Abstract
Objectives
To evaluate the role of highly active antiretroviral therapy (HAART) and chemotherapy on tumor response among persons with AIDS-related Kaposi sarcoma (KS) and identify factors associated with response in a clinic setting.
Design
Retrospective cohort.
Methods
114 patients from two HIV clinics with a diagnosis of KS were identified via a clinical database. Records were reviewed to confirm KS diagnosis and abstract clinical and chemotherapy information. Demographics, laboratory values, and HAART use were abstracted electronically. Cox's proportional hazards models identified predictors of KS improvement and resolution.
Results
Thirty-six months following KS diagnosis, the rate of improvement among 64 patients with confirmed KS was 77%, and the rate of complete resolution 51%. In univariate analyses, recent chemotherapy was associated with KS improvement, and recent HIV viral load and HAART were associated with both improvement and resolution. No measured baseline characteristics (tumor stage, diagnosis year, CD4 T-cell count, HIV viral load, or prior HAART history) or recent CD4 T-cell counts predicted improvement or resolution. In multivariate analyses, recent chemotherapy (HR=5.5, 95% CI: 2.7-11.2, p<0.001) and HAART (HR=4.1, 95% CI: 1.4-12.6, p=0.01) were predictors of improvement; only recent HAART was associated with resolution (HR=6.2, 95% CI: 1.5-26.4, p=0.01). Response was not associated with type of HAART regimen (NNRTI-based, PI-based, or ritonavir-boosted PI-based).
Conclusions
HAART and chemotherapy are important in clinical KS response. Despite widespread availability of HAART and chemotherapy, KS continues to be a clinical problem; only half the patients achieved complete resolution of disease. New therapeutic approaches are needed.
Keywords: Kaposi sarcoma, HAART, chemotherapy, human herpesvirus 8, HIV/AIDS
Introduction
Kaposi sarcoma (KS) remains the most common malignancy among persons with HIV despite dramatic declines in incidence associated with highly active antiretroviral therapy (HAART).[1] Prior to introduction of HAART, treatment strategies for AIDS-related KS (AIDS-KS) had been mainly palliative, involving chemotherapy with low or short-term response.[2] HAART use among persons with AIDS-KS has been associated with improved survival and prolonged time to treatment failure.[3-5] Cases of complete KS resolution with HAART alone have been documented,[6-10] but few studies have examined response to HAART in a clinical setting. Patients with advanced KS rarely respond to HAART alone, but HAART in combination with chemotherapy improves response rates to 50%-82%.[8, 11-14] Neither the relative roles of HAART and chemotherapy, nor predictors of response to both HAART and chemotherapy in persons with KS have been extensively described. Furthermore, evaluations of treatment with both HAART and chemotherapy in primary care settings, where adherence may be an issue, do not exist. Therefore, we sought to determine the role of HAART and chemotherapy on clinical response of KS during the 36 months following KS diagnosis among patients with AIDS-KS in routine primary care at two HIV clinics.
Methods
Study setting and participants
We examined all patients with a diagnosis of KS who were enrolled in the longitudinal study of HIV-infected patients at the University of Washington (UW) and Harborview Medical Center HIV clinics in Seattle, Washington between January 1, 1996 and December 31, 2005. Patients in the UW HIV Cohort receive primary care at UW clinics, provide informed consent, and are followed until death or relocation from UW. Information on sex, age, race, risk-group for HIV transmission, CD4 T-cell counts, HIV-1 plasma RNA levels (viral load), and antiretroviral treatment was obtained from the UW HIV Information System (UWHIS), which captures longitudinal data on the UW HIV Cohort from electronic medical records and other institutional data systems.[15, 16] In addition, we reviewed medical charts to confirm KS diagnosis, classify KS tumor stage at diagnosis, obtain chemotherapy administration dates, and document changes in clinical appearance of KS. KS stage was defined per AIDS clinical trials group (ACTG) tumor staging classification as T0 if disease was confined to the skin and/or lymph nodes or oral involvement was confined to the hard palate, or T1 if there was pulmonary or gastrointestinal involvement, tumor associated edema or ulceration, or extensive oral involvement.[17] We excluded patients who did not have clinically or histologically confirmed KS, were diagnosed with and/or received care for KS at institutions other than the UW HIV clinics, or had all KS lesions removed.
