Abstract
Malaria vectors can reach very high densities in villages near irrigated rice fields in Africa, leading to the expectation that malaria should be especially prevalent there. Surprisingly, this is not always the case. In Niono, Mali, villages from nonirrigated areas have higher malaria prevalence than those within the irrigated regions, which suffer from higher mosquito numbers. One hypothesis explaining this observation is that mosquitoes from irrigated fields with high densities are inefficient vectors. This could occur if higher larval densities lead to smaller mosquitoes that suffer elevated mortality. Three predictions of the hypothesis were studied. First, the effect of larval density on larval body size was measured for both Anopheles gambiae Giles and Anopheles funestus Giles. Second, the relationship between larval and adult body size was tested. Third, evidence of an effect of adult size on survivorship in both irrigated and nonirrigated villages during the wet and dry seasons was sought. There was a modest positive relationship between densities of immatures and larval size, and a strong relationship between larval and adult size. Furthermore, adult survivorship was higher in nonirrigated areas. However, there was no effect of size on survivorship between comparable samples from both the irrigated and nonirrigated zones. Although density may have a causal relationship with reduced transmission in the irrigated areas of Niono, it is unlikely to be because higher density leads to smaller body size and lower survivorship.
Keywords: Anopheles, irrigation, size, density, survivorship
Irrigation in Sub-Saharan Africa can be a mixed blessing. It contributes to greater food production and income; however, it may increase the incidence of diseases such as schistosomiasis and malaria (Service 1989a,b; Dzodzomenyo et al. 1999; van der Hoek 2004). It is surprising, then, that several investigators have reported malaria transmission to be the same, or even less, in irrigated areas with high vector densities than in nearby nonirrigated areas with lower numbers of mosquitoes (Ijumba and Lindsay 2001, Diuk-Wasser et al. 2005a). For example, Dolo et al. (2004) found densities of Anopheles gambiae Giles in the irrigated region of Niono, Mali, in excess of 550 bites per person per night, compared with “only” 30–50 bites per person per night in nearby nonirrigated villages. However, malaria prevalence measured by longitudinal surveys was lower in the irrigated areas (Sissoko et al. 2004). Reasons for this departure from expectation may include 1) the nuisance of so many mosquitoes might compel more people to use bednets; 2) the greater prosperity of irrigated areas might permit access to better health care, protection, or both; or, 3) the mosquitoes that emerge from the high-density irrigated areas during the rainy season might be less efficient at transmitting malaria, e.g., if they do not survive as well (Diuk-Wasser et al. 2005a).
This study tests the hypothesis that body size is the cause of reduced vector survivorship at high density, leading to lower malaria transmission measured by vectorial capacity (Diuk-Wasser et al. 2005a) and prevalence (Sissoko et al. 2004) in the irrigated areas of Niono. Several aspects of this hypothesis are supported by previous work; in the irrigated zone, high density during the larval stage leads to smaller larvae through competition for resources (Schneider et al. 2000, Gimnig et al. 2002). Because larval and adult sizes are correlated (Lyimo et al. 1992), competition results in smaller adults. Mosquitoes of smaller body size have been reported to survive less well than larger ones in several species (Nasci 1986a,b, 1987; Kitthawee et al. 1990, 1992; Lounibos and Conn 1991; Ameneshewa and Service 1996; Takken et al. 1998). A combination of these factors could explain a decrease in malaria transmission (Kitthawee and Edman 1995) within irrigated areas. If higher densities reduce the vectorial effectiveness of An. gambiae by decreasing body size and consequently reducing survivorship, we expect to find that densities of larvae, adults, or both have a negative correlation with adult body size and that adult body size in turn is positively correlated with adult survivorship.
Materials and Methods
Study Location
The study area was located around the town of Niono, which is surrounded by the largest irrigation project in Mali, first established in 1932 (Klinkenberg et al. 2003, Dolo et al. 2004). Field work was conducted in three villages in the irrigated zone of the Office du Niger: Tissana (5.91° W, 14.36° N), Ténégué (5.94° W, 14.34° N), and Niessoumana (5.97° W, 14.33° N). A fourth village, Kalanampala (l5.86° W, 14.15° N) is in the nonirrigated area, ≈30 km by road from Niono. A satellite image showing the location of the villages with reference to one another and to irrigation has been published by Diuk-Wasser et al. (2005a).
