Abstract
Adoptive T-cell immunotherapy has shown promise in the treatment of human malignancies, but the challenge of isolating T cells with high avidity for tumor antigens in each patient has limited application of this approach. The transfer into T cells of T-cell receptor (TCR) genes encoding high-affinity TCRs recognizing defined tumor-associated antigens can potentially circumvent this obstacle. Using a well-characterized murine model of adoptive T-cell immunotherapy for widely disseminated leukemia, we demonstrate that TCR gene–modified T cells can cure mice of disseminated tumor. One goal of such adoptive therapy is to establish a persistent memory response to prevent recurrence; however, long-term function of transferred TCR-transduced T cells is limited due to reduced expression of the introduced TCR in vivo in quiescent resting T cells. However, by introducing the TCR into a cell with a known endogenous specificity, activation of these T cells by stimulation through the endogenous TCR can be used to increase expression of the introduced TCR, potentially providing a strategy to increase the total number of tumor-reactive T cells in the host and restore more potent antitumor activity.
Introduction
The generation, isolation, and expansion of autologous high avidity, tumor antigen–specific T cells from cancer patients for use in autologous cell therapy is a laborious and, all too often, unsuccessful endeavor. Obstacles include central and peripheral tolerance to the targeted tumor cell antigens, which are commonly self-proteins,1,2 compromised immune systems in patients presenting for T-cell therapy, and high tumor antigen burdens in vivo. One strategy to circumvent these challenges would be to have a high-affinity, tumor antigen–specific T-cell receptor (TCR) available that could be used to transduce a patient's T cells and impart the desired specificity. Such tumor-reactive T cells could then potentially be rapidly expanded in vitro with well-defined techniques to the large numbers required for therapeutic infusions.3
A clinical trial employing TCR gene therapy to target the melanoma antigen recognized by T cells (MART-1) antigen in human patients has recently been reported.4 Although this study demonstrated proof of principle in humans, only 2 of 17 patients displayed clinical responses, which is far less than predicted if autologous nontransduced tumor-reactive T cells had been infused,5,6 suggesting the necessity for improving this strategy. At the time of infusion of the autologous polyclonal T cells transduced with a MART-1–specific TCR into lympho-depleted patients with melanoma, 42% of CD8+ T cells expressed the MART-1–specific TCR Vβ chain, but only 17% bound MART-1 tetramer. One month later, a molecular signature of retroviral transduction was detectable in 26% of patients' peripheral T cells, expression of the MART-1-specific TCR Vβ chain was detected on only 8%, and expression of both TCR chains sufficient to bind the MART-1 peptide/major histocompatibility complex tetramer averaged ~0.8% of peripheral T cells.4 This loss of expression of introduced TCR chains rendered the majority of persistent transduced cells no longer tumor reactive. Thus, advancing TCR gene therapy will require not only strategies to promote initial high levels of TCR gene expression to achieve immediate antitumor activity, but also methods to maintain TCR expression to establish long-term memory in the host. Using a well-described model for T-cell therapy of an established murine leukemia, we assessed the efficacy of candidate vectors for inducing and maintaining TCR expression prior to commencing human studies.
Our lab has extensively studied a murine model for adoptive T-cell therapy of disseminated leukemia with CD8+ T cells specific for the gag epitope derived from the Friend murine leukemia virus-induced erythroleukemia, FBL.7 Adoptive transfer of gag-specific CD8+ T cells from either FBL-immunized mice or TCR-transgenic mice expressing a Vα3Vβ12 gag–specific TCR (TCRαgag mice) into C57BL/6 (B6) mice bearing disseminated FBL tumor mediates tumor regression and long-lived protection from tumor challenge.8,9 Tumor eradication requires a prolonged response, and the antigen-specific T cells must persist for at least 30 days after transfer to be effective.10 Thus, this tumor therapy model provides a demanding setting for testing in vivo efficacy and persistence of adoptively transferred T cells.
We transduced the gag-specific Vα3Vβ12 TCR chains into CD8+ T cells from a TCR-transgenic mouse expressing a TCR specific for a viral antigen and assessed whether transduced, gag-specific TCR expression would be sufficiently maintained following transfer to mediate tumor regression and establish long-term memory. Our results demonstrate that transduced T cells can cure mice of FBL leukemia, but expression of transduced TCR chains is maintained in vivo only on a small subset of transferred T cells at 7 months. Downregulation of TCR expression largely reflected diminished promoter activity in quiescent cells rather than vector silencing, as activation via the endogenous TCR restored transduced TCR expression and tumor recognition.