Assessment of response
The interval between clinic visits varied in this population, but on average, patients are seen for routine care every 3-4 months.[16] We relied on clinicians to document changes in KS status in medical records. As this documentation was variable, we classified clinical response into three categories: no change/progression, improvement, and resolution. A patient was considered to have improvement if KS lesions improved without development of new lesions. Resolution of disease was defined as having no evidence of lesions consistent with KS noted on physical examination. Disease progression or the absence of documentation of KS status in clinical notes was recorded as no change/progression. For this study, we performed separate evaluations for improvement and resolution.
Therapeutic regimens
More than 90% of patients at the UW clinics obtain their medications at the on-site pharmacy, where medications must be refilled monthly.[18] Information regarding medications dispensed outside UW pharmacies are recorded in the chart review database and integrated within the UWHIS. We defined HAART use in a given month as prescription of ≥3 antiretroviral agents with at least one protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitor (NNRTI). In addition, we were interested in determining if specific HAART regimens, PI-based HAART regimens (PI-HAART) and regimens containing drugs found to be active against KS in vitro such as ritonavir (RTVB-HAART), were better predictors of clinical response than NNRTI-based HAART regimens (NNRTI-HAART).[19] HAART adherence was determined by dividing the number of months on HAART by the total number of follow-up months after HAART initiation. “Good adherence” was defined as ≥90% of HAART prescriptions filled as directed for the duration of observation. Chemotherapy use in a given month was defined as administration of chemotherapy during that month.
HIV treatment regimens are often dynamic; therefore, we determined the optimum treatment interval for prediction of improvement by comparing cumulative treatment time during various intervals between those who experienced improvement and those who did not. Persons with improvement were matched with persons with no change/progression according to follow-up time. Comparisons for the periods 3, 6, 9, and 12 months prior to improvement were conducted using Wilcoxon log-rank tests. Cumulative treatment months were dichotomized since this summary measure appeared to best explain the treatment impact. Accordingly, recent HAART use was defined as ≥2 months of HAART in the past 3 months and recent chemotherapy use was defined as any chemotherapy in the past 3 months when predicting improvement. The optimal treatment interval for prediction of resolution was determined in the same way; recent HAART use was defined as ≥5 months of HAART in the past 6 months and recent chemotherapy use was defined as any chemotherapy in the past 6 months.
Statistical methods
Kaplan-Meier survival analysis was used to estimate median time from KS diagnosis to first improvement and cumulative incidence of improvement 36 months after diagnosis; events were censored at the date of last visit, death, or 36 months following KS diagnosis. Univariate Cox's proportional hazards (Cox) models with time-varying covariates were used to quantify the risk for KS response with recent HAART and chemotherapy use and other possible predictive variables (age, KS stage, baseline CD4 T-cell count (≤200 versus >200 cells/mm3), baseline HIV viral load (<4 versus ≥4 log10 copies/ml), recent CD4 T-cell count, and recent HIV viral load). Recent CD4 T-cell count and HIV viral load were defined as the last recorded value within 12 months. Stepwise, backwards elimination was used to identify independent predictors of response. A p-value <0.05 was considered statistically significant in multivariate analyses. A term indicating PI-HAART use and RTVB-HAART use was added to the multivariate model to determine the additional effect of these regimens relative to NNRTI-HAART alone.
In a retrospective study such as ours, it is possible that patients were systematically prescribed specific treatment regimens based on characteristics of their HIV or KS disease. Thus, indication for which regimen was prescribed could confound the perceived effect of that regimen on KS response. To determine if confounding by indication was present, CD4 T-cell count and HIV viral load closest to HAART initiation within 3 months (at HAART initiation) was compared to an earlier value in the same participant 3-12 months prior to HAART initiation. McNemar's exact test was used to compare the proportion of patients with low CD4 T-cell count (≤200 cells/mm3) and high HIV viral load (>5 log10 copies/ml) at and prior to HAART initiation, and paired t-tests were used to compare mean changes in laboratory values.
