Abstract
Promoting angiogenesis via delivery of vascular endothelial growth factor (VEGF) and other angiogenic factors is both a potential therapy for cardiovascular diseases and a critical aspect for tissue regeneration. The recent demonstration that VEGF signaling is modulated by the Notch signaling pathway, however, suggests that inhibiting Notch signaling may enhance regional neovascularization, by altering the responsiveness of local endothelial cells to angiogenic stimuli. We tested this possibility with in vitro assays using human endothelial cells, as well as in a rodent hindlimb ischemia model. Treatment of cultured human endothelial cells with DAPT, a gamma secretase inhibitor, increased cell migration and sprout formation in response to VEGF stimulation with a biphasic dependence on DAPT concentration. Further, delivery of an appropriate combination of DAPT and VEGF from an injectable alginate hydrogel system into ischemic hindlimbs led to a faster recovery of blood flow than VEGF or DAPT alone; perfusion levels reached 80% of the normal level by week 4 with combined DAPT and VEGF delivery. Direct intramuscular or intraperitoneal injection of DAPT did not result in the same level of improvement, suggesting that appropriate presentation of DAPT (gel delivery) is important for its activity. DAPT delivery from the hydrogels also did not lead to any adverse side effects, in contrast to systemic introduction of DAPT. Altogether, these results suggest a new approach to promote angiogenesis by controlling Notch signaling, and may provide new options to treat patients with diseases that diminish angiogenic responsiveness.
Keywords: Angiogenesis, VEGF, Notch, ischemia, tissue engineering
Introduction
Promoting angiogenesis is both a potential effective therapy for cardiovascular diseases and a critical aspect for tissue regeneration [1–4]. Vascular endothelial growth factor (VEGF) plays a central role in angiogenesis and is involved in various stages of the neovascularization process, e.g., degradation of extracellular matrix, endothelial cell migration and proliferation, lumen formation, vessel branching and pruning[5]. Provision of exogenous VEGF, either as a recombinant protein or via gene delivery, has long been pursued in therapeutic angiogenesis[6–9]. However, clinical trials administrating exogenous VEGF did not result in consistent and significant benefits[10], likely due to the poor pharmacokinetics with bolus infusion[11, 12]. In addition, some diseases (e.g., diabetes) result in reduced VEGF receptor expression and signaling [13, 14], pointing to the need for a more precise control of angiogenic stimuli.
It has recently become clear that VEGF signaling is modulated by the Notch pathway[15]. Notch signaling is an evolutionary conserved mechanism involved in cell-cell interactions, and affects cell proliferation, differentiation and stem/progenitor cell fate decisions[16, 17]. Notch signaling plays crucial roles in the embryonic and postnatal development of various organs, including the vasculature[18]. It is required for arterial-venous differentiation, embryonic/postnatal angiogenesis and arteriogenesis, and tumor angiogenesis [19–23]. In postnatal tissues, Notch signaling also maintains the quiescent state of the endothelium by suppressing endothelial cell proliferation[22], inducing endothelial cell contact inhibition, and regulating endothelial tip cell formation and vessel branching[24–27]. Of the 4 Notch receptors (Notch 1, 2, 3 and 4) and 5 Notch ligands (Delta-like1, 3, 4 (Dll1,3,4), Jagged 1, 2) found in mammals, Dll4 and Notch 1 are found predominantly in the endothelium and Dll4 is specifically found only in the endothelium[17, 28, 29]. Inhibition of Notch signaling by blockade of Dll4[19, 20], or by inhibition of a Notch signaling enzyme complex (gamma secretase)[19] disrupts normal vessel structure and function, but does increase the number of endothelial cells participating in sprouting and vessel density[19, 20, 30]. In contrast, upregulation of the Notch ligand Dll4 inhibits VEGF-induced endothelial cell proliferation[30] and downregulates VEGFR2 expression[31]; this effect can be reversed by adding Notch inhibitors[30]. In addition, Notch signaling has been shown to affect the contribution of endothelial progenitor cells to neovascularization[32]. The effects of Notch signaling in angiogenesis may be partially explained by the observation that Notch signaling is in a negative feedback loop with the VEGF pathway[15, 17, 33, 34]. VEGF signaling lies upstream of the Notch pathway, and activation of VEGF signaling (e.g., binding of VEGF to its VEGFR2 receptor) activates Notch signaling by increasing the expression of Notch ligands such as Dll4[22, 33]. Upregulation of Notch ligands and their binding to neighboring Notch receptors in turn then downregulates VEGFR2 expression[30, 31]. Thus, Notch signaling is able to assist in pruning and patterning vascular networks by locally regulating endothelial cell responsiveness to global pro-angiogenic stimuli, particularly VEGF[15, 35, 36]. In contrast, inhibition of Notch/Dll4 can up-regulate VEGFR-2 mRNA expression[19, 30].
We postulated that controlled and local Notch inhibition might enhance regional neovascularization, by altering the responsiveness of local endothelial cells to angiogenic stimuli. This concept is in contrast to recent work in which inhibition of Notch signaling by systemic introduction of anti-Dll4 antibodies reduced tumor tissue growth by forming excessive yet dysfunctional vasculature, i.e., “non-productive angiogenesis”[19, 20]. In the current study, we tested our hypothesis with in vitro assays using human endothelial cells, as well as in a rodent hindlimb ischemia model in vivo. We chose a severe combined immunodeficiency (SCID) mouse model for in vivo studies as it exhibits a significantly reduced spontaneous recovery from hindlimb ischemia, as compared to normal mice[37]. Gamma secretase inhibitor (GSI) was used as the Notch inhibitor, as it has previously been shown to inhibit Notch 1 activity[22, 31]. Gamma secretase is required to cleave the Notch receptor and release the Notch intracellular domain into the nucleus to induce changes in the gene expression[26, 29, 30].