Results
Vector design affects introduced TCR gene expression
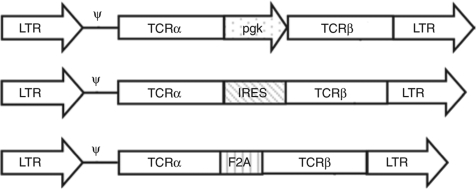
Expression levels of introduced TCR chains modulate antigen recognition, with T cells expressing higher levels of surface TCRα and β chains binding peptide–major histocompatibility complex tetramers more avidly, and responding better to antigen, than T cells with reduced expression of one or both of the introduced chains.11,12 In vitro studies comparing methods for expressing two genes from a single retroviral vector in T cells suggest that using the long terminal repeat (LTR) and an internal phosphoglycerate kinase (pgk) promoter, or the LTR with linking the genes via an internal ribosomal entry site (IRES) or 2A peptide, are among the best strategies for promoting high levels of both TCRα and β chain expression.13,14,15 However, previous studies have not directly compared efficacy of these strategies in tumor therapy, or in maintaining long-term expression of transduced TCRs. We, therefore, tested these approaches in a tumor therapy model, designing three different retroviral vectors that can simultaneous express the same TCRα and β chains (Figure 1). Vα3 and Vβ12 genes,9 encoding a high-affinity TCR recognizing an epitope within the Friend murine leukemia virus gag protein expressed by the FBL erythroleukemia, were inserted into retroviral vectors containing the same Moloney murine leukemia virus LTRs and backbone, but separated by a pgk promoter, an IRES, or the foot and mouth disease virus–derived 2A peptide (F2A) sequence that induces ribosomal skipping and has been used to create multicistronic vectors expressing equivalent levels of the TCRα and β chain genes.15 Retroviruses encoding these three different versions of the gag-specific TCR were transduced into naive CD8+ T cells from Thy1.1 congenic P14 TCR-transgenic B6 mice that express an endogenous Vα2Vβ8 TCR recognizing a gp33-derived epitope from the lymphocytic choriomeningitis virus.
Figure 1.
Design of retroviral constructs. The gag-specific Vα3 and Vβ12 TCR chains were cloned into pUC6S36 and separated by either an IRES element, murine phosphoglycerate kinase (pgk) promoter, or the 2A peptide sequence derived from the foot-and-mouth disease virus (F2A), and each entire cassette cloned into the BamHI and NotI sites of the retroviral vector pLZRSpBMN-Z, replacing the lacZ gene. Only the retrovirus portion of the vector is shown. IRES, internal ribosomal entry site; LTR, long terminal repeat; TCR, T-cell receptor.
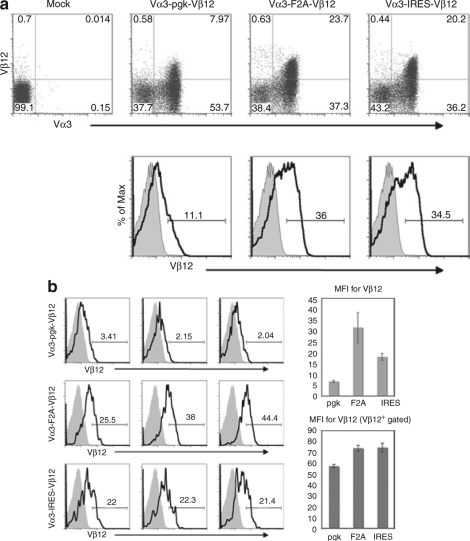
To assess relative levels of transduced TCR expression, T cells were incubated with antibodies to Vα3 and Vβ12 several days after transduction. Transduction efficiencies (based on Vα3 expression) were 62% for the pgk-containing construct, 61% for the F2A construct, and 56% for the IRES construct (Figure 2a). The mean fluorescence intensities of Vα3 expression (driven from the LTR in all constructs) were similar in all three T-cell populations and slightly lower than expression in TCRαgag T cells expressing the receptor from a germ-line transgene (data not shown). In contrast, the mean fluorescence intensity of Vβ12 expression differed among the constructs, with the weakest expression of Vβ12 in pgk-transduced cells (Figure 2b). A fraction of T cells in all three groups expressed low levels of Vβ12 despite strong expression of Vα3, which likely reflects noncoordinate reduced expression of the β chain compared to the α chain, and/or mismatched pairing of the introduced Vα3 chain with the endogenous Vβ8 chain in these cells. In comparison to cells from TCRαgag mice, we have previously shown that T cells from double-transgenic P14xTCRαgag mice also have reduced expression of Vβ12 relative to Vα3,16 suggesting the Vα3 chain from TCRαgag can efficiently pair with the Vβ8 chain of the P14 TCR.
Figure 2.
Transduction efficiency and T-cell receptor α and β chain expression in P14 T cells. (a) P14 T cells were transduced with one of the three retroviral constructs shown in Figure 1 and incubated with antibodies to CD8α, Vα3, and Vβ12 2 days post-transduction. Dot plots were gated on CD8+ lymphocytes, and histograms were gated on CD8+, Vα3+ lymphocytes to illustrate relative levels of Vβ12 expression in transduced T cells compared to mock-transduced controls. (b) Histograms were analyzed as in a above to compare Vβ12 expression in three separate experiments. Means and standard deviations for the mean fluorescence intensity (MFI) of Vβ12 expression for the transduced T cells gated on CD8+, Vα3+ lymphocytes from the three experiments were calculated (right, upper bar graph). The means and standard deviations for the MFI of Vβ12 expression in transduced T cells were also calculated after eliminating T cells expressing low or absent levels of Vβ12 by gating on CD8+, Vα3+, Vβ12+ lymphocytes (right, lower bar graph). F2A, foot and mouth disease virus–derived 2A peptide; IRES, internal ribosomal entry site; pgk, phosphoglycerate kinase.