To determine the impact of possible confounding by indication, we conducted an analysis using marginal structural (MS) models and compared it to a similar Cox model. MS models are used to estimate the effect of a time-varying exposure on time to response outcomes when confounding by indication is present. Here, confounding by indication was considered to be likely since the prescribing of antiretroviral therapy is commonly influenced by laboratory measures of immunosuppression such as CD4 T-cell count and HIV viral load, and HAART use impacts these same measures. The MS model uses inverse probability weights to create a risk set where CD4 T-cell count and HIV viral load are no longer confounders by indication.[20] Given that MS models are limited to non-dynamic treatment regimens, we modeled treatment for this model with the assumption that once a patient starts HAART he/she remained on HAART. All analyses were performed with SAS statistical software, version 9.1 (SAS Institute Inc, Cary, North Carolina).
Results
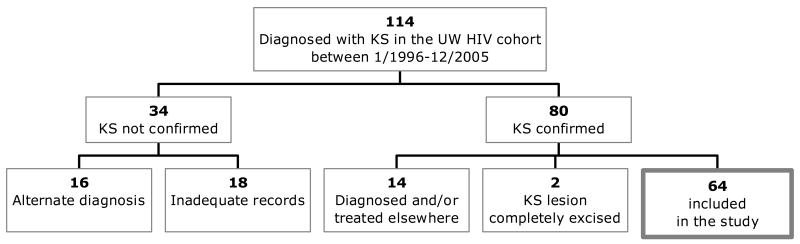
Between January 1996 and December 2005, 114 patients were identified as having KS. After careful review, 16 (14%) had an alternate diagnosis, 18 (16%) had insufficient information to confirm KS diagnosis, and 80 (70%) were confirmed to have KS. Of those with confirmed KS, 14 were diagnosed or treated elsewhere, 2 had their KS lesions completely excised, and the remaining 64 had sufficient information for inclusion in this study (Figure 1).
Figure 1. Selection of the study population.
Abbreviations: KS = Kaposi sarcoma, HIV = Human immunodeficiency virus, UW = Universityof Washington.
All patients were male; most were white (69%) and men who have sex with men (90%). Median age was 38 years (range: 26-44). Forty-four (69%) had T0 disease, 20 (31%) had T1 disease, and 10 (16%) were on HAART in the 12 months prior to diagnosis. Median CD4 T-cell count at diagnosis was 40 cells/mm3 (interquartile range (IQR): 10-155), and median HIV viral load was 5.0 log10 copies/ml (IQR: 4.1-5.4)(Table 1). Patients were followed a median of 6 months (range: 1-36), with 6 deaths (9%) during follow-up for improvement, and a median of 16.5 months (range: 2-36), with 15 deaths (23%) during follow-up for resolution.
Table 1. Characteristics of the study population.
| Baseline characteristics | N=64 |
|---|---|
| Sex | |
| Male | 64 (100) |
| Age, median (range) | 38 (26, 44) |
| Race | |
| White | 44 (69) |
| Black | 9 (14) |
| Hispanic/Latino | 7 (11) |
| Asian/Pacific Islander | 4 (6) |
| Risk group* | |
| MSM | 54 (90) |
| IDU | 18 (30) |
| KS stage | |
| T0 | 44 (69) |
| T1 | 20 (31) |
| KS diagnosis year | |
| 1996-1997 | 17 (27) |
| 1998-1999 | 12 (19) |
| 2000-2001 | 13 (20) |
| 2002-2003 | 12 (19) |
| 2004-2005 | 10 (16) |
| Median (interquartile range) Log plasma RNA levels | 5.0 (4.1, 5.4) |
| HIV plasma RNA levels (copies/ml) | |
| Missing | 5 (8) |
| 0-9,999 | 12 (19) |
| 10,000 – 99,999 | 16 (25) |
| ≥ 100,000 | 31 (48) |
| CD4 T-cell count (cells/mm3), median (interquartile range) | 45 (10,155) |
| Missing | 4 (6) |
| 0-50 | 32 (50) |
| 51-200 | 17 (27) |
| >200 | 11 (17) |
| Prior HAART use in past year | 10 (16) |
Missing data on 4 patients.
Abbreviations: MSM = men who have sex with men, IDU = Injection drug user, HIV = human immunodeficiency virus, RNA = ribonucleic acid, HAART = highly active antiretroviral therapy.
