Abstract
BMD does not entirely explain an individual's risk of fracture. The purpose of this study was to assess whether specific differences in spatially resolved bone composition also contribute to fracture risk. These differences were assessed using Fourier transform infrared spectroscopic imaging (FTIRI) and analyzed through multiple logistic regression. Models were constructed to determine whether FTIRI measured parameters describing mineral content, mineral crystal size and perfection, and collagen maturity were associated with fracture. Cortical and cancellous bone were independently evaluated in iliac crest biopsies from 54 women (32 with fractures, 22 without) who had significantly different spine but not hip BMDs and ranged in age from 30 to 83 yr. The parameters that were significantly associated with fracture in the model were cortical and cancellous collagen maturity (increased with increased fracture risk), cortical mineral/matrix ratio (higher with increased fracture risk), and cancellous crystallinity (increased with increased fracture risk). As expected, because of its correlation with cortical but not cancellous bone density, hip BMD was significantly associated with fracture risk in the cortical but not the cancellous model. This research suggests that additional parameters associated with fracture risk should be targeted for therapies for osteoporosis.
Key words: bone fracture, bone fragility, collagen cross-links, crystallinity, mineral maturity, multiple logistic regression
INTRODUCTION
The World Health Organization Consensus Conference defines osteoporosis as a condition of bone deterioration in which individuals have “a BMD that lies 2.5 SDs or more below the average value for young healthy women,”(1) and the Surgeon General's report adds to this definition increased risk of fracture.(2) BMD, whereas associated with fracture risk, is not fully predictive of who will experience a low impact (fragility) fracture.(3–5) Large epidemiological studies have shown that BMD accounts for only ∼60% of the fracture risk(6,7) and suggested that other “bone quality” parameters may account for why two individuals with similar lifestyles and equivalent BMDs may have different fragility fracture histories. Although measurable decreases in BMD in untreated patients have been associated with increased risk of fragility fracture,(8) areal BMD changes account for less than one half of the improvement in fracture risk seen in osteoporotic patients treated with antiresorptive and anabolic agents.(9)
The parameters generally considered to be representative of “bone quality” are geometry (including connectivity), presence of microcracks, and extent of mineralization.(5,9) The properties of the bone collagen matrix have been suggested to be equally important based on chemical,(10–14) spectroscopic,(15–20) and gene association(21,22) studies. The clinical methods routinely used to identify osteoporosis and fracture risk measure density, geometry, and mineral content.(22,23) These methods do not, however, provide information on the extracellular matrix. Infrared and Raman spectroscopic imaging have been used to describe the changes in both cortical and cancellous bone in biopsy tissues as a function of age, disease, and treatment for osteoporosis.(15–20,24–27) In contrast to clinical methodologies, these spectroscopic methods provide spatially resolved information (∼10 and ∼1 μm, respectively) on properties of both the mineral and the matrix.
Fourier transform infrared microspectroscopy (FTIRM) and imaging (FTIRI) of bone tissue use spectrometers coupled with light microscopes to examine nondecalcified sections of bone at ∼25- or ∼7-μm spatial resolution, respectively.(26) FTIRI allows changes in the bone mineral and matrix environment to be examined with morphological detail. The validated parameters that can then be calculated from hyperspectral images (where x and y indicate the location in the specimen and z the intensity at a specific wavenumber or a calculated parameter) include mineral/matrix ratio, carbonate/phosphate ratio, crystallinity, and collagen maturity (collagen cross-link ratio, XLR).(27) The purpose of this study was to test the hypothesis that some bone mineral and matrix bone properties calculated from FTIRI would explain some of the fragility fracture risk not predicted by BMD.
MATERIALS AND METHODS
All of the clinician co-authors (R.R.R., A.H., D.D., E.S., J.C.) provided biopsies for this study. Under an IRB-approved protocol, 54 iliac crest biopsies were obtained from women by these collaborating investigators. IRB approval was obtained at the individual research centers. Patients suffering low-trauma fractures who had not been treated for their osteoporosis before the time of biopsy or those who received only hormone replacement therapy (n = 8) were included. Patients with other conditions that would impact fracture risk (e.g., osteogenesis imperfecta, skeletal dysplasias) were excluded. The inclusion and exclusion criteria for each of the studies from which biopsies were provided are listed in Table 1. The following information was provided for each patient: code number, age at biopsy, hormonal replacement therapy (HRT; yes/no = 1/0), spine and hip BMD (Hologistic; if Lunar, the following equation was used to convert to Hologistic values: Hologic = 0.863 × Lunar − 0.048), T-score, and presence (1) or absence (0) of fractures at the time of biopsy. Patients who received PTH or antiresorptive therapies other than estrogen were excluded from this study. The biopsies were processed for FTIRI in a blinded fashion and the codes not broken until the time of statistical analysis.
