DOI] [PubMed] [Google Scholar]
- 204.Parver LM. Temperature modulating action of choroidal blood flow. Eye. 1991;5(Pt 2):181–185. doi: 10.1038/eye.1991.32. [DOI] [PubMed] [Google Scholar]
- 205.Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor2 receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145–152. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- 206.Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF(2) receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 207.Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- 208.Perrin MH, Sutton S, Bain DL, Berggren WT, Vale WW. The first extracellular domain of corticotropin releasing factor-R1 contains major binding determinants for urocortin and astressin. Endocrinology. 1998;139:566–570. doi: 10.1210/endo.139.2.5757. [DOI] [PubMed] [Google Scholar]
- 209.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 210.Petkova-Kirova PS, Gagov HS, Duridanova DB. Urocortin hyperpolarizes stomach smooth muscle via activation of Ca2+-sensitive K+ currents. J Muscle Res Cell Motil. 2000;21:639–645. doi: 10.1023/a:1005653218639. [DOI] [PubMed] [Google Scholar]
- 211.Peto CA, Arias C, Vale WW, Sawchenko PE. Ultrastructural localization of the corticotropin-releasing factor-binding protein in rat brain and pituitary. J Comp Neurol. 1999;413:241–254. [PubMed] [Google Scholar]
- 212.Petraglia F, Florio P, Benedetto C, Marozio L, Di Blasio AM, Ticconi C, Piccione E, Luisi S, Genazzani AR, Vale W. Urocortin stimulates placental adrenocorticotropin and prostaglandin release and myometrial contractility in vitro. J Clin Endocrinol Metab. 1999;84:1420–1423. doi: 10.1210/jcem.84.4.5585. [DOI] [PubMed] [Google Scholar]
- 213.Petraglia F, Florio P, Gallo R, Simoncini T, Saviozzi M, Di Blasio AM, Vaughan J, Vale W. Human placenta and fetal membranes express human urocortin mRNA and peptide. J Clin Endocrinol Metab. 1996;81:3807–3810. doi: 10.1210/jcem.81.10.8855842. [DOI] [PubMed] [Google Scholar]
- 214.Pisarchik A, Slominski A. Corticotropin releasing factor receptor type 1: molecular cloning and investigation of alternative splicing in the hamster skin. J Invest Dermatol. 2002;118:1065–1072. doi: 10.1046/j.1523-1747.2002.01770.x. [DOI] [PubMed] [Google Scholar]
- 215.Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem. 2004;271:2821–2830. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 217.Poliak S, Mor F, Conlon P, Wong T, Ling N, Rivier J, Vale W, Steinman L. Stress and autoimmunity: the neuropeptides corticotropin-releasing factor and urocortin suppress encephalomyelitis via effects on both the hypothalamic-pituitary-adrenal axis and the immune system. J Immunol. 1997;158:5751–5756. [PubMed] [Google Scholar]
- 218.Porcher C, Juhem A, Peinnequin A, Sinniger V, Bonaz B. Expression and effects of metabotropic CRF1 and CRF2 receptors in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1091–G1103. doi: 10.1152/ajpgi.00302.2004. [DOI] [PubMed] [Google Scholar]
- 219.Porcher C, Peinnequin A, Pellissier S, Meregnani J, Sinniger V, Canini F, Bonaz B. Endogenous expression and in vitro study of CRF-related peptides and CRF receptors in the rat gastric antrum. Peptides. 2006;27:1464–1475. doi: 10.1016/j.peptides.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 220.Rademaker MT, Cameron VA, Charles CJ, Richards AM. Integrated hemodynamic, hormonal, and renal actions of urocortin 2 in normal and paced sheep: beneficial effects in heart failure. Circulation. 2005;112:3624–3632. doi: 10.1161/CIRCULATIONAHA.105.561308. [DOI] [PubMed] [Google Scholar]
- 221.Rademaker MT, Charles CJ, Espiner EA, Fisher S, Frampton CM, Kirkpatrick CM, Lainchbury JG, Nicholls MG, Richards AM, Vale WW. Beneficial hemodynamic, endocrine, and renal effects of urocortin in experimental heart failure: comparison with normal sheep. J Am CollCardiol. 2002;40:1495–1505. doi: 10.1016/s0735-1097(02)02170-8. [DOI] [PubMed] [Google Scholar]