Materials and Methods
Cell proliferation and migration assays
For cell proliferation assays, human microvascular endothelial cells (EC) (Lonza, MD) cultured in EGM-2MV media (Lonza) were seeded in 24-well plates at a seeding density of 1×104/cm2. Media was supplemented with recombinant human VEGF-A165 (10ng/mL) (R&D systems, MN) and/or 0.1% (v/v) of solutions with different concentrations of gamma secretase inhibitor IX (also named DAPT (N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester) (EMD Chemicals, NJ). Media was then changed every other day and cells were counted at day 6 with a coulter counter. Recombinant human Dll4 (R&D systems, MN) was also used in some studies, and the 24-well plates were preincubated with 0.2% gelatin (w/v) in PBS containing 1μg/mL Dll4 at 4°C overnight, as described previously[30]. Cells were then seeded in media with or without VEGF, followed by new media containing DAPT. DAPT did not affect cell adhesion (data not shown). For cell migration analysis, 5×105 cells in 100μl EGM-2MV were seeded in the top well of transwell dishes (Costar, MA) with a pore size of 5 μm. Cell migration towards the bottom well (containing 600μl endothelial basal medium supplemented with 50ng/mL VEGF and 50ng/mL recombinant hepatocyte growth factor (HGF) (R&D systems, MN)) was quantified by a coulter counter after 24 hours.
Sprouting assay
The detailed method for the sprouting assay was described previously[38, 39]. In brief, human microvascular endothelial cells (Lonza, MD) cultured in EGM-2MV media were seeded onto Cytodex 3 microcarriers (Amersham Biosciences, NJ) at a ratio of 3× 106 cells per 40mg beads. Beads with adhered cells continued to be cultured on a shaker until cells reached confluence. The beads in EGM2-MV were then mixed with a solution of human fibrinogen solution (4mg/mL) and aprotinin (500μg/mL) at a volume ratio of 1: 3:0.4. The beads-fibrinogen mixture was then placed into 24-well plates to which human thrombin (25 units/mL) was added at a volume ratio of 4:5 and mixed thoroughly by pipetting. The mixtures of beads, fibrinogen, thrombin and aprotinin were kept in the wells at room temperature for 5 min before being transferred to a 37°C incubator for 10 min to form hydrogels. Fresh EGM-2 media was placed on top of the gel for 30min, removed and replaced with EGM basal medium containing different concentrations of VEGF and Notch inhibitor. Media was changed every day. After 6 days, media was removed and gels were washed twice with PBS, and fixed with 4% formaldehyde overnight at 4°C. The formaldehyde solution was then aspirated, the gels were washed twice with PBS, and the number of sprouts were counted and normalized to the number of beads. (150–250 beads analyzed for each experimental condition). Sprouts were defined as a linear extension containing more than one cell[39].
DAPT and VEGF release from alginate hydrogels
DAPT was radiolabeled with 3H by Vitrax (Placentia, CA) with a resulting specific activity of 8.4 Ci/mmol. Differential amounts of DAPT (containing a mixture of radiolabeled and unlabeled DAPT with a final activity of 150 cpm/ng), with or without VEGF, was incorporated within alginate gels prepared as previously described[38]. Triplicate samples (50microliter) of hydrogels were placed in 24-well plates, and 1mL PBS buffer supplemented with 0.1g/L MgCl2·6H2O and 0.132 g/L of CaCl2·2H2O was added and maintained at 37°C. An aliquot of buffer was collected at set time points, and the radioactivity was measured by a Perkin Elmer liquid scintillation analyzer (Tri-Carb 2800TR, Perkin Elmer Inc., MA) and normalized to determine the release kinetics. VEGF release from alginate gel was measured with a human VEGF ELISA kit (R&D systems, MN).
Western Blots
Human microvascular endothelial cells cultured in EGM-2MV were grown to 75–80% confluent, changed to medium containing 0.2% serum, and the medium containing 10ng/mL VEGF, 2.5μM DAPT, or a combination of both was added to the dishes for varying time as specified. Cell lysates were collected in two types of lysis buffer (LyA and LyB). LyA contained 35mM NaCl, 25mM HEPES pH7.4, 50% Glycerol, 5mM EDTA, 1% Triton X, 50mM Sodium Fluoride, 100μM Sodium Orthovandate and Complete Mini Protease inhibitor (Roche). LyB was LyA supplemented with 1% Tween 20. Cell lysates were electrophoresed in precast Tris_glycine gels (Invitrogen), and transferred onto 0.2μm nitrocellulose membranes (Amersham Pharmacia). Blots were incubated with primary antibodies for VEGFR2 (Cell Signaling), βActin (Chemicon) or Vinculin (Calbiochem) followed by appropriate species-specific secondary antibodies (Jackson Laboratory), and chemiluminescence (Pierce) was detected with X-ray film (Kodak MR). Densitometric evaluations were performed with Kodak MS software.