Table 1.
Inclusion and Exclusion Criteria
| Study | N | Inclusion | Exclusion |
| AH/DD | 14 | From among 17 postmenopausal women referred with symptomatic vertebral fractures in 1986, with radiological evidence of osteopenia. | |
| • BMD T-scores < −2.5 | |||
| • No prior antiresorptive treatment, including estrogen | |||
| • Signed informed consent to enter a small clinical trial exploring cyclical treatment with PTH(1-38) with/without sequential calcitonin, using pretreatment and post-treatment (200 days) bone biopsies as part of the primary outcomes | |||
| ES | 5 | From among patients with low BMD (T-score < −2.5 at any site) and/or one or more fragility fractures (excluding digits, skull) | Any secondary cause of osteoporosis, including |
| • Estrogen deficiency | |||
| • Steroid excess | |||
| • Ages 20–48 | • Antiepileptic drugs | ||
| • Normal menses throughout | • Celiac disease | ||
| JC | 8 | Part of an opportunistic study in which a group of postmenopausal women treated long term with high-dose estradiol therapy | |
| • These women did not have osteoporosis, their BMD was on the high side | |||
| • The dose of estradiol, given as an implant, was 50–100 mg approximately every 6 months | |||
| RRR | 27 | Part of a study of growth hormone releasing hormone | More than 15% below or 30% above ideal body weight as defined in the 1983 Metropolitan Life tables. |
| • Age 45–80 yr, postmenopausal for at least 5 yr, who had at least one low-trauma fracture or who had very low spinal BMD by DXA. | |||
| • In good general health based on medical history, physical and screening laboratory examination. or part of a study of menopause effects on bone | |||
| • Healthy with normal premenopausal E2 and FSH levels At least 46 yr of age, having regular menses |
Biopsies used in this study all had been previously fixed with alcohol and embedded in polymethyl methacrylate (PMMA). The embedded tissues were cut at 2–3 μm thickness and mounted on barium fluoride infrared windows (SpectraTech, Hopewell Junction, NY, USA). Three sections from each biopsy were examined using a Perkin Elmer Spotlight 300 Infrared Imaging system (Perkin Elmer Instruments, Waltham, MA, USA) at a spectral resolution of 4 cm−1. Images from cortical and cancellous regions of the biopsy were analyzed separately. Background (BaF2 window only) and PMMA spectra were collected for each section analyzed, and these spectra were used for correction of the sample spectral data. Spectra were baseline corrected, and the PMMA spectral contribution was subtracted using ISYS software (Spectral Dimensions, Olney, MD, USA). The mean and SDs from three to six cortical or cancellous regions per patient for all FTIRI parameters were calculated and saved in a database.
The following FTIRI parameters, reviewed in detail elsewhere,(26) were calculated using ISYS software. Mineral/matrix ratio, which measures bone mineral (correlated to ash weight) is calculated by integrated area of phosphate (916–1180 cm−1)/amide I (1592–1712 cm−1). Carbonate/mineral ratio (C/P), which reflects the level of carbonate substitution in the hydroxyapatite (HA) crystal, is calculated through the integrated area of the ν2 carbonate peak (840–892 cm−1) and that of the phosphate. Crystallinity (XST), which is related to mineral crystal size and perfection as determined by X-ray diffraction, is calculated as the 1030/1020 cm−1 peak intensity ratio. The collagen maturity (XLR) was estimated as the intensity ratio of amide I sub-bands at 1660 and 1690 cm−1
The FTIRI data were dichotomized based on the presence (1) or absence (0) of a fracture. Multiple logistic regressions using the equation:
were calculated from the indicated parameters separately in the cortical and cancellous bone. In this equation, BMD is hip BMD as spine BMD was not available for all patients, M/M is mineral/matrix ratio, XST is crystallinity, XLR is collagen maturity, C/P is carbonate to phosphate ratio (also not available for all patients), and RX is estrogen treatment (Y/N= 1/0). Logistic regression analyses were calculated with JMP 4.0 (Statistics Discovery Software; SAS Institute, Cary, NC, USA). Infrared parameters, age, and BMD were used as continuous variables, and fracture and estrogen treatment were used as nominal values. All parameters including age, BMD (hip and spine), and the FTIRI parameters were entered into the database, and bivariate (pairwise) analyses were run to estimate the significance of individual factors. The full model was run to see which individual effects remained significant, and the parameters with the highest p values were sequentially removed and the model rerun. Additional comparisons were based on two-sided t-tests.
