Abstract
A total of 600 healthy adults ≥ 65 years were randomized to receive 2 vaccinations 1 month apart of a subvirion avian influenza A/H5N1 vaccine containing 3.75, 7.5, 15, or 45 μg of hemagglutinin (HA) with or without aluminum hydroxide (AlOH). All formulations were safe. Groups given the vaccine with AlOH had more injection site discomfort. Dose-related increases in antibody responses were noted after the second vaccination. Antibody responses to the vaccine were not enhanced by AlOH at any HA dose level. A microneutralization titer ≥ 40 was observed in 36% and 40% of subjects who received 45 μg of HA with or without AlOH, respectively.
Keywords: Avian influenza vaccine, aluminum hydroxide, elderly
1. Introduction
The World Health Organization (WHO) has identified influenza A/H5N1 vaccine development as a cornerstone of pandemic preparedness [1]. Treanor, et al. [2] studied a two-dose regimen of a nonadjuvanted subvirion H5N1 vaccine that was subsequently approved by the United States Food and Drug Administration on 17 April 2007 [3]. Adults ≥ 65 years of age were not included in the Treanor, et al. [2] study. The immune responses of elderly adults to influenza vaccines are generally diminished as compared to those of younger adults [4]. Aluminum salts have been proposed as possible adjuvants for use in combination with pandemic influenza vaccine antigens to improve immune responses [5-7]. Therefore, in the current study, we evaluated this subvirion inactivated influenza H5N1 vaccine at a range of doses with or without aluminum hydroxide (AlOH) in healthy adults ≥ 65 years of age. This report describes our results with respect to the safety and immunogenicity of these vaccine formulations.
2. Materials and methods
2.1 Vaccines
Inactivated subvirion influenza A/H5N1 vaccine was prepared using the reassortant virus A/Vietnam/1203/2004 × A/Puerto Rico/8/34, derived by reverse-genetics techniques as previously described [2]. Four dose levels (3.75 μg of HA/0.25 mL; 7.5 μg of HA/0.5 mL; 15 μg of HA/0.5 mL; or 45 μg of HA/0.5 mL) were formulated with (+) or without (-) AlOH at 1200 μg (Al) per mL (sanofi pasteur, Swiftwater, PA).
2.2 Subjects
Subjects were 65 years of age or older and were ambulatory and judged to be medically stable for any underlying conditions, including acceptable vital signs (heart rate < 100 bpm and blood pressure ≤ 160 mm Hg systolic and ≤ 90 mm Hg diastolic), and no new or changes in prescription medications within 3 months of vaccination. Exclusion criteria included immunosuppression; known allergy to any component of the vaccines (including eggs); history of Guillain-Barré syndrome; prior receipt of an influenza A/H5 vaccine; and receipt of licensed inactivated or live vaccines within the preceding 2 weeks or 4 weeks, respectively. The protocol and consent forms were approved by the institutional review board of each participating study site.
2.3 Study design
We conducted a multicenter, randomized, dose-ranging clinical trial. All subjects as well as site and laboratory staff, except the vaccinators, were blinded to the vaccines administered. The vaccinators were not involved in the assessment of responses after immunization. Written informed consent was obtained from potential subjects prior to screening. Eligible subjects were randomly assigned to receive two doses of vaccine with approximately 60 subjects in each of the 3.75, 7.5, and 15 μg groups, and approximately 120 subjects in the 45 μg groups (Table 1). Each vaccination was administered into the deltoid muscle, and the two doses were administered approximately 28 days apart. Subjects were observed for at least 15 minutes after each immunization. For the seven days after each immunization, subjects recorded their oral temperature and the presence and severity of injection site findings (pain, tenderness, redness, and swelling) and systemic symptoms (feverishness, malaise, myalgias, headache, and nausea) on a memory aid. Subjects were seen in the clinic on days 2 and 8 after each vaccination, at which time their memory aids were reviewed by study staff. Twenty-eight days after each vaccination and 6 months after the second vaccination, the interim medical history was reviewed. Blood specimens for antibody assays were collected before and one month after each vaccination and 6 months after the second vaccination. Adverse events (AEs) and serious adverse events (SAEs) were defined, graded, and followed as previously reported in the Keitel, et al. [8] study of this vaccine in younger adults.
Table 1.
