Abstract
Correlations among bone strength, muscle mass, and physical activity suggest that these traits may be modulated by each other and/or by common genetic and/or environmental mechanisms. This study used structural equation modeling (SEM) to explore the extent to which select genetic loci manifest their pleiotropic effects through functional adaptations commonly referred to as Wolff's law. Quantitative trait locus (QTL) analysis was used to identify regions of chromosomes that simultaneously influenced skeletal mechanics, muscle mass, and/or activity-related behaviors in young and aged B6×D2 second-generation (F2) mice of both sexes. SEM was used to further study relationships among select QTLs, bone mechanics, muscle mass, and measures of activity. The SEM approach provided the means to numerically decouple the musculoskeletal effects of mechanical loading from the effects of other physiological processes involved in locomotion and physical activity. It was found that muscle mass was a better predictor of bone mechanics in young females, whereas mechanical loading was a better predictor of bone mechanics in older females. An activity-induced loading factor positively predicted the mechanical behavior of hindlimb bones in older males; contrarily, load-free locomotion (i.e., the remaining effects after removing the effects of loading) negatively predicted bone performance. QTLs on chromosomes 4, 7, and 9 seem to exert some of their influence on bone through actions consistent with Wolff's Law. Further exploration of these and other mechanisms through which genes function will aid in development of individualized interventions able to exploit the numerous complex pathways contributing to skeletal health.
Key words: quantitative trait locus, structural equation modeling, bone mechanics, physical activity, mice
INTRODUCTION
The development and maintenance of adequate bone strength is a complex process that involves both direct and indirect genetic and environmental pathways. Previous studies have shown that increases in loading frequency, strain, and strain rate, above maintenance thresholds, increase bone formation,(1,2) whereas decreases in strain magnitude result in increased bone resorption or bone loss.(3) Variations in peak bone mass and rates of bone loss are therefore thought to be influenced by variations in muscle mass or muscle strength,(4–6) as well as the frequency and duration of activity.(7,8)
The highest routinely applied loads experienced by bone come from the muscles used to maintain posture and produce movement. In general, the moment arms (i.e., distance to the center of joint rotation) through which muscles act are much shorter that those through which environmental forces act; thus, proportionately greater muscle forces are required to balance the external forces imposed on the body.(9) Frost(10) suggested that age-related changes in bone mass follow the age-related increases in muscle strength occurring during growth, the plateau in strength seen in young adults, and the decline after 30–40 yr of age. During the plateau, the skeleton has adapted to stronger young-adult muscles. With age, decreases in muscle mass, strength, and activity level produce corresponding declines in strain magnitude that eventually fall below a threshold required for bone maintenance, and bone loss ensues. Along these same lines of reasoning, interindividual variations in muscle mass and strength or the frequency and duration of activity may produce correlated variations in peak bone mass and rates of bone loss. Because peak bone mass has been shown to predict fracture risk, insight into the mechanisms through which genes influence both bone acquisition and the rate of bone loss could lead to new methods for preventing osteoporosis and its attendant increased risk of fracture late in life.
The continuous distribution of skeletal phenotypes such as BMD or whole bone breaking strength indicates that they are complex traits regulated through a myriad of interconnected pathways that originate through gene expression. Several such genes may affect bone through actions on muscle and behavior. Twin studies in humans(11) and studies using inbred mice(12–15) have confirmed that bone properties are highly heritable. Previous studies have also shown that muscle mass is significantly correlated with BMD in humans(4) and in mice(5) and that physical activity influences skeletal quality.(7,8) Kaye and Kusy(7) studied these relationships across several mouse strains and suggested that activity as well as muscle size and strength are genetically influenced and that part of the genetic influence on bone size and strength is modulated through activity and muscle size.
The study reported here used F2 mice derived from C57BL/6J (B6) and DBA/2J (D2) inbred strains. B6 mice have been shown to be more active compared with D2 mice.(16–19) This is exemplified in the findings of Lerman et al.,(19) who showed that B6 mice voluntarily ran the greatest exercise wheel distance, duration, and speed compared with several inbred mouse strains including D2 mice.(19) The genetic influence on skeletal differences observed in B6 and D2 mouse strains could therefore be at least partially modulated by genetically induced differences in activity level, as well as a differential skeletal response to loading and environmental factors. A pleiotropic approach to the study of musculoskeletal relationships is not a novel concept and has received more attention in recent years. For a recent review, the reader is referred to Karasik and Kiel,(20) who point out that the sources of covariation between muscle and bone could occur during patterning in embryonic life, allometric growth, and the homeostatic period of adult life.(21)
This report explores simultaneous relationships among measures of muscle strength (implied by muscle mass), skeletal integrity, physical activity–related behaviors, and genetic loci affecting these traits in F2 progeny of B6 and D2 inbred mice. Muscle mass, several skeletal phenotypes, and activity measures from F2 mice were used in quantitative trait locus (QTL) analyses. Chromosomal regions that were identified from the QTL analyses to have pleiotropic effects across at least two of the three domains (bone, muscle, or activity) were selected for follow-up analyses using structural equation modeling (SEM). Our aim was to learn the extent to which the gene or genes functioning within select pleiotropic loci contribute to bone strength through their actions on behavior and/or muscles, which is conveyed to bone through functional adaptations commonly referred to as Wolff's Law.
MATERIALS AND METHODS
Overview
Activity-related behavioral measures, muscle mass, and skeletal phenotypes were assessed in young adult (n = 191 males and 185 females) and aged (n = 187 males and 185 females) F2 progeny of DBA/2J (D2) and C57BL/6J (B6) inbred mouse strains. These measures were used for QTL analysis and SEM. The same measures were also obtained from a second population of 23 B×D recombinant inbred (RI) mouse strains with 10 male and 10 female mice per strain. RI data were used as a means of comparing our activity measures to various measures of activity found in the literature.
Animals
Breeding and maintenance of progenitor and RI strains, and F2 animals derived from the progenitor strains, were conducted in the barrier facility maintained by The Center for Developmental and Health Genetics at The Pennsylvania State University. Mice were weaned into like-sex sibling groups at ∼23 days of age with four animals per cage. They were fed a diet of autoclaved Purina Mouse Chow 5010 (content: 1.0% calcium, 0.67% phosphorus, 0.22% magnesium, and 4.4 IU/g vitamin D) ad libitum designed to be equivalent, after autoclaving, to Purina 5001 (content: 0.95% calcium, 0.67% phosphorus, 0.21% magnesium, and 4.5 IU/g vitamin D). The barrier facility was maintained under positive pressure with a temperature- and humidity-controlled environment and a 12-h light/dark cycle.
Animal activity measures
Several assessments of activity-related behaviors were recorded on each animal. Measurements began at ∼130 days of age for the F2 young animals, 150 days of age for the RI young animals (±2 wk), and 430 days of age for the F2 aged animals. Measurements of each activity were made on each F2 animal at 1-mo increments on three separate occasions. The mean of the three measurements was used for subsequent analyses in the F2 cohorts. For RI animals, the mean of each RI strain was used for comparison with relevant activity data found in the literature.