RESULTS
Evaluation of the patient demographics showed there were no differences in age between the fracture and nonfracture group (Table 2). The fracture group had significantly lower spine but not hip BMD, and this was reflected in their calculated T-scores.
Table 2.
Patient Characteristics
| Fracture (N = 32) | Nonfracture (N = 22) | p | |
| Age at biopsy (yr) | 59 ± 17 | 56 ± 5 | 0.31 |
| BMD spine (g/cm2) | 0.74 + 0.2 | 0.99 ± 0.16 | <0.005 |
| BMD T-score spine | −2.7 ± 1 | 0.12 ± 2 | <0.005 |
| BMD hip (g/cm2) | 0.70 ± 0.06 | 0.83 ± 0.26 | 0.15 |
| BMD T-score hip | −2.5 ± 0.6 | −1.0 + 2 | 0.16 |
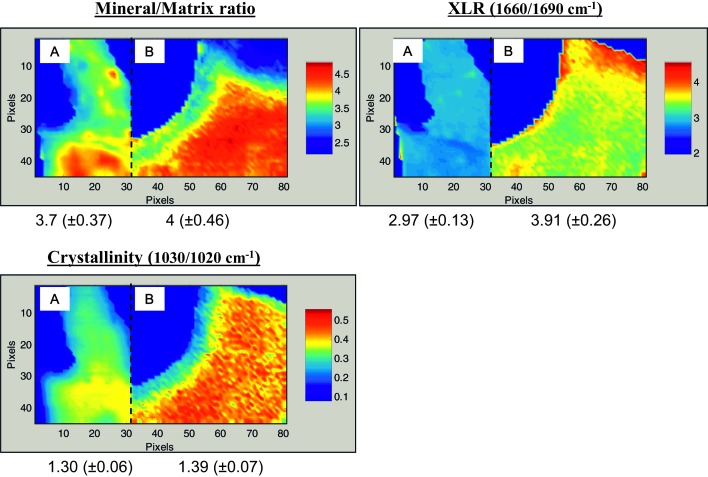
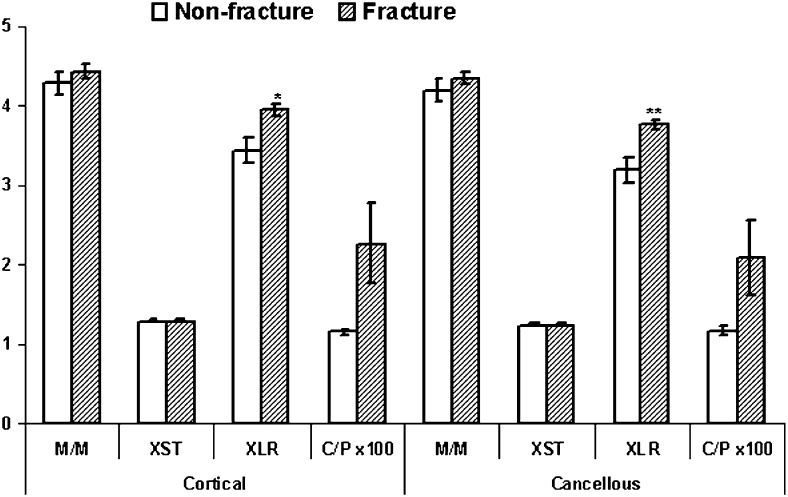
Typical FTIRI images are shown in Figs. 1A–1C with data for a T-score–matched pair (T = –1.3), one of whom sustained a fracture and one who did not. As can be seen, the fracture case looks different in terms of the means for the FTIR parameters, and the actual measured values for the images shown are different. When the mean values for all fracture cases were compared with the nonfracture cases (Fig. 2), statistically significant differences (p < 0.05) were observed in cortical and cancellous bone for the collagen maturity (XLR) and in cortical bone for carbonate/mineral ratio. The number of biopsies for which carbonate/phosphate ratios were available was smaller than that for the other parameters because of a change in the detector, which enabled us to directly acquire carbonate content information.
