Converging lines of evidence indicate that stress increases risk of addictive behaviors (1). Early life stress and child maltreatment, chronic cumulative adversity, major life trauma and negative emotionality and impulsivity/sensation seeking traits are each associated with increasing level of drug use and abuse (1). Persistent and uncontrollable stressful experiences interacts with individual genetic susceptibility to alter synthesis, expression and signaling in stress-related pathways (e.g. corticotrophin releasing factor (CRF), glucocorticoids, norepinephrine, GABA, neuorpeptide Y, BDNF, serotonin, gluatamate and dopamine), thereby resulting in individual differences in stress responses ([2], [3]. For example, genetic variation in the serotonin transporter gene interacts with early life stress resulting in hyperresponsivity to stressors that increase vulnerability to psychiatric disorders such as major depression. Similarly, individual variation in the NPY gene is associated with anxiety and emotional reactivity (1). Chronic adversity and early life stress is also associated with altered glucocortoid gene expression, increased CRF mRNA expression in the amygdala, and changes in serotonin function ([2], [3] and [4]).
Cumulative adversity and early life stress also has effects on mesolimbic dopamine transmission (1). Microarray analysis has shown distinctive signaling patterns and differential gene expression in CRF, GABA and dynorphin signaling in key limbic and striatal regions involved in stress and reward processing (5). Polygenic effects are also possible in which combination of genetic polymorphisms to affect molecular synthesis and expression in multiple signaling pathways to produce complex behavioral traits such as altered stress and reward sensitivity, which in turn, could affect stress-sensitive alterations in the rewarding effects of addictive substances (6). Other behavioral neuroscience studies document psychosocial stress-related effects on dopaminergic pathways (1). These findings are beginning to provide the molecular basis of how early life stress and cumulative adversity initiates epigenetic changes that increase susceptibility for emotional distress and also alter transmission of reward pathways to affect the reinforcing properties of addictive substances.
Drugs of abuse such as alcohol and nicotine profoundly affect the stress pathways (1). Acute and regular binge use of these drugs result in alterations in gene expression, with signaling effects on both reward and stress-related molecules (7), thereby affecting transmission in stress and reward pathways. Cellular and molecular changes associated with acute and regular binge, and chronic exposure to drugs of abuse such as alcohol and nicotine are now increasingly well documented (7). Such changes are likely associated with enhanced and persistent changes in hedonic state (i.e., increases in negative affect), increasing levels of craving and alterations in central and peripheral stress responses and finally, the effects of these changes on increasing drug use, and relapse risk which are increasingly well documented effects of chronic drug use in humans (1). This growing body of research combined with the previously cited research on epigenetic changes in stress and reward pathways highlight the intricate and complex web of interactions in the stress and reward circuits that ultimately affects individual responses to stress and drugs, addiction risk and relapse susceptibility (see Figure 1).
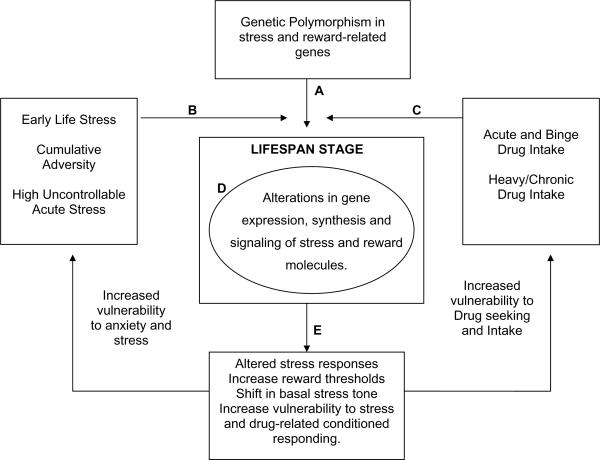
Figure 1.