Treatment regimens
Improvement
Prior to improvement, 48 (75%) of 64 patients received HAART, constituting 319 (46%) of 695 person-months of follow-up. Thirty-eight (60%) persons received PI-HAART (246 person-months), and 18 (28%) received RTVB-HAART (73 person-months) at some point during follow-up. The most common regimens were lamivudine-nelfinavir-zidovudine (19% of person-months) in 1996-1999, efavirenz-lamivudine-zidovudine (23%) in 2000-2003, and atazanavir-lamivudine-tenofovir-ritonavir (30%) in 2004-2006. Among those who started HAART, median time from KS diagnosis to HAART initiation was 1 month (range: ≥12 months prior to 15 months after diagnosis), with median duration of 4 months. Seven (15%) patients changed regimens ≥2 times during follow-up and 77% had good adherence.
Twenty-seven (42%) patients received chemotherapy, totaling 99 person-months. Liposomal doxorubicin was used the majority of the time (98%). The average time to start of chemotherapy was 3 months after KS diagnosis (range: 0-32 months).
Resolution
During the time at risk for resolution, 53 (83%) patients received HAART, constituting 732 (59%) of 1238 person-months of follow-up. Forty-four (69%) persons received PI-HAART (558 person-months), and 25 (39%) received RTVB-HAART (228 person-months) at some point during follow-up. The most common regimens were lamivudine-nelfinavir-stavudine (20% of person-months) in 1996-1999, efavirenz-lamivudine-zidovudine (19%) in 2000-2003, and atazanavir-lamivudine-tenofovir (34%) in 2004-2006. Among those who started HAART, median time from KS diagnosis to HAART initiation was 1 month (range: ≥12 months prior to 19 months after diagnosis), with median duration of 11 months. Sixteen (30%) patients changed regimens ≥2 times during follow-up and 57% had good adherence, lower than during the somewhat shorter periods of time prior to improvement.
Thirty-two (50%) patients received chemotherapy, totaling 256 person-months. Similar to the time prior to improvement, liposomal doxorubicin was used the majority of the time (96%). The average time to start of chemotherapy was 6 months after KS diagnosis (range: 0-32 months).
Response of KS to therapy
Improvement
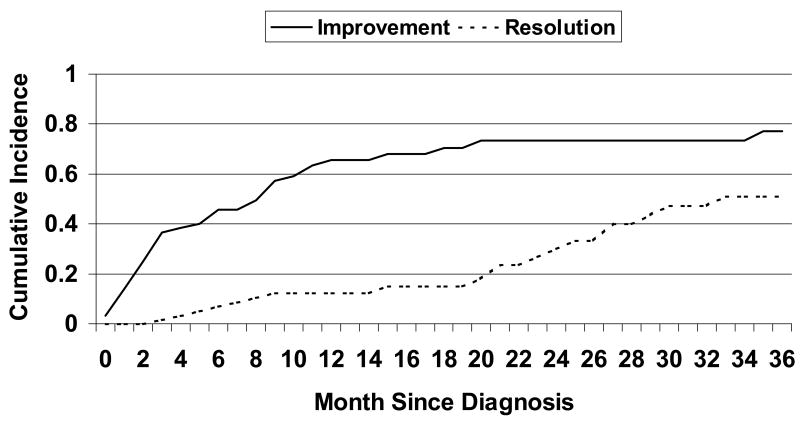
During the 36 months following KS diagnosis, 42 patients experienced improvement in their KS disease with an overall estimated median time to first improvement of 9 months (95% CI: 4-11) and 3-year cumulative improvement probability of 0.77 (95% CI: 0.64-0.90)(Figure 2). In univariate analysis, none of the baseline clinical characteristics, stage, diagnosis year, CD4 T-cell count, HIV viral load, or prior HAART history, was associated with improvement. However, risk of improvement was lower with higher levels of recent HIV viral load (hazard ratio (HR)=0.7 for a log10 copies/ml increase in HIV RNA levels, 95% CI: 0.6-0.9, p=0.005). Recent CD4 T-cell count was not associated with persistent KS (p=0.3). Both recent chemotherapy and HAART use were associated with improvement compared to those with no recent chemotherapy or HAART use, respectively (HR=5.0, 95% CI: 2.5-10.0, p<0.001 and HR=2.6, 95% CI: 1.3-5.1, p=0.006)(Table 2). Recent chemotherapy and HAART remained significant in multivariate analysis. The adjusted hazards for improvement was 4.1 times higher for those with recent HAART use compared to those with no recent HAART use (95% CI: 1.4-12.6, p=0.01), and 5.5 times higher for those with recent chemotherapy compared to those with no recent chemotherapy (95% CI: 2.7-11.2, p<0.001).