Activity was measured in a 40 × 40 × 15-cm-deep, black opaque plastic arena marked into four quadrants with a 15-mm hole cut in the center of each quadrant. Animals were consistently tested, starting early in the light phase of the 12/12 light/dark cycle, typically 8:00–9:00 a.m. The test room was dimly illuminated with the test equipment illuminated using a red light. Animals were individually placed into a clear plastic cylinder in the center of the arena. After 10 s, the cylinder was lifted, and each mouse was observed for 3 min. The number of quadrants entered and the number of times the animal reared onto its hind limbs were recorded. Hind limb rearings were not distinguished from wall-leanings (i.e., a rearing that touched the wall was counted as a rear). We interpreted the number of quadrants entered as a measure of general locomotor activity and hind limb rearing as more similar to resistance-type exercise or training.
Genotyping
F2 mice were genotyped in-house using 96 microsatellite markers distributed throughout the genome, with an average spacing of 15–20 cM. Marker analyses were conducted on purified DNA samples procured from tail snips using an automated, fluorescence-based detection system described in detail in Vandenbergh et al.(22)
Tissue harvesting and muscle mass measurement
Animal weights were recorded before death by cervical dislocation at ∼200 days of age for the young cohort and 500 days of age in the aged cohort. Nose-to-anus length was recorded immediately after death. The gastrocnemius, soleus, tibialis anterior, and extensor digitorum longus muscles were carefully removed from the right hind limb and weighed to the nearest hundredth of a milligram. The right hind limb of each animal was harvested, and the tibia and femur were cleaned, wrapped in saline soaked gauze, and stored at −20°C for future mechanical testing.
Skeletal assessment
At the time of testing, the tibia and femur were thawed at ambient temperature. The midshaft of the right tibia and femur were tested to failure in three-point bending using a Materials Testing System (MTS) MiniBionix testing apparatus with a support span of either 10 (tibia) or 8 mm (femur) and a deformation rate of 1 mm/min. Tibias were oriented so that the nosepiece was anteriorly directed with respect to the tibial shaft. This was necessary to prevent the tibia from rolling during testing. Femurs were loaded from anterior to posterior. Each tibia and femur were loaded to failure while recording load and actuator displacement at 20 Hz, and a load-deformation curve was generated using MATLAB scientific software (version 6.5, release 13; MathWorks). Yield load, yield deformation, energy absorbed at yield (area under the load-deflection curve), failure load, failure deformation, energy absorbed at failure, and stiffness (initial slope of the load-deflection curve) were determined.
Data analyses
Sex and age group differences and descriptive statistics:
Before analysis, all phenotypic data were screened for normality and, when necessary, transformed using either a natural log or square root transformation. Group differences were determined from a two-way analysis of variance (ANOVA) and covariance (ANCOVA) using a multivariate general linear model in SPSS 16.0 statistical software. The main effects of sex and age and the interaction of sex and age were determined for body size measures from ANOVA; for all other measures, an ANCOVA was performed with body weight and body length as covariates. Within each sex separately, pairwise comparisons for age differences were performed, and body size–adjusted means and SEs were obtained.
Assessment of activity-related behavioral measures:
To verify the suitability of our behavioral measures as measures of activity, we compared the within-strain means for quadrants entered and hind limb rears, from our B×D RI animals, with other measures of activity performed on the same B×D RI strains in previous studies (http://www.genenetwork.org/). The activity-related behaviors, as measured in our B×D RI strains, were significantly correlated to other more commonly used measures of activity performed in previous studies. The number of quadrants entered was significantly correlated with distance traveled (r = 0.62, p < 0.01(23); r = 0.74, p < 0.0005(24); r = 0.64, p < 0.01(25); and r = 0.67, p < 0.005(26)), and hind limb rears was significantly correlated with rearings (r = 0.65, p < 0.005).(24)
QTL analysis
Genome-wide QTL analyses were performed on the F2 cohort to locate chromosomal regions influencing the continuously distributed phenotypic measures, as previously reported for muscle and bone from individual age groups.(15, 27–30) QTL analyses were conducted using QTL Cartographer software to perform interval mapping.(31) LOD scores of 4.3 or greater were considered significant, whereas scores between 2.8 and 4.3 were considered suggestive.(32) Analyses were performed on pooled data from the 200- and 500-day-old male and female cohorts unadjusted for age or sex and also on pooled data for each sex separately. In each case, analyses were conducted using both raw, unadjusted data and then data adjusted for body weight and length through multiple regression.(33) QTLs identified as having significant pleiotropic effects on phenotypic measures from at least two of the three domains (activity, muscle, or bone) were selected to be included in subsequent structural equation models.
SEM specification and testing
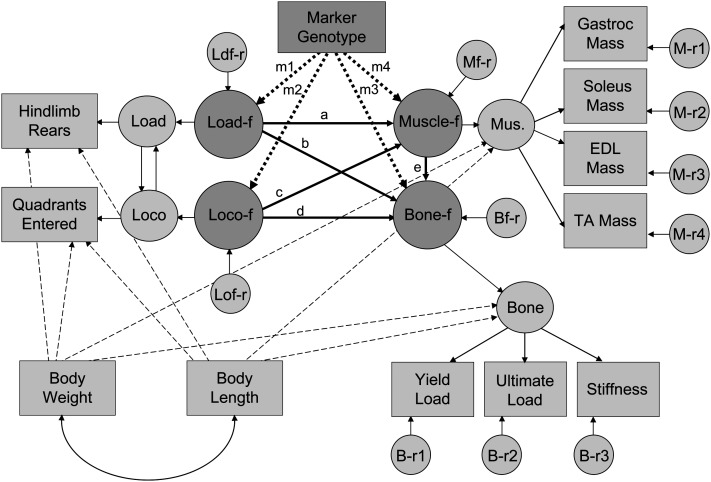
SEM was performed using Amos 7.0 statistical software, and parameter estimates were obtained using a full information maximum likelihood algorithm. A model was developed to study the hypothesized relationships among skeletal quality, muscle mass, and activity-related behavior (Fig. 1). The model included latent variables representing a bone factor (indicated by unadjusted measures of yield load, ultimate load, and stiffness), a muscle factor (indicated by unadjusted measures of gastrocnemius, soleus, extensor digitorum longus, and tibialis anterior muscle masses), locomotion-free loading (indicated by quadrants entered residualized from hind limb rearing), and loading-free locomotion (indicated by rearing residualized from quadrants entered). Quadrants entered and hind limb rearing were highly correlated (r = 0.73; p < 0.001).
FIG. 1.
Full path diagram for the tibia and femur SEM model. Model A (without genetic marker) does not include paths m1–m4. Model B (with genetic marker) includes all paths. Rectangles, measured variables; large circles, latent factors; small circles, residual variance.