- 222.Rademaker MT, Charles CJ, Espiner EA, Frampton CM, Lainchbury JG, Richards AM. Endogenous urocortins reduce vascular tone and renin-aldosterone/endothelin activity in experimental heart failure. Eur Heart J. 2005;26:2046–2054. doi: 10.1093/eurheartj/ehi227. [DOI] [PubMed] [Google Scholar]
- 223.Rademaker MT, Charles CJ, Espiner EA, Frampton CM, Lainchbury JG, Richards AM. Four-day urocortin-I administration has sustained beneficial haemodynamic, hormonal, and renal effects in experimental heart failure. Eur Heart J. 2005;26:2055–2062. doi: 10.1093/eurheartj/ehi351. [DOI] [PubMed] [Google Scholar]
- 224.Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Railson JE, Liao Z, Brar BK, Buddle JC, Pennica D, Stephanou A, Latchman DS. Cardiotrophin-1 and urocortin cause protection by the same pathway and hypertrophy via distinct pathways in cardiac myocytes. Cytokine. 2002;17:243–253. doi: 10.1006/cyto.2001.1011. [DOI] [PubMed] [Google Scholar]
- 226.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Richard D, Rivest R, Naimi N, Timofeeva E, Rivest S. Expression of corticotropin-releasing factor and its receptors in the brain of lean and obese Zucker rats. Endocrinology. 1996;137:4786–4795. doi: 10.1210/endo.137.11.8895348. [DOI] [PubMed] [Google Scholar]
- 228.Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology. 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- 229.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W. Potent and long acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 230.Robinson BM, Tellam DJ, Smart D, Mohammad YN, Brennand J, Rivier JE, Lovejoy DA. Cloning and characterization of corticotropin-releasing factor and urocortin in Syrian hamster (Mesocricetus auratus) Peptides. 1999;20:1177–1185. doi: 10.1016/s0196-9781(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 231.Roste GK, Dietrichs E. Cerebellar cortical and nuclear afferents from the Edinger-Westphal nucleus in the cat. Anat Embryol (Berl) 1988;178:59–65. doi: 10.1007/BF00305015. [DOI] [PubMed] [Google Scholar]
- 232.Ryabinin AE, Tsivkovskaia NO, Ryabinin SA. Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience. 2005;134:1317–1323. doi: 10.1016/j.neuroscience.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 233.Sajdyk TJ, Gehlert DR. Astressin, a corticotropin releasing factor antagonist, reverses the anxiogenic effects of urocortin when administered into the basolateral amygdala. Brain Res. 2000;877:226–234. doi: 10.1016/s0006-8993(00)02638-x. [DOI] [PubMed] [Google Scholar]
- 234.Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 235.Saruta M, Takahashi K, Suzuki T, Fukuda T, Torii A, Sasano H. Urocortin 3/stresscopin in human colon: possible modulators of gastrointestinal function during stressful conditions. Peptides. 2005;26:1196–1206. doi: 10.1016/j.peptides.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 236.Saruta M, Takahashi K, Suzuki T, Torii A, Kawakami M, Sasano H. Urocortin 1 in colonic mucosa in patients with ulcerative colitis. J Clin Endocrinol Metab. 2004;89:5352–5361. doi: 10.1210/jc.2004-0195. [DOI] [PubMed] [Google Scholar]
- 237.Scarabelli T, Knight R. Urocortins: take them to heart. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:335–342. doi: 10.2174/1568016043356174. [DOI] [PubMed] [Google Scholar]
- 238.Scarabelli TM, Pasini E, Ferrari G, Ferrari M, Stephanou A, Lawrence K, Townsend P, Chen-Scarabelli C, Gitti G, Saravolatz L, Latchman D, Knight RA, Gardin JM. Warm blood cardioplegic arrest induces mitochondrial-mediated cardiomyocyte apoptosis associated with increased urocortin expression in viable cells. J Thorac Cardiovasc Surg. 2004;128:364–371. doi: 10.1016/j.jtcvs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 239.Scarabelli TM, Pasini E, Stephanou A, Comini L, Curello S, Raddino R, Ferrari R, Knight R, Latchman DS. Urocortin promotes hemodynamic and bioenergetic recovery and improves cell survival in the isolated rat heart exposed to ischemia/reperfusion. J Am Coll Cardiol. 2002;40:155–161. doi: 10.1016/s0735-1097(02)01930-7. [DOI] [PubMed] [Google Scholar]
- 240.Schulman D, Latchman DS, Yellon DM. Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol. 2002;283:H1481–H1488. doi: 10.1152/ajpheart.01089.2001. [DOI] [PubMed] [Google Scholar]