FIG. 1.
Typical infrared images for the FTIR parameters recorded in trabecular bone from two patients, one with fractures and one without, who had comparable BMD T-scores of −1.3. Patient A had no fractures (t = −1.32) and was 50 yr old at time of biopsy. Patient B had a fracture history (T = −1.25) and was 58 yr old at time of biopsy. Numerical values below the images are the means ± SD for that parameter in the figure and indicate the range of data for the pixels shown. Note in these figures, 1 pixel = 6.25 μm.
FIG. 2.
Summary of measured FTIRI parameters for all cases; mean ± SD. *p < 0.05 vs. nonfracture controls; **p < 0.01 vs. nonfracture controls.
A model based on multiple logistic regression indicated that increasing crystallinity and collagen maturity were significantly associated with fracture in cancellous bone, whereas hip BMD, mineral/matrix ratio, and collagen maturity were significantly associated with fracture in cortical bone (Table 3). The cortical bone model converged with p < 0.0001, R 2 = 0.58 and the cancellous bone model with p = 0.0009, R 2 = 0.66. The data in Table 3 summarize the intercepts (βo) and β parameters for each of the variables and their significance levels. Decreased carbonate/mineral was significant and accounted for 44% of the variation noted in the 22 samples for which this parameter could be measured in the single logistic regression (Table 4). However, it dropped out of the multiple logistic regression model (with 54 samples) without a change in R for the model, indicating that either its effects were accounted for by some other factor (presumably crystallinity or mineral/matrix ratio) or that we had insufficient samples to determine its full effect.
Table 3.
Multiple Logistic Regression Results for Dependent Variables of Fracture
| Independent variables |
Cortical |
Cancellous |
||
| β-coefficient | p | β-coefficient | p | |
| Intercept | 19.51 | 0.03 | 14.76 | 0.04 |
| BMD (hip) | 10.9 | 0.009 | ||
| Crystallinity | NS | −5.24 | 0.03 | |
| XLR | −3.56 | 0.012 | −2.38 | 0.002 |
| Mineral/matrix | −3.37 | 0.04 | NS | |
| C/P | NS | NS | ||
p < 0.001 for both whole model tests, R2 = 0.58 (cortical); 0.66 (cancellous). A negative number for the coefficient indicates that a higher value is associated with the risk of fracture.
NS, not significant.
Table 4.
Single Logistic Regression
| Variable | Estimate | R2 | p | |||
| Age | −0.0063 | 0.001 | 0.81 | |||
| RX (n = 8) | 11.6 | 0.21 | 0.90 | |||
| BMD (hip) | 4.0 | 0.08 | 0.22 | |||
|
Cortical |
Cancellous |
|||||
| Estimate | R2 | p | Estimate | R2 | p | |
| Crystallinity | −2.22 | 0.017 | 0.69 | −9.26 | 0.09 | 0.04 |
| XLR | −1.37 | 0.08 | 0.046 | −0.511 | 0.17 | 0.30 |
| Mineral/Matrix | −0.92 | 0.0007 | 0.83 | −0.37 | 0.06 | 0.56 |
| C/P(×100)(n = 22) | 10.5 | 0.43 | 0.008 | 10.19 | 0.49 | 0.009 |
RX, estrogen treatment.
DISCUSSION
This study showed a significant association between FTIRI parameters related to mineral content, mineral composition (crystallinity and carbonate/phosphate ratio), and collagen maturity with bone fracture. These findings agree in part with previous reports that found elevated collagen cross-link ratio (collagen maturity) in cortical and cancellous bone of young women with unexpected fragility fractures(27) and with our earlier reports of increased crystallinity in bone that was histologically osteopenic.(16)
Multiple logistic regression is the most efficient way of examining the effects of various independent variables on a dichotomous dependent variable. Although it does assume that the independent variables, in this case, BMD, crystallinity, mineral/matrix ratio, and collagen cross-link ratio, are linearly related to the dependent variable, fracture, on a log scale, that assumption seems justified by the relatively high R 2 values of the fit. The multiple logistic regression models explain 58% and 66% of the difference between fracture and nonfracture patients in cortical and cancellous bone, respectively. Given that a great deal of fracture risk has to do with chance, we believe that this was a strong confirmation of the appropriateness of our analytical method.