Subject demographics according to vaccine group received*
| ALL (N=599) |
3.75 μg - (N=60) |
3.75 μg + (N=57) |
7.5 μg - (N=62) |
7.5 μg + (N=64) |
15 μg - (N=59) |
15 μg + (N=59) |
45 μg - (N=118) |
45 μg + (N=120) |
P-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender N(%) | 0.45** | |||||||||
| Male | 275 (46) | 24 (40) | 24 (42) | 29 (47) | 22 (34) | 31 (53) | 28 (47) | 59 (50) | 58 (48) | |
| Female | 324 (54) | 36 (60) | 33 (58) | 33 (53) | 42 (66) | 28 (47) | 31 (53) | 59 (50) | 62 (52) | |
| Ethnicity N(%) | 0.053** | |||||||||
| Non-Hispanic | 592 (99) | 57 (95) | 57 (100) | 60 (97) | 63 (98) | 59 (100) | 59 (100) | 117 (99) | 120 (100) | |
| Hispanic | 7 (1) | 3 (5) | 0 | 2 (3) | 1 (2) | 0 | 0 | 1 (1) | 0 | |
| Race N(%) | 0.057** | |||||||||
| White | 583 (97) | 59 (98) | 54 (95) | 57 (92) | 63 (98) | 59 (100) | 57 (97) | 116 (98) | 118 (98) | |
| Black | 7 (1) | 0 | 0 | 3 (5) | 1 (2) | 0 | 2 (3) | 0 | 1 (1) | |
| Asian | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other | 9 (2) | 1 (2) | 3 (5) | 2 (3) | 0 | 0 | 0 | 2 (2) | 1 (1) | |
| Age (Years) | 0.39*** | |||||||||
| Mean (STD) | 72.4 (5.4) | 71.2 (4.5) | 73.8 (6.1) | 72.1 (4.4) | 72.4 (6.0) | 72.2 (5.8) | 72.2 (5.6) | 72.3 (5.1) | 72.7 (5.5) | |
| Median | 71.4 | 70.6 | 72.2 | 71.6 | 71.7 | 70.2 | 71.2 | 71.5 | 71.8 | |
| Min, Max | 65.0, 91.2 | 65.0, 83.8 | 65.1, 90.3 | 65.6, 81.7 | 65.1, 87.9 | 65.2, 88.8 | 65.1, 89.2 | 65.1, 91.2 | 65.1, 87.8 |
- and + indicate without or with aluminum hydroxide adjuvant, respectively.
The p-value of comparisons among treatment groups in Gender was calculated by Chi-Square test.
The p-values of comparisons among treatment groups in Ethnicity and Race were calculated by Fisher's exact test.
****: The p-value of comparisons among treatment groups in Age was calculated by ANOVA.
2.4 Antibody assays
Microneutralization (Neut) and hemagglutination-inhibition (HAI) assays were performed at the Southern Research Institute as previously described [8]. Seroconversion was defined as a four-fold or greater increase in antibody titer after vaccination (if antibody was detectable in the pre-vaccination sample) or an increase in titer from < 10 before vaccination to ≥ 40 after vaccination.
2.5 Statistical considerations
Prior to analyzing the endpoints, tests of homogeneity were performed to evaluate any differences between groups with respect to age, race/ethnicity, and gender. Frequencies of reactogenicity after each vaccination were based on the most severe response reported. Overall comparisons between vaccine groups were based on Fisher's exact test in which reactogenicity was dichotomized as none to mild or moderate to severe. Logistic regression models, controlling for age and gender, were used to evaluate differences between vaccine groups for injection site/systemic reactogenicity.
This study was not designed to test a specific immunogenicity hypothesis. The goal was to achieve initial estimates of this vaccine's dose-dependent and adjuvant-dependent immune responses for future investigations. The sample size for the 45 μg group (n = 120) was larger than for other dose levels and was selected to test the significance of an adjuvant effect within this dose group, with an 80% power to detect a difference in the proportion of responders of 50%, provided the response rate was 40% or greater in the nonadjuvant group.
Immune responses were summarized in terms of H5-specific Neut and HAI antibody titers, transformed to a logarithmic scale for analyses. Analyses included the distribution of titers (emphasizing the proportion of subjects achieving titers that were ≥ 40 and a 4-fold rise over baseline) at 28 and 56 days after the first vaccination. Fisher's exact test and ANOVA were used to test the effect of AlOH for dichotomous (titer ≥ 40, 4-fold rise) and continuous [geometric mean titers (GMT)] measures, respectively. Univariate and multivariate logistic regression and linear regression were conducted for 4-fold responses and GMT, respectively. The Cochran-Armitage trend test was performed to explore the dose response. The scores of the doses for this test were defined as 3.75, 7.5, 15, and 45. One-sided exact p values were selected. No adjustments were made for multiple comparisons.
