Abstract
Increases in adiposity trigger metabolic and inflammatory changes that interfere with insulin action in peripheral tissues, culminating in beta cell failure and overt diabetes. We found that the cAMP Response Element Binding protein (CREB) is activated in adipose under obese conditions, where it promotes insulin resistance by triggering expression of the transcriptional repressor ATF3 and thereby down-regulating expression of the adipokine hormone adiponectin as well as the insulin-sensitive glucose transporter 4 (GLUT4). Transgenic mice expressing a dominant negative CREB transgene in adipocytes displayed increased whole body insulin sensitivity in the contexts of diet-induced and genetic obesity; and they were protected from the development of hepatic steatosis and adipose tissue inflammation. These results indicate that adipocyte CREB provides an early signal in the progression to Type 2 diabetes.
Introduction
During fasting, mammals maintain energy balance by shifting from glucose to fat burning. Circulating catecholamines mobilize triglyceride stores from white adipose tissue (WAT) by increasing lipolysis (Joost et al., 1988; Joost et al., 1986; Laakso et al., 1992), and by reducing glucose uptake in fat through activation of the cAMP pathway (Joost et al., 1988; Joost et al., 1986; Laakso et al., 1992). Although they decrease membrane targeting of the GLUT4 transporter, catecholamines also down-regulate adipocyte GLUT4 gene expression in adipose in response to fasting and Type 2 diabetes (Berger et al., 1989; Garvey et al., 1989; Kahn et al., 1989; Sivitz et al., 1989).
Superimposed on these metabolic effects, catecholamines also inhibit the expression and secretion of certain adipocytokine hormones, most notably adiponectin, a key modulator of systemic insulin sensitivity (Delporte et al., 2002; Fu et al., 2007; Ricci et al., 2005; Scriba et al., 2000). Depletion of adiponectin in adipose reduces insulin sensitivity (Kubota et al., 2002; Maeda et al., 2002; Nawrocki et al., 2006), while over-expression of adiponectin enhances it, in part through effects on hepatic glucose production (Berg et al., 2001; Combs et al., 2001; Kim et al., 2007; Satoh et al., 2005; Yamauchi et al., 2002b).
The CREB family of cAMP responsive transcription factors (CREB1, ATF1, CREM) has been found to mediate effects of catecholamines and other fasting hormones on cellular gene expression (Herzig et al., 2001; Zhang et al., 2005a). Following its phosphorylation at Ser133, CREB stimulates transcription through recruitment of the histone acetylase CBP and through an association with the CREB Regulated Transcriptional Coactivator (CRTC; also known as TORC2) family of latent cytoplasmic coactivators (Chrivia et al., 1993; Ravnskjaer et al., 2007; Xu et al., 2007). CREB promotes glucose homeostasis during fasting, for example, by triggering expression of the gluconeogenic program in liver (Herzig et al., 2001). Hepatic CREB activity is constitutively up-regulated in diabetes, where it contributes to hyperglycemia and insulin resistance (Dentin et al., 2008; Dentin et al., 2007). The extent to which adiponectin or other adipose-derived hormones modulate hepatic gluconeogenesis via CREB remains unclear, however.
In addition to its function in liver, CREB also appears to promote expression of the adipogenic program in pre-adipocytes (Zhang et al., 2004), although its role in mediating effects of cAMP in mature adipocytes has not been addressed. We found that adipocyte CREB is activated in obesity, when it disrupts insulin action and promotes systemic insulin resistance. Blocking CREB activity in adipocytes prevented the development of inflammatory infiltrates in adipose as well as systemic insulin resistance under obese conditions. These results point to a central role for adipose tissue in coordinating systemic insulin action and in modulating energy balance in part through obesity-related increases in CREB activity.
RESULTS
Insulin Sensitivity in Obese Mice with Reduced Adipocyte CREB Activity
CREB family members (CREB1, CREM, ATF1) exhibit considerable functional redundancy (Mayr and Montminy, 2001; Shaywitz and Greenberg, 1999); and mice with individual knockouts show only limited phenotypes (Bleckmann et al., 2002; Blendy et al., 1996; Hummler et al., 1994). To block the activity of all CREB family members in mature adipocytes, we generated transgenic mice that express the dominant negative CREB inhibitor ACREB, a synthetic polypeptide that heterodimerizes with and disrupts binding of CREB1, CREM, and ATF1, but not unrelated bZIP proteins to DNA (Ahn et al., 1998). Because CREB has been found to promote adipogenesis (Tseng et al., 2005; Zhang et al., 2004), we targeted ACREB expression specifically to mature adipocytes using the adipose-specific aP2 promoter.
ACREB mRNA was detected in adipocytes but not in peritoneal macrophages or in adipose-derived stromal vascular cells from two independent FAT-ACREB (F-ACREB) founder lines established on different genetic backgrounds (CB6 and C57/Bl6; Figures 1A, S1). Expression of ACREB, either acutely by adenoviral delivery or chronically in cells from F-ACREB transgenic mice, reduced mRNA amounts for the CREB target gene NR4A2 in primary adipocyte cultures exposed to the cAMP activator forskolin (FSK; Figure 1B). F-ACREB mice grow normally and are indistinguishable from control littermates under normal chow conditions (Figure S2).
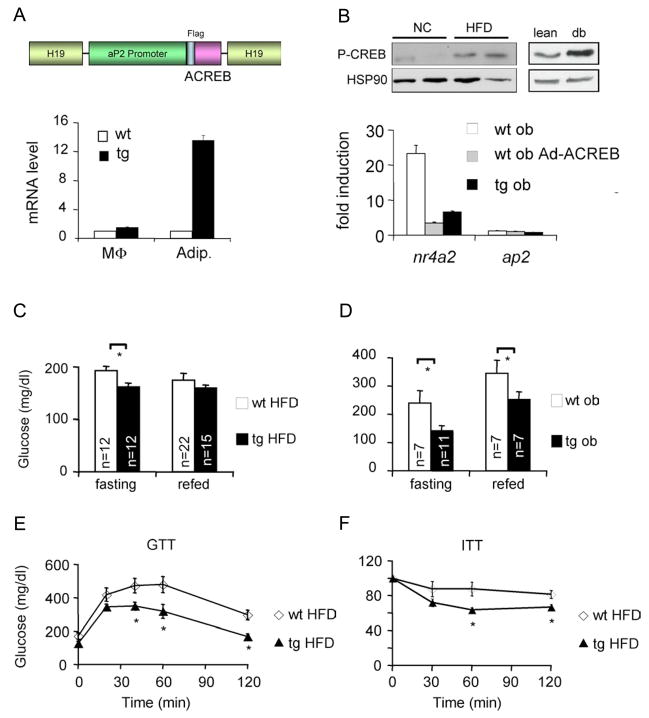
Figure 1.