A model depicting the dynamic interplay of individual genetic polymorphisms of stress and reward related genes (A), expressed in the context of the stress environment (B), and/or exposure to substances and level of drug intake (C). A dynamic interplay between these variables affects gene expression, synthesis and transcription of regulatory proteins and molecules with changes in the intracellular and extracellular environment (D), resulting in individual differences in responses to stress and to addictive substances (E). These changes and neuroadapatations are further affected by the lifespan stage of the nervous system. Altered stress responding, changes in reward threshold, increases in anxiety, craving and increased drug taking (E) represent the phenotypes that affect altered stress responses and increased anxiety, and increased vulnerability to drug intake. In the presence of high stress and/or increased drug intake, such phenotypes would increase risk of psychiatric disease onset. At each point in the feed-forward cascade between these variables there exists an opportunity for formal or informal interventions such as social support, education, training/rehabilitation, specific behavioral and pharmacological interventions, change in lifestyle (e.g., exercise) and environmental context/enrichment, which serve as resilience and recovery factors that potentially also affect cellular and molecular signaling with the possibility of redirecting and changing the dynamic interplay of these interactions towards prevention and/or recovery.
In this issue, the findings of Laucht et al (8) exemplify the effects of complex interactions between genes, environmental stress and drug exposure on drug use behaviors, as presented in figure 1. They examined whether genetic 5HTTLPR polymorphism interacts with stress to affect alcohol intake in young adults. The serotonin transporter gene that plays a key role in regulation of serotonin function is dependent on the environmental context for expression. While a number of studies have shown that the S allele interacts with stressful environment to increase risk of depression and negative emotionality, there is also evidence that the L allele is associated with alcohol intake, craving and binge alcohol consumption (8, 9). This research indicates 5HTTLPR polymorphisms show differential gene expression in the context of stress environments and alcohol intake. Consistent with previous research, Laucht et al (8) report main effects of genotype and current stressful environment on alcohol consumption, indicating that the LL group drank more than the other genotype groups and, as expected, the high stress groups consumed more alcohol as well. However, a key novel aspect of this paper is that using an elegant design and using both early life stress and recent stress assessments, the authors identify interactive effects of genotype and stressful environment on drinking behavior which is present only in young men and not women (men drank significantly more alcohol than the women). The results indicate that alcohol-related gene expression changes in the L-allele 5HTTLPR polymorphism show susceptibility to stress signaling such that the men who are L carriers with high stress show greatest alcohol intake and binge alcohol consumption. These findings are consistent with the growing literature on interactive epigenetic effects of multiple vulnerability factors to produce specific behavioral phenotypes.
Chronic use of nicotine and nicotine withdrawal as with other drugs of abuse are associated with cellular and molecular changes in brain stress and reward signaling that are consistent with altered peripheral stress responses, changes in corticostriatal-limbic circuits and increased negative affect, high craving and relapse susceptibility ([1] and [7]). In animal studies, these changes are associated with an upregulation of brain CRF and noradrenergic pathways and reductions in BDNF, NPY and other stress regulatory systems (1). Furthermore, such changes could last for several weeks, if not months, making drug craving and relapse susceptibility ongoing challenges in addiction treatment. Previous research has shown that nonspecific CRF antagonists decreases stress-induced reinstatement of drug-seeking in animals abusing alcohol, nicotine, cocaine and heroin (1). Furthermore, alpha-2adrenergic antagonists such as lofexidne, guanfacine and clonidine that decrease brain norepinephrine also decrease stress-induced reinstatement and stress-induced craving and relapse susceptibility (1).
Using pharmacological manipulation, Bruijnzeel et al. (10) in this issue conducted a careful assessment to determine whether the CRF1 or the CRF2 receptors are involved in the nicotine withdrawal-related negative affect and stress-induced reinstatement of nicotine seeking. Intracranial self stimulation (ICSS) procedures were used to assess negative affect associated with nicotine withdrawal, which was precipitated by administration of the nicotine receptor antagonist mecamylamine. Footshock-induced reinstatement was used to evaluate the effects of stress on nicotine seeking behavior. Using targets specific to each receptor subtype, the findings showed that the CRF1receptor antagonist, R278995/CRA0450, but not the specific CRF2 receptor antagonist, Astressin 2B, blocked both the effects of mecamylamine-precipitated nicotine withdrawal and stress-induced reinstatement of nicotine seeking. The findings elegantly demonstrate the potential utility of CRF1 receptor antagonists in decreasing withdrawal-related negative affect and stress-induced relapse in order to reduce the high smoking relapse rates. Indeed, the findings suggest that CRF1 receptor antagonists are likely to decrease upregulation of CRF mRNA expression in extrahypothalamic striatal-limbic regions known to modulate stress and reward sensitivity, and thereby affecting associated signaling cascades to confer benefit in susceptibility to drug seeking and relapse.