The poorer expression of Vβ12 in T cells transduced with the pgk vector was observed in multiple experiments (Figure 2b and data not shown) and was apparent even when Vα3+ cells expressing low or undetectable levels of Vβ12 were eliminated from the analysis (Figure 2b lower bar graph). Consistent with data demonstrating that decreased levels of TCR expression result in reduced responses to antigen,11,12 transduced T cells containing the pgk promoter proliferated in vitro at about one fourth the rate of cells transduced with the IRES- or F2A-containing constructs when stimulated with irradiated FBL (data not shown). As a result, it proved very difficult to expand pgk-transduced T cells that maintained expression of both chains, and we were unable to achieve the numbers necessary for in vivo therapy, as this required a large number of repeated stimulations and resulted in terminal differentiation of the cells. Thus, for in vivo analysis of therapeutic efficacy, we only compared cells transduced with the IRES and F2A vectors that yielded roughly equivalent expression of both chains and expanded similarly.
In vitro expansion of TCR gene–modified T cells
Protocols for generating the large numbers of T cells required for adoptive therapy in humans typically rely upon isolating small numbers of antigen-specific T cells followed by multiple cycles of in vitro stimulation with αCD3.3 Two to three months of in vitro culture with repeated stimulations are generally required to isolate and expand a T-cell line or clone to sufficient numbers for therapy, which for retrovirally transduced cells may lead to progressive loss of gene expression over time.17 Although improved efficiency of TCR gene transduction and selection might ultimately shorten this period by increasing the frequency of reactive T cells, we modeled the current prototypic conditions for human T-cell therapy by expanding Vα3Vβ12 TCR-transduced and mock transduced P14 T cells in vitro for nine cycles (~2.5 months) in the presence of cells expressing costimulatory molecules to minimize changes in T-cell function.18,19 The transduced and control TCRαgag T cells were repetitively stimulated with irradiated FBL tumor cells, using antigen recognition to select T cells expressing the introduced, gag-specific TCR rather than incorporating a drug selection enzyme in the vectors, as this assures functional TCR expression and avoids enzymes that can serve as neoantigens that stimulate host immune responses resulting in rejection of transferred T cells.20
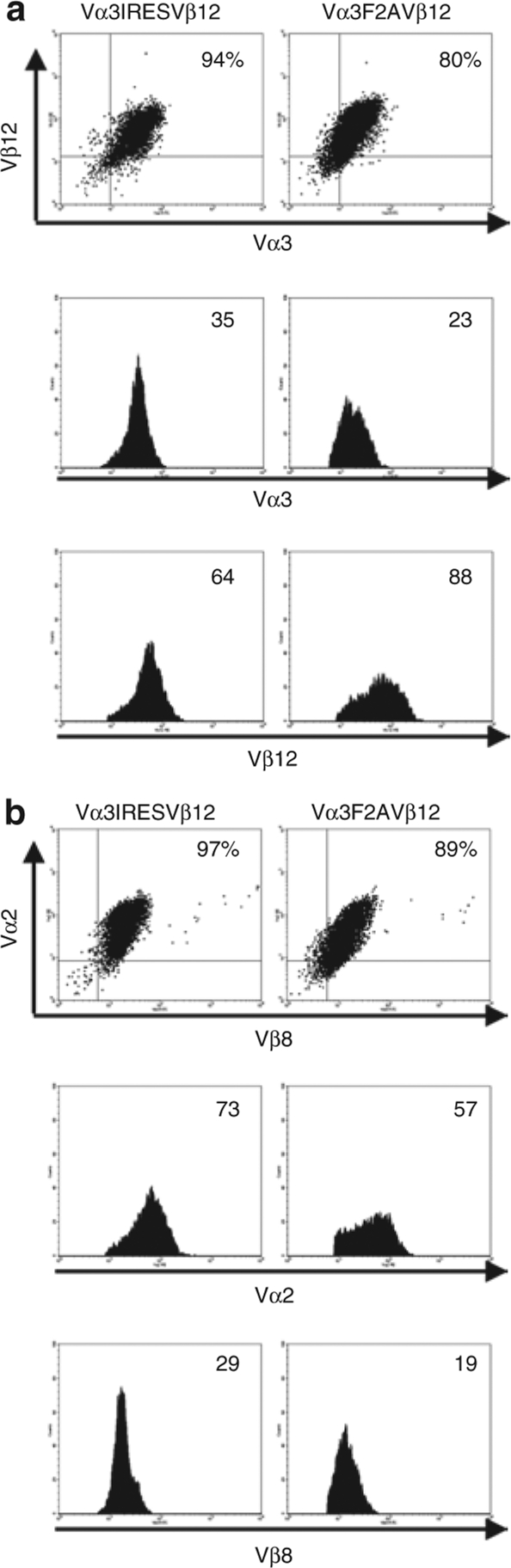
After eight cycles of in vitro stimulation, transduced T cells were incubated with antibodies to both the introduced (Vα3 and Vβ12) and endogenous (Vα2 and Vβ8) TCR chains to reassess surface expression (Figure 3a,b). The Vα3IRESVβ12-transduced T-cell population maintained expression of both the introduced and endogenous TCR chains at high levels, with >90% of the cells expressing all four TCR chains. On Vα3F2AVβ12-transduced T cells, high levels of the introduced TCR were found on 80% of cells and high levels of the endogenous TCR on 89%. Thus, TCR expression appeared to be better retained in the Vα3IRESVβ12-transduced T-cell population. The mean fluorescence intensity of expression of the introduced TCR α-chain was slightly higher in the Vα3IRESVβ12-transduced T cells, whereas the mean fluorescence intensity of expression of the introduced TCR β−chain was somewhat higher in the Vα3F2AVβ12-transduced T cells (Figure 3a).
Figure 3.