- 241.Seasholtz AF, Burrows HL, Karolyi IJ, Camper SA. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22:743–751. doi: 10.1016/s0196-9781(01)00387-4. [DOI] [PubMed] [Google Scholar]
- 242.Sehringer B, Zahradnik HP, Simon M, Ziegler R, Noethling C, Schaefer WR. mRNA expression profiles for corticotrophin-releasing hormone, urocortin, CRH-binding protein and CRH receptors in human term gestational tissues determined by real-time quantitative RT-PCR. J Mol Endocrinol. 2004;32:339–348. doi: 10.1677/jme.0.0320339. [DOI] [PubMed] [Google Scholar]
- 243.Seres J, Bornstein SR, Seres P, Willenberg HS, Schulte KM, Scherbaum WA, Ehrhart-Bornstein M. Corticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab. 2004;89:965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- 244.Singh LK, Boucher W, Pang X, Letourneau R, Seretakis D, Green M, Theoharides TC. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J Pharmacol Exp Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- 245.Skelton KH, Nemeroff CB, Knight DL, Owens MJ. Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci. 2000;20:1240–1248. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Slominski A, Wortsman J. Neuroendocrinology of the skin. EndocrRev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 248.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 249.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206:780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Smith JE, Jansen AS, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- 252.Solinas G, Summermatter S, Mainieri D, Gubler M, Montani JP, Seydoux J, Smith SR, Dulloo AG. Corticotropin-releasing hormone directly stimulates thermogenesis in skeletal muscle possibly through substrate cycling between de novo lipogenesis and lipid oxidation. Endocrinology. 2006;147:31–38. doi: 10.1210/en.2005-1033. [DOI] [PubMed] [Google Scholar]
- 253.Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 254.Spina MG, Merlo-Pich E, Akwa Y, Balducci C, Basso AM, Zorrilla EP, Britton KT, Rivier J, Vale WW, Koob GF. Time-dependent induction of anxiogenic-like effects after central infusion of urocortin or corticotropin-releasing factor in the rat. Psychopharmacology. 2002;160:113–121. doi: 10.1007/s00213-001-0940-y. [DOI] [PubMed] [Google Scholar]
- 255.Stenzel P, Kesterson R, Yeung W, Cone RD, Rittenberg MB, Stenzel-Poore MP. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol Endocrinol. 1995;9:637–645. doi: 10.1210/mend.9.5.7565810. [DOI] [PubMed] [Google Scholar]
- 256.Sutton SW, Behan DP, Lahrichi SL, Kaiser R, Corrigan A, Lowry P, Potter E, Perrin MH, Rivier J, Vale WW. Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology. 1995;136:1097–1102. doi: 10.1210/endo.136.3.7867564. [DOI] [PubMed] [Google Scholar]
- 257.Swinny J, Kalicharan D, Gramsbergen A, van der Want JJ. The localisation of urocortin in the adult rat cerebellum: a light and electron microscopic study. Neuroscience. 2002;114:891–903. doi: 10.1016/s0306-4522(02)00311-1. [DOI] [PubMed] [Google Scholar]
- 258.Tache Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tache Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gend Med. 2005;2:146–154. doi: 10.1016/s1550-8579(05)80043-9. [DOI] [PubMed] [Google Scholar]
- 260.Tache Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 261.Taché Y, Martinez V, Million M, Maillot C. Role of corticotropin-releasing factor receptor subtype 1 in stress-related functional colonic alterations: implications in irritable bowel syndrome. Eur J Surg Suppl. 2002;587:16–22. [PubMed] [Google Scholar]
- 262.Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- 263.Takahashi K. Letter regarding article by Rademaker et al, “integrated hemodynamic, hormonal, and renal actions of urocortin 2 in normal and paced sheep: beneficial effects in heart failure”. Circulation. 2006;113:e710. doi: 10.1161/CIRCULATIONAHA.105.612309. [DOI] [PubMed] [Google Scholar]
