Abstract
Between 65 and 175 K, nitroxyl radicals with spirocyclohexyl groups at the 2- and 6-positions of the piperidine ring exhibit spin echo dephasing rates that are slower than for nitroxyl radicals with 2,5-gem-dimethyl or 2,6-gem-dimethyl substituents that are currently used as spin labels, and are slow enough to permit DEER measurements at temperatures up to about 125 K for spin labels with analogous ring structures.
Pulsed double electron-electron resonance (DEER or PELDOR) spectroscopy is increasingly being used to measure electron-electron interspin distances in biomolecules and polymers.1-4 This method is particularly powerful for systems such as membrane proteins that are difficult to crystallize and that are too large for solution NMR studies. Analysis of DEER data permits determination of both the mean distance and the width of the distance distribution. Faster spin echo dephasing rates restrict the measurements to shorter interspin distances and limit the accuracy to which the width of the distribution can be determined.1
In addition to molecules with native paramagnetic centers, distance measurements are made possible for other biomolecules by introduction of cysteines via site-directed mutagenesis and subsequent attachment of thiol-specific nitroxyl spin labels.5 Polymers also can be spin labeled. All the currently available labels have gem-dimethyl groups adjacent to the nitroxyl N-O moiety, as in MTSSL. Rotation of these methyl groups at rates comparable to the anisotropy in the electron-proton hyperfine coupling to the methyl protons enhances the spin echo dephasing rates at temperatures above about 60 K,6-8 so the optimum temperature for DEER measurements with these labels is 50 to 60 K.1 It would be significantly less expensive if the experiments could be performed at higher temperatures with liquid nitrogen. However, for the methyl-containing spin labels, DEER experiments at higher temperatures have substantially poorer signal-to-noise and are limited to shorter distances because of the faster spin echo dephasing rates.1
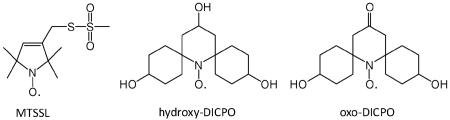
Recently, the synthesis of nitroxyl radicals, oxo-DICPO and hydroxy-DICPO, with 4′-hydroxyspirocyclohexyl groups at the 2,6 positions instead of the usual methyl groups as in MTSSL has been reported.9 The 4′-hydroxy groups on the spirocyclohexyl groups provide water solubility. To minimize the impact on protein structures, it is important to keep the size of spin labels as small as possible.10 The Connolly’s solvent excluded volume estimated from chem 3D pro (CambridgeSoft) is 251 Å3 for the central portion of hydroxy-DICPO or oxo-DICPO, excluding the hydroxy and oxo groups, is about 70% greater than the 146 Å3 for the nitroxyl ring containing portion of MTSSL.
In this communication we report the electron spin relaxation properties of hydroxy-DICPO and oxo-DICPO at X-band (∼9.5 GHz). The favorable relaxation properties of these nitroxyls demonstrate that derivatives of these radicals with 4-position sidechains appropriate for attachment to a protein will make it possible to perform DEER measurements in the liquid nitrogen temperature range with signal-to-noise and upper distance limitation similar to what is currently available only by using liquid helium.
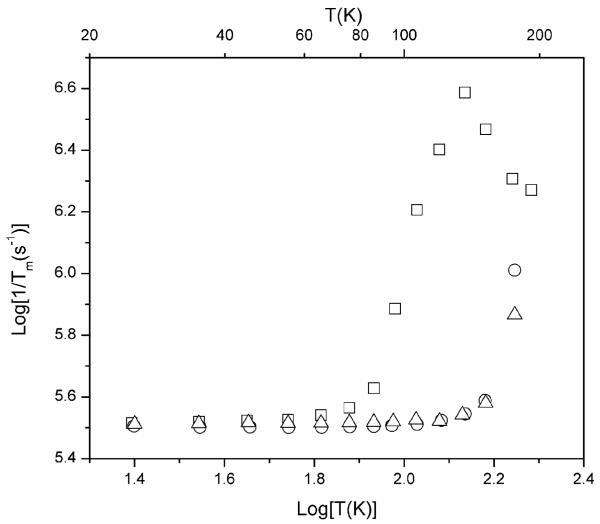
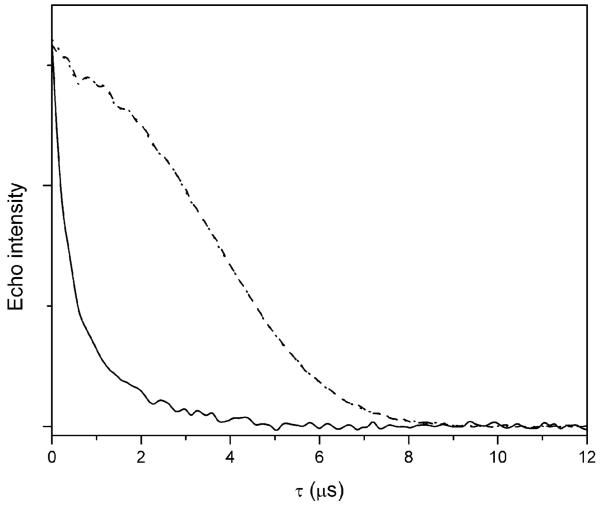