3. Results
3.1 Subjects
A total of 600 subjects were enrolled between March and August 2006. The reasons that subjects were withdrawn from the study are shown in Figure 1. The subject who received an incorrect dose as the first vaccine was excluded from the safety and immunogenicity analyses. Two subjects who did not receive the second vaccine and did not provide post-vaccination blood samples were excluded from all immunogenicity analyses. One subject did not return for a blood sample 28 days after the first vaccination and was consequently not included in the immunogenicity analyses for this time point, but this subject was included in all other analyses for which data were available. Baseline demographic characteristics of enrolled subjects are shown in Table 1. No significant differences in baseline gender, ethnicity, race, or age were noted between the vaccine groups.
Figure 1.
Profile of the clinical trial and the reasons for subject withdrawal.
3.2 Safety and Reactogenicity
Serious Adverse Events
Thirty-seven serious adverse events, including two deaths, were reported during the study. None of these SAEs was considered to be associated with vaccination. One subject with a history of hypertension and a transient ischemic attack died of natural causes 46 days after receiving the second vaccination. A second subject died of an astrocytoma 151 days after receiving the second vaccination.
Injection Site Reactogenicity
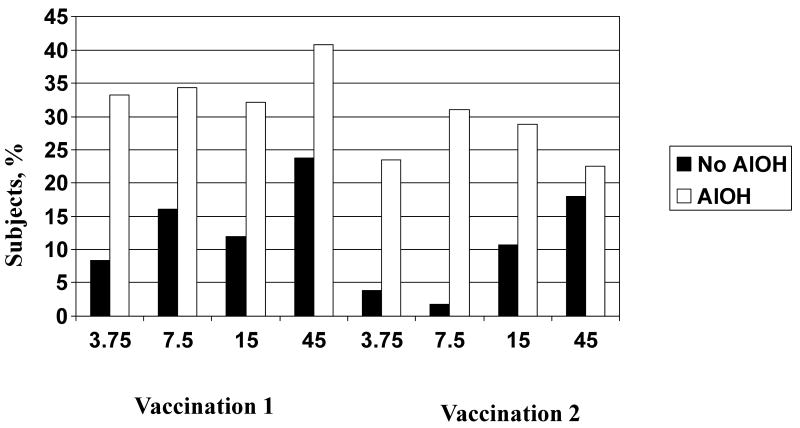
Tenderness at the injection site was the most common solicited adverse event. Most injection site symptoms were mild and peaked on day 0 or 1. No severe injection site reactions were reported following either vaccination. For the nonadjuvanted groups after both vaccinations, a dose-related increase in the frequency of injection site tenderness was observed (p = 0.05 and 0.002 after the first and second vaccinations, respectively) (Figure 2). Tenderness was significantly higher in the AlOH group compared to the nonadjuvanted group for all dose levels and both vaccinations (p ≤ 0.03 for all comparisons) except the 45 μg group after the second vaccination (p = 0.5). The frequencies of pain and tenderness were both significantly greater after the first vaccination than after the second vaccination for the 45 μg with AlOH group (p = 0.02 and 0.003, respectively). In logistic regression analyses, the inclusion of AlOH was independently associated with a higher frequency of tenderness after both vaccinations and of pain after the first vaccination only. Increased HA dose and younger age were associated with a higher frequency of tenderness after the first vaccination only; no gender-related differences were noted (data not shown).
Figure 2.
Percentage of subjects with injection site tenderness during the 7 days after receipt of different doses of inactivated influenza A/H5N1 vaccine with (white bars) or without (black bars) aluminum hydroxide (AlOH) adjuvant.
Systemic Reactogenicity
No severe systemic reactogenicity symptoms were reported following either vaccination. Malaise was the most prevalent systemic reaction reported across all groups. For the AlOH or nonadjuvanted groups after either vaccination, no dose-related increases in systemic symptoms were observed. No significant differences in individual symptoms were observed when comparing the frequencies of systemic reactions between the AlOH and nonadjuvanted groups at the same dose level. A comparison of the frequencies of any systemic reaction between the first and second vaccinations at the same dose level found that malaise and headache were more frequent after the first vaccination for the 3.75 μg group (p = 0.04 and 0.01, respectively; data not shown). In logistic regression analyses, younger age was independently associated with a higher frequency of headache after both vaccinations and female gender was associated with an increased frequency of headache after the second vaccination only (data not shown).