- 264.Takahashi K, Totsune K, Murakami O, Saruta M, Nakabayashi M, Suzuki T, Sasano H, Shibahara S. Expression of urocortin III/stresscopin in human heart and kidney. J Clin Endocrinol Metab. 2004;89:1897–1903. doi: 10.1210/jc.2003-031663. [DOI] [PubMed] [Google Scholar]
- 265.Takahashi K, Totsune K, Murakami O, Shibahara S. Urocortins as cardiovascular peptides. Peptides. 2004;25:1723–1731. doi: 10.1016/j.peptides.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 266.Takahashi K, Totsune K, Saruta M, Fukuda T, Suzuki T, Hirose T, Imai Y, Sasano H, Murakami O. Expression of urocortin 3/stresscopin in human adrenal glands and adrenal tumors. Peptides. 2006;27:178–182. doi: 10.1016/j.peptides.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 267.Tanaka Y, Makino S, Noguchi T, Tamura K, Kaneda T, Hashimoto K. Effect of stress and adrenalectomy on urocortin II mRNA expression in the hypothalamic paraventricular nucleus of the rat. Neuroendocrinology. 2003;78:1–11. doi: 10.1159/000071700. [DOI] [PubMed] [Google Scholar]
- 268.Terui K, Higashiyama A, Horiba N, Furukawa KI, Motomura S, Suda T. Coronary vasodilation and positive inotropism by urocortin in the isolated rat heart. J Endocrinol. 2001;169:177–183. doi: 10.1677/joe.0.1690177. [DOI] [PubMed] [Google Scholar]
- 269.Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci USA. 2004;101:9468–9473. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 271.Theoharides TC, Singh LK, Boucher W, Pang X, Letourneau R, Webster E, Chrousos G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology. 1998;139:403–413. doi: 10.1210/endo.139.1.5660. [DOI] [PubMed] [Google Scholar]
- 272.Timofeeva E, Richard D. Functional activation of CRH neurons and expression of the genes encoding CRH and its receptors in food-deprived lean (Fa/?) and obese (fa/fa) Zucker rats. Neuroendocrinology. 1997;66:327–340. doi: 10.1159/000127256. [DOI] [PubMed] [Google Scholar]
- 273.Todorovic C, Radulovic J, Spiess J. Interrelationship between anxiety-like behavior and fear conditioning-the role of corticotropin-releasing receptor 2 Program No.370.15. 2002 Abstract Viewer/Itinerary Planner; Washington, DC. Society for Neuroscience; 2002. Online. 2002. [Google Scholar]
- 274.Torricelli M, Ignacchiti E, Giovannelli A, Merola A, Scarpetti E, Calonaci G, Picciolini E, Florio P, Reis FM, Linton EA, Petraglia F. Maternal plasma corticotrophin-releasing factor and urocortin levels in post-term pregnancies. Eur J Endocrinol. 2006;154:281–285. doi: 10.1530/eje.1.02091. [DOI] [PubMed] [Google Scholar]
- 275.Tsatsanis C, Androulidaki A, Alissafi T, Charalampopoulos I, Dermitzaki E, Roger T, Gravanis A, Margioris AN. Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J Immunol. 2006;176:1869–1877. doi: 10.4049/jimmunol.176.3.1869. [DOI] [PubMed] [Google Scholar]
- 276.Tsatsanis C, Androulidaki A, Dermitzaki E, Charalampopoulos I, Spiess J, Gravanis A, Margioris AN. Urocortin 1 and Urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett. 2005;579:4259–4264. doi: 10.1016/j.febslet.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 277.Turnbull AV, Vale W, Rivier C. Urocortin, a corticotropin-releasing factor-related mammalian peptide, inhibits edema due to thermal injury in rats. Eur J Pharmacol. 1996;303:213–216. doi: 10.1016/0014-2999(96)00141-0. [DOI] [PubMed] [Google Scholar]
- 278.Turnbull AV, Vaughan J, Rivier JE, Vale WW, Rivier C. Urocortin is not a significant regulator of intermittent electrofootshock-induced adrenocorticotropin secretion in the intact male rat. Endocrinology. 1999;140:71–78. doi: 10.1210/endo.140.1.6419. [DOI] [PubMed] [Google Scholar]
- 279.Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 280.Uzuki M, Sasano H, Muramatsu Y, Totsune K, Takahashi K, Oki Y, Iino K, Sawai T. Urocortin in the synovial tissue of patients with rheumatoid arthritis. ClinSci (Lond) 2001;100:577–589. [PubMed] [Google Scholar]
- 281.Valdenaire O, Giller T, Breu V, Gottowik J, Kilpatrick G. A new functional isoform of the human CRF2 receptor for corticotropin-releasing factor. Biochim Biophys Acta. 1997;1352:129–132. doi: 10.1016/s0167-4781(97)00047-x. [DOI] [PubMed] [Google Scholar]