Whereas both quadrants entered and hind limb rearing are indicators of the animals' behavioral activities, we reasoned that the two types of activity could have different effects on the musculoskeletal system, particularly if the more intense skeletal loading associated with hind limb rearing could be effectively separated from the other physiological effects of routine ambulation. We therefore removed the correlation between hind limb rearing and quadrants entered by regressing them on each other to isolate unique aspects of rearing that are separate from quadrants entered and unique aspects of quadrants entered that are separate from rearing. In the first case, the unique aspects should include enhanced skeletal loading without the repetitive low-load component associated with normal ambulation, a model deemed to be more representative of resistance type exercise. The latter case includes routine ambulatory motions devoid of high skeletal loads, which is more representative of aerobic type activity. Regressing one on the other allowed the separate effects of loading and locomotion to be independently analyzed.
In contrast to the QTL analyses, unadjusted musculoskeletal and activity measures were used in the SEM model; however, body weight and body length were included in the model as independent predictors to account for the known association of body size with musculoskeletal measures. The body size adjustment was made at an intermediate level between the phenotypic measures and the main structural factors (Fig. 1).
Two different SEM models were evaluated. Model A examined structural paths without accounting for the genotype, whereas model B included animal genotype at selected loci. As shown in Fig. 1, the main structural paths connecting the primary factors after the body size adjustments are labeled a–e. The primary relationships of interest in model A included the structural paths for loading and locomotion on the muscle factor (paths labeled a and c, respectively), loading and locomotion on the bone factor (paths labeled b and d, respectively), and the muscle factor on the bone factor (path labeled e). Model B tested additional structural paths, m1–m4, by adding the genotype the genetic marker closest to select QTL identified as having potentially pleiotropic effects across at least two of the three domains of activity, muscle, and bone. Models A and B were evaluated using skeletal measures from femur and tibia separately.
Paths a–e and m1–m4, as well as the influence of body weight and length on the muscle and bone factors and the quadrants entered and hind limb rearing measurements, were estimated. Standardized (SRW) and unstandardized regression weights (URW), SEs, and critical ratios (CR=URW/SE) for the tibia and femur models were determined. The critical ratio was tested against a t-distribution to determine whether the path coefficient was significantly different than zero.
RESULTS
General trends in measured phenotypes
Female mice had smaller muscles, were less active (fewer quadrants entered and rearings), and had bones that were weaker than their male counterparts. Body weight increased, and hind limb rearing decreased as a function of age in both sexes (Table 1). When controlling for body weight and length, muscle mass as well as yield and ultimate load in the tibia and femur decreased with age in both males and females. The stiffness of both bones increased as consequence of age.
Table 1.
Descriptive Statistics: Means and SEs for Young and Old Female and Male Cohorts
|
Females |
Males |
||||
| Young | Old | Young | Old | ||
| Phenotypic measure | Group differences | (n = 185) | (n = 185) | (n = 191) | (n = 187) |
| Body weight (g) | a s i | 28.19 | 36.30 | 39.30 | 47.20 |
| (0.44) | (0.44) | (0.43) | (0.44) | ||
| Body length (cm) | a s | 9.75 | 10.33 | 10.36 | 10.98 |
| (0.03) | (0.03) | (0.03) | (0.03) | ||
| Body mass index (kg/m2) | a s i | 2.95 | 3.35 | 3.66 | 3.90 |
| (0.03) | (0.03) | (0.03) | (0.03) | ||
| Cohort means adjusted for size covariates (body weight and length) | |||||
| Hind limb rears (#) | a s i | 11.09 | 9.81 | 14.43 | 11.49 |
| (0.56) | (0.56) | (0.64) | (0.68) | ||
| Quadrants entered (#) | s i | 20.05 | 23.32 | 23.83 | 23.99 |
| (0.86) | (0.85) | (0.91) | (0.96) | ||
| Gastrocnemius (mg) | a s i | 118.7 | 111.3 | 153.4 | 135.0 |
| (0.90) | (0.91) | (1.21) | (1.28) | ||
| Soleus (mg) | a s | 8.31 | 7.68 | 10.62 | 9.60 |
| (0.12) | (0.12) | (0.14) | (0.15) | ||
| Extensor digitorum longus (mg) | a s | 9.15 | 9.07 | 11.79 | 10.82 |
| (0.10) | (0.10) | (0.12) | (0.13) | ||
| Tibialis anterior (mg) | a s | 42.74 | 40.99 | 54.14 | 51.12 |
| (0.31) | (0.31) | (0.42) | (0.44) | ||
| Tibia yield load (N) | a i | 11.94 | 11.04 | 14.00 | 11.16 |
| (0.17) | (0.17) | (0.24) | (0.25) | ||
| Tibia ultimate load (N) | a s | 13.66 | 12.01 | 15.94 | 13.65 |
| (0.18) | (0.18) | (0.24) | (0.25) | ||
| Tibia stiffness (N/mm) | a s i | 56.55 | 62.07 | 70.58 | 81.80 |
| (0.90) | (0.90) | (1.40) | (1.48) | ||
| Femur yield load (N) | a s i | 15.80 | 15.23 | 16.49 | 14.40 |
| (0.26) | (0.26) | (0.24) | (0.26) | ||
| Femur ultimate load (N) | a s i | 19.62 | 18.98 | 20.53 | 18.21 |
| (0.30) | (0.30) | (0.26) | (0.27) | ||
| Femur stiffness (N/mm) | a s i | 110.3 | 154.0 | 108.6 | 139.2 |
| (2.7) | (2.7) | (2.3) | (2.4) | ||
Group Differences (p ≤ 0.05) from ANOVA/ANCOVA as a function of age (a), sex (s), and sex by age interactions (i). Shading indicates significant within-sex age differences (p ≤ 0.05).
Structural equation model A: pooled data
Table 2 presents the standardized regression weights from tibial and femoral models run on pooled data and data from the four individual cohorts. Models run on combined data from all groups revealed significant positive associations of both body weight and length with the muscle factor, but only body weight was significantly associated with the bone factor. Body weight was negatively associated with quadrants entered indicating that larger animals were less active. Hind limb rearing was not significantly associated with either body weight or length. When controlling for body weight and length, locomotion-free loading was positively associated with tibial bone mechanics (β = 0.215) and muscle mass (β = 0.255), and muscle was positively associated with tibial bone mechanics (β = 0.365).
Table 2.