The spin echo dephasing rates for hydroxy-DICPO and oxo-DICPO exhibit negligible temperature dependence up to about 125 K, and are strikingly slower than for MTSSL between about 65 and 150 K (Fig. 1). The pulse sequence for the spin echo decay experiments is 90°-τ-180°-τ-echo. For a slower dephasing rate, the echo decays more slowly as the time τ between the pulses increases (Fig. 2). The values of Tm are calculated by fitting a single exponential to the echo decay curves. Greater echo intensity at longer values of τ permits measurement of longer values of interspin distance and greater accuracy in the widths of the distance distributions.1 The relatively slow and temperature independent echo decay rates for oxo-DICPO and hydroxy-DICPO at temperatures up to about 125 K demonstrate that in the absence of ring methyl groups, the echo dephasing for nitroxyls is long enough up to about 125 K to be suitable for DEER experiments. Therefore design of analogous radicals with a substitutent at the 4-position that is suitable for selectively labeling a protein will permit DEER measurements up to about 125 K. The faster dephasing rates at temperatures above about 150 K are due to the onset of motion as the 1 : 1 water-glycerol glass softens. The temperature dependence of Tm for piperidinyl nitroxyl radicals with 2,6-gem-dimethyls is similar to that shown for MTSSL.11 The use of a matrix that remains rigid up to higher temperatures could permit DEER measurements with spin labels analogous to oxo-DICPO and hydroxy-DICPO at temperatures higher than 125 K.
Fig. 1.
Temperature dependence of spin echo dephasing rates in 1 : 1 water-glycerol for MTSSL (□), oxo-DICPO (○), and hydroxy-DICPO (△).
Fig. 2.
Echo decay curves in 1 : 1 water-glycerol at 106 K for MTSSL (solid line), oxo-DICPO (dotted line) and hydroxy-DICPO (dashed line).
The electron spin lattice rate, 1/T1, determines the repetition rate that must be used to allow for the spins to relax sufficiently between pulse sequences. Slower relaxation rates require slower repetition rates. The repetition rate for the DEER experiments is selected to obtain the best signal-to-noise per time. Between about 60 and 125 K, 1/T1 for nitroxyls is dominated by the Raman process and is in the high temperature limit above 100 K, where it increases proportional to T2.12 As a result, if temperature is doubled, 4 times as many echoes can be recorded in the same time period. Signal-to-noise increases proportional to the square root of the number of scans averaged, so if the signal amplitude were independent of temperature, averaging 4 times as many echoes would improve signal-to-noise by a factor of 2. In this temperature region the Boltzmann populations of the spin energy levels vary as 1/T, so the signal amplitude decreases proportional to 1/T. Thus in the temperature range where 1/T1 varies as T2 the effects of the temperature dependences of the Boltzmann populations and of T1 cancel out, and the average signal amplitude per unit time would beindependent of temperature if the spin echo dephasing rate were independent of temperature.
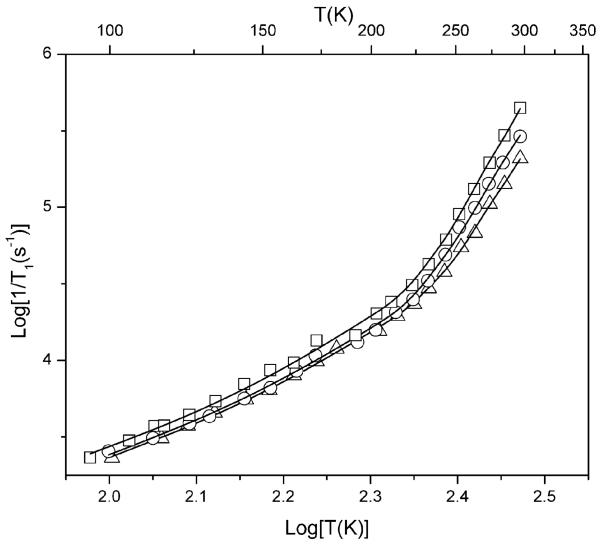
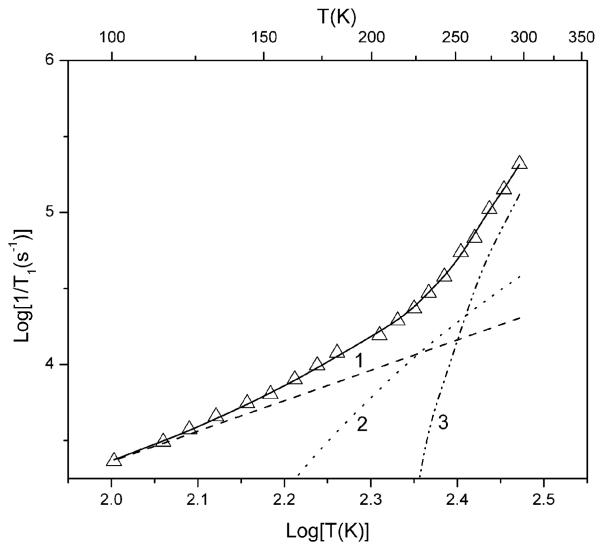