Mice deficient in adipocyte CREB activity remain insulin sensitive under obese conditions. A. Top, schematic of dominant negative ACREB transgene expressed from the fat specific aP2 promoter in FAT-ACREB (F-ACREB) C57/Bl6 mice. Bottom, Q-PCR analysis of ACREB mRNA amounts in peritoneal macrophages (MΦ) and adipocytes from wild-type (wt) or transgenic (tg) F-ACREB littermates. B. Top, immunoblot showing relative amounts of phospho (Ser133) CREB (P-CREB) in adipose from mice maintained on normal chow (NC) or high fat diet (HFD). Right, relative P-CREB amounts in adipose from lean or genetically obese (db) mice. Bottom, effect of forskolin (FSK) exposure (4 hours) on CREB target gene expression (NR4A2) in cultured adipocytes expressing ACREB, through adenoviral infection (ob Ad-ACREB) or chronically, in cells from F ACREB transgenic (tg ob) mice. C. and D. Relative circulating blood glucose concentrations in control (wt) and F-ACREB (tg) mice under HFD (C) and genetically obese conditions (ob/ob) (D; wt ob and tg ob). Glucose levels were evaluated in overnight fasted and 2 hour refed mice. E. and F. Glucose (E) and insulin (F) tolerance testing of F-ACREB mice relative to wild-type littermates maintained under HFD conditions for 9.5 weeks. Unless stated otherwise, mice were maintained on HFD for 8–12 weeks, while ob/ob mice were analyzed at 12–16 weeks of age. (*; P<0.05). Data are means ± s.e.m.
In the insulin resistant state, chronic elevations in circulating insulin paradoxically potentiate catecholamine effects on cAMP signaling in adipocytes (Hupfeld et al., 2003; Zhang et al., 2005a). Indeed, amounts of Ser133 phosphorylated, active CREB were increased following high fat diet (HFD) feeding and in genetically obese (db/db) mice relative to lean controls (Figure 1B). To determine the consequence of CREB activation in this setting, we performed metabolic studies on F-ACREB mice following HFD feeding or after breeding them onto a genetically obese (ob/ob) background. Compared to controls, F-ACREB mice had lower circulating blood glucose concentrations, despite similar food intake, body temperature, physical activity, weight gain, and fat mass in each group (Figures 1C,D; S3). Whole-body insulin sensitivity was also improved in F-ACREB animals after 9.5 weeks after HFD feeding, as revealed by glucose (GTT) and insulin (ITT) tolerance testing (Figure 1E,F).
Systemic Insulin Sensitivity in F-ACREB Mice
To determine the relative effects of adipose-specific ACREB expression on insulin resistance in muscle and liver, we performed euglycemic-hyperinsulinemic clamp studies. After 24 weeks of HFD feeding, basal glucose infusion rates (GIR) during the clamp studies were elevated 5-fold in F-ACREB mice relative to controls; and insulin-stimulated glucose disposal rates (IS-GDR), measures of muscle insulin sensitivity, were increased 10-fold (Figure 2A–C). Adipose-specific expression of ACREB caused similar changes in liver, where insulin infusion during the clamp study inhibited hepatic glucose production (HGP) 3-fold greater in transgenic compared to wild-type littermates (Figure 2D). Consistent with an increase in insulin signaling, amounts of phosphorylated, active AKT were also up-regulated in both skeletal muscle and liver from F-ACREB animals following insulin injection (Figures 2E,F; S4).
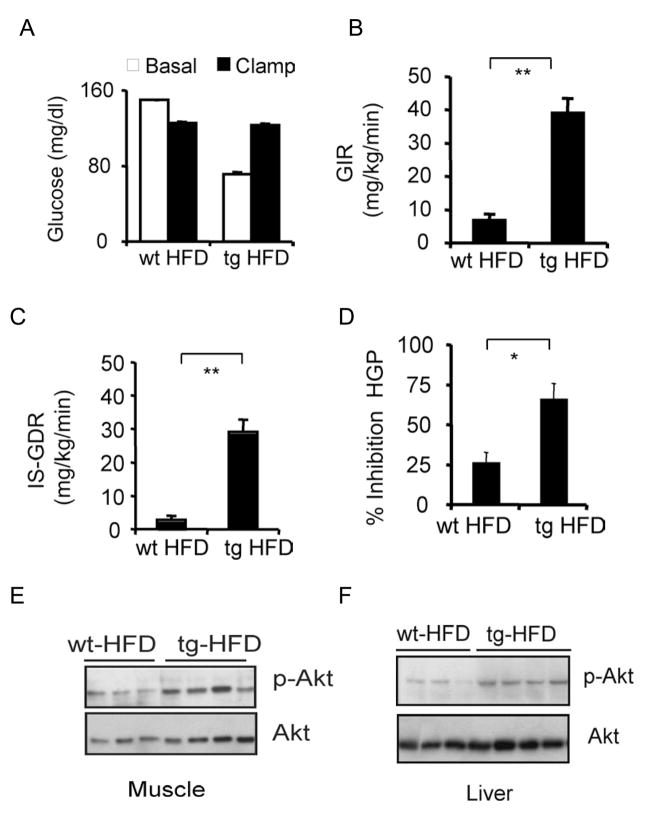
Figure 2.
Adipose-specific disruption of CREB activity enhances insulin sensitivity in muscle and liver. Hyperinsulinemic-euglycemic clamp studies of wild-type (n=8) and F-ACREB (n=9) mice that were maintained on a HFD for 24 weeks. A. Blood glucose concentrations in high fat diet (HFD) fed F-ACREB (tg) and wild-type (wt) littermates prior to (basal) or after euglycemic clamping (clamp). B. and C. Basal (GIR; panel B) and insulin-stimulated (IS-GDR; panel C) glucose infusion rates in F-ACREB and control mice under HFD conditions. (**; P<0.00001) D. Relative inhibition of hepatic glucose production (HGP) by insulin in F-ACREB and control mice. (*; P<0.003) E. and F. Immunoblots of phospho-AKT and total AKT protein amounts in skeletal muscle (panel E) and liver (panel F) lysates from F-ACREB and control littermates following insulin injection. Data are means ± s.e.m.
Despite the elevations in circulating glucose levels in the control group, plasma insulin concentrations were comparable between transgenic and control littermates (Figure S5), suggesting an increased efficiency of glucose-stimulated insulin release in F-ACREB animals. To test this idea, we monitored circulating insulin concentrations under hyperglycemic clamp conditions. In line with their relative insulin sensitivity, glucose infusion rates were increased by 75% in HFD-fed transgenic mice compared to controls (Figure S6). Pointing to an overall improvement in pancreatic islet function, circulating insulin levels rose substantially in glucose-stimulated F-ACREB mice but not in control animals. Taken together, these results indicate that the adipose-specific disruption of CREB activity prevents the development of beta cell failure in the context of obesity.
White adipose stores from obese individuals are often characterized by immune cell infiltrates that further aggravate insulin resistance through the release of inflammatory chemokines (Weisberg et al., 2003; Xu et al., 2003). Although they were abundant in fat pads from HFD-fed controls, immune cells were relatively absent in WAT from F-ACREB mice (Figure 3A). As well, inflammatory pathway gene expression was substantially reduced in WAT from HFD-fed F-ACREB mice compared to wild-type (Figure S7). Consistent with these improvements, insulin-stimulated glucose uptake was increased in adipocytes from HFD-fed F-ACREB mice (Figure 3B). As well mRNA and protein amounts for the insulin-sensitive GLUT4 transporter were also elevated.