Larval Collections
Larval collections were conduced in Tissana, Ténégué, and Niessoumana by using 350-ml dippers (BioQuip Products, Rancho Dominguez, CA) along the edge of rice fields. Two groups of three adjacent rice fields per village were sampled two times each in August and September 2003 (total 36 samples or fields per visit). There was at least a 2-wk period between successive samplings of any particular field to render the samples roughly independent. The same villages were visited in September 2004 when 12 fields in each were sampled, including the six visited in 2003 (total 36 fields). Larval collections were not conducted in Kalanampala because we did not locate habitats with more than a few An. gambiae larvae in or immediately around that village.
Two workers collected larvae at intervals of 1–2 m along the edge of each field following standard technique (Service 1993). Density was estimated as the number of dips required to collect 25 larvae up to a maximum of 45 dips by the same worker. A fixed number of larvae was collected from each field in an attempt to standardize the variance of size estimates between fields. The sample collected by the first worker will be referred to as sample A; this was the sample used to estimate larval density and the instar composition in the field. The second worker collected sample B, which also numbered ≈25 larvae. The number of fourth instars in sample B was enriched through an extra collecting effort after the initial 25 larvae were captured with the aim of capturing at least 10 individuals of this stage.
Samples A and B were taken back to the laboratory in separate plastic bags filled with water from the fields. Larvae in sample A were sorted by instar and fixed in Carnoy’s solution for later measurement. All larvae from sample B were placed in one growth chamber per field (BioQuip Products) filled with tap water. Pupation of fourth instar larvae generally occurred within 24 h. Because pupae do not feed, any effects of habitat on adult size should be reflective of conditions in the rice field and not the artificial growth chamber. Early each morning, for up to 3 d after the collection, any emerged adults in the growth chambers were fixed in Carnoy’s solution.
Density Estimation in Rice Fields
To estimate the reliability of our measures of larval density, transects were conducted along the middle of the length (≈20 m) and width (≈8 m) of three rice fields, two in Ténégué and one in Niessoumana during September 2003. This resulted in a total of six transects. For each of these, a single dip sample was taken roughly every meter. These data were collected to assess the representativeness of edge dips to counts from elsewhere in the field.
We also used an existing data set (Diuk-Wasser et al. 2005b) of 1,448 rice fields that had each been sampled with 20 dips from April 2000 to January 2001. For that data set, five dips were taken at regular intervals along each side of the fields, and the number of larvae in each dip was recorded.
Adult Collections
Collections of adults were made by night landing catches from Tissana and Ténégué in the irrigated zone and from Kalanampala during September 2004. A dry season collection also was made in March 2004 from only Ténégué. These villages all show the pattern of adult mosquito abundance typical to this region (Table 1). In nonirrigated villages An. gambiae is common during the rainy season but apparently absent during the dry season; in the irrigated villages, An. gambiae is very abundant during the rainy season and moderately abundant during the dry part of the year, when some farmers irrigate for a second rice crop in March—April.
Table 1.
Number of adult An. gambiae captured per collector by landing catch
| Sample | n | No. of nights |
Interior: mean (SE) |
Exterior: mean (SE) |
|---|---|---|---|---|
| Ténégué (dry season) | 8 | 2 | 33 (10.0) | 12 (2.5) |
| Ténégué (rainy season) | 4 | 1 | 41 (7.8) | 40 (2.8) |
| Tissana (rainy season) | 4 | 1 | 57 (4.2) | 37 (0.7) |
| Kalanampalaa (rainy season) | 10 | 2 | 23 (7.6) | 7 (2.2) |
Nonirrigated village; n, Number of human-nights (one interior and one exterior per station per night).
Densities were high during the September collections, as expected, so sufficiently large sample sizes were obtained with one or two night catches. During the dry season there were fewer mosquitoes, so it was necessary to supplement night catches with daytime collections of unfed females by using mouth aspirators from inside homes and other structures. Night landing catches were conducted from 1800 to 0600 hours at two collecting stations over 300 m apart in the village, each with one collector indoors and one outdoors. Standard methods were employed for these collections (Service 1993).
Estimation of Survivorship and Size
Adult females were dissected between 0900 and 1200 hours on the morning after night catches. Parity rates were estimated by examination of the ovaries following the method of Detinova (1962). Under the assumption of no net change in population size (i.e., constant recruitment and death rate), survivorship can be estimated as the gth root of the parity rate (Davidson and Draper 1953, Davidson 1954), where g is the number of days between a bloodmeal and oviposition (the gonotrophic cycle; WHO 1975). Numerous laboratory experiments by Y. T. Touré and C.E.T. (unpublished data) have found that g of An. gambiae s.l. in Mali is very close to 2.0, identical to other estimates from West Africa (Thompson 1948).