Murine ischemic hindlimb model
All protocols were approved by Harvard's Institutional Animal Care and Use Committee. The animals used were 6-week old severe combined immunodeficiency (SCID) mice on a C57BL/6J background (Jackson Laboratory, ME). Unilateral hindlimb ischemia was created as previously described[40, 41]. In brief, the animals were anesthetized by intraperitoneal injections of ketamine (80mg kg−1) and xylazine (5 mg kg−1). The external iliac and femoral artery and vein were ligated, and 50μL alginate hydrogel (prepared by the protocol described previously[38]) incorporating 3μg VEGF and/or 86–8600ng DAPT was injected near the distal end of the ligation site. As controls, VEGF and DAPT in PBS were also injected intramuscularly or intraperitoneally (bolus delivery). Incisions were closed by 5–0 Ethilon sutures (Johnson & Johnson, NJ). Blood flow in the hindlimb was monitored by a laser Doppler perfusion imaging (LDPI) system (Perimed AB, Sweden) and the results were normalized to the control unligated limb of the same animal.
Histology and immunohistochemistry
Hindlimb muscle tissues between the two suture knots defining the ligation site were dissected and fixed by Z-fix (Anatech, MI) overnight and changed into 70% EtOH for storage prior to histology processing. Samples were embedded in paraffin and sectioned (5 μm thick) onto slides by Paragon (Paragon Bioservices, MD). Sections were incubated with primary anti-mouse CD31 antibody (1:250) (Pharmingen, CA), followed by incubation with an anti-rat mouse biotinylated secondary (1:200) (Vector Laboratories, CA), and amplified by a Tyramide Signal Amplification (TSA) Biotin System (Perkin Elmer Life Sciences, MA). Staining was developed using DAB+ substrate chromogen (DAKO, CA) and counterstained with Mayer's Hematoxylin. Capillary densities were quantified by counting the CD31 positive capillary numbers, normalized to the tissue area, in 30 randomly chosen high-power (200x, 400x) fields. Images were captured with an Olympus-IX81 light microscope connected to an Olympus DP70 digital image capture system, as previously described[38, 41].
Histology and immunohistochemistry techniques for small intestines were described previously[19, 42]. In brief, the small intestine was dissected from mice, and formalin-fixed and paraffin-embedded. 3 μm-thick sections were pre-treated with peroxidase blocking buffer, and then stained by hematoxylin and eosin, Alcian blue (pH 2.5, PolyScientific), anti-Ki67, or HES-1 antibody. For Ki-67 staining, sections were pretreated with Target Retrieval Solution (S1700, DAKO), incubated with rabbit anti-Ki67 (1:200, clone SP6, Neomarkers), followed by a biotinylated goat anti-rabbit secondary (7.5 μg/mL, Vector Laboratories), and stained with the Vectastain ABC Elite Kit (Vector Laboratories). For HES-1 staining, sections were pretreated with Target Retrieval Solution (S1700, DAKO), incubated with an anti-rat HES-1 antibody (1 μg/mL, clone NM1, MBL International), followed by a biotinylated rabbit anti-rat secondary (2.5 μg/mL) and TSA-HRP Kit (T20931, Invitrogen). All immunoperoxidase-stained sections were counterstained with Mayer's Haematoxylin. Quantification of nuclear Ki67 and HES-1 staining was based on the assessment of 300 jejunum crypt cells per animal.
Statistical analysis
All statistical comparisons were performed using Student's t-test (two-tail comparisons). Differences between conditions were considered significant only for p< 0.05.
Results
Effect of DAPT on EC in vitro
The effect of DAPT on endothelial cell proliferation, migration and sprout formation in vitro was first examined. In the absence of VEGF, DAPT had little effect on cell proliferation at any concentration tested (Fig. 1A) (only 2μM shown here, but similar for 0.2μM and 20μM). VEGF alone increased cell proliferation, as compared to blank controls, and adding a low concentration (0.2μM) of DAPT to VEGF exerted no effect on cell proliferation. However, increasing the concentration of DAPT (2μM or 20μM) in the presence of the same concentration of VEGF inhibited EC proliferation substantially (56% and 62% respectively), indicating that in 2-D cultures excessive Notch inhibition suppresses EC proliferation. To confirm that DAPT affected endothelial cells via the Notch signaling specifically, we activated Notch signaling of EC with a Notch ligand (Delta-like ligand 4, Dll4, specifically found on endothelium[30]) and examined if DAPT could reverse the effect of Notch activation. Initial cell adhesion was not affected by the presence of Dll4 (data not shown). However, cells adherent to surfaces with pre-adsorbed Dll4 showed a decreased proliferation, which was offset by the addition of DAPT (Fig 1B). This result was consistent with previous reports[30] and confirmed that DAPT influenced the Notch/Dll4 signaling pathway. The ability of DAPT to influence EC migration was next examined (Fig. 1C). Endothelial cells exhibited minimal migration when the chemoattractants (VEGF and HGF) were not present, but adding chemoattractants induced a 8-fold increase in the number of migrated cells. A low concentration of DAPT (0.25μM) exerted little effect on cell migration. Increasing the DAPT concentration to 2.5μM enhanced EC migration, yet an even higher concentration of DAPT (25μM) reduced this effect, indicating a biphasic response to Notch inhibition. Lastly, the effect of DAPT on the ability of endothelial cells to form sprouts in vitro (analogous to capillary formation), a process integrating cell proliferation, migration and differentiation, was examined (Fig. 1D). Again, DAPT had little effect when VEGF was not present, as sprouting was minimal in this condition. Adding 2.5μM DAPT to 10ng/mL VEGF containing media greatly increased the sprout number, as compared to using VEGF alone. However, a higher dose of DAPT (10μM) did not enhance sprouting over that obtained with VEGF alone.
Figure 1.