Figure 2. Cumulative Incidence of improvement and resolution.
Table 2. Hazard ratios associated with improvement and resolution using univariate Cox Regression with time varying covariates.
| Improvement | Resolution | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95 %CI) | p | HR (95 %CI) | p | HR (95 %CI) | p | HR (95 %CI) | p | |
| Demographic characteristics | ||||||||
| Age | 1.0 (1.0, 1.1) | 1.0 | 1.0 (0.9, 1.1) | 0.5 | ||||
| Baseline Clinical Characteristics | ||||||||
| KS stage | ||||||||
| Moderate | 1.0 | 1.0 | ||||||
| Extensive | 1.0 (0.5, 2.0) | 1.0 | 1.1 (0.4, 2.8) | 0.9 | ||||
| KS diagnosis year | ||||||||
| 1996-2000 | 1.0 | 1.0 | ||||||
| 2001-2005 | 1.3 (0.7, 2.5) | 0.4 | 0.8 (0.6, 1.2) | 0.4 | ||||
| CD4 T-cell count | ||||||||
| 0-200 | 1.6 (0.6, 3.9) | 0.9 (0.3, 2.8) | ||||||
| ≥ 200 | 1.0 | 0.3 | 1.0 | 0.9 | ||||
| HIV viral load | ||||||||
| 0-10,000 | 1.0 | 1.0 | ||||||
| ≥ 10,000 | 1.4 (0.6, 3.4) | 0.5 | 0.8 (0.3, 2.3) | 0.6 | ||||
| Prior HAART history | ||||||||
| No | 1.0 | 1.0 | ||||||
| Yes | 0. 8 (0.3, 1.9) | 0.5 | 0.7 (0.2, 3.2) | 0.7 | ||||
| Laboratory Characteristics | ||||||||
| CD4 T-cell count | ||||||||
| 50 unit difference (between subjects) | 1.0 (0.8, 1.1) | 0.3 | 1.1 (0.9, 1.2) | 0.4 | ||||
| Log HIV RNA viral load | ||||||||
| 1 unit difference (between subjects) | 0.7 (0.6, 0.9) | 0.005 | 0.7 (0.5, 1.0) | 0.02 | ||||
| Treatment Characteristics | ||||||||
| Recent chemotherapy | ||||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Yes | 5.0 (2.5, 10.0) | <0.001 | 5.5 (2.7, 11.2) | <0.001 | 0.6 (0.2, 1.7) | 0.3 | 0.6 (0.2, 1.8) | 0.3 |
| Recent HAART use | ||||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Yes | 2.6 (1.3, 5.1) | 0.006 | 4.1 (1.4, 12.6) | 0.01 | 4.7 (1.4, 15.8) | 0.01 | 6.2(1.5, 26.4) | 0.01 |
| PI-HAART vs. NNRTI-HAART | 0.4 (0.1, 1.3) | 0.1 | 0.8 (0.2, 2.4) | 0.6 | ||||
| RTVB-HAART vs NNRTI-HAART | 1.0 (0.3, 3.6) | 1.0 | 0.6 (0.1, 2.6) | 0.5 | ||||
| RTVB-HAART vs PI-HAART | 2.7 (0.9, 8.2) | 0.08 | 0.8 (0.2, 3.2) | 0.8 | ||||
Abbreviations: HR = hazard ratio, CI = confidence interval, p = p-value, KS = Kaposi sarcoma, HIV = human immunodeficiency virus, RNA = ribonucleic acid, HAART = highly active antiretroviral therapy, PI-HAART= protease inhibitor based HAART, NNRTI-HAART = nonnucleoside reverse transcriptase inhibitor based HAART, RTVB-HAART = ritonavir-boosted HAART.
Resolution
The estimated median time to resolution was 33 months and 3-year cumulative resolution probability was 0.51 (95% CI: 0.34-0.68). None of the 19 patients with disease resolution had disease recurrence during the median 45 months of follow-up after resolution (range: 0-111 months). Like KS improvement, the only clinical variable associated with resolution was recent HIV viral load (HR=0.7, 95% CI: 0.5-1.0, p=0.02). With regard to treatment, recent HAART use was associated with resolution (HR=4.7, 95% CI: 1.4-15.8, p=0.01), while chemotherapy was not (HR=0.6, 95% CI: 0.2-1.7, p=0.3). Only recent HAART use was statistically significant in multivariate analyses.