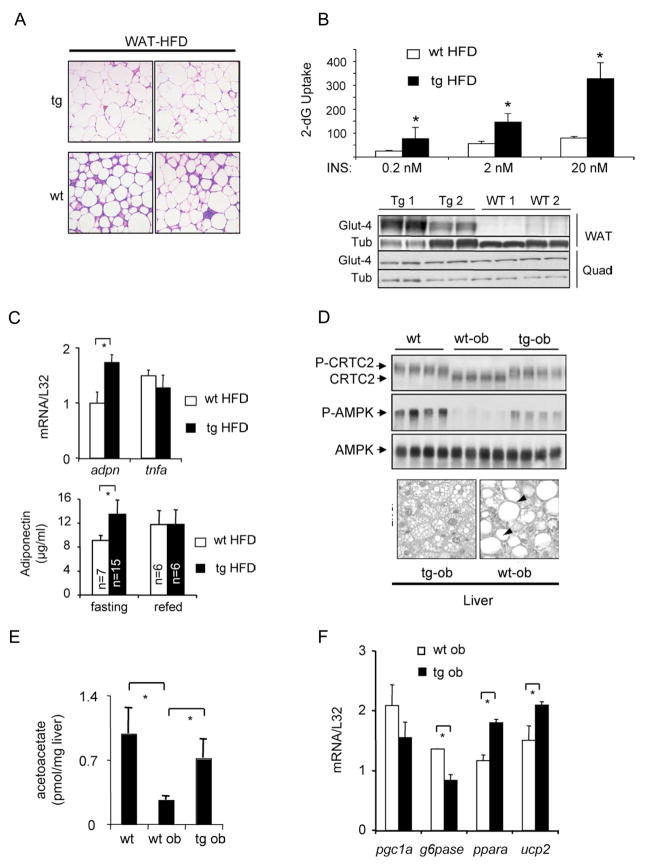
Figure 3.
Reduced adipose tissue inflammation and hepatic steatosis in F-ACREB mice. A. Histological analysis of hematoxylin-eosin (H&E) stained WAT sections from HFD-fed F-ACREB (tg) and control (wt) littermates showing relative accumulation of inflammatory cell infiltrates. B. Top, insulin-stimulated glucose uptake in primary cultured adipocytes from F-ACREB transgenic (tg HFD) and control (wt HFD) littermates under HFD conditions. Relative uptake of 3H-2 deoxyglucose (4μCi/ml; expressed as cpm/106 cells) into cultured adipocytes from HFD-fed mice exposed to various concentrations of insulin for 20 minutes followed by incubation with 3H-2 deoxyglucose for 5 minutes. ( *; P<0.002 relative to wild-type HFD fed mice; data are means ± s.e.m.) Bottom, immunoblot of GLUT4 protein amounts in adipose and quadriceps muscle from F-ACREB (Tg1, Tg2) and wild-type (WT1, WT2) littermates maintained under HFD conditions. C. Top, Q-PCR analysis of adiponectin mRNA amounts in adipose tissue from F-ACREB (tg HFD) and wild-type (wt HFD) littermates maintained under high fat diet conditions (n=3 per group; *, P<0.05). Bottom, circulating plasma adiponectin concentrations in fasted or 2 hour refed F-ACREB and control mice. D. Top, immunoblot showing phospho-AMPK amounts in liver lysates from wild-type (wt), ob/ob (wt-ob) and F-ACREB transgenic (tg-ob) mice. Amounts of unphospho- and phospho-CRTC2 also indicated. Bottom, hepatic sections from F-ACREB transgenic ob/ob and control ob/ob mice showing relative accumulation of lipid droplets. E. Acetoacetate content in livers of F-ACREB ob/ob and control ob/ob mice relative to lean wild-type animals (n=4). ( *; P<0.05 transgenic relative to control mice; n=4; data are means ± s.e.m.). F. Q-PCR analysis of beta oxidation (PGC1α, PPARα, UCP2) and gluconeogenic (PEPCK, G6Pase) gene expression in livers from ob/ob F-ACREB transgenic mice relative to ob/ob controls.
Enhanced Adiponectin Expression in F-ACREB Mice
We considered that ACREB expression in adipocytes may improve systemic insulin sensitivity in F-ACREB mice by altering the profile of circulating adipo-cytokine hormones. Although plasma concentrations of resistin, RBP4, TNFα, and IL-1β were similar in both groups (Figure S8), circulating levels of high molecular weight, active adiponectin protein were elevated in F-ACREB transgenic compared to wild type controls; adiponectin mRNA amounts in F-ACREB WAT were also increased (Figures 3C, S9). Adiponectin has been found to enhance insulin signaling in liver and other tissues through induction of the Ser/Thr kinase AMPK (Nawrocki et al., 2006; Ruderman et al., 2003; Yamauchi et al., 2002a). Supporting this notion, amounts of phosphorylated, active AMPK were up-regulated in livers from F-ACREB mice compared to controls (Figure 3D, top).
AMPK is thought to improve hepatic insulin sensitivity in part by preventing the abnormal accumulation of triglycerides, a condition known as hepatic steatosis. Indeed, large lipid droplets were evident in livers from ob/ob controls, but they were relatively scarce in F-ACREB ob/ob mice (Figure 3D, bottom). Consistent with these changes, hepatic ketone levels and fatty acid oxidation gene expression were elevated, while lipogenic gene expression was decreased in F-ACREB ob/ob animals compared to controls (Figures 3E, F; S10).
Superimposed on its role in hepatic lipid metabolism, AMPK also reduces glucose production by the liver in part through phosphorylation of the CREB coactivator CRTC2 (Foretz et al., 1998; Lochhead et al., 2000) (Koo et al., 2005; Shaw et al., 2005). Indeed, amounts of phosphorylated, inactive CRTC2 were increased in livers from F-ACREB mice, while mRNA amounts for gluconeogenic genes were reduced (Figures 3D, F; S10).
Adiponectin Inhibits CRTC2 Activity
Having seen that hepatic CRTC2 activity is curtailed in F-ACREB animals, we wondered whether adiponectin directly modulates the gluconeogenic program via AMPK-mediated phosphorylation and inactivation of CRTC2. Exposure of cultured primary hepatocytes to FSK triggered CRTC2 dephosphorylation, leading to increases in cAMP response element (CRE)-luciferase reporter activation, gluconeogenic gene expression, and glucose production (Figure 4A–D). Demonstrating the importance of CRTC2 dephosphorylation, CRE-luciferase activity and glucose output were constitutively elevated in cells expressing phosphorylation-defective, active S171A CRTC2.