Maintenance of transduced T-cell receptor (TCR) expression with repetitive in vitro stimulation. P14 T cells transduced with the TCRαgag receptor chains were incubated with antibodies to CD8α and either the introduced, gag-specific TCR chains (Vα3 and Vβ12) or the endogenous gp33-specific TCR chains (Vα2 and Vβ8). (a) Expression of the gag-specific TCR on day 9 following the 8th in vitro stimulation when cells had been in culture for ~2.5 months. (b) Expression of the gp33-specific TCR at the same time point as in a. All plots shown are gated on CD8+ lymphocytes and the numbers in the histograms indicate the mean fluorescence intensity of α- and β−chain expression. F2A, foot and mouth disease virus–derived 2A peptide; IRES, internal ribosomal entry site.
TCR gene–modified T cells can cure mice of disseminated tumor
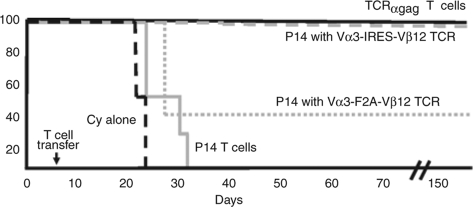
To test whether Vα3Vβ12 TCR–transduced T cells can cure disseminated leukemia, B6 mice were inoculated with FBL and 5 days later, when leukemia was widely disseminated, received cyclophosphamide and TCR-transduced T cells, followed by interleukin (IL)-2 for 10 days to promote T-cell viability and in vivo expansion.7 As a positive control, tumor-bearing mice received IL-2 plus T cells from a similarly maintained TCRαgag T-cell line;9 as negative control, mice received IL-2 plus mock-transduced P14 T cells. All mice that received cells from the in vitro-cultured TCRαgag T-cell line rejected their tumors and survived disease-free for >150 days (Figure 4). Similarly, the five mice that received Vα3IRESVβ12-transduced P14 T cells rejected tumor and survived. Of the five mice that received Vα3F2AVβ12-transduced P14 T cells, three succumbed to tumor on day 25, while two mice rejected tumor and survived long-term. All mice that received mock-transduced P14 T cells (4/4) died of tumor between days 23 and 30.
Figure 4.
Therapy of mice bearing disseminated FBL leukemia with P14 T cells transduced with a gag-specific TCR. B6 mice were injected with 2 × 106 live FBL tumor cells intraperitoneal on day 0 and cyclophosphamide (Cy) on day 5. Six hours following Cy treatment, mice received no T cells (dashed black line) or 6 × 106 mock-transduced P14 T cells (solid gray line), P14 T cells transduced with a Vα3F2AVβ12 retrovirus (dotted gray line), P14 T cells transduced with a Vα3IRESVβ12 retrovirus (dashed gray line), or TCRαgag transgenic T cells (solid black line). The transduced T cells had been expanded in vitro for nine stimulation cycles and were compared for in vivo efficacy to similarly expanded transgenic T cells expressing the same TCR. Mice were euthanized if progressive tumor growth became evident (mortality anticipated in 48 h). F2A, foot and mouth disease virus–derived 2A peptide; IRES, internal ribosomal entry site; TCR, T-cell receptor.
Small numbers of transduced T cells persist long-term, but are largely unresponsive to gag
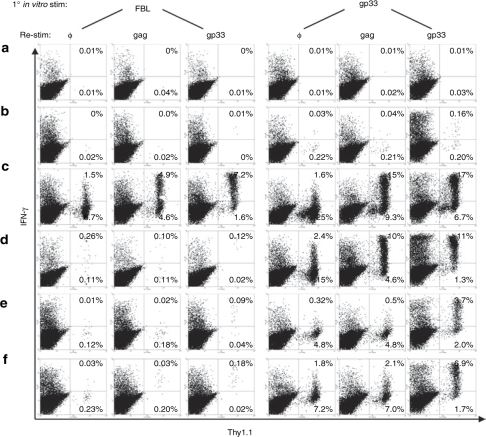
Effective immunotherapy in humans will, in some settings, require long-term persistence of adoptively transferred cells with maintenance of function. To ascertain whether TCR gene–modified T cells maintain functional expression of the introduced TCR long after adoptive transfer, mice cured of tumor (Figure 4) were euthanized on day 214 after tumor challenge (295 days after cells were activated for retroviral transduction) and the splenocytes were stimulated in vitro with either irradiated FBL or gp33 peptide to assess the ability of persisting transferred T cells to recognize the antigens targeted by the introduced and endogenous TCR chains. T cells from P14xTCRαgag mice expressing both sets of TCR chains exhibit equivalent proliferative responses to irradiated FBL and gp33 peptide when stimulated in vitro for 6 days (data not shown). We could only evaluate splenocytes from six of the seven cured mice as one mouse treated with Vα3IRESVβ12 T cells died from unknown causes without recurrent tumor on day 197. Six days following stimulation with irradiated FBL or gp33 peptide, transferred cells that had been capable of responding/expanding to each antigen were enumerated by restimulating cells for 6 h with no peptide, gag peptide, or gp33 peptide, and analyzed for interferon-γ (IFN-γ) production by flow cytometry (gag peptide induces more detectable cytokine responses than FBL in this brief in vitro assay).