Effect of DAPT on endothelial cell phenotype in vitro. (A) Fractional change in cell number for cells cultured in the media supplemented with (+) or without (−) 10ng/mL VEGF, and without (−) or with 0.25μM(+), 2.5 μM (++), or 25μM (+++) DAPT. (B) Fractional change in cell number for cells exposed to surface-coated 1μg/mL Dll4 (− − + +), Dll4 with 10ng/mL VEGF (− + + +), or Dll4, VEGF and 0.25μMDAPT (− − − +). Cell number ratio was calculated based on the number of seeded cells. (C) Cell migration towards medium containing VEGF and HGF (each 50ng/mL) as influenced by 0.25μM (+), 2.5μM (++) or 25μM (+++) DAPT. (D) Sprout formation of endothelial cells, normalized to the total bead number. Mean values are presented with standard deviations (n=4–6). * represents P<0.05 as compared to other noted conditions. ** represents P<0.05 as compared to all other conditions. NS, not statistically significant.
It has been reported that Notch signaling can modulate VEGF signaling by regulating the expression level of VEGFR2 (KDR/Flk-1)[17], the key tyrosine kinase receptor responsible for multiple angiogenesis events. We next examined the effect of DAPT on EC VEGFR2. The total level of VEGFR2 with short time treatment (15min) remained constant for either VEGF or DAPT treatment (Fig. 2), as indicated by western blot analysis of cell lysates obtained using lysis buffer LyB (see Methods) that retrieved all the membrane-bound and intracellular proteins. However, analysis of cell lysates obtained with LyA lacking Tween 20 revealed a reduction in VEGFR2 with VEGF treatment. In contrast, DAPT treatment demonstrated increased VEGFR2 levels with the same lysis buffer, indicating DAPT reversed the reduction of VEGFR2 induced with the VEGF exposure (Fig 2).
Figure 2.
Effects of VEGF and DAPT exposure on VEGFR2 expression of endothelial cells. Cells were untreated (control), or treated with 10ng/mL VEGF, 2.5μM DAPT, or a combination of both (VEGF+DAPT) for 15min. Cell lysates extracted using either lysis buffer A (LyA), or lysis buffer B (LyB) buffer that extract all cellular components, were examined for the different treatment conditions and analyzed using western blotting. A control protein, vinculin was also shown for comparison.
Effect of VEGF and DAPT in vivo
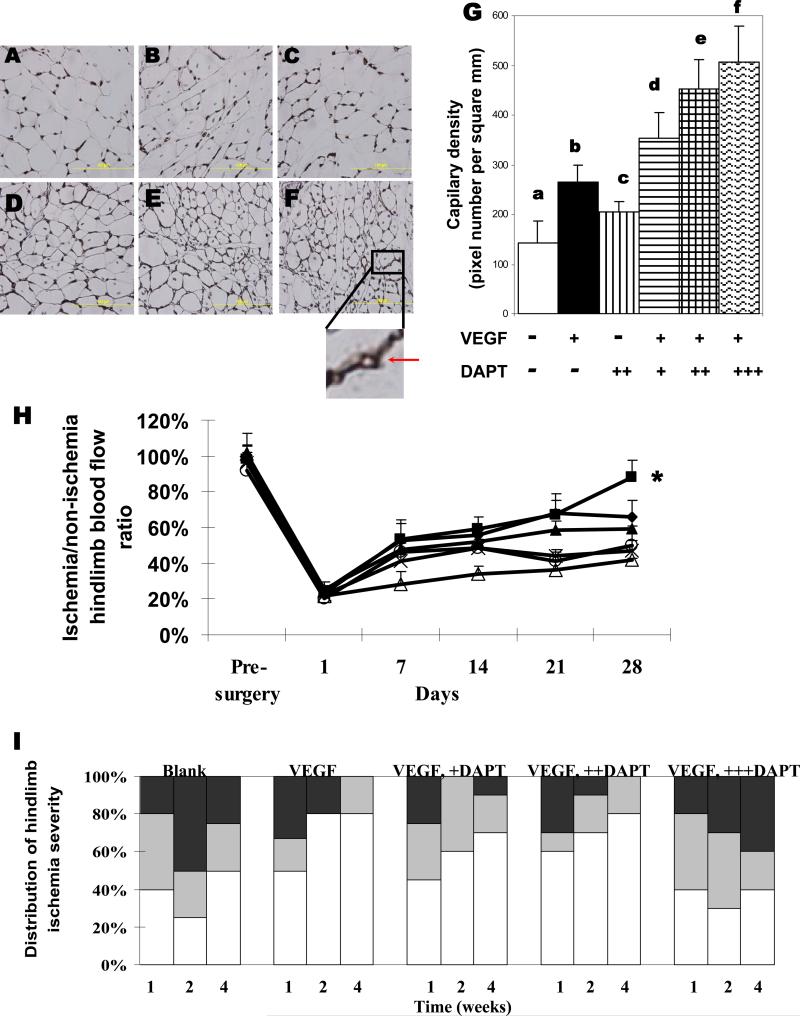
We then examined the effect of DAPT on the angiogenesis process in vivo. An injectable alginate hydrogel delivery system was previously developed to provide a sustained and localized delivery of VEGF, leading to improved blood perfusion recovery[38], and this system was used to examine the effect of combining VEGF and DAPT delivery in vivo. The in vitro release profile of incorporated DAPT from the alginate gel system was first examined (Fig. 3). The majority (ca. 90%) of incorporated DAPT was released in the first day and the remaining (ca. 10%) DAPT was slowly released over the next 3–4 days, in a manner largely independent of the total dose of DAPT. This rapid release was expected for a small molecule encapsulated in the gel, and was desired to prime cells for subsequent activation by VEGF. DAPT release was not influenced by the presence of VEGF in the gel (Fig. 3). VEGF release from alginate gels exhibited a smaller initial burst, and a more sustained release profile, which was not affected by the presence of DAPT. The ability of single and joint delivery of DAPT and VEGF to promote new blood vessel formation and relieve ischemia was then tested in murine hindlimb ischemia model. Examination of tissue sections indicated that sustained VEGF delivery increased the blood vessel density in the originally ischemic muscle tissue, as expected (Fig. 4A-4G). Delivery of DAPT alone from gels did not appear to significantly increase the vessel density. However, combining DAPT and VEGF increased the vessel density, in a manner dependent on the dose of DAPT (Fig 4A-4F). Quantification of blood vessel densities confirmed the qualitative observations (Fig 4G).