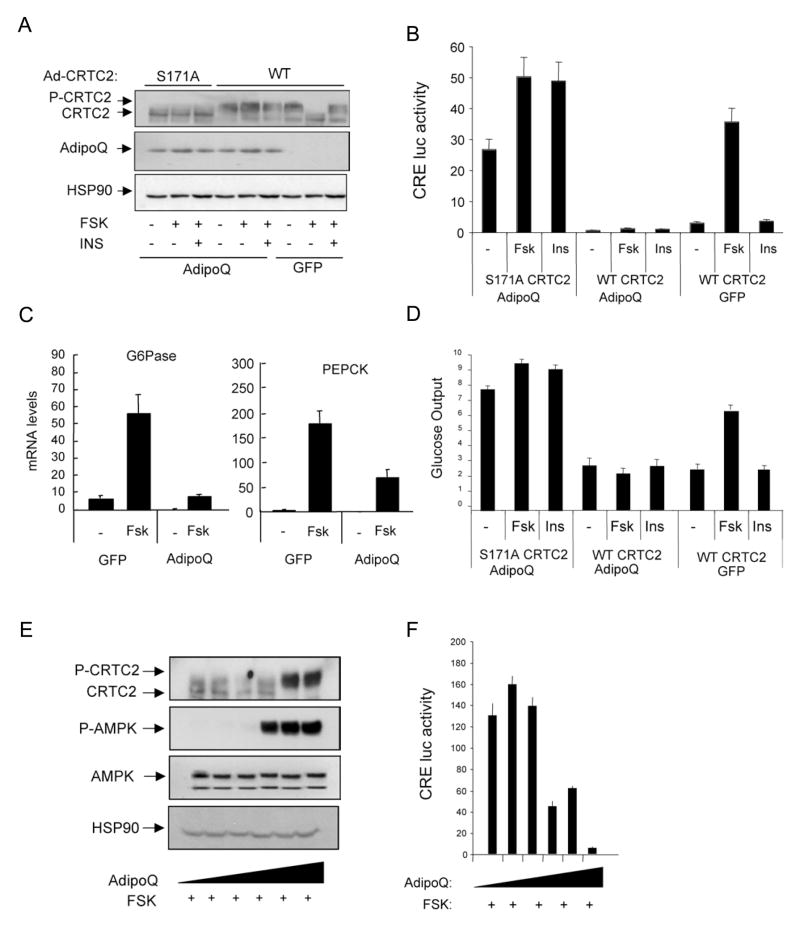
Figure 4.
Adiponectin modulates hepatic gluconeogenesis via AMPK-mediated phosphorylation of CRTC2. A–D. Effect of adenoviral adiponectin (AdipoQ) or green fluorescent protein (GFP) expression on amounts of phospho-CRTC2 (A), CRE-luciferase reporter activity (B), gluconeogenic gene expression (C), and glucose secretion (D) from transduced primary hepatocytes exposed to FSK (10uM) and/or insulin (100nM). Co-infection with adenoviruses encoding wild-type (WT) or phosphorylation-defective (S171A) CRTC2 indicated. Data are means ± SEM; n=3 per group. E–F. Phospho-CRTC2 amounts (E) and CRE-luciferase reporter activity (F) in primary hepatocytes exposed to increasing concentrations of adiponectin protein (2–15μg/ml). Ad-CRE luc activity was measured in cells treated with FSK (10μM) plus resistin (15μg/ml) for 5 hours. Representative of 3 independent experiments shown. Data are means ± SEM; n=3.
Based on the ability for adiponectin (Ad-adiponectin) to lower glucose production following adenoviral delivery into liver (Satoh et al., 2004; Satoh et al., 2005), we examined effects of Ad-adiponectin expression in primary hepatocytes on CRTC2 activity. Indeed, Ad-adiponectin blocked the dephosphorylation of CRTC2 and correspondingly lowered CRE-luciferase reporter activation in cells exposed to FSK (Figure 4A–D). As a result, adiponectin also down-regulated gluconeogenic gene expression (G6Pase, PEPCK) and glucose production. But Ad-adiponectin did not reduce CRE-luciferase activity or glucose output in hepatocytes expressing the phosphorylation-defective S171A CRTC2, confirming the importance of Ser171 phosphorylation for these effects. Indeed, exposure of primary mouse hepatocytes to physiologic concentrations of adiponectin (2–15μg/ml) (Kadowaki et al., 2006) also blocked CRTC2 dephosphorylation and CRE-luciferase reporter activation in cells exposed to FSK (Figure 4E, F). Taken together, these results support the notion that adiponectin reduces hepatic gluconeogenesis via the AMPK-mediated phosphorylation of CRTC2 at Ser171.
CREB Stimulates ATF3 Expression in Obesity
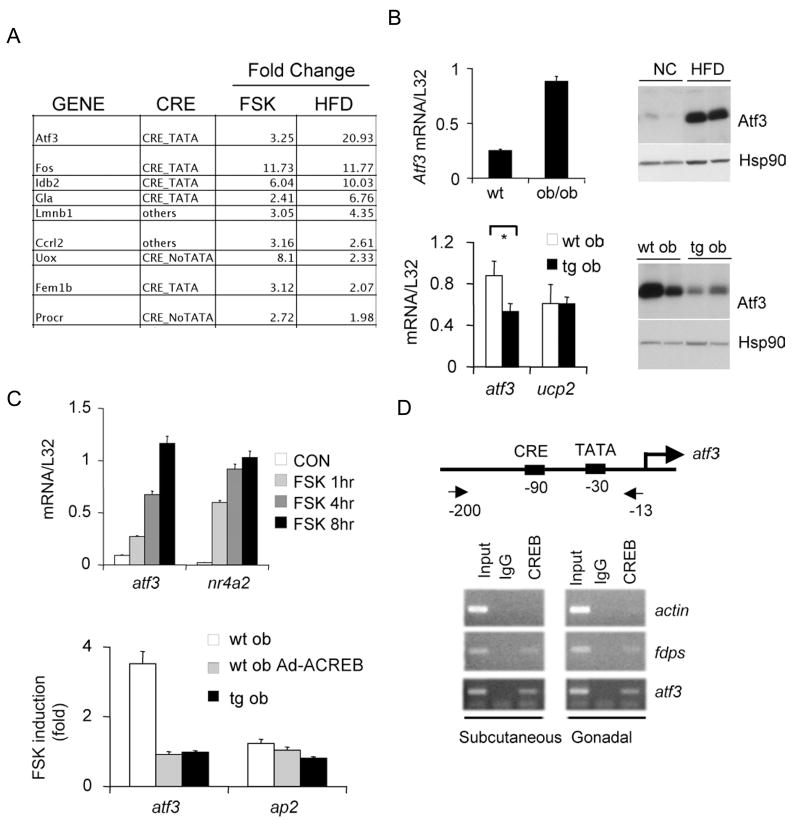
Having seen that GLUT4 and adiponectin gene expression in WAT is relatively higher in obese F-ACREB mice over controls, we considered that CREB could inhibit these genes directly or through up-regulation of a transcriptional repressor. Arguing against a direct effect, CREB binding over the adiponectin and GLUT4 promoters was not detectable by chromatin immunoprecipitation assay (Zhang et al., 2005b) (not shown). In gene profiling assays to characterize putative CREB target genes that are up-regulated 2-fold or better in primary adipocytes exposed to FSK and in WAT from HFD-fed compared to NC-fed mice, we identified the transcriptional repressor ATF3 (Chen et al., 1994) as the top scoring gene (Figure 5A). ATF3 mRNA and protein amounts were increased nearly 10-fold in WAT from HFD-fed and genetically obese ob/ob mice relative to lean controls (Figure 5B, top). Conversely, ATF3 protein and mRNA amounts were decreased in WAT from F-ACREB mice compared to wild-type littermates (Figure 5B, bottom).
Figure 5.