Other Adverse Events
One hundred thirty-four unsolicited AEs associated with vaccination were reported by 94 (15.7%) subjects. Ninety-five subjects had AEs due to abnormal vital signs as assessed prior to receipt of the second vaccination. The most common abnormal vital sign was systolic hypertension. There did not appear to be a significant dose-related difference in the occurrence of vital sign AEs across the vaccine groups.
3.3 Immunogenicity
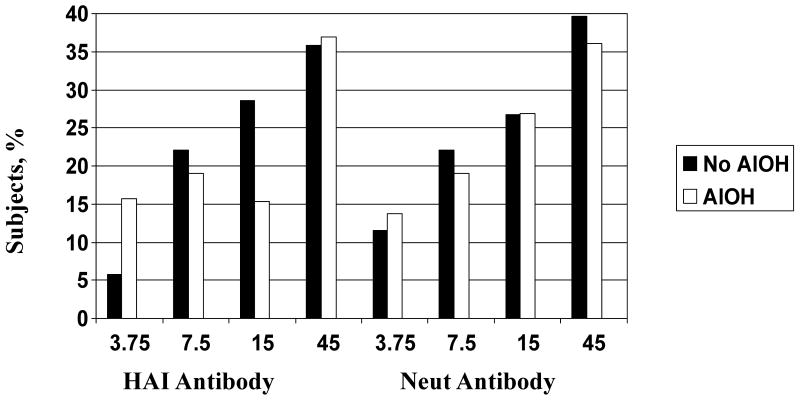
Serum HAI and Neut antibody responses before the first vaccination and 28 days after the first and second vaccinations are shown in Table 2. The GMTs of serum HAI and Neut antibodies before vaccination were similar among all groups. The percentages of subjects achieving a serum HAI or Neut antibody titer ≥ 40 twenty-eight days after the second vaccination are shown in Figure 3.
Table 2.
Serum hemagglutination inhibition (HAI) and microneutralization (Neut) antibody responses according to vaccine group received*
| Before Vaccination | After First Vaccination | After Second Vaccination | ||||||
|---|---|---|---|---|---|---|---|---|
| Assay and Group | No. of Subjects (N=597) | GMT (95% CI)** | No. of Subjects (N=596) | GMT (95% CI) | % Seroconversion*** (95% CI) | No. of Subjects (N=545) | GMT (95% CI) | % Seroconversion (95% CI |
| HAI | ||||||||
| 3.75- | 60 | 5.3 (4.9-5.7) | 60 | 6.7 (5.3-8.4) | 5.0 (1.0-13.9) | 52 | 6.6 (5.3-8.1) | 5.8 (1.2-15.9) |
| 3.75+ | 56 | 6.5 (5.2-8.1) | 56 | 8.1 (5.8-11.2) | 5.4 (1.1-14.9) | 51 | 8.9 (6.3-12.7) | 7.8 (2.2-18.9) |
| 7.5- | 62 | 6.6 (5.4-8.2) | 62 | 8.8 (6.4-12.0) | 11.3 (4.7-21.9) | 59 | 11.0 (7.5-16.1) | 15.3 (7.2-27.0) |
| 7.5+ | 64 | 6.2 (5.2-7.3) | 64 | 7.5 (5.8-9.5) | 4.7 (1.0-13.1) | 58 | 9.8 (7.2-13.2) | 12.1 (5.0-23.3) |
| 15- | 58 | 6.5 (5.5-7.8) | 58 | 9.3 (6.8-12.7) | 12.1 (5.0-23.3) | 56 | 13.0 (9.1-18.8) | 21.4 (11.6-34.4) |
| 15+ | 59 | 5.8 (5.1-6.7) | 58 | 8.1 (6.1-10.7) | 6.9 (1.9-16.7) | 52 | 10.1 (7.2-14.3) | 13.5 (5.6-25.8) |
| 45- | 118 | 5.9 (5.3-6.5) | 118 | 13.7 (10.4-17.9) | 24.6 (17.1-33.4) | 106 | 19.2 (14.1-26.0) | 34 (25.0-43.8) |
| 45+ | 120 | 6.1 (5.4-6.9) | 120 | 13.0 (9.8-17.2) | 20.8 (14.0-29.2) | 111 | 19.6 (14.5-26.4) | 33.3 (24.7-42.9) |
| Neut | ||||||||
| 3.75- | 60 | 5.8 (5.2-6.4) | 60 | 7.8 (6.2-9.8) | 5.0 (1.0-13.9) | 52 | 8.1 (6.4-10.2) | 9.6 (3.2-21.0) |
| 3.75+ | 56 | 6.7 (5.5-8.3) | 56 | 8.9 (6.8-11.8) | 5.4 (1.1-14.9) | 51 | 10.6 (7.8-14.4) | 7.8 (2.2-18.9) |
| 7.5- | 62 | 7.0 (5.7-8.7) | 62 | 9.8 (7.3-13.2) | 8.1 (2.7-17.8) | 59 | 13.7 (9.5-19.6) | 13.6 (6.0-25.0) |
| 7.5+ | 64 | 6.7 (5.6-7.9) | 64 | 8.1 (6.5-10.0) | 3.1 (0.4-10.8) | 58 | 14.2 (10.9-18.5) | 10.3 (3.9-21.2) |
| 15- | 58 | 6.6 (5.6-7.8) | 58 | 10.2 (7.7-13.7) | 10.3 (3.9-21.2) | 56 | 14.9 (10.8-20.5) | 17.9 (8.9-30.4) |