As previous research has shown that wing length is highly correlated to the first two principal components of adult size (Petrarca et al. 1998), this measure was used to estimate adult body size. Samples were dried in individual tubes for 24–48 h before dissection of a single wing from each individual, which was mounted on a slide with a small drop of ethanol. Coverslips were attached with nail varnish and wing length measured from the alular notch to the wing tip excluding fringe using a filar micrometer and dissecting microscope. This procedure was repeated to estimate the body size of adult An. gambiae from the growth chambers.
Larvae also were measured using a filar micrometer and dissecting microscope. The larvae were measured for total length, from the distal point of the head to the end of the anal segment excluding antennae, feeding brush, and caudal hair. We distinguished instars of An. gambiae and separated these from those of Anopheles funestus Giles (Gilles and De Meillon 1968, Gilles and Coetzee 1987) with the aid of a dissecting microscope.
Statistical Testing
Using the transect data, the number of larvae per dip at field edges were compared with counts from field interiors with a Kruskal—Wallis tests to ascertain whether sampling only from the edge of fields would bias density estimates. In addition, the general reliability of density estimation by dipping was tested using the Diuk-Wasser et al. (2005b) data set with a one-way intraclass correlation on average measure [ICC(k) in the terminology of McGraw and Wong 1996]. This test produces a measure of the variance that can be attributed to the rice field itself (the object whose density we wish to estimate) as a proportion of the overall variance in the dip data. Thus, if each dip exactly measured the actual density in the field (agreed with all other dips), then ICC(k) = 1.
The effect of larval density on larval body size was tested using the mean values for length measurements per field. Mean length was calculated for each instar and species when more than one was present. Density (necessarily a single value per field) was estimated by the number of dips required to collect 25 larvae, as described above.
A relationship between adult size and survivorship was sought by comparing the body size distribution of parous versus nulliparous female An. gambiae per village sample with a Kolmogorov—Smirnov two-sample test (Sokal and Rohlf 1969). This approach allows the detection of significant differences in any size class and thus is more sensitive than testing the means alone. It also controls for other factors affecting survivorship between villages that may be confounded with adult size.
Results
Reliability of Larval Density Estimates
The number of larvae captured per dip in transects varied between zero and six. A statistically significant difference in number of larvae was not found between edge and interior dips within fields (Kruskal—Wallis test, field 1: nedge = 8, ninterior = 17; χ21 = 1, P = 0.16; field 2: nedge = 8, ninterior = 19; χ21 = 0.085, P = 0.77; and field 3: nedge = 8, ninterior = 17; χ21 = 0.070, P = 0.79).
The intraclass correlation showed that 86% of the variance in the data were between fields, indicating a high level of reliability (n = 1448 fields, 20 observations per field; ICC F1,447, 27,512 = 7.38; P < 0.001).
Density and Larval and Adult Body Size
Differences in instar composition or length per instar between years were tested to see whether pooling was justified. Instar composition varied significantly between 2003 and 2004 (Fisher exact test; P < 0.035) as did length per instar based on analysis of variance (ANOVA) (R Development Core Team 2004): instar: F2, 673 = 671.4; P < 0.001; year: F1, 673 = 4.2; P = 0.04; species: F1, 673 = 3.2; P = 0.08. This suggests a need to include year as a blocking variable when examining the effect of density on larval size in this data set.
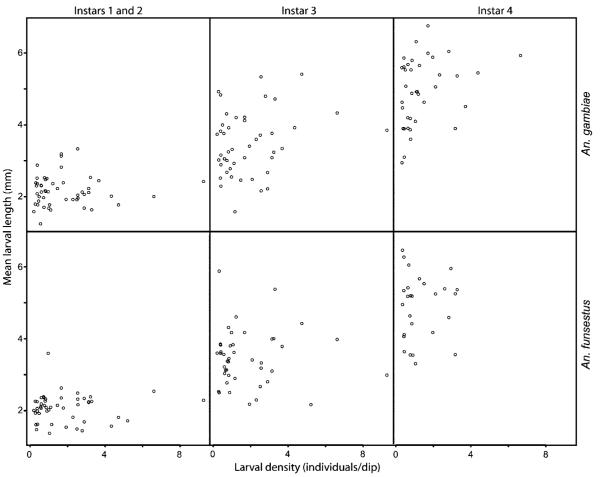
An analysis of covariance model (StataCorp 2003) of these data found a significant effect of density on mean larval size (Table 2), but this effect disappears when one or more of the other terms are removed (data not shown), significant or not (such as year or species). This indicates a significant (and positive: see Fig. 1) but slight effect of density on mean larval size, which can easily be missed if other factors are not controlled.