Figure 3.
In vitro factor release. DAPT release was analyzed from alginate hydrogels containing 86ng DAPT alone (diamond), 860ng DAPT alone (square), 8600ng DAPT alone (triangle), or 860ng DAPT and 3μg VEGF (horizontal line). VEGF release was monitored from gels containing 3μg VEGF alone (star). Mean values are presented with standard deviations (n=6).
Figure 4.
Analysis of angiogenesis in ischemic hindlimbs. Representative images (A-F) and quantities of capillaries in muscle tissues adjacent to the injection site, measured from tissues immunostained for CD31 (G). Tissues were retrieved from animals treated with either blank gels (A, a), VEGF (3μg) (B, b), 860ng DAPT(C, c), VEGF+86ng DAPT (D, d), VEGF+860ng DAPT (E, e), or VEGF+8600ng DAPT (F, f) delivered from alginate hydrogels. The insert shows the lumen structure of a positively stained capillary. Scale bar, 100μm. (H) Blood perfusion profile of hindlimbs treated with alginate hydrogels incorporating 860ng DAPT and 3μg VEGF (square), 86ng DAPT+VEGF (diamond),, VEGF alone (triangle), 860ng DAT alone (open circle), 8600ng DAPT +VEGF (star), or blank gels (open triangle). Mean values are presented with standard deviations (n=6). *, statistically significant difference (P<0.05), as compared to other conditions. (I) Quantification and distribution of hindlimb ischemia severity observed in different experimental groups over time. Tissue necrosis of hindlimbs subjected to surgery were visually examined, and grouped as normal (displaying limb integrity from non-ischemic hindlimbs of the same animal, white regions), or presenting one necrotic toe (gray), or multiple necrotic toes (black). Animals were treated with blank gels, 3μg VEGF, or combinations of VEGF with 86ng DAPT (+DAPT), 860ng DAPT (++DAPT), or 8600ng DAPT (+++DAPT).
The perfusion resulting from angiogenesis was subsequently tested, as vessel densities may not correlate with vascular function, as shown recently[19, 20]. Compared to the delivery of blank gels, sustained delivery of VEGF (3μg) led to a significant recovery of perfusion (Fig 4H), consistent with previous findings[38]. Combining delivery of DAPT (860ng) with VEGF (3μg) from the injectable alginate hydrogel system led to a significantly greater recovery of blood flow than the same dose of VEGF or DAPT alone; perfusion levels reached >80% of the normal level by week 4 (Fig 4H). In contrast, delivery of VEGF with a higher (8600ng) dose of DAPT decreased perfusion levels to below that of VEGF alone, in spite of the finding that this condition led to the highest capillary density. Because severe ischemia can lead to limb necrosis, the ability of VEGF and DAPT gel delivery to prevent or reverse necrosis was also analyzed (Fig 4I). Mice without any treatment (blank) exhibited a high initial level of toe necrosis, and minimal spontaneous recovery by week 4. In contrast, administering VEGF or a combination of VEGF and DAPT, decreased the severity of ischemia at week 1, and led to better recovery at later time-points (week 2 and week 4). The biphasic dose effect of DAPT, when combined with VEGF, shown in the perfusion analysis, was also observed in the necrosis measurements. The highest DAPT dose (8600ng) combined with VEGF exhibited a slightly higher level of toe necrosis than lower doses of DAPT (86ng and 860ng), or delivery of VEGF alone. DAPT delivery alone, however, failed to induce any improvement over time, although a lower initial level of necrosis, as compared to no treatment, was observed (data not shown). Altogether, these results suggest an optimal dose of DAPT can facilitate VEGF-induced angiogenesis and relieve ischemia, but excessive Notch inhibition may lead to non-functional angiogenesis as has been previously reported in other models[19, 20].