| 15+ | 59 | 6.7 (5.8-7.8) | 58 | 8.7 (6.8-11.1) | 3.5 (0.4-11.9) | 52 | 14.3 (10.6-19.3) | 19.2 (9.6-32.5) |
| 45- | 118 | 6.3 (5.6-7.0) | 118 | 13.5 (10.8-16.9) | 12.7 (7.3-20.1) | 106 | 21.8 (17.3-27.6) | 35 (25.9-44.8) |
| 45+ | 120 | 6.7 (5.9-7.6) | 120 | 13.5 (10.6-17.1) | 14.2 (8.5-21.7) | 111 | 26.5 (20.7-33.9) | 31.5 (23.0-41.0) |
- and + indicate without or with aluminum hydroxide adjuvant, respectively.
Geometric mean titer (95% confidence interval).
Percentage of subjects with a ≥ 4-fold rise in titer or increase from < 10 to ≥ 40 at the indicated time.
Figure 3.
Percentage of subjects achieving a serum HAI or Neut titer ≥ 40 approximately 28 days after receipt of two vaccinations with inactivated influenza A/H5N1 vaccine with (white bars) or without (black bars) aluminum hydroxide (AlOH) adjuvant.
Dose-response relationships after vaccination
Significant dose-response relationships for serum HAI and Neut antibody responses (GMTs, proportions of subjects with seroconversion, and the proportions of subjects with a titer ≥ 40) were observed after receipt of the second vaccination in both the adjuvanted and nonadjuvanted vaccine groups (p < 0.01 for all comparisons).
Effect of aluminum hydroxide on antibody responses
No significant differences in serum HAI or Neut antibody responses (GMTs, proportions of subjects with seroconversion, and the proportions of subjects with a titer ≥ 40) were observed between adjuvanted and nonadjuvanted groups given similar doses of vaccine after the first or second vaccination (p = not significant for all comparisons). In regression analyses, increasing HA dose was associated with higher serum HAI and Neut antibody responses (GMTs and proportions of subjects with seroconversion) after the first and second vaccinations (data not shown).
Baseline antibody responses
At baseline, 9% of subjects had detectable HAI antibody (titer ≥ 10), and 15% had detectable Neut antibody. To gain insight into whether these low levels of antibody reflected antibody to H5 or were nonspecific background, we compared the antibody response in these subjects after the first vaccination. We reasoned that those with true H5 antibody should be primed and have a higher response to the vaccine. When the HAI and Neut titers 28 days after the first vaccination were compared, the subjects with preexisting antibody had higher GMTs than subjects without preexisting antibody (94.0 vs. 7.8 for HAI; 49.6 vs. 8.0 for Neut; p < 0.0001 for both comparisons, Wilcoxon rank test) (Table 3). Subjects with preexisting antibody were also more likely to have seroconversion after the first vaccination (38% vs. 11% for HAI; 24% vs. 6.3% for Neut; p < 0.0001 for both comparisons, Fisher's exact test) (Table 3). These significant differences remained when adjusting for HA dose and adjuvant.