Transduced and transferred P14 T cells, identified by Thy1.1 expression, were detected in cultures from four of the six evaluable mice (Figure 5c–f). In three of the four mice with persisting transduced T cells, <0.5% of CD8+ T cells present 6 days after FBL stimulation were derived from Vα3Vβ12 TCR–transduced P14 precursors (%Thy1.1+ T cells without restimulation) (Figure 5d–f, first column). In contrast, primary stimulation with gp33 peptide resulted in 2.5- to 50-fold greater numbers of transduced P14 T cells (5–27% of total CD8+ T cells) compared to primary FBL stimulation (Figure 5c–f, fourth column versus first column). Similar results were obtained with both the IRES (Figure 5c,d) and F2A (Figure 5e,f) containing vectors. In one mouse transduced P14 T cells represented ~10% of CD8+ cells after stimulation with FBL (Figure 5c), but even in this mouse the number of cells that responded to gp33 was ~2.5 times greater. This data suggest the majority of transduced T cells persisting in treated mice had downregulated expression of the introduced, gag-specific TCR such that the cells were only marginally responsive to gag, but retained the ability to respond to gp33 through the endogenous TCR.
Figure 5.
Analysis of long-term persistence and function of transferred cells in treated mice. On day 214 following tumor challenge, splenocytes from surviving treated mice from Figure 4 were stimulated in vitro with irradiated FBL or gp33 peptide. Six days later, cells were restimulated with no peptide (φ), gag peptide, or gp33 peptide for 6 h and analyzed for production of IFN-γ and expression of Thy1.1. All transduced and adoptively transferred P14 T cells were derived from T cells expressing the Thy1.1 congenic marker. (a–d) Mice received Vα3IRESVβ12-transduced P14 T cells and (e,f) mice received Vα3F2AVβ12-transduced T cells. All plots are gated on CD8+ lymphocytes. IFN-γ, interferon-γ.
Stimulation via the endogenous TCR augments the number of T cells that can recognize tumor
The poor response of transduced T cells to FBL stimulation could reflect downregulation of the introduced TCR via methylation-induced silencing of the integrated vector or decreased transcription of vector-encoded genes when T cells are in a quiescent state. To differentiate between these possibilities, and determine whether it might be possible to rescue TCR expression and the antitumor activity of transduced T cells, splenocytes from euthanized mice were stimulated with either FBL or gp33, and restimulated with gag peptide to assess tumor antigen recognition as reflected by IFN-γ production. Initial stimulation with gp33 resulted in 3–100 times more T cells producing IFN-γ in response to gag peptide than if cells were first stimulated with FBL (Figure 5c–f, second and fifth columns). Thus, activating persistent, quiescent, transduced T cells via the endogenous TCR increases expression of the transduced TCR and may provide a strategy to increase the number of T cells that can recognize tumor cells in vivo.
Discussion
Several studies have compared activities of different promoters for expressing genes in T cells. Previously we found that in retrovirally transduced primary T cells the Moloney murine leukemia virus LTR and internal pgk promoters were superior to internal β-actin, ubiquitin, cytomegalovirus, and SV-40 promoters.13 Two reports directly comparing the pgk promoter to a bicistronic IRES strategy suggested that resulting gene expression levels were equivalent,14,21 and a third report demonstrated only slightly reduced expression with a pgk promoter compared to an IRES.22 A comparison of an IRES versus a 2A peptide for expressing codon-optimized TCRs driven by a retroviral LTR in primary T cells demonstrated equivalent TCR surface expression and cellular responses to antigen with both constructs.23 However, as none of these studies assessed long-term expression of TCRs in vivo, we compared these different methods to determine whether one of these strategies promotes better TCR gene expression levels and T-cell function in vivo.
In distinction to previous reports, our construct using an internal pgk promoter yielded reduced expression of the pgk-regulated β chain compared to the IRES- and F2A-containing constructs. This disparity might be species related, as the previous studies evaluating the pgk promoter were performed in human, not mouse, T cells.13,14,21,22 However, our results do suggest that, even with positive selection for cells expressing functional levels of the introduced TCR chains by using repeated antigen stimulation as the proliferative trigger, transduction with a vector employing an internal pgk promoter to drive independent expression of the β chain appears inefficient for sustaining strong expression of both TCR chains in murine T cells.
Among T cells transduced with IRES- and F2A-containing constructs the Vα3F2AVβ12-transduced T cells appeared somewhat less effective in adoptive immunotherapy, with only two of five treated mice surviving, whereas all five mice that received Vα3IRESVβ12-transduced T cells eliminated tumor and survived. The reasons for this discrepancy in vivo are unclear and might merely reflect the distinct properties of cells expanded extensively in culture from a small starting population. One potential problem with F2A containing vectors is that the F2A peptide contains a sequence that fits the motif of an H2-Db-binding epitope (KQTLNFDLL;24) and adoptively transferred T cells expressing novel epitopes from transgenes can induce potent immune responses.20 To test whether mice might be rejecting Vα3F2AVβ12-transduced P14 T cells, we injected six additional mice with these cells and IL-2 for 10 days and tested splenocytes from these mice for recognition of the KQTLNFDLL peptide by analysis of IFN-γ production in response to peptide stimulation. None of the mice mounted a detectable response to this peptide (data not shown), suggesting the potential epitope derived from F2A, even if recognizable, is not highly immunogenic in this context. Alternatively, the observed differences in in vivo antitumor activity may simply reflect differences in the total number of gag-specific T cells transferred, as flow cytometric analysis demonstrated that the population of Vα3F2AVβ12-transduced T cells contained ~14% fewer T cells expressing high levels of both introduced TCR chains compared to Vα3IRESVβ12-transduced T cells (Figure 3). Future studies will be necessary to further clarify these issues. Nonetheless, the potential for 2A peptides to present novel epitopes to the immune system, in concert with the observed efficacy of the IRES construct, suggests IRES-based vectors should be useful in TCR gene therapy for humans.