Table 2.
Analysis of covariance of mean larval size per field as determined by density
| Source | Partial SS | df | MS | F | P |
|---|---|---|---|---|---|
| Model | 314.26 | 7 | 44.89 | 88.74 | <0.01 |
| Density | 2.56 | 1 | 2.56 | 5.06 | 0.03 |
| Instar | 126.40 | 2 | 63.20 | 124.92 | <0.01 |
| Species | 0.07 | 1 | 0.07 | 0.14 | 0.71 |
| Yr | 1.33 | 1 | 1.33 | 2.62 | 0.11 |
| Density × instar | 1.36 | 2 | 0.68 | 1.35 | 0.26 |
| Residual | 123.95 | 245 | 0.51 | ||
| Total | 438.22 | 252 | 1.74 |
Larvae per dip is a continuous variable. Larval stage, species, and collection year were discrete variables. The sum of squares (SS) are partial because the factors in the model are not perfectly orthogonal.
Fig. 1.
Estimated larval density versus mean length of larvae per growth stage and species. Each point represents the mean for a separate field from the 2003 and 2004 collections.
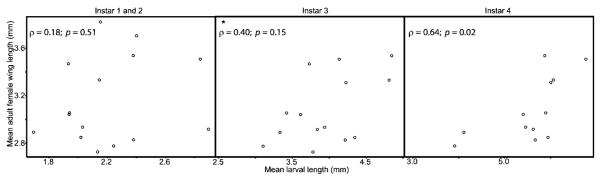
The mean body size of female adult An. gambiae emerging from the fourth instar of sample B had a strong relationship to the mean size of larvae of the same instar and species from sample A paired by field (Fig. 2). The correlation between the two measurements is significant only for the comparison of fourth instars, and not significant between sample B adults and earlier instars of sample A. This test was only possible for the 2003 samples because no adult An. gambiae emerged from the growth chambers in 2004, possibly due to contaminated water.
Fig. 2.
Mean larval length for An. gambiae (sample A; n per mean = 1–12 individuals, mean number of observations = 3.13) versus mean adult An. gambiae female wing length from growth chambers collected from the same field (sample B; n per mean = 1–3 individuals, mean number of observations = 1.44). Spearman rank correlations were conducted for each of the three stage classes, with results shown in the figure panels. The point marked with an asterisk (*) was considered an outlier and was not included in the analysis.
The wing lengths of all the emerged adult females of sample B (n = 27 individuals; mean = 3.14, SE = 0.07) were compared with those of the nulliparous females collected in the irrigated villages during the wet season (n = 186 individuals; mean = 3.07, SE = 0.01). There was not a statistically significant difference in size between the two samples, which supports the assumption that nulliparous females collected from the village are a representative sample of the size of adults produced in the fields (t27.5 = 2.04, P > 0.05).
Adult Body Size and Survival
Differences between samples in parity rates and wing lengths were tested with Bonferroni corrected pairwise chi-square tests (R Development Core Team 2004) and a Student—Newman—Keuls multiple range grouping test (StataCorp 2003), respectively. The two rainy season irrigated village samples (Ténégué [rainy season] and Tissana) were the only ones which did not differ significantly in either of these tests, and so were combined for a joint irrigated rainy season estimate (Table 3).
Table 3.