Tibia and Femur Model A (Without Genetic Marker)
|
Tibia model
|
Femur model
|
||||||||||||
| All Groups |
Females
|
Males
|
All Groups |
Females
|
Males
|
||||||||
| SEM model paths: model A | Young | Older | Young | Older | Young | Older | Young | Older | |||||
| Bone | ← | Loading | (b) | 0.215 | 0.127 | 0.246 | 0.211 | 0.129 | 0.049 | 0.230 | −0.066 | −0.010 | 0.267 |
| Bone | ← | Locomotion | (d) | −0.055 | 0.000 | 0.191 | −0.131 | −0.152 | −0.061 | −0.020 | 0.055 | 0.028 | −0.163 |
| Muscle | ← | Loading | (a) | 0.255 | 0.004 | 0.039 | −0.075 | 0.037 | 0.258 | 0.008 | 0.041 | −0.076 | 0.038 |
| Muscle | ← | Locomotion | (c) | 0.059 | 0.107 | 0.066 | 0.134 | −0.007 | 0.059 | 0.104 | 0.065 | 0.135 | −0.005 |
| Bone | ← | Muscle | (e) | 0.365 | 0.274 | 0.110 | 0.267 | 0.260 | 0.073 | 0.224 | 0.232 | 0.246 | 0.114 |
| Muscle | ← | Length | 0.123 | 0.303 | 0.228 | 0.379 | 0.163 | 0.122 | 0.301 | 0.230 | 0.379 | 0.161 | |
| Muscle | ← | Weight | 0.611 | 0.295 | 0.380 | 0.303 | 0.340 | 0.612 | 0.300 | 0.378 | 0.303 | 0.339 | |
| Bone | ← | Length | −0.080 | 0.036 | 0.153 | −0.014 | 0.143 | −0.051 | 0.178 | 0.251 | 0.130 | −0.056 | |
| Bone | ← | Weight | 0.293 | 0.112 | 0.192 | 0.254 | −0.006 | 0.260 | 0.125 | 0.044 | 0.360 | 0.289 | |
| Quadrants | ← | Length | 0.157 | 0.122 | −0.057 | 0.046 | 0.165 | 0.157 | 0.123 | −0.056 | 0.044 | 0.163 | |
| Rears | ← | Length | 0.017 | 0.057 | 0.044 | 0.026 | 0.043 | 0.018 | 0.058 | 0.047 | 0.024 | 0.041 | |
| Quadrants | ← | Weight | −0.162 | −0.153 | −0.047 | −0.268 | −0.342 | −0.162 | −0.154 | −0.047 | −0.266 | −0.341 | |
| Rears | ← | Weight | −0.048 | −0.047 | −0.081 | −0.195 | −0.228 | −0.048 | −0.048 | −0.082 | −0.193 | −0.227 | |
| Loading | ↔ | Locomotion | 0.360 | 0.370 | 0.395 | 0.297 | 0.316 | 0.360 | 0.370 | 0.395 | 0.297 | 0.316 | |
Standardized regression weights for pooled data and young and old female and male cohorts. Shading indicates paths that were significant at p ≤ 0.05; bold italics indicates paths that were suggestive at p ≤ 0.10. Paths a–e are indicated on Fig. 1. and were allowed to be free across groups.
Structural equation model A: sex- and age-dependent analyses
As with the pooled data set, body weight and length were significant predictors of the muscle factor in all age and sex subgroups except older males (Table 2). Body weight was also a significant predictor of the tibial factor in older females and young males and the femoral factor in young and older males, whereas body length was a significant predictor of femoral bone mechanics in older females. Body weight and length were not associated with hind limb rearing or quadrants entered in females, but body weight significantly predicted these activity measures in young and older males.
The associations between loading and the muscle factor that were noted for the pooled data set were not evident in models run separately for each sex and age; however, loading was positively associated with the tibial factor in older females and young males and the femoral factor in young females and older males. A positive association of muscle mass with bone mechanics was identified in all cohorts except older female tibia and older male femur. Perhaps the most striking result, found in both the tibial and femoral models for older males, was that locomotion was negatively associated with bone mechanics.
QTL analyses
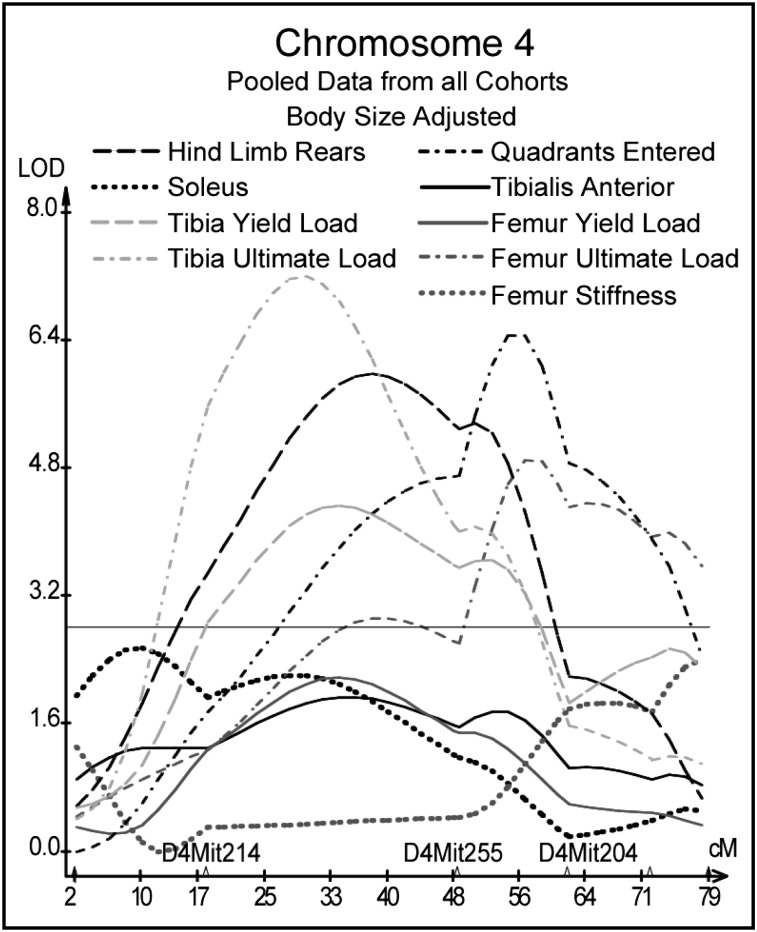
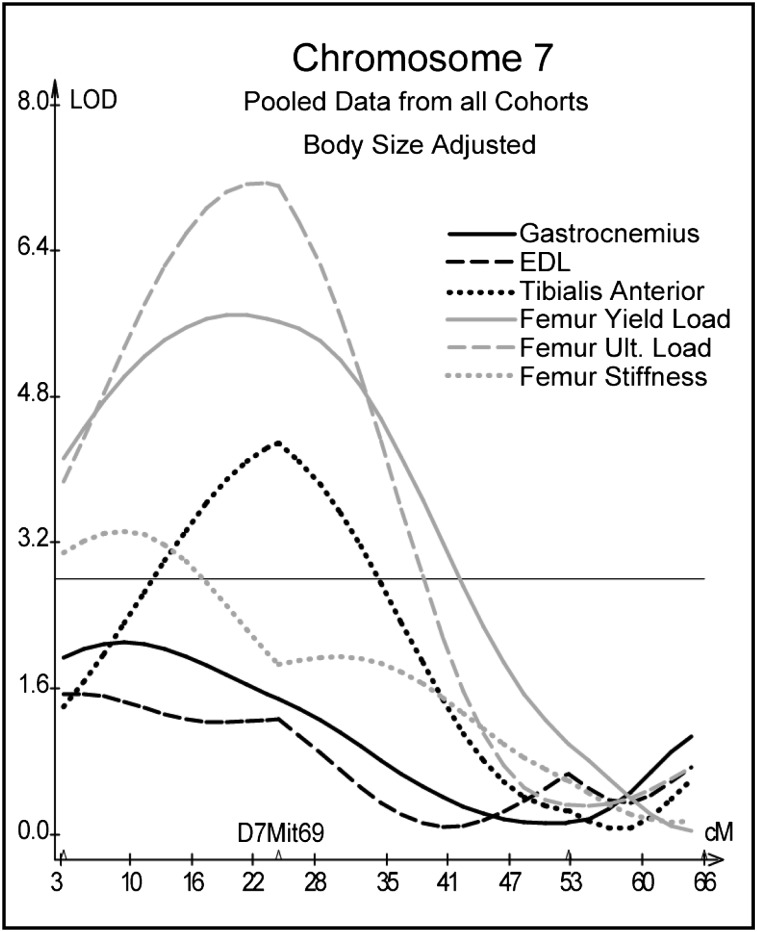
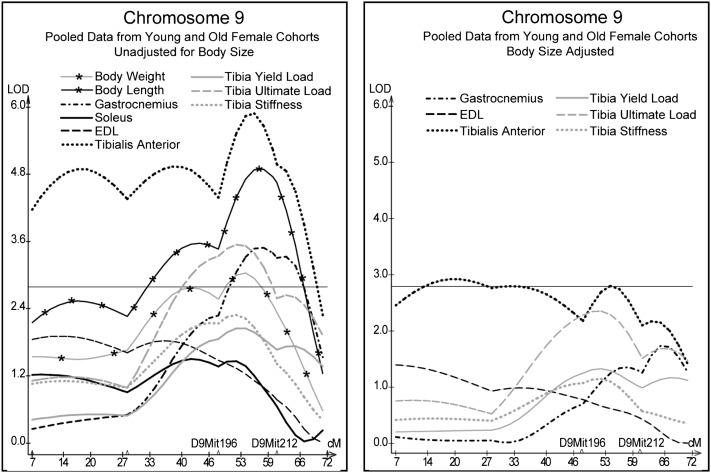