The retroviral vector used for these studies is an early generation Moloney murine leukemia virus vector, and modifications to the TCR genes and/or vector backbone should further enhance transgene expression.25 Gene modifications that can increase expression include codon optimization and removal of RNA instability motifs and cryptic splice sites,23 and insertion in the 3′–untranslated region of the post-transcriptional regulatory element from the woodchuck hepatitis virus.26 Vector changes such as insertion of the β-IFN scaffold attachment region13,27 and/or chicken beta-globin insulator28 may facilitate long-term gene expression by inhibiting integration-site methylation and maintaining chromatin in a more open conformation. The potential benefits of such modifications will need to be evaluated in in vivo models, such as employed in our study, which require prolonged responses and permit assessment of long-term memory.
Long-term persistence of adoptively transferred, TCR gene–modified T cells with retention of function in vivo will be necessary in some therapeutic settings. Although lymphodepletion of hosts prior to adoptive T-cell therapy promotes the persistence of transferred cells,29 there is no evidence that lymphodepletion promotes long-term expression of transduced TCR chains after the proliferative drive has terminated.4 To enhance transferred T-cell persistence in the context of TCR gene therapy, Heemskerk and colleagues proposed transducing tumor-specific TCRs into T cells expressing endogenous receptors specific for latent viruses such as cytomegalovirus and Epstein-Barr virus.30 They reasoned that in vivo triggering of virus-specific T cells by endogenous viral reactivation may intermittently trigger proliferation of transferred T cells. Our presented data, as well as data from experiments not shown, suggest this strategy should be further explored as it might provide the additional benefit of maintaining tumor reactivity by restoring expression of the transduced TCR.
Among murine tumor immunotherapy studies examining TCR gene transfer into mature T cells,31,32,33,34,35 only one has shown functional maintenance of transduced T cells in immunocompetent mice.34 In that study, transduced T cells proliferated in response to tumor rechallenge 3 months after initial tumor challenge. Our results extend these observations, demonstrating that small numbers of transduced T cells can be maintained in most mice for up to 7 months, but that with the commonly used retroviral vector the majority of these T cells lose expression of the transduced TCR and cannot respond to the tumor antigen at this time. Moreover, our in vitro data suggest that periodic restimulation of such cells via the endogenous TCR may provide a means to induce proliferation and restore transduced TCR expression and tumor reactivity. Periodic in vivo stimulation of TCR gene–modified T cells through an endogenous receptor of known specificity, either by relying on natural, though unpredictable, reactivation of chronic viruses in the setting of using virus-specific T cells as recipients of the TCR genes, or by intentional vaccination, may not only help to maintain the number of transferred T cells but may also increase the fraction of these T cells capable of responding to the outgrowth of residual tumor cells.
Materials and Methods
Mice. C56BL/6 (B6) and congenic Thy1.1 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). P14 mice, expressing a Vα2Vβ8 TCR transgene, bred on the B6 background were a kind gift from Dr. Murali Krishna-Kaja (University of Washington) and were bred with congenic Thy1.1 mice to generate P14+/–xThy1.1+/– heterozygotes as a source of P14 Thy1.1+ T cells. TCRαgag mice, expressing a Vα3Vβ12 TCR transgene, also bred on the B6 background, were generated in our lab.9 P14xTCRαgag mice were generated in our lab by crossing P14 and TCRαgag mice. All mouse experiments were approved by the University of Washington Institutional Animal Care and Use Committee.
Peptides, antibodies, and media. The peptide epitopes from Friend murine leukemia virus gag recognized by CD8+ T cells from TCRαgag mice [CCLCLTVFL with the cysteines replaced by aminobutyric acid to improve solubility while retaining specificity (data not shown)] and from lymphocytic choriomeningitis virus-gp33 recognized by CD8+ T cells from P14 mice (KAVYNFATM) were synthesized and purified by high pressure liquid chromatography by Synpep (Dublin, CA). Antibodies to Vα2, Vα3, Vβ8, Vβ12, CD8α, Thy1.1, IFN-γ, and FcγIII/IIR were purchased from BD Pharmingen (San Diego, CA). All cell culture was performed with RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 25 mmol/l HEPES, 2 µmol/l L-glutamine, 100 U/ml penicillin/streptomycin, 10% fetal calf serum, and 30 µmol/l 2-mercapatoethanol (complete medium).