- 282.Valdez GR, Inoue K, Koob GF, Rivier J, Vale WW, Zorrilla EP. Human urocortin II: Mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- 283.Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- 284.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 285.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 286.Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- 287.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. JComp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 288.Vasconcelos LA, Donaldson C, Sita LV, Casatti CA, Lotfi CF, Wang L, Cardinouche MZ, Frigo L, Elias CF, Lovejoy DA, Bittencourt JC. Urocortin in the central nervous system of a primate (Cebus apella): sequencing, immunohistochemical, and hybridization histochemical characterization. J Comp Neurol. 2003;463:157–175. doi: 10.1002/cne.10742. [DOI] [PubMed] [Google Scholar]
- 289.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 290.Venihaki M, Majzoub J. Lessons from CRH knockout mice. Neuropeptides. 2002;36:96–102. doi: 10.1054/npep.2002.0906. [DOI] [PubMed] [Google Scholar]
- 291.Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, Liapakis G, Graf T, Majzoub JA. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviours. J Neuroendocrinol. 2004;16:411–422. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- 292.Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heinemann SF, Vale W, Lee KF. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- 293.Vita N, Laurent P, Lefort S, Chalon P, Lelias JM, Kaghad M, Le Fur G, Caput D, Ferrara P. Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 1993;335:1–5. doi: 10.1016/0014-5793(93)80427-v. [DOI] [PubMed] [Google Scholar]
- 294.Wang L, Martinez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1401–R1410. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- 295.Wang L, Martinez V, Vale W, Taché Y. Fos induction in selective hypothalamic neuroendocrine and medullary nuclei by intravenous injection of urocortin and corticotropin-releasing factor in rats. Brain Res. 2000;855:47–57. doi: 10.1016/s0006-8993(99)02200-3. [DOI] [PubMed] [Google Scholar]
- 296.Wang X, Su H, Copenhagen LD, Vaishnav S, Pieri F, Shope CD, Brownell WE, De Biasi M, Paylor R, Bradley A. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control but an impaired acoustic startle response. Mol Cell Biol. 2002;22:6605–6610. doi: 10.1128/MCB.22.18.6605-6610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus alter food and water consumption. Behav Neurosci. 2005;119:1235–1243. doi: 10.1037/0735-7044.119.5.1235. [DOI] [PubMed] [Google Scholar]
- 298.Weitemier AZ, Tsivkovskaia NO, Ryabinin AE. Urocortin 1 distribution in mouse brain is strain-dependent. Neuroscience. 2005;132:729–740. doi: 10.1016/j.neuroscience.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 299.Weninger SC, Peters LL, Majzoub JA. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinology. 2000;141:256–263. doi: 10.1210/endo.141.1.7277. [DOI] [PubMed] [Google Scholar]
- 300.Westphal NJ, Seasholtz AF. CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci. 2006;11:1878–1891. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
- 301.Wetzka B, Sehringer B, Schafer WR, Biller S, Hor C, Benedek E, Deppert WR, Zahradnik HP. Expression patterns of CRH, CRH receptors, and CRH binding protein in human gestational tissue at term. Exp Clin Endocrinol Diabetes. 2003;111:154–161. doi: 10.1055/s-2003-39778. [DOI] [PubMed] [Google Scholar]
- 302.Wiesner B, Roloff B, Fechner K, Slominski A. Intracellular calcium measurements of single human skin cells after stimulation with corticotropin-releasing factor and urocortin using confocal laser scanning microscopy. J Cell Sci. 2003;116:1261–1268. doi: 10.1242/jcs.00301. [DOI] [PubMed] [Google Scholar]