The temperature dependence of 1/T1 for MTSSL, hydroxy-DICPO and oxo-DICPO (Fig. 3) is similar for the three radicals, which shows that the pulse repetition times required for hydroxy-DICPO, oxo-DICPO and related derivatives are similar to those for MTSSL. Above 125 K the spin lattice relaxation rates increase more quickly with increasing temperature than the T2 dependence of the Raman process. This behavior is characteristic of nitroxyl radicals and is assigned to a local mode.12 The modeling of the temperature dependence of 1/T1 for hydroxy-DICPO is shown in Fig. 4. Figures showing analogous modeling of the temperature dependence of 1/T1 for MTSSL and oxo-DICPO are included in the ESI.† The signal-to-noise per unit time in DEER experiments for spin labels with methyl-less structures similar to that of hydroxy-DICPO or oxo-DICPO are expected to be at least as good at about 125 K as have been obtained for MTSSL at ∼60 K.
Fig. 3.
Temperature dependence of spin lattice relaxation rates in 1 : 1 water-glycerol measured in the center of the X-band spectrum for MTSSL (□), oxo-DICPO (○), and hydroxy-DICPO (△). The solid lines are the least-squares fits to the data of a model that is described in the ESI.†
Fig. 4.
Modeling of the temperature dependence of spin lattice relaxation rates for hydroxy-DICPO in 1 : 1 water-glycerol, measured in the center of the X-band spectrum. The solid line is the sum of contributions from the Raman process (line 1), the local mode (line 2), and modulation of g and A anisotropy by tumbling (line 3). Although spin-rotation was included in the fit line, the contribution is so small that a line depicting that contribution is not included in the figure.
Replacement of the gem-dimethyl groups that are present on the carbons adjacent to the nitroxyl N-O group in traditional spin labels such as MTSSL by spirocyclohexyl groups removes a major spin echo dephasing mechanism and makes the spin echo dephasing time long enough up to about 125 K to permit DEER measurements of interspin distances in the liquid nitrogen range.
Footnotes
Electronic supplementary information (ESI) available: Preparation of samples, EPR spectroscopy, determination of tumbling correlation times, modeling of the temperature dependence of 1/T1, and orientation dependence of relaxation rates.
Notes and references
- 1.Jeschke G, Polyhach Y. Phys. Chem. Chem. Phys. 2007;9:1895–1910. doi: 10.1039/b614920k. [DOI] [PubMed] [Google Scholar]
- 2.Jeschke G, Schlick S. Phys. Chem. Chem. Phys. 2006;8:4095–4103. doi: 10.1039/b607826e. [DOI] [PubMed] [Google Scholar]
- 3.Chiang Y-W, Borbat PP, Freed JH. J. Magn. Reson. 2005;177:184–196. doi: 10.1016/j.jmr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Chiang Y-W, Borbat PP, Freed JH. J. Magn. Reson. 2005;172:279–295. doi: 10.1016/j.jmr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Hubbell WL, Fross A, Langren R, Lietzow MA. Curr. Opin. Struct. Biol. 1998;8:649–656. doi: 10.1016/s0959-440x(98)80158-9. [DOI] [PubMed] [Google Scholar]
- 6.Dzuba SA, Maryasov AG, Salikhov AK, Tsvetkov YD. J. Magn. Reson. 1984;58:95–117. [Google Scholar]
- 7.Nakagawa K, Candelaria MB, Chik WWC, Eaton SS, Eaton GR. J. Magn. Reson. 1992;98:81–91. [Google Scholar]
- 8.Zecevic A, Eaton GR, Eaton SS, Lindgren M. Mol. Phys. 1998;95:1255–1263. [Google Scholar]
- 9.Okazaki S, Mannan MA, Sawai K, Masumizu T, Miura Y, Takeshita K. Free Radical Res. 2007;41:1069–1077. doi: 10.1080/10715760701449302. [DOI] [PubMed] [Google Scholar]
- 10.Feix JB, Klug CS. Biol. Magn. Reson. 1998;14:251–281. [Google Scholar]
- 11.Eaton SS, Eaton GR. Biol. Magn. Reson. 2000;19:29–154. [Google Scholar]
- 12.Sato H, Kathirvelu V, Fielding AJ, Bottle SE, Blinco JP, Micallef AS, Eaton SS, Eaton GR. Mol. Phys. 2007;105:2137–2151. [Google Scholar]