QTL analyses of pooled data from the 200-day and 500-day F2 cohorts revealed QTL for phenotypic measures from at least two of the three domains of activity, muscle, and bone on chromosomes 4, 7, and 9. On Chr 4, hind limb rears and tibial yield and ultimate load co-located near marker D4Mit255 at 48.5 cM; quadrants entered and femur ultimate load co-located near marker D4Mit204 at 61.9 cM (Fig. 2). Muscle and skeletal measures for the femur mapped near marker D7Mit69 at 24.5 cM on Chr 7 (Fig. 3). Unadjusted muscle and skeletal measures for females mapped to Chr 9 between markers D9Mit196 at 48 cM and D9Mit212 at 61 cM. Although present, corresponding peaks for size adjusted data were much less impressive and not significant (Fig. 4).
FIG. 2.
Interval mapping results on Chr 4 for body size–adjusted residuals for activity, muscle, tibia, and femur measures pooled from all cohorts. LOD scores are plotted against centimorgan position along the chromosome. The horizontal line indicates the suggestive LOD score threshold of 2.8.
FIG. 3.
Interval mapping results on Chr 7 for body size–adjusted residuals for muscle and femur measures pooled from all cohorts. See Fig. 2 legend.
FIG. 4.
Interval mapping results on Chr 9 for unadjusted and body size–adjusted residuals for muscle and tibia measures pooled from young and old female cohorts. See Fig. 2 legend.
Structural equation model B
Tables 3 and 4 present standardized regression weights from the tibial models incorporating genotype at markers D4Mit255 and D9Mit212 and the femoral models incorporating genotype at markers D4Mit204 and D7Mit69. Within the tibial model, marker D4Mit255 was positively linked to loading in young females, locomotion in young and older females, and bone mechanics in older females. This marker was negatively linked to muscle and positively linked to bone mechanics in young males (Table 3). Within the femoral models, marker D4Mit204 was positively linked to locomotion in young and older females and bone mechanics in older females and young and older males (Table 4). The markers on Chr 7 and 9 were significantly linked to both muscle and bone mechanics in some of the subgroups. Marker D7Mit69 was positively linked to both muscle and bone mechanics in older females and young males and bone mechanics in older males (Table 4). Marker D9Mit212 was negatively linked to muscle and bone mechanics in young females, bone mechanics in older females, and muscle in older males (Table 3).
Table 3.
Tibia Model B (With Genetic Marker)
|
Marker: D4Mit255
|
Marker: D9Mit212
|
|||||||||
|
Females
|
Males
|
Females
|
Males
|
|||||||
| SEM for tibia model B paths | Young | Old | Young | Old | Young | Old | Young | Old | ||
| Loading | ← | Marker | 0.191 | 0.131 | 0.069 | 0.048 | −0.059 | 0.049 | 0.050 | 0.050 |
| Locomotion | ← | Marker | 0.201 | 0.204 | 0.012 | 0.017 | 0.013 | 0.003 | 0.001 | −0.051 |
| Muscle | ← | Marker | −0.131 | −0.139 | −0.221 | −0.122 | −0.189 | −0.023 | −0.093 | −0.389 |
| Bone | ← | Marker | 0.078 | 0.173 | 0.308 | 0.132 | −0.189 | −0.146 | −0.048 | 0.041 |
| Bone | ← | Loading | 0.106 | 0.220 | 0.187 | 0.111 | 0.109 | 0.262 | 0.215 | 0.122 |
| Bone | ← | Locomotion | −0.022 | 0.142 | −0.141 | −0.152 | 0.013 | 0.190 | −0.132 | −0.148 |
| Muscle | ← | Loading | 0.037 | 0.055 | −0.048 | 0.048 | −0.012 | 0.042 | −0.069 | 0.091 |
| Muscle | ← | Locomotion | 0.139 | 0.104 | 0.136 | −0.007 | 0.112 | 0.066 | 0.132 | −0.048 |
| Bone | ← | Muscle | 0.283 | 0.135 | 0.341 | 0.282 | 0.238 | 0.111 | 0.262 | 0.276 |
Standardized regression weights with genetic marker D4Mit255 (left) and marker D9Mit212 (right) for young and old female and male data. Shading indicates paths that were significant at p ≤ 0.05; bold italics indicates paths that were suggestive at at p ≤ 0.10.
Table 4.