Table 3.
Comparisons of geometric mean titer (GMT) at Day 0 and after the first vaccination and proportions with seroconversion 28 days after the first vaccination, for subjects with a baseline titer < 10 and those with baseline titer ≥ 10
| Baseline Titer <10 | Baseline Titer ≥ 10 | P value | ||
|---|---|---|---|---|
| HAI | N | 540 | 56 | |
| Baseline GMT | 5.0 | 38.1 | <.0001* | |
| Post Dose 1 GMT | 7.8 | 94.0 | <.0001* | |
| Number (%) w/ 4-Fold Rise | 60 (11) | 21 (38) | <.0001** | |
| Neut | N | 509 | 87 | |
| Baseline GMT | 5.2 | 25.4 | <.0001* | |
| Post Dose 1 GMT | 8.0 | 49.6 | <.0001* | |
| Number (%) w/ 4-Fold Rise | 32 (6.3) | 21 (24) | <.0001** |
Wilcoxon rank test
Fisher's exact test
4. Discussion
Our results confirm and extend previous observations [7,8,9] related to the use of AlOH to improve the immunogenicity of H5N1 vaccines. The frequency of injection site tenderness was increased in groups receiving vaccine with AlOH. Dose-related increases in injection site tenderness also were noted. The addition of AlOH did not clinically improve antibody responses for the antigen doses tested. Our observations are also in agreement with those of Treanor, et al. [10] who studied this same nonadjuvanted vaccine among healthy elderly adults but also included a 90 μg dose group.
Previous studies [2,9] have also detected HAI, and more often Neut, antibodies to H5N1 virus prior to vaccination. The concern is whether these results represent true antibody to H5 or antibody to a cross-reacting epitope from H1, H2, or H3 viruses. Throsby, et al. [11] recently described a panel of monoclonal antibodies recovered from combinatorial display libraries that were constructed from human IgM+ memory B cells of recent seasonal influenza vaccinees. These monoclonal antibodies have broad heterosubtypic neutralizing activity against antigenically diverse H1, H2, H5, and H9 influenza subtypes. Neutralizing antibody also may be due to N1 specific antibody, as H1N1 viruses have circulated and been part of seasonal influenza vaccines. Subjects were not questioned regarding their past contacts with poultry or aquatic fowl. Subjects with preexisting antibody responded better to the first vaccination than subjects without preexisting antibody, an observation also made by Bernstein, et al. [9]. This improved response may be more suggestive of a booster response. This interesting observation deserves additional study.
One strategy to improve the immunogenicity of this H5N1 vaccine could be a prime-boost regimen whereby the H5N1 vaccine is included in annual vaccinations to prime the population and then a booster dose provided at the start of a pandemic. Recent reports by Zangwill, et al. [12] and Goji, et al. [13] suggest that the prime-boost strategy may be feasible. In the study by Zangwill and colleagues [12], healthy subjects 18-64 years of age who had previously received two doses (7.5-, 15-, 45-, or 90-μg each) of a nonadjuvanted subvirion inactivated H5N1 influenza vaccine received a third vaccination six months later of the same dose. The third dose of vaccine stimulated a Neut antibody response that was of greater magnitude than that seen 28 days after the second vaccination [2,12]. Available elderly subjects in our study who received two doses of H5N1 vaccine are currently being enrolled in a clinical trial that will evaluate their immune responses to a third dose of H5N1 vaccine from a different clade administered more than two years after initial receipt of an H5N1 vaccine.
A second strategy could be the use of other adjuvants to improve immunogenicity. MF59 is an oil-in-water emulsion adjuvant that has been demonstrated to increase antibody titers to avian influenza H9N2 [14] and H5N1 [9] vaccines in healthy adults. These trials did not include adults ≥ 65 years of age. MF59 has been approved for use in seasonal influenza vaccines for the elderly in several countries in Europe [15]. Thus, this strategy also deserves additional study.
Improving the immunogenicity of influenza vaccines will only partially meet the challenge of preparing an aging population for future epidemics and pandemics. By 2020, individuals ≥ 65 years of age will comprise about 16% of the projected population of the United States [16]. The impact of pandemic influenza on this population group may depend on their past exposures to similar influenza viruses, either by natural infection or vaccination. The promotion of seasonal influenza vaccination among the elderly is an important first step toward pandemic preparedness.