Parity rates, estimated daily survivorship, and wing length measurements for all adult female An. gambiae collected in 2003 and 2004
| Wing length: mean (SE) |
K-S test |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sample name | n | PR | ES | Parous | Nulliparous | All females/ SNK range |
D | P |
| Ténégué (dry season) | 157 | 0.45b | 0.672 | 3.24 (0.25) | 3.25 (0.26) | 3.24 (0.26)/A | 0.143 | 0.41 |
| Ténégué (rainy season) | 117 | 0.35 | 0.592 | 3.01 (0.17) | 3.03 (0.17) | 3.02 (0.17)/B | 0.182 | 0.34 |
| Tissana (rainy season) | 153 | 0.28b | 0.530 | 3.00 (0.16) | 2.99 (0.15) | 2.99 (0.15)/B | 0.102 | 0.90 |
| Kalanampalaa (rainy season) | 142 | 0.43 | 0.655 | 2.82 (0.23) | 2.89 (0.19) | 2.86 (0.21)/C | 0.139 | 0.52 |
| Irrigated zone (rainy season) | 270 | 0.31 | 0.558 | 3.00 (0.16) | 3.01 (0.16) | 0.140 | 0.21 | |
Significant differences in parity rates between individual samples were tested with Boneferroni corrected chi-square tests. Comparisons were only significant between Ténégué (dry season) and Tissana (χ2 = 9.77, P = 0.02). Differences in wing length of all females from each sample were tested with a Student—Newman—Keuls (SNK) Multiple range grouping test (subsets for α = 0.05) on a one-way ANOVA (wing length ≈ sample: F3, 565 = 94.47; P < 0.001). For this test groupings are represented by the letters A, B, and C; samples with the same grouping do not differ significantly in wing length. Differences in wing length between parous and nulliparous females within each sample were tested with two-sample Kolmogorov—Smirnoff (K-S) tests.
Nonirrigated village; n, sample size; PR, parity rate; ES, estimated daily survivorship.
Significantly different parity rates at α = 0.05
Estimated daily survival rates ranged from 0.53 in Tissana (rainy) to 0.67 in Ténégué (dry). Importantly, when the mosquito densities are lower (irrigated villages during the dry season and nonirrigated villages), daily survival is higher, and when mosquito densities are high (irrigated villages during the rainy season), daily survival is lower. Both this result and the estimates of survivorship obtained here are similar to those found by others (Dolo et al. 2004). Finally, Table 3 also shows that the distribution of wing lengths between parous and nulliparous females were in all cases not significantly different by Kolmogorov-Smirnov two sample tests.
Discussion
Although some portions of the size based explanation of the link between density and transmission are substantiated by these results, the entire chain of events is not likely in Niono. The results of this study support alternate causes for the previously observed decrease of malaria prevalence in villages with high vector density (Dolo et al. 2004, Sissoko et al. 2004). The potential role of bednet use is noteworthy and is discussed below.
An association between larval density and larval body size was seen, but it was weak and sensitive to other variables. This may be the result of small sample sizes per field. Surprisingly, the relationship observed was positive in the case of An. gambiae, the opposite of what is expected under resource competition (Gimnig et al. 2002). The positive relationship suggests that some fields are simply superior larval habitats to others and that these produce both more and slightly larger larvae. Density independent factors that determine field quality seem to be important: adjacent fields of the same rice growth stage that shared a water source were often seen to have very different larval densities. Agricultural practices such as fertilizer usage have been shown to affect larval densities (Victor and Reuben 2000, Mutero et al. 2004) and have been proposed to be a factor in Niono (Diuk-Wasser et al. 2005b). In addition, predation may affect larval composition and numbers (Service 1977).
Density dependence and competition among larval anophelines has been shown under controlled conditions without predation (Gimnig et al. 2002) and under strict laboratory conditions (Schneider et al. 2000). The density of larvae in such studies was in the hundreds per liter, however, whereas the highest estimated density observed in this study was under 20 per liter. This agrees with densities reported from other, much more extensive, sampling efforts in this area (Diuk-Wasser et al. 2005b). We have found the accuracy of density estimation in these rice fields to be better than generally reported (WHO 1975, Service 1993) and that edge samples are reflective of density in other parts of the field; it is therefore unlikely that densities ever get high enough for there to be resource limitation.
A significant relationship between larval and adult body size was found for fourth instars. This was absent from earlier instars collected from the same fields, as expected, because other cohorts are the product of a different environmental and parental composition than the one from which adults were produced for comparison. Because body size may affect multiple aspects of the mosquito vector competence (Lounibos and Conn 1991, Suwanchaichinda and Paskewitz 1998, Takken et al. 1998, Ng’habi et al. 2005) apart from survival, body size will remain of interest to malaria researchers (Mwangangi et al. 2004). For this study, however, the importance of body size to transmission rests on it having a significant effect on adult survivorship.