CREB stimulates expression of the transcriptional repressor ATF3 in adipose under obese conditions. A. Results from gene profiling studies showing genes induced 2-fold or greater in primary adipocytes following exposure to FSK and in WAT harvested from high fat diet (HFD) relative to normal chow fed mice. Presence of conserved CREB binding site (CRE) and TATA box indicated. B. Top left, relative ATF3 mRNA amounts in WAT from lean and ob/ob mice. Top right, effect of normal chow (NC) and high fat diet feeding (HFD) on ATF3 protein amounts in white adipose from wild-type mice. Bottom, relative ATF3 mRNA (left) and protein (right) amounts in WAT from F-ACREB ob/ob mice compared to control ob/ob animals. C. Q-PCR analysis of ATF3 mRNA in HEK293T cells (top) and in cultured primary adipocytes of ob/ob mice (bottom) following exposure to forskolin. Effect of ACREB expression, either acutely through adenoviral infection (ob Ad-ACREB), or chronically in cells from ACREB transgenic mice (tg ob) indicated. D. Chromatin Immunoprecipitation (ChIP) assay of subcutaneous or gonadal WAT showing CREB occupancy over the ATF3 promoter in vivo. CREB binding to positive control (FDPS) and negative control (actin) promoters shown for comparison. Relative recovery of ATF3 promoter from immunoprecipitates of CREB or non-specific IgG also indicated. Position of CREB binding site and TATA box relative to transcription start site on the ATF3 promoter shown.
Realizing that the ATF3 promoter contains a conserved CRE in the proximal promoter (Liang et al., 1996), we tested the role of CREB in modulating this gene directly in adipocytes. Exposure of cultured primary adipocytes or HEK293T cells to FSK increased ATF3 mRNA amounts; these effects were blocked following expression of adenovirally encoded ACREB (Figure 5C). ATF3 mRNA amounts- elevated in primary adipocytes from ob/ob mice- were down-regulated in cells from F-ACREB ob/ob littermates. Pointing to a direct role for CREB in this process, we recovered the ATF3 promoter from immunoprecipitates of CREB prepared from gonadal as well as subcutanenous adipose tissue by ChIP analysis (Figure 5D).
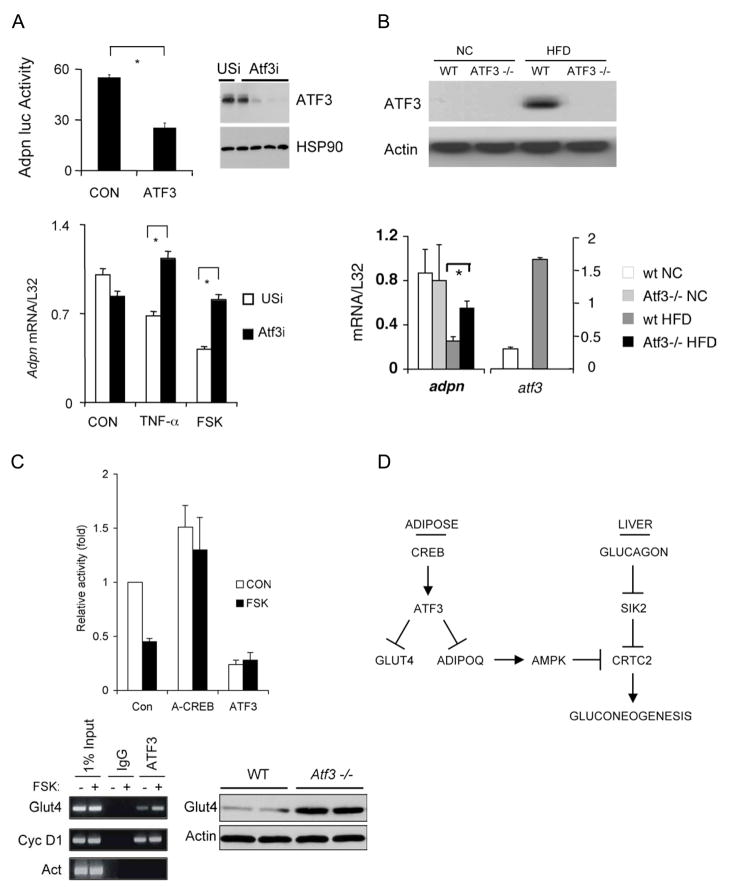
We examined whether CREB inhibits adiponectin gene expression in adipocytes through the induction of ATF3. Consistent with this idea, ATF3 over-expression disrupted adiponectin promoter activity in transient transfection assays with an adiponectin luciferase reporter (Figure 6A, top) (Kim et al., 2006). Exposure of primary adipocytes to FSK also reduced adiponectin mRNA amounts; these effects were suppressed when cells were depleted of ATF3 using adenovirally encoded ATF3 RNAi (Figure 6A, bottom). We also employed ATF3−/− mice to determine whether ATF3 mediates the HFD-associated decrease in adiponectin gene expression. In line with the up-regulation of ATF3 mRNA and protein, HFD feeding reduced adiponectin gene expression in WAT from wild-type littermates (Figure 6B). But adiponectin mRNA amounts remained high in HFD fed ATF3−/− mice, demonstrating the importance of ATF3 in this setting.
Figure 6.
ATF3 mediates inhibitory effects of CREB on GLUT4 and adiponectin gene expression in adipose under obese conditions. A. Top left, transient assay showing adiponectin-luciferase reporter activity in control and ATF3 over-expressing HEK293T cells. Top right, immunoblot showing effect of adenovirally encoded ATF3 RNAi or control (USi) RNAi on amounts of ATF3 protein in primary adipocytes. Bottom, Q-PCR analysis of adiponectin mRNA amounts in primary adipocytes infected with adenovirally encoded unspecific (USi) or ATF3 RNAi. Cells exposed to TNFα or FSK for 24 hours indicated. B. Top, immunoblot of ATF3 protein amounts in WAT from wild-type and Atf3−/− mice under normal chow (NC) and high fat diet (HFD) feeding conditions. Bottom, Q-PCR analysis of adiponectin and ATF3 mRNA amounts in WAT from Atf3−/− and control littermates maintained on NC or HFD. C. Top, transient assay of HEK293T cells showing effect of A-CREB or ATF3 over-expression on GLUT4-luciferase reporter activity in HEK293T cells under basal conditions and following exposure to FSK for 4 hours. Bottom left, ChIP assay showing recovery of GLUT4 promoter from immunoprecipitates of ATF3 or non-specific IgG prepared from HEK293T cells following exposure to FSK as indicated. Recovery of positive control (cyclin D1) or negative control (Actin) promoters in immunoprecipitates of ATF3 included for comparison. Bottom right, GLUT4 protein amounts in white adipose from Atf3−/− and wild-type littermates. D. Adipocyte CREB promotes insulin resistance in obesity. Under lean conditions, increases in circulating adiponectin reduce hepatic glucose output during fasting by triggering the AMPK-mediated phosphorylation of the CREB coactivator CRTC2 in hepatocytes. Adipocyte CREB is activated in obesity, where it promotes insulin resistance via the ATF3-mediated inhibition of adiponectin and GLUT4 gene expression.