- 303.Wiley KE, Davenport AP. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Br J Pharmacol. 2004;143:508–514. doi: 10.1038/sj.bjp.0705985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wille S, Sydow S, Palchaudhuri MR, Spiess J, Dautzenberg FM. Identification of amino acids in the N-terminal domain of corticotropin-releasing factor receptor 1 that are important determinants of high-affinity ligand binding. J Neurochem. 1999;72:388–395. doi: 10.1046/j.1471-4159.1999.0720388.x. [DOI] [PubMed] [Google Scholar]
- 305.Wiltshire S, Hattersley AT, Hitman GA, Walker M, Levy JC, Sampson M, O’Rahilly S, Frayling TM, Bell JI, Lathrop GM, Bennett A, Dhillon R, Fletcher C, Groves CJ, Jones E, Prestwich P, Simecek N, Rao PV, Wishart M, Bottazzo GF, Foxon R, Howell S, Smedley D, Cardon LR, Menzel S, McCarthy MI. A genomewide scan for loci predisposing to type 2 diabetes in a U.K. population (the Diabetes UK Warren 2 Repository): analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q. Am J Hum Genet. 2001;69:553–569. doi: 10.1086/323249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wu X, Cooper RS, Borecki I, Hanis C, Bray M, Lewis CE, Zhu X, Kan D, Luke A, Curb D. A combined analysis of genomewide linkage scans for body mass index from the National Heart, Lung, and Blood Institute Family Blood Pressure Program. Am J Hum Genet. 2002;70:1247–1256. doi: 10.1086/340362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wu Y, Xu Y, Zhou H, Tao J, Li S. Expression of urocortin in rat lung and its effect on pulmonary vascular permeability. J Endocrinol. 2006;189:167–178. doi: 10.1677/joe.1.06607. [DOI] [PubMed] [Google Scholar]
- 308.Wu Y, Zhou H, Xu Y, Li S. Enhanced expression of urocortin in lung tissues of rats with allergic asthma. Biochem Biophys Res Commun. 2006;341:532–540. doi: 10.1016/j.bbrc.2005.12.214. [DOI] [PubMed] [Google Scholar]
- 309.Xu J, Hennebold JD, Stouffer RL. Dynamic expression and regulation of the corticotropin-releasing hormone/urocortin-receptor-binding protein system in the primate ovary during the menstrual cycle. J Clin Endocrinol Metab. 2006;91:1544–1553. doi: 10.1210/jc.2005-2776. [DOI] [PubMed] [Google Scholar]
- 310.Yamamoto H, Maeda T, Fujimura M, Fujimiya M. Urocortin-like immunoreactivity in the substantia nigra, ventral tegmental area and Edinger-Westphal nucleus of rat. Neurosci Lett. 1998;243:21–24. doi: 10.1016/s0304-3940(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 311.Yamauchi N, Otagiri A, Nemoto T, Sekino A, Oono H, Kato I, Yanaihara C, Shibasaki T. Distribution of urocortin 2 in various tissues of the rat. J Neuroendocrinol. 2005;17:656–663. doi: 10.1111/j.1365-2826.2005.01354.x. [DOI] [PubMed] [Google Scholar]
- 312.Zhao L, Donaldson CJ, Smith GW, Vale WW. The structures of the mouse and human urocortin genes (Ucn and UCN) Genomics. 1998;50:23–33. doi: 10.1006/geno.1998.5292. [DOI] [PubMed] [Google Scholar]
- 313.Zhao Y, Fekete EM, Brennan M, Mattock M, Rivier J, Vale WW, Koob GF, Zorrilla EP. Intracerebroventricular urocortin 3 lacks the anxiety-like properties of corticotropin-releasing factor1 receptor agonists in rats Program No.1027.14. 2004. Abstract Viewer/Itinerary Planner; Washington, DC. Society for Neuroscience; 2004. Online 2004. [Google Scholar]
- 314.Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- 315.Zorrilla EP, Koob GF. The roles of urocortins 1, 2 and 3 in the brain. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of Stress and the Brain (series title: Techniques in the Behavioral and Neural Sciences, vol. 15) Elsevier Science; New York: 2005. pp. 179–203. [Google Scholar]
- 316.Zorrilla EP, Reinhardt LE, Valdez GR, Inoue K, Rivier JE, Vale WW, Koob GF. Human urocortin 2, a corticotropin-releasing factor (CRF)2 agonist, and ovine CRF, a CRF1 agonist, differentially alter feeding and motor activity. J Pharmacol Exp Ther. 2004;310:1027–1034. doi: 10.1124/jpet.104.068676. [DOI] [PubMed] [Google Scholar]
- 317.Zorrilla EP, Schulteis G, Ling N, Koob GF, De Souza EB. Performance-enhancing effects of CRF-BP ligand inhibitors. Neuroreport. 2001;12:1231–1234. doi: 10.1097/00001756-200105080-00035. [DOI] [PubMed] [Google Scholar]
- 318.Zorrilla EP, Taché Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]