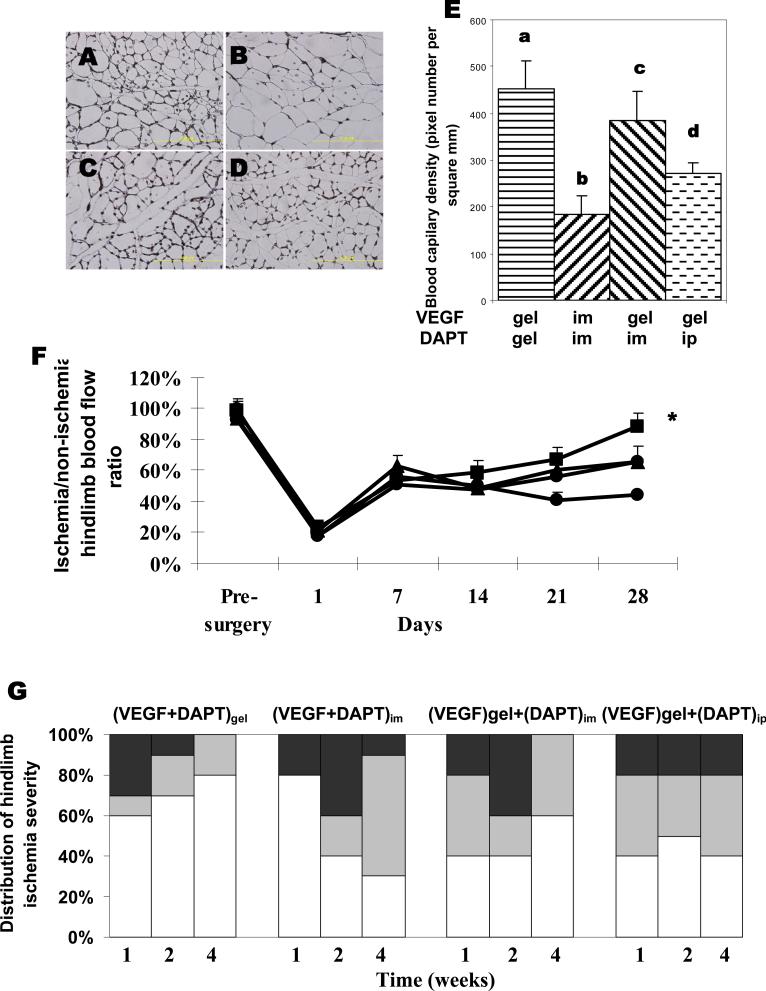
The importance of sustained and localized delivery of VEGF and DAPT was next probed by examining various combinations of bolus (intramuscular or intraperitoneal injection) and gel delivery. In contrast to the hydrogel delivery, bolus injection of VEGF and DAPT led to little increase in vessel densities, as compared to blank gel controls (Fig 5A-5E); vessel densities were much lower than those obtained with gel delivery of these factors. When VEGF was delivered from the hydrogel, simultaneous intramuscular or intraperitoneal injection of DAPT led to a small increase in the vessel densities, but neither condition resulted in the same level of increase as VEGF and DAPT delivery together from the gel system (Fig 5E). Not surprisingly, direct muscle injection of DAPT and VEGF, or gel delivery of VEGF combined with intramuscular or intraperitoneal injection of DAPT significantly reduced perfusion recovery (Fig. 5F). Tissue necrosis was also not relieved as effectively by IM or IP delivery of DAPT combined with VEGF gel delivery (Fig. 5G)
Figure 5.
Effects of the delivery method of DAPT on angiogenesis in vivo. Representative images (A-D) and quantities of capillaries in tissues adjacent to the injection site measured by CD31 immunostaining of tissue samples (E). Tissues were retrieved from animals treated with gel delivery of both VEGF and DAPT(A, a), intramuscular injection of VEGF and DAPT (B, b), or gel delivery of VEGF combined with intramuscular injection of DAPT (C, c), or combined with intraperitoneal injection of DAPT (D, d). The doses are 860ng for DAPT and 3μg for VEGF respectively. Scale bar, 100μm. (F) Blood perfusion profile of hindlimbs treated with gel delivery of VEGF and DAPT (diamond), gel delivery of VEGF and intramuscular delivery of DAPT (triangle), gel delivery of VEGF and intraperitoneal delivery of DAPT (square), or intramuscular delivery of DAPT and VEGF (circle). Mean values are presented with standard deviations (n=6). *, statistically significant difference (P<0.05), as compared to other conditions. (G) Quantification and distribution of hindlimb ischemia severity observed in different experimental groups over time. Tissue necrosis of hindlimbs subjected to surgery were visually examined, and grouped as normal (displaying limb integrity from non-ischemic hindlimbs of the same animal, white regions), or presenting one necrotic toe (gray), or multiple necrotic toes (black). Animals were treated with gel delivery of VEGF and DAPT ((VEGF+DAPT)gel), gel delivery of VEGF and intramuscular delivery of DAPT ((VEGF)gel+(DAPT)im), gel delivery of VEGF and intraperitoneal delivery of DAPT ((VEGF)gel+(DAPT)ip), or intramuscular delivery of DAPT and VEGF ((VEGF+DAPT)im).
Side effects of DAPT in vivo
A key concern with angiogenesis approaches that manipulate Notch signaling is side-effects at distant sites, due to the broad impact of Notch signaling in many tissues and organs. The influence of gel and intraperitoneal (IP) DAPT delivery on other tissues was probed by examining intestinal tissue, as a significant limitation of past approaches to the delivery of Notch inhibitors was their undesirable impact on the proliferation and differentiation of crypt cells in the small intestine[19, 42]. The morphology of the small intestine, as well as several molecular markers of phenotype were examined to determine how IP and gel DAPT delivery affected the crypt cells. Expression of HES-1 (hairy and enhancer of split 1), a member of basic helix-loop-helix family of transcription factors and a known Notch target gene in crypts[43] was first examined. IP delivery of DAPT significantly decreased HES-1 expression as compared to control tissues (Fig 6c). Approximately 80% of cells in control tissues, and tissues from animals with gel delivery of DAPT were HES-1 positive, but this was reduced to 50% for mice subjected to intraperitoneal injection of DAPT. Loss of Notch signaling can alter the proliferation rate of crypt cells, as shown by Ki-67 staining. IP delivery of DAPT led to a cellular proliferation rate (40%), which was markedly reduced as compared to control and gel delivery (70%) (Fig 6d-6f). In addition, Notch inhibition has been reported to alter the balance between proliferative crypt cells and goblet cells, resulting in more deposition of glycosaminoglycan molecules, as characterized by alcian blue staining. IP delivery of DAPT led to greater glycosaminoglycan deposition in intestinal tissues than control tissues or tissues from animals with gel delivery of DAPT (Fig 6g-6i), again indicating suppressed Notch signaling with IP delivery of DAPT. Lastly, IP DAPT delivery resulted in a significant alteration of the morphology of the small intestine as compared to controls, as demonstrated by hematoxylin and eosin staining (Fig 6j, 6l). Gel delivery of DAPT, however, did not lead to significant changes in gross tissue structure (Fig 6k). Altogether, these results suggested that localized DAPT delivery from the alginate gel delivery system did not lead to adverse systemic effects.