The finding of an association between increased crystallinity (and altered crystal composition—i.e., decreased carbonate/mineral ratio) and fracture risk is also in agreement with several other studies. Increased particle sizes (larger clumps of crystals) were visible optically in thin sections obtained from biopsies of patients that had fractured femoral necks as contrasted with controls.(28) Larger crystal particles were also associated with aging, and the presence of larger crystals suggested to be related to bone fragility.(29) Our initial studies of biopsies from patients with osteoporosis also found increased crystal sizes in their biopsies as contrasted with control tissues,(16,30) as did a recent Raman study.(31) In the Raman study,(31) increasing tissue level strength and stiffness was reported to increase parallel to the crystallinity, whereas ductility decreased. Using Raman spectroscopy, McCreadie et al.(32) found elevated carbonate/phosphate levels in fracture cases, as we saw with FTIRI in both high and low turnover osteoporosis.(16)
There are several possible reasons for the association between crystal size and fracture risk. First, the larger more perfect bone mineral crystals may represent those that remain when bone is remodeled, and hence may reflect increased tissue turnover and the loss of younger, newly formed bone. The smaller crystals are generally more soluble and are dissolved first. Smaller crystals also reflect newly formed bone; hence, a loss of smaller crystals means less formation and/or more resorption. Second, from physicochemical principles, larger particles in general tend to be more brittle and weaker, because when a force is applied, the atoms generally try to move in relation to the adjacent layer of atoms. In metals, for example, making the particles (grains) smaller generally strengthens the materials. Broadening the size distribution also strengths the material.(33) Finally, larger crystals may not be able to align as well with the collagen matrix, weakening the crystal–mineral interactions, and making the composite weaker.
Cortical and cancellous bone showed a significant association between increasing collagen maturity and fracture risk. This is mostly likely because of the more stable nature of the older bone and may represent an effect rather than a cause. Similar to the crystallinity measurements, alterations in collagen maturity and collagen composition have also been previously associated with whole bone mechanical properties and fracture risk. For example, alterations in collagen cross-linking, based on Raman analyses, were associated with mechanical changes in human cortical bones of different ages.(15) A study of intracapsular hip fracture cases as contrasted with age-matched postmortem controls also found reduced collagen enzymatic cross-links in high-density bone and increased pentosidine in both low- and high-density bone and higher plasma homocysteine and lower pyridoxal levels than in controls.(13) The collagen cross-linking structure was also altered in microdamaged areas of dog bone,(12) consistent with ruptured cross-links and reduced fracture resistance. Fracture cases also showed increased lysyl hydroxylation.(14) Based on chemical analysis of secreted markers of bone turnover, bone collagen maturation varied with different antiresorptive treatments.(34) This has potential implications for treatment.
It is also possible that the altered collagen maturity predisposes the bone to fracture. Proper collagen content, structure, and maturity are all important for mechanical integrity; this can be seen from analyses of the brittle bone found in children and subclinical models of osteogenesis imperfecta, as reviewed elsewhere.(35,36) Studies of young women with unexplained fractures have also found increased XLR values in their bones.(27)
The proper collagen matrix is important for regulation of mineral deposition and is a major contributor to the strength of the composite tissue.(37) Increased collagen cross-linking has been correlated with resistance to fracture in chickens,(37) and defects in collagen organization are associated with decreased mechanical strength, although in the study in question, alterations in cross-links were not reported.(38) Increased collagen cross-linking may reflect the older nature of the matrix associated with decreased new bone formation and/or increased loss of younger bone. However, even in young individuals, impaired collagen production and maturation is known to lead to brittle bone disease.(33)
This study thus showed that mineral crystal size and composition and matrix maturity are associated with fracture risk. This association is independent of age and, for cancellous bone, of BMD. In cortical bone, BMD and mineral/matrix ratio were also independent predictors, an unexpected finding that may reflect the use of a global hip BMD to compare with iliac crest mineral content. The FTIRI parameters most likely come into play, after microarchitecture and the presence of microcracks, have been taken into consideration, but this preliminary study did not have sufficient samples to investigate those other markers of bone quality. We can speculate, however, that if architecture and the presence of microcracks were comparable in two samples, the one where the crystals were larger (leading to increased brittleness) or the collagen excessively cross-linked (decreasing its resilience), would be more likely to fracture.