Comparison of HAART Regimens
The HR for improvement and resolution of KS associated with the different HAART regimens are shown in the multivariate models in Table 2. Among persons on HAART, those on RTVB-HAART were 2.7 times more likely to experience improvement than those on PI-HAART, although this did not reach statistical significance (95% CI: 0.9-8.2, p=0.08). Compared to those on NNRTI-HAART, risk for improvement did not differ for those on PI-HAART (p=0.12) or RTVB-HAART (p=1.0). For KS resolution, neither PI-HAART nor RTVB-HAART were significantly different than NNRTI-HAART (p=0.6 and p=0.5).
Indication for HAART
The proportion of patients with low CD4 T-cell count was not different 3-12 months prior to versus at HAART initiation (p=0.08), however, CD4 T-cell counts from the period prior to HAART initiation were significantly lower than counts at HAART initiation (mean decrease=62 cells/mm3, p=0.01). Similarly, the proportion of patients with high HIV viral load (>5 log10 copies/ml) was higher at HAART initiation than prior to HAART initiation (100% versus 33%, p=0.05), while there was no significant change in HIV viral load when considered as a continuous measure (p=0.2).
Cox models adjusting for chemotherapy, recent CD4 T-cell count, and recent HIV viral load and MS models that account for confounding by indication with CD4 T-cell count and HIV viral load, chemotherapy, and baseline CD4 T-cell count and HIV viral load resulted in similar risk estimates for HAART for both improvement (Cox HR=2.0, 95% CI: 0.9-4.6 and MS HR=1.7, 95% CI: 0.8-3.5) and resolution (Cox HR=3.1, 95% CI: 0.4-26.1 and MS HR=3.9, 95% CI: 0.3-45.1).
Discussion
We evaluated clinical response of AIDS-KS to HAART and chemotherapy among a large cohort of HIV patients in routine primary care, and found that half completely resolved their disease within 36 months of diagnosis. This figure is similar to observed resolution rates of 44%-60% reported in smaller observational studies and clinical trials.[21-24] However, we found the median time to complete response (33 months) to be considerably longer than previously reported (6 months).[7, 9, 10, 23, 25] Additionally, the median time to first improvement was 9 months, demonstrating that KS response is slow. Time to response may be longer in clinical settings compared to trials as treatment may not be started immediately, and clinicians may delay treatments such as cytotoxic chemotherapy in non-severe cases. Taken together, our findings suggest that even in the era of extremely effective therapy for HIV infection and wide availability to chemotherapy, KS remains a persistent disease.
Both HAART and chemotherapy appear to have important roles in influencing the clinical course of AIDS-KS. Recent HAART use was the only factor associated with resolution and both HAART and chemotherapy were independently associated with improvement, suggesting introduction of either HAART or chemotherapy can induce initial response to KS disease, while only HAART was associated with complete resolution of disease.
A number of studies have attempted to identify predictors of KS response, but no factor has been consistently identified. CD4 T-cell count, HIV viral load, human herpesvirus-8 (HHV-8) DNA levels, or KS stage have each intermittently been associated with KS response.[6-8, 11, 14, 21, 22, 24, 26-28] Apart from recent HAART and chemotherapy use, the only other predictor of KS response identified in this study was low HIV viral load. This finding is consistent with multiple reports of KS improvement or resolution associated with significant decrease or undetectable HIV viral load.[7, 22, 26] It appears that controlling HIV viral load is essential for clinical improvement, disease resolution of KS, and perhaps decreased risk of relapse. Like other studies, we did not find an association between CD4 T-cell count and KS response, suggesting suppression of HIV replication, and not immune reconstitution, may play a more important role in the resolution of KS.[14, 22, 26, 27] Support for this hypothesis comes from several sources. First, in endemic areas, KS occurs in the absence of identifiable T-cell deficiency.[28] Second, it has been demonstrated that HIV induces lytic replication of HHV-8, and HIV Tat enhances the activity of HHV-8 genes involved in angiogenesis, proliferation, and viral replication.[29]