The two samples with the highest estimated survivorship had very large or very small size compared with the other samples. This indicates that there is a low linkage between adult body size and survivorship. The survivorship estimates are only rough, so this result is not conclusive. Independent from estimated survivorship, a better test is to compare adult body sizes of older and younger females within samples (villages). Results presented here suggest that nulliparous females are representative in size of females being produced in the rice fields. They are predominantly those that have not yet oviposited but will do so and have been successful only in flying to the village and landing on a human to feed. Parous females are necessarily older and have been successful in feeding, laying eggs and returning to feed again at least once. If adult size is associated with survivorship and larger females have an advantage, then the size of parous (older) females should be larger than that of the nulliparous (younger) females. Comparison of the sizes of these females showed no difference between older (parous) females and younger (nulliparous) ones. Overall, adult body size in these samples was not closely related to female daily survival—by either test—and so is unlikely to underlie the reduction in malaria transmission that occurs in irrigated villages during the rainy season (Sissoko et al. 2004).
The densities of adult mosquitoes observed from night catches are substantially less than those reported by Dolo et al. (1999, 2004). The largest number of bites per person per night recorded here was 57 compared with >500 in the earlier study. Although the rank order of density was similar in both studies, the reason for this difference in maximum density is not clear. Possibilities include the choice of collecting locations within villages, collectors with varying levels of experience or simple year-to-year differences in mosquito abundance.
Despite the low relationship between density and larval size, significant variation in adult body size between dry versus rainy season and irrigated versus nonirrigated zones was seen. Based on our results, this reflects varying larval sizes, but causes at the larval stage remain unclear. Others have observed a difference in size of adult Anopheles dirus Peyton and Harrison between rainy and dry seasons, perhaps because of nutritional stress on larvae from the dilution of aquatic habitats or physiological changes with temperature (Kitthawee et al. 1992).
Species other than An. gambiae were excluded from our samples and so did not interfere with adult size measurements. An. funestus were not abundant and are easily distinguishable. Dolo et al. (1999) further reported that Anopheles arabiensis Patton, although present, is not common in this area. Within An. gambiae s.s., almost 100% have been reported as belonging to the Mopti chromosomal form over the year (Dolo et al. 1999). Consequently, our samples are very likely to be almost all An. gambiae s.s., Mopti chromosomal form, with some An. arabiensis and other chromosomal forms present in very low numbers (Dolo et al. 2004).
Size does not seem to be the causal factor linking higher vector density to lower malaria prevalence through survivorship. Survivorship is generally an important determinant of vector competence, however, and may be affected by other factors in Niono. Of the hypotheses enumerated in the introduction and in previous work(Dolo et al. 2004, Diuk-Wasser et al. 2005a), increased bednet use with high mosquito density is a likely causative factor because anthropophily very clearly decreases with increasing density (Diuk-Wasser et al. 2005a). Bednet use is a focus of future research for our group. This factor alone may be sufficient to explain the reduction in malaria prevalence, or it may be a product of lower survivorship combined with increased zoophily in these anophelines.
Overall, the results presented here agree qualitatively with previous entomological studies in the Niono region (Dolo et al. 2004). They do not support the hypothesis that density effects on size are responsible for reduced malaria transmission in Niono through lower vector survivorship. A modest and unexpectedly positive effect of density on larval size is suggested by the data and a relationship between larval and adult size in An. gambiae were detected, but there was no evidence of reduced survivorship for smaller females in any of the samples. Previous findings about estimated survivorship were confirmed: during periods of high mosquito abundance daily survival is higher in the nonirrigated villages than in the irrigated area. It is also apparent from these results that the adult size of An. gambiae varies significantly between zones and between seasons.
Previous research indicated that female An. gambiae from nonirrigated villages are more effective vectors of malaria than those from the rainy season irrigated villages, when the number of mosquito bites per night per person can be extremely high (Dolo et al. 2004). This study indicates this difference is not mediated by differences in body size produced by larval crowding.
Acknowledgments
We thank the residents of Ténégué, Tissana, and Kalanampala; the village guides; and Kefa Diarra for assistance. We also thank Adama Dao, Sibiry Samake, and Boubacar Guindo for assistance in the field as well as Yeya Touré and Robert Gwadz. We acknowledge the collaboration of the Niono Health Center, the Office du Niger, the Institute d’Economie Rurale as well as the members of the Malaria Research and Training Center GIS Laboratory in Bamako for advice and help. This work was supported by National Institutes of Health Grant 5RO1AI51633.