One advantage of our study is that we had untreated patients with a wide range of BMDs both in the fracture and control groups. Earlier studies using other techniques such as MRI(39) had only patients with low BMD, a limited number of subjects, or lacked sufficient untreated controls.
Despite the fact that we had 54 patient biopsies, our study had several limitations. First, the biopsies were obtained retrospectively from other investigators. This means both the fracture and the control data might be biased by the subjects' willingness to participate in the study at the various collaborating institutions. Second, because of the small sample size, it was difficult to tell if the reason that many FTIRI parameters dropped out of the model was because of lack of variability or because of interdependence. Those parameters included age, spine BMD, and carbonate/mineral ratio. We did not have data on the observed heterogeneity(19) of each of the variables for the majority of these samples, although we know that the heterogeneity does vary with treatment. Future studies with larger sample numbers will address these additional parameters. The third limitation was that we did not know whether a patient with a fracture had a single, or more than one, fracture. It is well established that patients with osteoporosis have an increased risk of sustaining a second fracture after the first.(40,41) Fourth, the biopsies used in this study were not pre- and postfracture tissues from the same person. Ideally prefracture tissue from fracture prone patients would be compared with tissue from controls. Finally, the iliac crest biopsies are not taken from a clinically relevant site, nor are they taken from the site at which the individuals in the sample differed in BMD or in fracture status. However the fact that we can detect differences in patients with and without osteoporosis and with and without treatment for osteoporosis using FTIRI analyses of iliac crest biopsies(16,18,19,27,30) implies that this is not a major limitation of the study. However, to address this concern, we are currently performing a necropsy study to determine the site to site variation in FTIRI properties in individuals with no evidence of bone disease, and have data in baboon osteons that show consistency in the tissue age–dependent variation in FTIRI parameters.(42)
In conclusion, based on this first analysis, we suggest that collagen maturity and crystallinity contribute to bone weakening and hence fracture. Furthermore, we suggest that analyses of these parameters in therapy trials could be used provide greater insight into treatment efficacy than clinical measures alone.
ACKNOWLEDGMENTS
This study was supported by NIH Grant R01AR041325 and P30AR046121 (NIH Core Center Grant) to A.L.B. and AR049896 to E.S.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.World Health Organization. Geneva, Switzerland: World Health Organization; 2003. Prevention and Management of Osteoporosis. [Google Scholar]
- 2.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 3.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCreadie BR, Goldstein SA. Biomechanics of fracture: Is bone mineral density sufficient to assess risk? J Bone Miner Res. 2000;15:2305–2308. doi: 10.1359/jbmr.2000.15.12.2305. [DOI] [PubMed] [Google Scholar]
- 5.Perilli E, Baleani M, Ohman C, Fognani R, Baruffaldi F, Viceconti M. Dependence of mechanical compressive strength on local variations in microarchitecture in cancellous bone of proximal human femur. J Biomech. 2008;41:438–446. doi: 10.1016/j.jbiomech.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Siris ES, Brenneman SK, Miller PD, Barrett-Connor E, Chen YT, Sherwood LM, Abbott TA. Predictive value of low BMD for 1-year fracture outcomes is similar for postmenopausal women ages 50-64 and 65 and Older: Results from the National Osteoporosis Risk Assessment (NORA) J Bone Miner Res. 2004;19:1215–1220. doi: 10.1359/JBMR.040508. [DOI] [PubMed] [Google Scholar]
- 7.Hillier TA, Stone KL, Bauer DC, Rizzo JH, Pedula KL, Cauley JA, Ensrud KE, Hochberg MC, Cummings SR. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: The study of osteoporotic fractures. Arch Intern Med. 2007;167:155–160. doi: 10.1001/archinte.167.2.155. [DOI] [PubMed] [Google Scholar]