Figure 6.
Effect of DAPT delivery method on the structure and phenotype of small intestine. Representative images of tissue sections from small intestines isolated from mice without any treatment(control), mice treated with 860ng DAPT delivered from alginate gel ((DAPT)gel), or 860ng DAPT injected intraperitoneally ((DAPT)ip), which were stained against Notch gene HES-1 expression (A, B, C), Ki67 expression (D, E, F), alcian blue staining for glycosaminoglycans (G, H, I), or hematoxylin and eosin staining (J, K, L). Scale bar, 100μm.
Discussion
Our studies demonstrate that optimal Notch inhibition combined with VEGF can enhance functional angiogenesis, as indicated by accelerated recovery of tissue perfusion and reduction of necrosis in the murine hindlimb ischemia model, as compared to VEGF alone. Further, delivery of Notch inhibitors via the alginate system did not lead to significant side effects at distant organs. These findings are in sharp contrast to the previous tumor angiogenesis studies in which Notch inhibition, via bolus systemic injection of Notch inhibitors, led to excessive and dysfunctional vasculature[19, 20]. We believe the differences between the current and past studies relate to the local and optimal level of Notch inhibition accomplished with localized gel delivery in the current study. Our observation that an excessive amount of Notch inhibitors, even with gel delivery, led to increased capillary densities, but failed to enhance tissue perfusion, is consistent with past tumor angiogenesis studies[19, 20].
The in vitro studies demonstrated that angiogenic behavior induced by VEGF exposure could be enhanced by an optimal level of the Notch inhibitor DAPT, yet excessive DAPT inhibited EC proliferation, migration and sprout formation. The angiogenesis assay studied in the experiments, sprout formation in a 3-D fibrin-based artificial ECM, recapitulates the integrated cellular behavior of proliferation, migration and differentiation required to form capillaries[19, 44], and thus provides as a useful model to assess the effect of Notch inhibition. Our results suggest that the relative strength of VEGF to Notch inhibition may be important in determining endothelial cells' sprouting capability. The lack of an effect of Notch inhibition on EC proliferation, migration and sprout formation in the absence of VEGF confirms previous findings that Notch signaling acts downstream of VEGF signaling[21, 22]. Previous studies have also shown that Notch inhibition promoted endothelial cell proliferation and sprout formation[19, 22], and that activation of Notch signaling by the Notch ligand Dll4 inhibited endothelial cell proliferation and migration[24, 30]. In contrast, other studies have suggested that inhibiting Notch signaling decreases endothelial cell proliferation and has an inhibitory effect on migration[29, 45]. These apparently contradictory findings likely indicate that the exact role of Notch signaling in angiogenesis is highly dependent on the temporal and spatial presentation of Notch signaling molecules. The effect of DAPT may be partially explained by its effect on VEGFR2 localization. DAPT was found to upregulate VEGFR2 availability, and counter the effect of VEGF exposure, and this may be related to the ability of Notch signaling to provide feedback control of VEGF signaling. The biphasic relationship between DAPT concentration and endothelial cell response in vitro correlated with the influence of DAPT on the functionality of blood vessels in vivo.
A combination of an optimal level of DAPT and VEGF delivered from the gel led to increased blood vessel densities, accelerated recovery of blood flow, and reduced necrosis in a murine hindlimb ischemia model. While the blood vessel density increased monotonically with the DAPT dose, the vessel density did not directly correlate to the blood flow and reversal of tissue necrosis. An intermediate dose of DAPT together with VEGF generated an intermediate blood vessel density, but this resulted in the most accelerated perfusion recovery and the least necrosis. The dose of VEGF in the in vivo studies was based on previous studies with this gel system[38] and we postulate that varying the dose of VEGF will alter the optimal DAPT dose, as it is likely the relative strength of VEGF signaling to Notch signaling will be critical to the angiogenesis response. The results of this study indicated that the delivery method used for DAPT and VEGF is also crucial. Bolus delivery of DAPT and VEGF did not lead to as significant of blood flow recovery, or necrosis recovery. Bolus delivery of DAPT also led to side effects at distant organs while gel delivery of DAPT did not. In the future, fine-tuning Notch signaling to regulate angiogenesis may also be accomplished by varying the spatial and temporal presentation of exogenous Notch ligands. This process may require synergistic effects with other peri-vascular cells, such as vascular smooth muscle cells and pericytes, as Notch signaling has been found to be important in mediating the behavior of these cells as well[46, 47]. Altogether, these findings suggest the system and approach presented in this work could be useful in treating diseases that result in local tissue ischemia, such as coronary and peripheral ischemia.