References Cited
- Ameneshewa B, Service MW. The relationship between female body size and survival rate of the malaria vector Anopheles arabiensis in Ethiopia. Med. Vet. Entomol. 1996;10:170–172. doi: 10.1111/j.1365-2915.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Davidson G. Estimation of the survival rate of anopheline mosquitoes in nature. Nature (Lond.) 1954;174:792–793. doi: 10.1038/174792a0. [DOI] [PubMed] [Google Scholar]
- Davidson G, Draper C. Field studies of some of the basic factors concerned in the transmission of malaria. Trans. R. Soc. Trop. Med. Hyg. 1953;49:522–535. doi: 10.1016/s0035-9203(53)80005-2. [DOI] [PubMed] [Google Scholar]
- Detinova T. Age-grouping methods in Diptera of medical importance with special reference to some vectors of malaria. World Health Organization; Geneva, Switzerland: 1962. [PubMed] [Google Scholar]
- Diuk-Wasser MA, Touré MB, Dolo G, Bagayoko M, Sogoba N, Traoré SF, Manoukis N, Taylor CE. Vector abundance and malaria transmission in rice-growing villages in Mali. Am. J. Trop. Med. Hyg. 2005a;72:725–731. [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Dolo G, Bagayoko M, Sogoba N, Touré MB, Moghaddam M, Manoukis N, Rian S, Traoré SF, Taylor CE. Patterns of irrigated rice growth and malaria vector breeding in Mali using multitemporal ERS-2 synthetic aperture radar. Int. J. Remote Sens. 2005b;27:535–548. doi: 10.1080/01431160500104350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolo G, Dao A, Traoré S, Bouaré M, Sogoba N, Niaré O, Bagayogo M, Sangaré D, Touré YT. Technical report. West African Rice Development Association; Cotonou, Benin: 1999. Rapport de l’étude entomologique sur la transmission du paludisme dans six villages (aout 1995-fevrier 1998) [Google Scholar]
- Dolo G, Briet OJT, Dao A, Traoré SF, Bouaré M, Sogoba N, Niaré O, Bagayogo M, Sangaré D, Teuscher T, Touré YT. Malaria transmission in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop. 2004;89:147–159. doi: 10.1016/j.actatropica.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Dzodzomenyo M, Dunyo SK, Ahorlu CK, Coker WZ, Appawu MA, Pedersen EM, Simonsen PE. Bancroftian filariasis in an irrigation project community in southern Ghana. Trop. Med. Int. Health. 1999;4:13–18. doi: 10.1046/j.1365-3156.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Gilles MT, Coetzee M. A supplement to the Anophelinae of Africa south of the Sahara. Johannesburg: 1987. Publications of the South African Institute of Medical Research No. 55. [Google Scholar]
- Gilles MT, De Meillon B. The Anophelinae of Africa, south of Sahara (Ethiopian Zoogrographical Region) 2nd ed. Johannesburg: 1968. Publications of the South African Institute of Medical Research No. 54. [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J. Med. Entomol. 2002;39:162–172. doi: 10.1603/0022-2585-39.1.162. [DOI] [PubMed] [Google Scholar]
- Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: paddies paradox. Med. Vet. Entomol. 2001;15:1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- Kitthawee S, Edman JD. Adult body size and biting activity of field populations of Anopheles dirus (Diptera: Culicidae) Southeast Asian J. Trop. Med. Public Health. 1995;26:582–585. [Google Scholar]
- Kitthawee S, Edman JD, Sattabongkot J. Evaluation of survival potential and malaria susceptibility among different size classes of laboratory-reared Anopheles dirus. Am. J. Trop. Med. Hyg. 1990;43:328–332. doi: 10.4269/ajtmh.1990.43.328. [DOI] [PubMed] [Google Scholar]
- Kitthawee S, Edman JD, Upatham ES. Relationship between female Anopheles dirus (Diptera: Culicidae) body size and parity in a biting population. J. Med. Entomol. 1992;29:921–926. doi: 10.1093/jmedent/29.6.921. [DOI] [PubMed] [Google Scholar]
- Klinkenberg E, Takken W, Huibers F, Touré YT. The phenology of malaria mosquitoes in irrigated rice fields in Mali. Acta Trop. 2003;85:71–85. doi: 10.1016/s0001-706x(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Conn J. Fecundity, parity and adult feeding relationships among Nyssorhynchus malaria vectors from Venezuela. Mem. Inst. Oswaldo Cruz. 1991;86:57–66. doi: 10.1590/s0074-02761991000100010. [DOI] [PubMed] [Google Scholar]
- Lyimo EO, Takken W, Koella JC. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomol. Exp. Appl. 1992;63:265–271. [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Pyschol. Methods. 1996;1:30–46. [Google Scholar]