- 8.Berger C, Langsetmo L, Joseph L, Hanley DA, Davison S, Josse RG, Prior JC, Kreiger N, Tenenhouse A, Goltzman D The CaMos Research Group. Association between change in BMD and fragility fracture in women and men. J Bone Miner Res. 2009;24:361–370. doi: 10.1359/jbmr.081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benhamou CL. Effects of osteoporosis medications on bone quality. Joint Bone Spine. 2007;74:39–47. doi: 10.1016/j.jbspin.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Byrjalsen I, Leeming DJ, Qvist P, Christiansen C, Karsdal MA. Bone turnover and bone collagen maturation in osteoporosis: Effects of antiresorptive therapies. Osteoporos Int. 2008;19:339–348. doi: 10.1007/s00198-007-0462-5. [DOI] [PubMed] [Google Scholar]
- 11.Karsdal MA, Byrjalsen I, Leeming DJ, Delmas PD, Christiansen C. The effects of oral calcitonin on bone collagen maturation: Implications for bone turnover and quality. Osteoporos Int. 2008;19:1355–1361. doi: 10.1007/s00198-008-0603-5. [DOI] [PubMed] [Google Scholar]
- 12.Ruppel ME, Burr DB, Miller LM. Chemical makeup of microdamaged bone differs from undamaged bone. Bone. 2006;39:318–324. doi: 10.1016/j.bone.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int. 2006;79:160–168. doi: 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- 14.Saito M, Mori S, Mashiba T, Komatsubara S, Marumo K. Collagen maturity, glycation induced-pentosidine, and mineralization are increased following 3-year treatment with incadronate in dogs. Osteoporos Int. 2008;19:1343–1354. doi: 10.1007/s00198-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 15.Ager JW, Nalla RK, Breeden KL, Ritchie RO. Deep-ultraviolet Raman spectroscopy study of the effect of aging on human cortical bone. J Biomed Opt. 2005;10:034012–034017. doi: 10.1117/1.1924668. [DOI] [PubMed] [Google Scholar]
- 16.Boskey AL, DiCarlo E, Paschalis E, West P, Mendelsohn R. Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: An FT-IR microspectroscopic investigation. Osteoporos Int. 2005;16:2031–2038. doi: 10.1007/s00198-005-1992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtland HW, Nasser P, Goldstone AB, Spevak L, Boskey AL, Jepsen KJ. Fourier Transform Infrared Imaging Microspectroscopy and Tissue-Level Mechanical Testing Reveal Intraspecies Variation in Mouse Bone Mineral and Matrix Composition. Calcif Tissue Int. 2008;83:342–353. doi: 10.1007/s00223-008-9176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paschalis EP, Boskey AL, Kassem M, Eriksen EF. Effect of hormone replacement therapy on bone quality in early postmenopausal women. J Bone Miner Res. 2003;18:955–959. doi: 10.1359/jbmr.2003.18.6.955. [DOI] [PubMed] [Google Scholar]
- 19.Boskey AL, Spevak L. Weinstein RS 2009 Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int. 20:793–800. doi: 10.1007/s00198-008-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoehrer R, Dempster DW, Bilezikian JP, Zhou H, Silverberg SJ, Shane E, Roschger P, Paschalis EP, Klaushofer K. Bone quality determined by Fourier transform infrared imaging analysis in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2008;93:3484–3489. doi: 10.1210/jc.2008-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP, Aspden RM, Ralston SH. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest. 2001;107:899–907. doi: 10.1172/JCI10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dargent-Molina P, Benhamou CL, Cortet B, Sutter B, Thomas T. Devising global strategies for fracture-risk evaluation. Joint Bone Spine. 2007;74:240–244. doi: 10.1016/j.jbspin.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Miller LM, Vairavamurthy V, Chance MR, Mendelsohn R, Paschalis EP, Betts F, Boskey AL. In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the nu(4) PO(4)(3-) vibration. Biochim Biophys Acta. 2001;1527:11–19. doi: 10.1016/s0304-4165(01)00093-9. [DOI] [PubMed] [Google Scholar]
- 24.Nagy G, Lorand T, Patonai Z, Montsko G, Bajnoczky I, Marcsik A, Mark L. Analysis of pathological and non-pathological human skeletal remains by FT-IR spectroscopy. Forensic Sci Int. 2008;175:55–60. doi: 10.1016/j.forsciint.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Huang RY, Miller LM, Carlson CS, Chance MR. In situ chemistry of osteoporosis revealed by synchrotron infrared microspectroscopy. Bone. 2003;33:514–521. doi: 10.1016/s8756-3282(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 26.Boskey A, Mendelsohn R. Infrared analysis of bone in health and disease. J Biomed Opt. 2005;10:031102–031106. doi: 10.1117/1.1922927. [DOI] [PubMed] [Google Scholar]