Femur Model B (With Genetic Marker)
|
Marker: D4Mit204
|
Marker: D7Mit69
|
|||||||||
|
Females
|
Males
|
Females
|
Males
|
|||||||
| SEM for femur model B paths | Young | Old | Young | Old | Young | Old | Young | Old | ||
| Loading | ← | Marker | 0.100 | 0.038 | 0.041 | 0.042 | 0.032 | −0.031 | −0.077 | −0.069 |
| Locomotion | ← | Marker | 0.182 | 0.199 | 0.083 | 0.075 | −0.049 | 0.084 | −0.014 | 0.030 |
| Muscle | ← | Marker | −0.121 | −0.087 | −0.054 | −0.097 | 0.028 | 0.342 | 0.246 | 0.142 |
| Bone | ← | Marker | 0.088 | 0.164 | 0.215 | 0.172 | 0.146 | 0.274 | 0.289 | 0.238 |
| Bone | ← | Loading | 0.218 | −0.070 | −0.010 | 0.258 | 0.219 | −0.035 | 0.051 | 0.310 |
| Bone | ← | Locomotion | −0.045 | 0.006 | −0.005 | −0.176 | −0.007 | 0.019 | 0.046 | −0.174 |
| Muscle | ← | Loading | 0.021 | 0.040 | −0.073 | 0.042 | 0.006 | 0.076 | −0.033 | 0.062 |
| Muscle | ← | Locomotion | 0.132 | 0.093 | 0.146 | 0.005 | 0.107 | 0.013 | 0.139 | −0.014 |
| Bone | ← | Muscle | 0.232 | 0.245 | 0.259 | 0.127 | 0.220 | 0.145 | 0.182 | 0.081 |
Standardized regression weights with genetic marker D4Mit204 (left) and marker D7Mit69 (right) for young and old female and male data. Shading indicates paths that were significant at p ≤ 0.05; bold italics indicates paths that were suggestive at at p ≤ 0.10.
DISCUSSION
Structural equation model A
Mechanical loading, from both aerobic and resistance exercise, has been shown in both human(34,35) and animal(36) studies to produce an osteogenic response. We therefore expected that higher levels of activity would be associated with stronger bone and indeed we found that both hind limb rearing and quadrants entered were positively correlated with all three tibial measures. SEM modeling using residualized scores for locomotion-free loading and loading-free locomotion confirmed a positive association between loading and bone mechanics in both sexes, but also revealed an interesting sex-dependent association between locomotion and bone mechanics that was positive in females and negative in males.
These findings suggest a differential response of bone to locomotion and loading as a function of sex and age. The positive effects of loading on bone are well documented; the negative effects of locomotion are more difficult to interpret. One possible explanation could be that a greater level of general locomotion (quadrants entered) results in a higher level of energy expenditure, thereby reducing nonlean mass (fat). Supporting this argument is the observed negative association between body weight and activity measures in males. More active animals had lower body weight, presumably because of enhanced energy use with attendant decreases in fat mass. Previous studies have shown within-subject reductions in bone mass as a consequence of weight loss,(37–38) but the extent to which body weight induces alterations in skeletal loading and bone mass across individuals is unknown.
A study by Stewart et al.(39) examined the relationships of physical activity level, fitness, and body composition on BMD in older men and women. In women, body weight, fat mass, abdominal total fat, and lower and upper body strength were all highly correlated with BMD at the spine, hip, and total skeleton. In men, abdominal total fat was positively correlated with lumbar spine BMD, lower body strength was positively correlated with total hip BMD, and, similar to our findings in mice, daily physical activity was negatively correlated with lumbar spine BMD. The authors suggested that this finding could be explained by a trend of lower energy expenditure in more obese men.
A positive association between loading and bone mechanics was evident in the tibia of young females but only reached significance in the older female cohort. With age, rearing decreased in both sexes, although only significant in males, as did muscle mass after correcting for body size. Human studies have shown sex differences in the decline of muscle force and cross-sectional area with a significant decline in women around menopause and men at age 75.(40) A decrease in muscle strength with age was suggested by Frost(10) to place bones previously adapted to stronger young-adult muscles in a state of partial and gradual-onset disuse. In this study, the decrease in muscle mass and activity level may have precipitated these types of adaptations, thereby strengthening the relationship between loading and bone mechanics in older females. The decrease in rearing and muscle mass combined with a significant increase in quadrants entered could have contributed to the strengthened relationship of locomotion with bone mechanics in older females.
QTL analyses
Correlated phenotypes of muscle mass, bone mechanics, and activity often mapped in close proximity to each other indicating potential pleiotropic genetic effects. However, co-localization of correlated phenotypes to the same broad chromosomal region is not definitive evidence that all are under the control of any single gene or even a subset of genes. The broad marker spacing used in this QTL analyses is a limiting factor in teasing out pleiotropic affects and in the identification of potential genes of interest. The chromosomal regions identified here could include many genes and the exact locations of the gene or genes influencing the quantitative traits examined are unknown. High-resolution mapping will be required to distinguish between true pleiotropy and linkage of multiple QTLs. Nevertheless, anonymity of the gene or genes at work does not prevent exploration of the phenotypic relationships that they modulate.
Genetic influence of chromosome 4: markers D4Mit255 and D4Mit204 (model B)
A QTL for activity measures was identified near marker D4Mit255 (48.5 cM) in this study. Henderson et al.(41) previously reported a QTL on Chr 4 at 52 cM that influenced general locomotor activity levels in 70-day-old male and female F2 mice derived from inbred mice that were bidirectionally selected for open field activity. Results from the tibial model suggest that a gene or genes near marker D4Mit255 on Chr 4 has an influence on activity in females throughout young adulthood into old age, but the associated loading and locomotion does not produce a measurable effect on bone until old age, consistent with findings from model A.
Marker D4Mit255 was linked to bone mechanics in young males, with a similar trend in older males, but was not linked to loading or locomotion, suggesting that genes in this vicinity do not exert their effects on bone through mechanisms related to Wolff's Law. Interestingly, the adjacent marker, D4Mit204 at 61.9 cM, was significantly linked to tibial bone mechanics (results not shown) and femoral bone mechanics in both young and older males and femoral mechanics in older females.
Genetic influence of chromosome 7: marker D7Mit69 (model B)
Both muscle mass and femoral measures peaked near marker D7Mit69 on Chr 7. Body weight also mapped to this site. The D2 allele had an increasing effect on the muscle and skeletal measures, whereas the B6 allele had an increasing additive effect on body weight. When adjusting muscle and skeletal measures for body size, the LOD scores increased. For example, the LOD score increased from 4.3 to 7.2 for femoral ultimate load and from 0.3 to 4.3 for tibialis anterior muscle mass. Removing the variance associated with body size increased the significance of the skeletal QTL by decreasing the residual “error” variance relative to the true phenotypic value.(33)
Marker D7Mit69 was significantly linked to both muscle and femoral bone mechanics in older females. There was also the suggestion of a positive linkage between the muscle factor and bone mechanics in model B and a definite linkage when the marker is omitted (model A). Thus, it seems that this site affects female bone mechanics through muscle action but also through other as yet undefined pathways. In males, the results suggest that the influence of this QTL on muscle size was much stronger early in growth and development and acted on bone through muscle as evidenced by the significant path from the muscle factor to the bone factor in young males. With age, muscle mass could change more rapidly than bone mechanics irrespective of genotype, thereby reducing the association between the marker and muscle while maintaining a significant linkage between the marker and bone.