Acknowledgments
The authors thank the research subjects and staff: Vicki Smith, Linda Jamison, Michelle Dickey, Jesse LePage, Toni Cunningham, and David Bernstein at Cincinnati Children's Hospital; Carrie Nolan at University of Rochester; Lisreina Toro at Baylor College of Medicine; Melissa Billington, Lisa Chrisley, and Melissa Rosenberg at University of Maryland; Nancy Wagner at University of Iowa; and Karla Mosby, Carolyn Stefanski, and Joan Cannon at St. Louis University. We also thank Roland Levandowski, Shy Shorer, Katherine Muth, and Jean Hu-Primmer at DMID; Megan Sanza and Bernadette Jolles at EMMES; the Southern Research Institute; the PPD monitoring staff; and members of the Safety Monitoring Committee [Margo Schilling (Chair), Jeffrey Meier, Michael Keefer, Ann Falsey, Carol Tacket, Ward Bullock, Pamposh Kaul, and George Taffet] for their assistance in this study.
Funding: Research supported by the authors and summarized in this report was supported by Public Health Service Contracts N01-AI-25459, N01-AI-25465, N01-AI-25460, N01-AI-25461, N01-AI-45250, and N01-AI-30068 from the National Institute of Allergy and Infectious Diseases. Partial support was also provided by the University of Maryland General Clinical Research Center Grant M01-RR-16500, General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health. Wilbur Chen, M.D. receives support from K12RR023250.
Footnotes
Trial registration: ClinicalTrials.gov identifier: NCT00294099.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization (WHO) WHO strategic action plan for pandemic influenza. [April 25, 2009]; Available at: http://www.who.int/csr/resources/publications/influenza/StregPlanEPR_GIP_2006_2.pdf.
- 2.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 3.United States Food and Drug Administration. FDA approves first U.S. vaccine for humans against the avian influenza virus H5N1. [April 25, 2009]; Available at: http://www.fda.gov/bbs/topics/NEWS/2007/NEW01611.html.
- 4.Beyer WE, Palache AM, Baljet M, Masurel N. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine. 1989;7(5):385–94. doi: 10.1016/0264-410x(89)90150-3. [DOI] [PubMed] [Google Scholar]
- 5.Hehme N, Engelmann H, Künzel W, Neumeier E, Sänger R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med Microbiol Immunol. 2002;191(34):203–8. doi: 10.1007/s00430-002-0147-9. [DOI] [PubMed] [Google Scholar]
- 6.Hehme N, Engelmann H, Kuenzel W, Neumeier E, Saenger R. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 2004;103(12):163–71. doi: 10.1016/j.virusres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367(9523):1657–64. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 8.Keitel WA, Campbell JD, Treanor JJ, Walter EB, Patel SM, He F, et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I-II randomized clinical trial. J Infect Dis. 2008;198(9):1309–16. doi: 10.1086/592172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis. 2008;197(5):667–75. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 10.Treanor J, Bernstein D, Edwards K, Zangwill K, Noah D. Evaluation of inactivated monovalent rgA/Vietnam/1203/04 × PR8 subvirion vaccine in healthy elderly adults (abstract P731). Program and abstracts of the Options for the Control of Influenza VI Conference; 17-23 June 2007; Toronto, Canada. [Google Scholar]
- 11.Throsby M, van den Brink E, Jongeneelen M, Poon LL, Alard P, Cornelissen L, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PloS ONE. 2008;3(12):e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zangwill KM, Treanor JJ, Campbell JD, Noah DL, Ryea J. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J Infect Dis. 2008;197(4):580–3. doi: 10.1086/526537. [DOI] [PubMed] [Google Scholar]
- 13.Goji NA, Nolan C, Hill H, Wolff M, Noah DL, Williams TB, et al. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietman/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis. 2008;198(5):635–41. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 14.Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006;43(9):1135–42. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 15.Podda A, Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines. 2003;2(2):197–203. doi: 10.1586/14760584.2.2.197. [DOI] [PubMed] [Google Scholar]
- 16.United States Census Bureau. U.S. interim projections by age, sex, race, and Hispanic origin. [April 25, 2009];2004 March 18; Available at: http://www.census.gov/ipc/www/usinterimproj.