- 27.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent GN, Dodds RA, Klenerman L, Watts RW, Bitensky L, Chayen J. Changes in crystal size and orientation of acidic glycosaminoglycans at the fracture site in fractured necks of femur. J Bone Joint Surg Br. 1983;65:189–194. doi: 10.1302/0301-620X.65B2.6826629. [DOI] [PubMed] [Google Scholar]
- 29.Chatterji S, Wall JC, Jeffery JW. Age-related changes in the orientation and particle size of the mineral phase in human femoral cortical bone. Calcif Tissue Int. 1981;33:567–574. doi: 10.1007/BF02409493. [DOI] [PubMed] [Google Scholar]
- 30.Paschalis EP, Betts F, DiCarlo E, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissue Int. 1997;61:487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- 31.Yerramshetty JS, Akkus O. The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone. 2008;42:476–482. doi: 10.1016/j.bone.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 32.McCreadie BR, Morris MD, Chen TC, Sudhaker Rao D, Finney WF, Widjaja E, Goldstein SA. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone. 2006;39:1190–1195. doi: 10.1016/j.bone.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Li ZY, Lam WM, Yang C, Xu B, Ni GX, Abbah SA, Cheung KM, Luk KD, Lu WW. Chemical composition, crystal size and lattice structural changes after incorporation of strontium into biomimetic apatite. Biomaterials. 2007;28:1452–1460. doi: 10.1016/j.biomaterials.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Byrjalsen I, Leeming DJ, Qvist P, Christiansen C, Karsdal MA. Bone turnover and bone collagen maturation in osteoporosis: Effects of antiresorptive therapies. Osteoporos Int. 2008;19:339–348. doi: 10.1007/s00198-007-0462-5. [DOI] [PubMed] [Google Scholar]
- 35.Blank RD, Boskey AL. Genetic collagen diseases: Influence of collagen mutations on structure and mechanical behavior. In: Fratzl P, editor. Collagen Mechanics. New York, NY, USA: Springer Science; 2008. p. 448ff. [Google Scholar]
- 36.Miller E, Delos D, Baldini T, Wright TM, Pleshko Camacho N. Abnormal mineral-matrix interactions are a significant contributor to fragility in oim/oim bone. Calcif Tissue Int. 2007;81:206–214. doi: 10.1007/s00223-007-9045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparke AJ, Sims TJ, Avery NC, Bailey AJ, Fleming RH, Whitehead CC. Differences in composition of avian bone collagen following genetic selection for resistance to osteoporosis. Br Poult Sci. 2002;43:127–134. doi: 10.1080/00071660120109962. [DOI] [PubMed] [Google Scholar]
- 38.Silva MJ, Brodt MD, Wopenka B, Thomopoulos S, Williams D, Wassen MH, Ko M, Kusano N, Bank RA. Decreased collagen organization and content are associated with reduced strength of demineralized and intact bone in the SAMP6 mouse. J Bone Miner Res. 2006;21:78–88. doi: 10.1359/JBMR.050909. [DOI] [PubMed] [Google Scholar]
- 39.Majumdar S, Kothari M, Augat P, Newitt DC, Link TM, Lin JC, Lang T, Lu Y, Genant HK. High-resolution magnetic resonance imaging: Three-dimensional trabecular bone architecture and biomechanical properties. Bone. 1998;22:445–454. doi: 10.1016/s8756-3282(98)00030-1. [DOI] [PubMed] [Google Scholar]
- 40.Owen RA, Melton LJ, III, Ilstrup DM, Johnson KA, Riggs BL. Colles' fracture and subsequent hip fracture risk. Clin Orthop Relat Res. 1982;171:37–43. [PubMed] [Google Scholar]
- 41.Stewart A, Walker LG, Porter RW, Reid DM, Primrose WR. Predicting a second hip fracture. The potential role of dual X-ray absorptiometry, ultrasound and other risk factors in targeting of preventive therapy. J Clin Densitom. 1999;2:363–370. doi: 10.1016/s1094-6950(06)60401-0. [DOI] [PubMed] [Google Scholar]
- 42.Gourion-Arsiquaud S, Burket J, van der Meulen M, Mendelsohn R, Doty S, DiCarlo S, Havill L, Boskey AL. Spatial variation in osteonal bone properties relative to tissue and animal age. J Bone Miner Res. 2009 doi: 10.1359/JBMR.090201. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]