Vector construction and virus preparation. The pLZRSpBMN-Z retroviral vector (pLZRS) was a kind gift from Dr. Gary Nolan (Stanford University). The pgk promoter was cloned from pMSCVpuro (Clontech, Palo Alto, CA), the IRES from pIRES2EGFP (Clontech), and the 22 amino acid picornavirus–derived F2A sequence (from the foot and mouth disease virus) created using primers as described.15 The F2A segment adds 21 amino acids to the C-terminus of the TCRα chain and 1 amino acid to the N-terminus of the TCRβ chain. In addition, a G-S-G linker was placed between the TCRα chain and F2A sequence. The TCRα and β chain cDNAs (Vα3 and Vβ12) used for retroviral gene transfer were identical to those used to create TCRαgag TCR transgenic mice.9 Three different constructs, each containing both the Vα3 and Vβ12 cDNAs in the pLZRS vector were created in which the lacZ gene was removed and replaced with the genes for the TCR chains inserted downstream of the 5′–LTR and separated by either an internal murine pgk promoter, an IRES element, or the F2A sequence. The three vectors were independently transfected into Phoenix E packaging cells using Lipofectamine and continuously selected with puromycin (1 µg/ml). To produce retroviral supernantant, 2 × 107 selected packaging cells were plated without puromycin in T-225 flasks at 37 °C and viral supernatant harvested 2 days later. Fresh media was added to the flasks, and after a 20-h incubation at 32 °C additional viral supernatant was harvested. To concentrate the virus, 10 ml of PEG solution (100 g polyethylene glycol MW 8,000 + 6 g NaCl in 250 ml H2O with pH adjusted to 7.2 with NaOH) was added to 40 ml of the combined virus supernatant, stored at 4 °C for 1 to 2 days and centrifuged at 2,500 rpm (~1,500g) for 45 min at 4 °C. Each virus pellet was suspended in 500 µl complete medium, pellets from both harvest time points were pooled, and the concentrated virus was then either used immediately for transduction or stored at –80 °C for later use.
T-cell transduction and in vitro cell culture protocol. 4 × 107 splenocytes from P14xThy1.1 mice were stimulated in vitro with gp33 peptide (3 µmol/l final concentration) and 20 U/ml IL-2 in 12 ml complete medium in T-25 flasks (upright) for 25 h. Cells were washed, resuspended at 1 × 108 cells/ml, and 50 µl placed in each well of a 6-well plate with 1.5 ml concentrated retroviral supernatant, IL-2 (20 U/ml), and polybrene (2 ug/ml). The cells were then spinfected in a table-top centrifuge at 1,500 rpm (470g) for 1 h at 32 °C, and incubated at 37 °C for 16 h. Cells were washed, resuspended in fresh medium with IL-2 (20 U/ml) for the remainder of the 10-day cycle, and then restimulated with antigen every 9–10 days. Mock-transduced P14 T cells were repetitively restimulated with 5 × 106 irradiated (3,000 rads) B6 splenocytes as APC and feeder cells in 12 ml complete medium with 0.16 µg/ml gp33 peptide and 20 U/ml IL-2, whereas TCRαgag transgenic T cells and P14 T cells transduced to express the TCRαgag Vα3 and Vβ12 TCR chains were repetitively restimulated with 5 × 106 irradiated B6 splenocytes as feeder cells, 2 × 106 irradiated (10,000 rads) FBL stimulator cells, and 20 U/ml IL-2 in 12 ml complete medium. Data from our lab suggest that all CD8+ T cells cultured long-term in this fashion become effector/effector-memory T cells and that cells with phenotypic and functional central-memory qualities are not detected in such long-term cultures (data not shown).
Tumor therapy protocol. Adoptive immunotherapy was performed as previously described.7 Briefly B6 mice received 2 × 106 live FBL tumor cells from fresh ascites intraperitoneal. Five days later, when tumor was widely disseminated, mice were administered 180 mg/kg cyclophosphamide intraperitoneal and 6 h later, after clearance of the drug, 6 × 106 T cells intravenous. On days 5–15, mice received 1 × 104 U IL-2 intraperitoneal to promote survival and proliferation of the transferred cells. Mice were monitored for ascites and euthanized if the detectable tumor burden increased to a size that predictably led to morbidity/mortality within 24–48 hours and/or the mice exhibited unexplained severe morbidity.
In vitro stimulation and intracellular cytokine staining. 20–30 × 106 splenocytes from surviving mice cured of FBL tumor were stimulated in T-25 flasks with 20 U/ml IL-2, 5 × 106 irradiated (10,000 rads) FBL or 0.04 µg/ml gp33 peptide in 12 ml complete media for 6 days. 1 × 106 live cells from each of these cultures were stimulated a second time with gag (2.5 µg/ml), gp33 (2.5 µg/ml), or no peptide in 96-well plates with 10 U/ml IL-2 for 6 h, in the presence of Golgiplug (BD Pharmingen) for the last 5 h. Cells were incubated with antibodies to CD8α, Thy1.1, and FcγIII/IIR, fixed and permeabilized (cytofix/cytoperm, BD Pharmingen), washed (perm/wash, BD Pharmingen), incubated with antibody to IFN-γ, and visualized by flow cytometry.
Acknowledgments
We thank Claes Öhlén and Joseph Blattman for helpful discussions, Maria Huang for assistance with maintenance of the mouse colony, and Michael Kalos for initial cloning of the gag-specific TCR. The authors declare no financial conflicts of interest. This work was supported by grants from the NIH (R37 CA033084 and P01 CA18029) and Leukemia and Lymphoma Society (FND 7040). M.L.D. was supported as a Poncin Scholar.