In addition to its effects on adiponectin, exposure to FSK also inhibited GLUT4 promoter activity; these effects were reversed in cells expressing dominant negative ACREB protein (Figure 6C). In keeping with the presence of ATF3 binding sites at –508 and –555 on this promoter, exposure to FSK increased ATF3 occupancy over the GLUT4 gene. Correspondingly, ATF3 over-expression reduced GLUT4-luciferase reporter activity, while disruption of the ATF3 gene increased GLUT4 mRNA and protein amounts in WAT from ATF3−/− mice (Figures 6C, S11). Taken together, these results support the notion that the CREB-mediated induction of ATF3 in obesity promotes insulin resistance through the subsequent down-regulation of adiponectin and GLUT4 expression in adipose (Figure 6D).
Discussion
Obesity triggers a number of perturbations that alter adipose tissue homeostasis (de Luca and Olefsky, 2008; Lumeng et al., 2007). Increases in adipose tissue mass promote local areas of micro-hypoxia that activate stress and inflammatory pathways within the adipocytes and surrounding stromal vascular cells. The subsequent release of chemokines by adipocytes is thought to cause a large and sustained influx of circulating monocytes that become adipose tissue macrophages (ATMs). Because they also release a variety of cytokines, ATMs further exacerbate the chronic inflammatory state in adipose, by promoting lipolysis as well as insulin resistance in adipocytes (Xu et al., 2003). This insulin resistant state in adipose can then be communicated to the periphery through increases in circulating FFAs and through changes in the repertoire of secreted adipokines, culminating in systemic insulin resistance in both liver and muscle.
We found that the activation of CREB in adipose represents an important step in the development of insulin resistance in obesity. When CREB activity is inhibited, adipose tissue and systemic insulin sensitivity are relatively preserved in obese mice. Because the ACREB transgene is selectively expressed in adipocytes, the systemic insulin sensitive phenotype must originate within adipose itself. Despite these improvements in insulin sensitivity, F-ACREB animals gain weight comparably to controls in the context of high fat diet feeding or in ob/ob mice, indicating that obesity can be uncoupled from insulin resistance.
In parallel with the increases in adipose tissue insulin sensitivity and glucose transport, pancreatic islet function is also relatively preserved in F-ACREB mice. Although the underlying mechanism is unclear, we imagine that the associated improvements in glucose and lipid homeostasis may protect against deterioration of beta cell function often associated with obesity. Indeed, the systemic changes in F-ACREB mice are reminiscent of those observed in fatty acid binding protein (FABP4, FABP5) knockout mice, which remain insulin sensitive under obese conditions (Cao et al., 2008). Despite comparable circulating concentrations of free fatty acids, FABP4−/−; FABP5−/− knockout mice were protected from hepatic steatosis due to increases in circulating concentrations of the “lipokine” hormone palmitolineate. Further studies should reveal whether the composition of circulating free fatty acids is similarly altered in F-ACREB animals.
Adipose tissue macrophage content and inflammatory gene expression were also reduced in F-ACREB mice, and these animals were protected from the subsequent development of insulin resistance in the other major insulin target tissues, muscle and liver. The secretion of tumor necrosis factor (TNF) by adipose tissue macrophages has been shown to increase adipocyte insulin resistance through the activation of the Ser/Thr kinase JNK1, which in turn phosphorylates the insulin receptor substrate 1 (IRS1) on serine (Sabio et al., 2008). These localized changes in adipocyte insulin resistance are transmitted to the liver through the release of interleukin 6 (IL6) from adipocytes, leading to hepatic up-regulation of SOCS3, an inhibitor of insulin receptor signaling. Although we did not observe changes in circulating concentrations of IL6 in F-ACREB relative to control mice, it is interesting to note that the IL6 promoter contains a number of CREB binding sites that can potentially mediate induction of this gene in response to cAMP signals (Zhang et al., 2005b).
CREB was found to compromise adipocyte function in part by stimulating the expression of ATF3, a repressor that binds to and inhibits transcription of the adiponectin and GLUT4 genes. Supporting this idea, ATF3 has been found to inhibit adiponectin gene expression in 3T3-L1 adipocytes in response to ER stress signals (Kim et al., 2006; Koh et al., 2007). Disrupting CREB activity in adipocytes was sufficient to promote relatively normal glucose homeostasis in the contexts of dietary and genetic obesity. Indeed, modest over-expression of adiponectin or GLUT4 in fat also appears to enhance glucose tolerance and insulin sensitivity and, in the case of adiponectin, to reduce inflammatory pathway signaling (Berg et al., 2001; Carvalho et al., 2005; Combs et al., 2001; Kim et al., 2007; Satoh et al., 2005; Yamauchi et al., 2002b).
In addition to its effects on ATF3, CREB may modulate the expression of other cellular genes that influence lipid metabolism in adipocytes. For example, cAMP appears to increase mRNA amounts for the Inhibitor of DNA binding 2 (Id2), a helix loop helix protein that has been found to promote adipogenesis by stimulating the expression of PPAR gamma in adipose (Park et al., 2008). Indeed, adipocyte Id2 gene expression is increased in obesity, while mice with a knockout of Id2 gene have reduced adipose stores at birth. Based on the presence of conserved CREB binding sites in the Id2 gene and the ability for cAMP to stimulate Id2 gene expression, CREB may modulate the lipogenic program in adipocytes through up-regulation of this transcription factor.
Taken together, these results show that targeting therapies to adipose tissue, and in particular to the CREB signaling system, could have important therapeutic benefits in a variety of insulin resistant states. Further analysis of other adipocyte genes that are modulated by CREB under obese conditions should provide greater insight into this process.
Experimental Procedures
Mice
A 5.6 kilobase fragment of the aP2 promoter and a 256bp fragment encoding flag-ACREB were subcloned into the pWhere vector containing two H19 chromatin insulators (Invivogen). Fat-ACREB transgenic mice were generated on the B6 and CB6 backgrounds. Mice were genotyped using the following primer sets: for ACREB transgene: F tg- GTCCAGTGATCATTGCCAGG, F wt- TGCTGCCGCATCAGGCAAT and R- AGCACTGCCACTCTGTTCTC (wt= 230bp, tg=380bp). Ob/+ mice were purchased from Jackson Laboratory and were bred in the Salk transgenic facility. Transgenic mice were crossed to ob/+ mice to obtain tg-ob/+ mice and wt-ob/+ mice; this intercross generated tg; ob/ob (tg-ob) and wt; ob/ob (wt-ob) mice. For HFD studies, 8-week old male mice were fed on 60% HFD for 12–24 weeks. ATF3−/− mice (Hartman et al., 2004) were maintained on 45% HFD or 12% normal chow diet for 12-weeks.
Cells and Reagents
HEK293T cells were maintained in DMEM supplemented with 10% FBS. Adenoviruses encoding TORC2, GFP, Adiponectin, ATF3, ACREB, and ATF3i were generated using the Ad-Track-Ad-easy system (Herzig et al., 2001). Target sequence for ATF3 RNAi is AAGGAACATTGCAGAGCTAAG (nt. 527–546). pGL3-Adiponectin-luc (Iwaki et al., 2003) contains 1kb of the human adiponectin promoter fused to luciferase cDNA. TNF-α (Calbiochem) and FSK (Sigma) were used at 10ng/ml (in PBS) and 10μM (in DMSO), respectively. Antibodies include adiponectin (Sigma, 1:8000), Atf3 (Santa Cruz, 1:500), HSP90 (Santa Cruz, 1:5000); p-AMPK and AMPK, p- ACC and ACC, p-AKT and AKT (Cell Signaling) at 1:1000.