Conclusion
Our studies demonstrate that optimal Notch inhibition combined with VEGF can enhance functional angiogenesis, as indicated by accelerated recovery of tissue perfusion and reduction of necrosis in the murine hindlimb ischemia model, as compared to VEGF or DAPT alone. Further, delivery of Notch inhibitors via the alginate system did not lead to significant side effects at distant organs.
Acknowledgement
The authors acknowledge the financial support from NIH (R01 HL069957), and the Biological Resources Branch of the National Cancer Institute for providing VEGF for our studies. Dmitry Shvartsman is acknowledged for his comments. LC is a recipient of the Juvenile Diabetes Research Foundation International Postdoctoral Fellowship.
Footnotes
Conflict of Interest Disclosures None.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Cao L, Mooney DJ. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv Drug Deliv Rev. 2007;59(13):1340–1350. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12(8):2093–2104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 4.Brey EM, Uriel S, Greisler HP, McIntire LV. Therapeutic neovascularization: contributions from bioengineering. Tissue Eng. 2005;11(3–4):567–584. doi: 10.1089/ten.2005.11.567. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 6.Isner JM. Therapeutic angiogenesis: a new frontier for vascular therapy. Vasc Med. 1996;1(1):79–87. doi: 10.1177/1358863X9600100114. [DOI] [PubMed] [Google Scholar]
- 7.Freedman SB, Isner JM. Therapeutic angiogenesis for ischemic cardiovascular disease. J Mol Cell Cardiol. 2001;33(3):379–393. doi: 10.1006/jmcc.2000.1329. [DOI] [PubMed] [Google Scholar]
- 8.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2(11):863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 9.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109(21):2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 10.Epstein SE, Fuchs S, Zhou YF, Baffour R, Kornowski R. Therapeutic interventions for enhancing collateral development by administration of growth factors: basic principles, early results and potential hazards. Cardiovasc Res. 2001;49(3):532–542. doi: 10.1016/s0008-6363(00)00217-0. [DOI] [PubMed] [Google Scholar]
- 11.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 12.Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, et al. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72(1):20–32. doi: 10.1067/mcp.2002.126179. [DOI] [PubMed] [Google Scholar]
- 13.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105(3):373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 14.Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, et al. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46(5):827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 16.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 17.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 18.Shawber CJ, Kitajewski J. Notch function in the vasculature: insights from zebrafish, mouse and man. BioEssays. 2004;26(3):225–234. doi: 10.1002/bies.20004. [DOI] [PubMed] [Google Scholar]
- 19.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444(7122):1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 20.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale N, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444(7122):1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 21.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: Implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23(1):14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, et al. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005;19(8):1027–1029. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- 23.Limbourg AP, M, Elligsen D, Sorensen I, Ziegelhoeffer T, Gossler A, Drexler H, et al. Notch ligand Delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007;100(3):363–371. doi: 10.1161/01.RES.0000258174.77370.2c. [DOI] [PubMed] [Google Scholar]
- 24.Noseda M, Chang L, McLean G, Grim JE, Clurman BE, Smith LL, et al. Notch activation induces endothelial cell cycle arrest and participates in contact inhibition: role of p21Cip1 repression. Mol Cell Biol. 2004;24(20):8813–8822. doi: 10.1128/MCB.24.20.8813-8822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445(7129):776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 27.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104(9):3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who Is talking to whom about what? Circ Res. 2007;100:1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 29.Takeshita K, Satoh M, Ii M, Silver M, Limbourg FP, Mukai Y, et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100(1):70–78. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107(3):931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, et al. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res. 2008;75(2):144–154. doi: 10.1016/j.mvr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Kwon SM, Eguchi M, Wada M, Iwami Y, Hozumi K, Iwaguro H, et al. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation. 2008 Jul 8;118(2):157–165. doi: 10.1161/CIRCULATIONAHA.107.754978. [DOI] [PubMed] [Google Scholar]
- 33.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104(9):3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 35.Uyttendaele H, Ho J, Rossant J, Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci U S A. 2001;98(10):5643–5648. doi: 10.1073/pnas.091584598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shawber CJ, Funahashi Y, Francisco E, Vorontchikhina M, Kitamura Y, Stowell S, et al. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J Clin Invest. 2007;117(11):3369–3382. doi: 10.1172/JCI24311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen RR, Snow JK, Palmer JP, Lin AS, Duvall CL, Guldberg RE, et al. Host immune competence and local ischemia affects the functionality of engineered vasculature. Microcirculation. 2007;14(2):77–88. doi: 10.1080/10739680601131101. [DOI] [PubMed] [Google Scholar]
- 38.Silva EA, Mooney DJ. Spatiotemporal control of VEGF delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5(3):590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen RR, Silva EA, Yuen WW, Brock AA, Fischbach C, Lin AS, et al. Integrated approach to designing growth factor delivery systems. FASEB J. 2007;21(14):3896–3903. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 40.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154(2):355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24(2):258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 42.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 43.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24(1):36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 44.Nakatsu MN, Sainson RCA, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 45.Chigurupati S, Arumugam TV, Son TG, Lathia JD, Jameel S, Mughal MR, et al. Involvement of notch signaling in wound healing. PLoS ONE. 2007;2(11):e1167. doi: 10.1371/journal.pone.0001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sainson RC, Harris AL. Regulation of angiogenesis by homotypic and heterotypic notch signalling in endothelial cells and pericytes: from basic research to potential therapies. Angiogenesis. 2008;11(1):41–51. doi: 10.1007/s10456-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 47.High FALM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2008;105(6):1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]