Whereas QTL analyses are limited in the precision with which a locus is mapped, identifying potential candidate genes within the region of a QTL is often the first step taken to elucidate the underlying mechanisms and genes responsible for the association. There are two candidate genes near marker D7Mit69 (23 cM) worth mentioning. Myogenic differentiation factor 1 (MyoD1) is located at 23.5 cM (MGI database 7–15–08) on Chr 7. MyoD1 is a muscle regulatory factor with the ability to convert fibroblasts to muscle cell lineages.(42) Variations in muscle cell differentiation could be contributing to variations in muscle mass and the corresponding covariation with bone strength. A second candidate gene, IGFI receptor (Igf1r), is located at 33 cM on Chr 7. IGF1 is associated with increased bone strength and muscle volume and strength,(43) and variations in the Igf1 receptor at the locus on Chr 7 could be contributing to the covariance of muscle and bone.
Genetic influence of chromosome 9: marker D9Mit212 (model B)
Similar to the site on Chr 7, body weight and body length also co-located with muscle mass and skeletal phenotypes; however, on Chr 9, the B6 genotype contributed the increasing allele for all phenotypes. In this case, the most convincing QTL for muscle and skeletal measures on Chr 9 were from female data that were unadjusted for body size. When muscle and skeletal measures were corrected for body size, the LOD scores lost significance. Despite the negative impact of size correction in the QTL analysis, marker D9Mit212 remained significantly linked to the muscle and bone factors in the SEM analysis even when controlling for body weight and length. Similar to the results for marker D7Mit69 (in males), this QTL could be influencing muscle size and strength during growth and development resulting in larger and or stronger bones; with age, muscle mass varies because of other factors, whereas bone mechanics maintains its linkage with marker D9Mit212.
Conclusions
The complex system responsible for bone adaptation, and ultimately fracture resistance, extends beyond the skeletal system to encompass other physiologic processes. Principal among these may be components related to muscle mass and force generation as well as components dictating locomotion and activity-related behaviors, because these contribute substantially to the loads borne by the skeleton.
QTL results indicate that a gene or genes on Chr 4 has pleiotropic effects on muscle mass, activity, and bone mechanics and genes on Chrs 7 and 9 affect muscle mass and bone mechanics. Follow-up SEM showed that the effects of frequency and magnitude of loading on the skeleton were both sex and age dependent. Muscle mass was the best predictor of bone mechanics in young female mice, whereas loading was a better predictor in older females, suggesting that skeletal loading through resistance type activities is closely linked to skeletal health in older females. Both loading and muscle mass predicted bone mechanics in males. Interestingly, locomotion was negatively associated with bone mechanics in older males, suggesting again that more resistance type activities may be better able to maintain bone health in older individuals. Further exploration of these and other mechanisms through which genes function will aid in the development of individualized interventions able to exploit the numerous complex pathways contributing to skeletal health.
ACKNOWLEDGMENTS
This work was supported by Grants P01 AG14731 and R01 AG21559 and Training Grant AG00276 from the National Institute on Aging of the National Institutes of Health.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Turner CH, Forwood MR, Otter MW. Mechanotransduction in bone: Do bone cells act as sensors of fluid flow? FASEB J. 1994;8:875–878. doi: 10.1096/fasebj.8.11.8070637. [DOI] [PubMed] [Google Scholar]
- 2.Turner CH, Owan I, Takano Y. Mechanotransduction in bone: Role of strain rate. Am J Physiol. 1995;269:E438–E442. doi: 10.1152/ajpendo.1995.269.3.E438. [DOI] [PubMed] [Google Scholar]
- 3.Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- 4.Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: A twin study. J Bone Miner Res. 1997;12:2076–2081. doi: 10.1359/jbmr.1997.12.12.2076. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Mohan S, Gu W, Wergedal J, Baylink DJ. Quantitative assessment of forearm muscle size, forelimb grip strength, forearm bone mineral density, and forearm bone size in determining humerus breaking strength in 10 inbred strains of mice. Calcif Tissue Int. 2001;68:365–369. doi: 10.1007/s00223-001-0004-7. [DOI] [PubMed] [Google Scholar]
- 6.De Jong Z, Munneke M, Lems WF, Zwinderman AH, Kroon HM, Pauwels EK, Jansen A, Ronday KH, Dijkmans BA, Breedveld FC, Vlieland TP, Hazes JM. Slowing of bone loss in patients with rheumatoid arthritis by long-term high-intensity exercise. Arthritis Rheum. 2004;50:1066–1076. doi: 10.1002/art.20117. [DOI] [PubMed] [Google Scholar]
- 7.Kaye M, Kusy RP. Genetic lineage, bone mass, and physical activity in mice. Bone. 1995;17:131–135. doi: 10.1016/s8756-3282(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 8.Gordon KR, Perl M, Levy C. Structural alterations and breaking strength of mouse femora exposed to three activity regimens. Bone. 1989;10:303–312. doi: 10.1016/8756-3282(89)90068-9. [DOI] [PubMed] [Google Scholar]
- 9.Lu T-W, Taylor SJG, O'Connor JJ, Walker PS. Influence of muscle activity on the forces in the femur: An in vivo study. J Biomech. 1997;30:1101–1106. doi: 10.1016/s0021-9290(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 10.Frost M. Perspective On our age-related bone loss: Insights from a new paradigm. J Bone Miner Res. 1997;12:1539–1546. doi: 10.1359/jbmr.1997.12.10.1539. [DOI] [PubMed] [Google Scholar]
- 11.Dequeker J, Nijs J, Verstraeten A, Geusens P, Gevers G. Genetic determinants of bone mineral content at the spine and radius: A twin study. Bone. 1987;8:207–209. doi: 10.1016/8756-3282(87)90166-9. [DOI] [PubMed] [Google Scholar]
- 12.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 13.Klein RF, Mitchell SR, Phillips TJ, Belknap JK, Orwoll ES. Quantitative trait loci affecting peak bone mineral density in mice. J Bone Miner Res. 1998;13:1648–1656. doi: 10.1359/jbmr.1998.13.11.1648. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu M, Higuchi K, Kasai S, Tsuboyama T, Matsushita M, Mori M, Shimizu Y, Nakamura T, Hosokawa M. Chromosome 13 locus, Pbd2, regulates bone density in mice. J Bone Miner Res. 2001;16:1972–1982. doi: 10.1359/jbmr.2001.16.11.1972. [DOI] [PubMed] [Google Scholar]