Adiponectin protein was collected in serum free medium from primary hepatocytes transduced with Ad-adiponectin virus. Multimeric forms of this hormone were confirmed by SDS-PAGE under reducing and non-reducing conditions (Pajvani et al., 2004). Adiponectin protein concentrations were determined by Elisa assay (Linco Research Inc, St. Charles, MO).
Primary hepatocyte culture
Mouse hepatocytes were harvested, cultured, and infected with adenoviruses as previously described (Dentin et al., 2004). For reporter studies, Ad-CRE luc infected primary hepatocytes were exposed to FSK for 5 hours, followed by exposure to control medium or insulin for 3 hours. Luciferase activity was normalized to β-gal activity from co-infected Ad-RSV β-gal vector. For glucose output studies, cells exposed for 5 hours to FSK, followed by 1 hour INS or control (G5 medium); glucose output then collected for 1 hour in control medium supplemented with 10mM lactate and 1mM pyruvate. For studies with adiponectin protein, active multimeric forms of adiponectin confirmed by SDS-PAGE analysis under non-reducing conditions.
Primary adipocyte culture and glucose uptake
Adipocytes were isolated from mouse visceral adipose tissue as described (Karnieli et al., 1981) with the following modifications. Adipose tissue was incubated for 30–45 minutes with Type I collagenase (InVitrogen) in Ringer Bicarbonate-Hepes (KRBH) buffer (10 mM bicarbonate, 30 mM Hepes, 200 nM adenosine, 2.5 mM glucose 1% fatty-acid-free BSA (pH 7.4)) at 37 ° C. The digestion mixture was filtered through a nylon strainer and centrifuged at 400 × g. Adipocytes in the supernatant were resuspended in glucose-free KRBH. Glucose uptake was measured using 3H-2-deoxyglucose. Cells were pretreated with insulin for 20 minutes and incubated at 37 ° C with 100 nM labeled deoxyglucose (4 mCi/ml) for 5 minutes. Labeled cells were collected after centrifugation at 10,000 × g for 10 sec. Cells were counted directly by scintillation. Cell aliquots were used to determine protein content and cell number for normalization.
Hyperinsulinemic-euglycemic clamps
Mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. Clamp studies on catheterized mice were performed as described(Hevener et al., 2003). HPLC purified [3-3H]-glucose (0.05 μCi/min, NEN) was infused for 2 hr (basal infusion period) into 6 hour fasted mice using micro-dialysis pumps, and blood samples were collected from the tail vein. Following the basal infusion period, a 120 min hyperinsulinemic-euglycemic clamp period was initiated with a continuous infusion of human insulin (Humulin R) at 12.5 mU/Kg/min. Blood samples were collected at 10 min intervals for the measurement of plasma glucose concentrations. A 50% glucose solution was infused at variable rates to maintain euglycemia during the clamp. Plasma was de-proteinized with ZnSO4 and Ba(OH)2, dried to remove 3H2O, resuspended in H2O, and counted in scintillation fluid for detection of 3H.
RNA Analysis
RNA extractions were carried out using Trizol followed by purification over a Qiaeasy RNA column. RT- and Q-PCR were carried out as described. The primer sets for atf3: AAGACAGAGTGCCTGCAGAA and GTGCCACCTCTGCTTAGCTC (232bp); nr4a2: CGCCGAAATCGTTGTCAGTA and CGACCTCTCCGGCCTTTTA (101bp); adpn: AAGGACAAGGCCGTTCTCTT and GAAAGCCAGTAAATGTAGAG (180bp); pgc1a: CAAGTCTAACTATGCAGACC and ACTTGCTCTTGGTGGAAGCA (67bp); ucp2: GATGGCTTGGCAGTCAAGAA and GAACTCCTGGAACTCGAGTTA (110bp); l32: TCTGGTGAAGCCCAAGATCG and CTCTGGGTTTCCGCCAGTT (100bp).
Blood metabolites
Glucose, FFA, TG, and ketones were measured using one-touch Ultra glucometer, NEFAC (WAKO Chemicals USA 99475409) and Serum TG determination kit (Sigma TR0100), respectively. Insulin, glucagon, adiponectin and resistin were measured using kits from Alpco (10115001 and 48-GLUCA-90) and Linco Research Inc (EZMADP-60K and CYT292), respectively.
Chromatin immunoprecipitation (CHIP)
CHIP in 293T cells and tissues were carried out as described (Koo et al., 2005). Lysates were immunoprecipitated with antibodies to CREB (244) as well as ATF3. Primers are as follows: atf3 CCGAACTTGCATCACCAGT and CGTTGCATCACCCCTTTTAT, which produce a 198bp band following amplification. Controls used in this experiment: positive control (fdps): GTAAGACAGGCAGCCAAAGC and CCACACTAAGGGCGGAAATA (267bp); negative control (actin): TGCTATCCCTGTACGCCTCT and CTCCTTAATGTCACGCACGA (227bp). Annealing temperature Tm=56 ° C.
Microarray
Total RNA samples were amplified, labeled, and hybridized to Affymetrix Mouse genome 430A 2.0 arrays (Affymetrix, Santa Clara, CA) using standard protocols. Scanned images were analyzed by using DCHIP software. Lower bounds of the 90% confidence intervals of fold changes (LFC) were used to identify cAMP-inducible genes. CRE site assignments have been described (Zhang et al., 2005b) and appear on the website http://natural.salk.edu/CREB. Microarray data have been deposited and can be accessed at NCBI GEO (accession number: GSE14363).
Metabolism
Liver glycogen and ketones were determined as described (Qi et al., 2006). Body temperature, food intake, metabolic cages and histology were carried out as reported (Qi et al., 2006). Effects of insulin on AKT phosphorylation in muscle and liver were determined following administration of insulin IP at 0.75U/kg body weight. Tissues were harvested 15 min after injection.
Statistical analysis
Results are expressed as mean ± s.e.m. Comparisons between groups were made by unpaired two-tailed Student t-test. P<0.05 was considered as statistically significant. All experiments were conducted on at least two independent occasions.