Finally, both of these two papers importantly contribute to the current research indicating that stress plays an integral role in the development and course of addiction. Such data necessitates a need to re-frame the landscape of addiction neurobiology to include the effects of stress in the expression of addiction vulnerability and course of the illness, i.e., relapse. These two studies jointly highlight the utility of genetic, molecular, pharmacological, psychosocial and behavioral approaches to understand the complexity underlying addiction. Using such multifactorial approaches can also have important implications for addiction prevention and treatment. For example, recent data indicates that ondansetron, the 5HT3 antagonist, could be of benefit in treating alcoholism but only in individuals who have the LL genotype ([9] and B.A. Johnson, personal communication). These data combined with Laucht et al's (8) findings raise the possibility that perhaps ondansetron may be used for secondary prevention with L carrier individuals who are binge drinkers and at-risk for developing alcoholism. Similarly, as pointed out by Bruijnzeel et al., small molecule CRF1 receptor antagonists need further development in the treatment of nicotine dependence, but given previous work by Shaham and colleagues (see [1] for review of this work), also for their potential benefit in other addictive disorders. Consistent with the existent literature on sex playing a key role in stress and reward signaling (4), the Laucht et al paper (8), points to sex as a critical variable in the molecular pathways involved in the risk of developing addiction. Such research underscores the need for sex-specific mechanistic and intervention development studies to truly address the pathophysiology of addictive disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sinha R. Chronic stress, drug use and vulnerability to addiction. Annals of the New York Academy of Sciences: Addiction Reviews. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 3.Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let's call the whole thing off. Epigenetics. 2007;2:22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- 4.Ren-Patterson RF, Cochran LW, Holmes A, Lesch KP, Lu B, Murphy DL. Gender-dependent modulation of brain monoamines and anxiety-like behaviors in mice with genetic serotonin transporter and BDNF deficiencies. Cell Mol Neurobiol. 2006;26:755–780. doi: 10.1007/s10571-006-9048-6. [DOI] [PubMed] [Google Scholar]
- 5.Olsen CM, Huang Y, Goodwin S, Ciobanu DC, Lu L, Sutter TR, Winder DG. Microarray analysis reveals distinctive signaling between the bed nucleus of the stria terminalis, nucleus accumbens, and dorsal striatum. Physiol Genomics. 2008;32:283–298. doi: 10.1152/physiolgenomics.00224.2006. [DOI] [PubMed] [Google Scholar]
- 6.Goldman D, Oroszi G, Ducci F. The genetics of addictions, Uncovering the genes. Nature Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 7.Renthal W, Nestler E. Epigenetic mechanisms in drug addiction. Trends in Molecular Medicine. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laucht M, Treutlein J, Schmid B, Blomeyer D, Buchmann AF, Schmidt MH, Esser G, Jennen-Steinmetz C, Rietschel M, Zimmermann US, Banaschewski T. Impact of Psychosocial Adversity on Alcohol Intake in Young Adults: Moderation by the LL Genotype of the Serotonin Transporter Polymorphism. Biol Psychiatry. 2009 Apr 7; doi: 10.1016/j.biopsych.2009.02.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Johnson BA, Javors MA, Roach JD, Seneviratne C, Bergeson SE, Ait-Daoud N, Dawes MA, Ma JZ. Can serotonin transporter genotype predict serotonergic function, chronicity, and severity of drinking? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:209–216. doi: 10.1016/j.pnpbp.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruijnzeel AJ, Prado M, Isaac S. Corticotropin-Releasing Factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009 Feb 11; doi: 10.1016/j.biopsych.2009.01.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]