REFERENCES
- Ho WY, Blattman JN, Dossett ML, Yee C., and , Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Morris EC, Bendle GM., and , Stauss HJ. Prospects for immunotherapy of malignant disease. Clin Exp Immunol. 2003;131:1–7. doi: 10.1046/j.1365-2249.2003.02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell SR., and , Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PD., and , Cheever MA. Treatment of disseminated leukemia with cyclophosphamide and immune cells: tumor immunity reflects long-term persistence of tumor-specific donor T cells. J Immunol. 1984;133:3401–3407. [PubMed] [Google Scholar]
- Öhlén C, Kalos M, Hong DJ, Shur AC., and , Greenberg PD. Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. J Immunol. 2001;166:2863–2870. doi: 10.4049/jimmunol.166.4.2863. [DOI] [PubMed] [Google Scholar]
- Öhlén C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, et al. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PD, Cheever MA., and , Fefer A. Detection of early and delayed antitumor effects following curative adoptive chemoimmunotherapy of established leukemia. Cancer Res. 1980;40:4428–4432. [PubMed] [Google Scholar]
- Cooper LJ, Kalos M, Lewinsohn DA, Riddell SR., and , Greenberg PD. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. J Virol. 2000;74:8207–8212. doi: 10.1128/jvi.74.17.8207-8212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowski JJ, Yu DC, Rubinstein MP, McKee MD, Cole DJ., and , Nishimura MI. CD8-independent tumor cell recognition is a property of the T cell receptor and not the T cell. J Immunol. 2003;170:2582–2589. doi: 10.4049/jimmunol.170.5.2582. [DOI] [PubMed] [Google Scholar]
- Cooper LJ, Topp MS, Pinzon C, Plavec I, Jensen MC, Riddell SR, et al. Enhanced transgene expression in quiescent and activated human CD8+ T cells. Hum Gene Ther. 2004;15:648–658. doi: 10.1089/1043034041361217. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA., and , Morgan RA. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174:4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving' 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, et al. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–674. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- Coito S, Sauce D, Duperrier A, Certoux JM, Bonyhadi M, Collette A, et al. Retrovirus-mediated gene transfer in human primary T lymphocytes induces an activation- and transduction/selection-dependent TCR-B variable chain repertoire skewing of gene-modified cells. Stem Cells Dev. 2004;13:71–81. doi: 10.1089/154732804773099272. [DOI] [PubMed] [Google Scholar]
- Deschamps M, Robinet E, Certoux JM, Mercier P, Sauce D, De Vos J, et al. Transcriptome of retrovirally transduced CD8+ lymphocytes: influence of cell activation, transgene integration, and selection process. Mol Immunol. 2008;45:1112–1125. doi: 10.1016/j.molimm.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten KB, Kramer D, Kueter EW, Graf M, Schoedl T, Meijer CJ, et al. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clin Immunol. 2006;119:135–145. doi: 10.1016/j.clim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Rammensee H, Bachmann J, Emmerich NP, Bachor OA., and , Stevanović S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Sauter SL., and , McCray PB., Jr Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors—design, biosafety, and production. Gene Ther. 2005;12:1089–1098. doi: 10.1038/sj.gt.3302570. [DOI] [PubMed] [Google Scholar]
- Engels B, Cam H, Schuler T, Indraccolo S, Gladow M, Baum C, et al. Retroviral vectors for high-level transgene expression in T lymphocytes. Hum Gene Ther. 2003;14:1155–1168. doi: 10.1089/104303403322167993. [DOI] [PubMed] [Google Scholar]
- Dang Q, Auten J., and , Plavec I. Human beta interferon scaffold attachment region inhibits de novo methylation and confers long-term, copy number-dependent expression to a retroviral vector. J Virol. 2000;74:2671–2678. doi: 10.1128/jvi.74.6.2671-2678.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani A, Hawley TS., and , Hawley RG. Performance- and safety-enhanced lentiviral vectors containing the human interferon-beta scaffold attachment region and the chicken beta-globin insulator. Blood. 2003;101:4717–4724. doi: 10.1182/blood-2002-09-2991. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk MH, Hoogeboom M, Hagedoorn R, Kester MG, Willemze R., and , Falkenburg JH. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J Exp Med. 2004;199:885–894. doi: 10.1084/jem.20031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Wolkers MC, van den Boom MD, van der Valk MA., and , Schumacher TN. Immunotherapy through TCR gene transfer. Nat Immunol. 2001;2:957–961. doi: 10.1038/ni1001-957. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Chamoto K, Funamoto H, Kosaka A, Matsuzaki J, Abe H, et al. An efficient method to prepare T cell receptor gene-transduced cytotoxic T lymphocytes type 1 applicable to tumor gene cell-therapy. Cancer Sci. 2003;94:389–393. doi: 10.1111/j.1349-7006.2003.tb01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoto K, Tsuji T, Funamoto H, Kosaka A, Matsuzaki J, Sato T, et al. Potentiation of tumor eradication by adoptive immunotherapy with T-cell receptor gene-transduced T-helper type 1 cells. Cancer Res. 2004;64:386–390. doi: 10.1158/0008-5472.can-03-2596. [DOI] [PubMed] [Google Scholar]
- Morris EC, Tsallios A, Bendle GM, Xue SA., and , Stauss HJ. A critical role of T cell antigen receptor-transduced MHC class I-restricted helper T cells in tumor protection. Proc Natl Acad Sci USA. 2005;102:7934–7939. doi: 10.1073/pnas.0500357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte MA, Coccoris M, Wolkers MC, van den Boom MD, Mesman EM, Song JY, et al. Targeting self-antigens through allogeneic TCR gene transfer. Blood. 2006;108:870–877. doi: 10.1182/blood-2005-08-009357. [DOI] [PubMed] [Google Scholar]
- Vieira J., and , Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]