- Mutero CM, Ng’anga PN, Wekoyela P, Githure J, Konradsen F. Ammonium sulphate fertilizer increases larval populations of Anopheles arabiensis and culicine mosquitoes in rice fields. Acta Trop. 2004;89:187–192. doi: 10.1016/j.actatropica.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Mwangangi JM, Mbogo CM, Nzovu JG, Kabiru EW, Mwanbi H, Githure JI, Beier JC. Relationships between body size of anopheles mosquitoes and Plasmodium falciparum sporozoite rates along the Kenya coast. J. Am. Mosq. Control Assoc. 2004;20:390–394. [PubMed] [Google Scholar]
- Nasci RS. The relationship between adult mosquito body size and parity in field populations. Environ. Entomol. 1986a;15:874–876. [Google Scholar]
- Nasci RS. The size of emerging and host-seeking Aedes aegypti and the relation of size to blood feeding success in the field. J. Am. Mosq. Control Assoc. 1986b;2:61–62. [PubMed] [Google Scholar]
- Nasci RS. Adult body size and parity in field populations of the mosquitoes Anopheles crucians, Aedes taeniorhychus and Aedes sollicitans. J. Am. Mosq. Control Assoc. 1987;3:636–637. [PubMed] [Google Scholar]
- Ng’habi KR, John B, Nkwengulila G, Knols BGJ, Killeen GF, Ferguson HM. Effect of larval crowding on mating competitiveness of Anopheles gambiae mosquitoes. Malar. J. 2005;4:49. doi: 10.1186/1475-2875-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrarca V, Sabatinelli G, Touré YT, Di Deco MA. Morphometric multivariate analysis of field samples of adult Anopheles arabiensis and Anopheles gambiae s.s (Diptera: Culicidae) J. Med. Entomol. 1998;35:16–25. doi: 10.1093/jmedent/35.1.16. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2004. ( http://www.Rproject.org) [Google Scholar]
- Schneider P, Takken W, McCall PJ. Interspecific competition between sibling species larvae of Anopheles arabiensis and An. gambiae. Med. Vet. Entomol. 2000;14:165–170. doi: 10.1046/j.1365-2915.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- Service MW. Mortalities of immature stages of species-b of Anopheles gambiae complex in Kenya: comparison between rice fields and temporary pools, identification of predators, and effects of insecticidal spraying. J. Med. Entomol. 1977;13:535–545. doi: 10.1093/jmedent/13.4-5.535. [DOI] [PubMed] [Google Scholar]
- Service MW. Irrigation: boon or bane. In: Service MW, editor. Demography and vector-borne diseases. CRC; Boca Raton, FL: 1989a. pp. 238–254. [Google Scholar]
- Service MW. Rice, a challenge to health. Parasitol. Today. 1989b;5:162–165. doi: 10.1016/0169-4758(89)90083-5. [DOI] [PubMed] [Google Scholar]
- Service MW. Mosquito ecology: field sampling methods. 2nd ed Elsevier; London, United Kingdom: 1993. [Google Scholar]
- Sissoko MS, Dicko A, Briet OJT, Sissoko M, Sagara I, Keita HD, Sogoba M, Rogier C, Touré YT, Doumbo OK. Malaria incidence in relation to rice cultivation in the irrigated sahel of Mali. Acta Trop. 2004;89:161–170. doi: 10.1016/j.actatropica.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 1st ed W. H. Freeman and Company; San Francisco, CA: 1969. [Google Scholar]
- StataCorp . Stata statistical software: release 8. Stata-Corp LP; College Station, TX: 2003. [Google Scholar]
- Suwanchaichinda C, Paskewitz SM. Effects of larval nutrition, adult body size and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize sephadex beads. J. Med. Entomol. 1998;35:157–161. doi: 10.1093/jmedent/35.2.157. [DOI] [PubMed] [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J. Med. Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Thompson RCM. Studies on Anopheles gambiae and A. melas in and around Lagos. Bull. Entomol. Res. 1948;36:395–417. doi: 10.1017/s0007485300023221. [DOI] [PubMed] [Google Scholar]
- van der Hoek W. How can better farming methods reduce malaria? Acta Trop. 2004;89:95–97. doi: 10.1016/j.actatropica.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Victor TJ, Reuben R. Effects of organic and inorganic fertilizers on mosquito populations in rice fields of Southern India. Med. Vet. Entomol. 2000;14:361–368. doi: 10.1046/j.1365-2915.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization . Manual on practical entomology in malaria. World Health Organization Division of Malaria and Other Parasitic Diseases; Geneva, Switzerland: 1975. part II of WHO offset publication No. 13. [Google Scholar]