Supplementary Material
Acknowledgments
We thank Ichi Shimomura for adiponectin luciferase constructs and Anh-Khoi for technical assistance. M.M. is supported by NIH grant DK-049777, the Foundation for Medical Research, and by the Kieckhefer Foundation. This work was supported by a University of California Discovery Biostar Grant and NIH grants DK033651 (to J.M.O.) and DK074868 (to C.K.G. and J.M.O.). L.Q. is supported by a JDRF fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty D, Vinson C. A dominant negative inhibitor of CREB reveals that it is a general mediator stimulus-dependent transcription of c-fos. Molec Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Berger J, Biswas C, Vicario PP, Strout HV, Saperstein R, Pilch PF. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989;340:70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- Bleckmann SC, Blendy JA, Rudolph D, Monaghan AP, Schmid W, Schutz G. Activating transcription factor 1 and CREB are important for cell survival during early mouse development. Mol Cell Biol. 2002;22:1919–1925. doi: 10.1128/MCB.22.6.1919-1925.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blendy J, Kaestner K, Weinbauer G, Nieschlag E, Schutz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab. 2005;289:E551–561. doi: 10.1152/ajpendo.00116.2005. [DOI] [PubMed] [Google Scholar]
- Chen BP, Liang G, Whelan J, Hai T. ATF3 and ATF3 delta Zip. Transcriptional repression versus activation by alternatively spliced isoforms. J Biol Chem. 1994;269:15819–15826. [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delporte ML, Funahashi T, Takahashi M, Matsuzawa Y, Brichard SM. Pre-and post-translational negative effect of beta-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem J. 2002;367:677–685. doi: 10.1042/BJ20020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319:1402–1405. doi: 10.1126/science.1151363. [DOI] [PubMed] [Google Scholar]
- Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, 3rd, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- Dentin R, Pegorier JP, Benhamed F, Foufelle F, Ferre P, Fauveau V, Magnuson MA, Girard J, Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- Foretz M, Carling D, Guichard C, Ferre P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- Fu L, Isobe K, Zeng Q, Suzukawa K, Takekoshi K, Kawakami Y. beta-adrenoceptor agonists downregulate adiponectin, but upregulate adiponectin receptor 2 and tumor necrosis factor-alpha expression in adipocytes. Eur J Pharmacol. 2007;569:155–162. doi: 10.1016/j.ejphar.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Huecksteadt TP, Birnbaum MJ. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989;245:60–63. doi: 10.1126/science.2662408. [DOI] [PubMed] [Google Scholar]
- Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J, Wang X, Frankel WL, Guttridge D, Prentki M, et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004;24:5721–5732. doi: 10.1128/MCB.24.13.5721-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala U, Hedrick S, Quinn R, Bauer A, Schutz G, Yoon C, Puisgever P, Spiegelman B, Montminy M. CREB Regulates Hepatic Gluconeogenesis via the Co-activator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- Hummler E, Cole TJ, Blendy JA, Ganss R, Aguzzi A, Schmid W, Beermann F, Schutz G. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci USA. 1994;91:5647–5651. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupfeld CJ, Dalle S, Olefsky JM. Beta -Arrestin 1 down-regulation after insulin treatment is associated with supersensitization of beta 2 adrenergic receptor Galpha s signaling in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2003;100:161–166. doi: 10.1073/pnas.0235674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- Joost HG, Habberfield AD, Simpson IA, Laurenza A, Seamon KB. Activation of adenylate cyclase and inhibition of glucose transport in rat adipocytes by forskolin analogues: structural determinants for distinct sites of action. Mol Pharmacol. 1988;33:449–453. [PubMed] [Google Scholar]
- Joost HG, Weber TM, Cushman SW, Simpson IA. Insulin-stimulated glucose transport in rat adipose cells. Modulation of transporter intrinsic activity by isoproterenol and adenosine. J Biol Chem. 1986;261:10033–10036. [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Cushman SW, Flier JS. Regulation of glucose transporter-specific mRNA levels in rat adipose cells with fasting and refeeding. Implications for in vivo control of glucose transporter number. J Clin Invest. 1989;83:199–204. doi: 10.1172/JCI113859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E, Zarnowski MJ, Hissin PJ, Simpson IA, Salans LB, Cushman SW. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981;256:4772–4777. [PubMed] [Google Scholar]
- Kim HB, Kong M, Kim TM, Suh YH, Kim WH, Lim JH, Song JH, Jung MH. NFATc4 and ATF3 negatively regulate adiponectin gene expression in 3T3-L1 adipocytes. Diabetes. 2006;55:1342–1352. doi: 10.2337/db05-1507. [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56:2973–2981. doi: 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- Laakso M, Edelman SV, Brechtel G, Baron AD. Effects of epinephrine on insulin-mediated glucose uptake in whole body and leg muscle in humans: role of blood flow. Am J Physiol. 1992;263:E199–204. doi: 10.1152/ajpendo.1992.263.2.E199. [DOI] [PubMed] [Google Scholar]
- Liang G, Wolfgang CD, Chen BP, Chen TH, Hai T. ATF3 gene. Genomic organization, promoter, and regulation. J Biol Chem. 1996;271:1695–1701. doi: 10.1074/jbc.271.3.1695. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Tanscriptional Regulation by the Phosphorylation Dependent Factor CREB. Nature Reviews-Molecular Cell Biology. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- Park KW, Waki H, Villanueva CJ, Monticelli LA, Hong C, Kang S, MacDougald OA, Goldrath AW, Tontonoz P. Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-gamma expression and adipocyte differentiation. Mol Endocrinol. 2008;22:2038–2048. doi: 10.1210/me.2007-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science. 2006;312:1763–1766. doi: 10.1126/science.1123374. [DOI] [PubMed] [Google Scholar]
- Ravnskjaer K, Kester H, Liu Y, Zhang X, Lee D, Yates JR, 3rd, Montminy M. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. Embo J. 2007;26:2880–2889. doi: 10.1038/sj.emboj.7601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci MR, Lee MJ, Russell CD, Wang Y, Sullivan S, Schneider SH, Brolin RE, Fried SK. Isoproterenol decreases leptin release from rat and human adipose tissue through posttranscriptional mechanisms. Am J Physiol Endocrinol Metab. 2005;288:E798–804. doi: 10.1152/ajpendo.00446.2004. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Saha AK, Kraegen EW. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology. 2003;144:5166–5171. doi: 10.1210/en.2003-0849. [DOI] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Miles PD, Imamura T, Usui I, Olefsky JM. Adenovirus-mediated chronic “hyper-resistinemia” leads to in vivo insulin resistance in normal rats. J Clin Invest. 2004;114:224–231. doi: 10.1172/JCI20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005;54:1304–1313. doi: 10.2337/diabetes.54.5.1304. [DOI] [PubMed] [Google Scholar]
- Scriba D, Aprath-Husmann I, Blum WF, Hauner H. Catecholamines suppress leptin release from in vitro differentiated subcutaneous human adipocytes in primary culture via beta1- and beta2-adrenergic receptors. Eur J Endocrinol. 2000;143:439–445. doi: 10.1530/eje.0.1430439. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science. 2005 doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: A Stimulus-Induced Transcription Factor Activated by A Diverse Array of Extracellular Signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Sivitz WI, DeSautel SL, Kayano T, Bell GI, Pessin JE. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989;340:72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Butte AJ, Kokkotou E, Yechoor VK, Taniguchi CM, Kriauciunas KM, Cypess AM, Niinobe M, Yoshikawa K, Patti ME, Kahn CR. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005;7:601–611. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Kasper LH, Lerach S, Jeevan T, Brindle PK. Individual CREB-target genes dictate usage of distinct cAMP-responsive coactivation mechanisms. Embo J. 2007;26:2890–2903. doi: 10.1038/sj.emboj.7601734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002a;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Oike Y, Kamon J, Waki H, Komeda K, Tsuchida A, Date Y, Li MX, Miki H, Akanuma Y, et al. Increased insulin sensitivity despite lipodystrophy in Crebbp heterozygous mice. Nat Genet. 2002b;30:221–226. doi: 10.1038/ng829. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005a;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Klemm DJ, Vinson C, Lane MD. Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein beta gene during adipogenesis. J Biol Chem. 2004;279:4471–4478. doi: 10.1074/jbc.M311327200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005b;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.