- 15.Lang DH, Sharkey NA, Mack HA, Vogler GP, Vandenbergh DJ, Blizard DA, Stout JT, McClearn GE. Quantitative trait loci analysis of structural and material skeletal phenotypes in C57BL/6J and DBA/2 second generation and recombinant inbred mice. J Bone Miner Res. 2005;20:88–99. doi: 10.1359/JBMR.041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayeda AR, Hofstetter JR. A QTL for the genetic variance in free-running period and level of locomotor activity between inbred strains of mice. Behav Genet. 1999;29:171–176. doi: 10.1023/a:1021639901679. [DOI] [PubMed] [Google Scholar]
- 17.Tang X, Orchard SM, Sanford LD. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002;136:555–569. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 18.Koyner J, Demarest K, McCaughran J, Jr, Cipp L, Hitzemann R. Identification and time dependence of quantitative trait loci for basal locomotor activity in the BXD recombinant inbred series and a B6D2 F2 intercross. Behav Genet. 2000;30:159–170. doi: 10.1023/a:1001963906258. [DOI] [PubMed] [Google Scholar]
- 19.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92:2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 20.Karasik D, Kiel DP. Perspective genetics of the musculoskeletal system: A pleiotropic approach. J Bone Miner Res. 2008;23:788–802. doi: 10.1359/jbmr.080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orestes-Cardoso SM, Nefussi JR, Hotton D, Mesbah M, Orestes-Cardoso MD, Robert B, Berdal A. Postnatal Msx1 expression pattern in craniofacial, axial, and appendicular skeleton of transgenic mice from the first week until the second year. Dev Dyn. 2001;221:1–13. doi: 10.1002/dvdy.1120. [DOI] [PubMed] [Google Scholar]
- 22.Vandenbergh DJ, Heron K, Peterson R, Shpargel KB, Woodroofe A, Blizard DA, McClearn GE, Vogler GP. Simple tests to detect errors in high-throughput genotype data in the molecular laboratory. J Biomol Tech. 2003;14:9–16. [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips TJ, Huson MG, McKinnon CS. Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. J Neurosci. 1998;18:3023–3034. doi: 10.1523/JNEUROSCI.18-08-03023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buck K, Lischka T, Dorow J, Crabbe J. Mapping quantitative trait loci that regulate sensitivity and tolerance to quinpirole, a dopamine mimetic selective for D(2)/D(3) receptors. Am J Med Genet. 2000;96:696–705. doi: 10.1002/1096-8628(20001009)96:5<696::aid-ajmg17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Demarest K, McCaughran J, Jr, Mahjubi E, Cipp L, Hitzemann R. Identification of an acute ethanol response quantitative trait locus on mouse chromosome 2. J Neurosci. 1999;19:549–561. doi: 10.1523/JNEUROSCI.19-02-00549.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolivar V, Flaherty L. A region on chromosome 15 controls intersession habituation in mice. J Neurosci. 2003;23:9435–9438. doi: 10.1523/JNEUROSCI.23-28-09435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lionikas A, Blizard DA, Vandenbergh DJ, Glover MG, Stout JT, Vogler GP, McClearn GE, Larsson L. Genetic architecture of fast- and slow-twitch skeletal muscle weight in 200-day-old mice of the C57BL/6J and DBA/2J lineage. Physiol Genomics. 2003;16:141–152. doi: 10.1152/physiolgenomics.00103.2003. [DOI] [PubMed] [Google Scholar]
- 28.Lionikas A, Blizard DA, Gerhard GS, Vandenbergh DJ, Stout JT, Vogler GP, McClearn GE, Larsson L. Genetic determinants of weight of fast- and slow-twitch skeletal muscle in 500-day old mice of the C57BL/6J and DBA/2J lineage. Physiol Genomics. 2005;21:184–192. doi: 10.1152/physiolgenomics.00209.2004. [DOI] [PubMed] [Google Scholar]
- 29.Lionikas A, Blizard DA, Vandenbergh DJ, Stout JT, Vogler GP, McClearn GE, Larsson L. Genetic determinants of weight of fast- and slow-twitch skeletal muscles in old mice. Mamm Genome. 2006;17:615–628. doi: 10.1007/s00335-005-0177-x. [DOI] [PubMed] [Google Scholar]
- 30.Foreman JE, Lionikas A, Lang DH, Gyekis JP, Krishnan M, Sharkey NA, Gerhard GS, Grant MD, Vogler GP, Mack HA, Stout JT, Griffith JW, Lakoski JM, Hofer SM, McClearn GE, Vandenbergh DJ, Blizard DA. Genetic architecture for hole-board behaviors across substantial time intervals in young, middle-aged, and old mice. Genes Brain Behav. doi: 10.1111/j.1601-183X.2009.00516.x. (in press). [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Basten CJ, Zeng Z-B. Raleigh, NC: Department of Statistics, North Carolina State University; 2001–2004. Windows QTL Cartographer 2.5. [Google Scholar]
- 32.Lander ES, Kruglyak L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 33.Lang DH, Sharkey NA, Lionikas A, Mack HA, Larsson LG, Vogler GP, Vandenbergh DJ, Blizard DA, Stout JT, Stitt JP, McClearn GE. Adjusting data to body size: A comparison of methods as applied to quantitative trait loci (QTL) analysis of musculoskeletal phenotypes. J Bone Miner Res. 2005;20:748–757. doi: 10.1359/JBMR.041224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ., Jr Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med. 1988;108:824–828. doi: 10.7326/0003-4819-108-6-824. [DOI] [PubMed] [Google Scholar]
- 35.Menkes A, Mazel S, Redmond RA, Koffler K, Libanati CR, Gundberg CM, Zizic TM, Hagberg JM, Pratley RE, Hurley BF. Strength training increases regional bone mineral density and bone remodeling in middle-aged and older men. J Appl Physiol. 1993;74:2478–2484. doi: 10.1152/jappl.1993.74.5.2478. [DOI] [PubMed] [Google Scholar]
- 36.Notomi T, Okazaki Y, Okimoto N, Saitoh S, Nakamura T, Suzuki M. A Comparison of resistance and aerobic training for mass, strength and turnover of bone in growing rats. Eur J Appl Physiol. 2000;83:469–474. doi: 10.1007/s004210000316. [DOI] [PubMed] [Google Scholar]
- 37.Chao D, Espeland MA, Farmer D, Register TC, Lenchik L, Applegate WB, Ettinger WH. Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc. 2000;48:753–759. doi: 10.1111/j.1532-5415.2000.tb04749.x. [DOI] [PubMed] [Google Scholar]
- 38.Gossain VV, Rao DS, Carella MJ, Divine G, Rovner DR. Bone mineral density (BMD) in obesity effect of weight loss. J Med. 1999;30:367–376. [PubMed] [Google Scholar]
- 39.Stewart KJ, Deregis JR, Turner KL, Bacher AC, Sung J, Hees PS, Tayback M, Ouyang P. Fitness, fatness and activity as predictors of bone mineral density in older persons. J Intern Med. 2002;252:381–388. doi: 10.1046/j.1365-2796.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 40.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci. 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 41.Henderson ND, Turri MG, DeFries JC, Flint J. QTL Analysis of multiple behavioral measures of anxiety in mice. Behav Genet. 2004;34:267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- 42.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 43.Kostek MC, Delmonico MJ, Reichel JB, Roth SM, Douglass L, Ferrell RE, Hurley BF. Muscle strength response to strength training is influenced by insulin-like growth factor 1 genotype in older adults. J Appl Physiol. 2005;98:2147–2154. doi: 10.1152/japplphysiol.00817.2004. [DOI] [PubMed] [Google Scholar]