Surprisingly, KS tumor stage was not associated with response in this study. Lack of relationship between disease stage and response to treatment is an unusual paradigm in cancer care, but is supported by other studies of KS treatment.[11, 14, 26] Only one study found KS tumor stage to be associated with response, but the analysis was restricted to patients on HAART alone, without any systemic treatment.[21] Certain factors may have limited our ability to explore the KS tumor stage and response relationship. First, approximately 30% of patients had severe disease, but staging was preformed only at diagnosis. It is conceivable that lesions could have progressed to more severe disease prior to treatment, though it is unlikely this would have affected treatment urgency or regimen. Second, we extrapolated staging based on clinical notes. It is possible that “true” staging may have been confounded by failure to document extensive cutaneous or asymptomatic visceral disease in those scored with T0 KS. Alternatively, conventional ACTG tumor staging classification may not provide useful information with regards to tumor response in the setting of HAART and chemotherapy. Tumor extent continues to be an important predictor of survival,[27] but none of the studies evaluating KS response in the presence of both HAART and chemotherapy found tumor stage associated with response.[11, 14, 26, 30] One study noted a trend toward KS response for patients with T0 KS, however, this was only observed among antiretroviral naïve patients.[26]
We found no differences in time to response among patients receiving NNRTI-HAART, PI-HAART, or RTVB-HAART, similar to what has previously been described.[7, 26] None of these regimens have been found to be more effective in the prevention of KS,[31, 32] but in vitro models support a direct effect of specific antiretroviral agents on HHV-8 replication, production of HIV-1 Tat, and KS angiogenesis and apoptosis.[19, 33-37] These studies support the concept that certain components of a HAART regimen may impact response of KS. Unfortunately, we lacked power to evaluate the effects of specific antiretroviral therapy on KS due to the heterogeneity of treatment regimens prescribed and limited number of subjects. Additional studies on the differential impact of antiretroviral agents on HHV-8 replication and KS resolution deserves further study.
The current study has limitations. First, we relied on objective responses noted in clinical records for our outcome, as uniform staging was not completed for all patients at diagnosis or follow-up. Comparison of improvement of KS disease to other studies was difficult since many studies used ACTG staging criteria for partial response, and most looked at best possible response, not time to first response. Second, 23% of patients died during follow-up. We suspect that death is not independent of response and therefore may have inflated our response rates. High death rates may bias the effect of treatment if treatment is associated with advanced HIV disease and death. However, we had insufficient power to show that treatment prescribing changes prior to death and therefore are unable to ascertain whether those who died are poorly representative of those censored at similar times. Finally, treatment effects may also be biased using Cox models because of confounding by indication. Since the proportion of patients with high HIV viral load was higher at HAART initiation than in the preceding 3-12 months, clearly some of those on HAART may be sicker than those off and therefore at a different risk of KS resolution. However, the treatment coefficients in the MS models, which account for confounding by indication, differed little from those of the Cox model. Unfortunately, we were unable to identify a statistical method that adjusts for both confounding by indication and allows for dynamism in HIV therapy, and the application of such a model to retrospective studies in the future will permit more proper modeling of the scientific question.
Prior to HAART, few people experienced complete resolution of KS with chemotherapy alone and in those who responded, response was short-lived.[39-51] Today, use of both HAART and chemotherapy was associated with a durable and complete resolution in half the patients studied. However, in sub-Saharan Africa, an area without widespread access to HAART and chemotherapy, KS continues to be a common malignancy, with an estimated 57,000 cases in 2002.[38-40] Poor adherence and resulting drug resistance may also limit future treatment options with HAART. KS remains an important clinical problem today in both resource-poor and -rich settings for which new therapeutic approaches are urgently needed.
Acknowledgments
The authors thank Peggie Griffith for compilation of the electronic medical files and Thomas Davis for assistance with data collection. This research was supported by NIH AI54162, Doris Duke Charitable Foundation Clinical Scientist Development Award, Centers for AIDS Research, and K24 AI071113 (AW).
Grant Support: NIH AI54162, Doris Duke Charitable Foundation Clinical Scientist Development Award, CFAR, K24 AI071113 (AW)
Footnotes
Data presented in part at the 10th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiences, North Bethesda, MD, October 16-17, 2006.
References
- 1.Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi's sarcoma and non-Hodgkin's lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94:1204–1210. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- 2.Vanni T, Sprinz E, Machado MW, Santana RD, Fonseca BA, Schwartsmann G. Systemic treatment of AIDS-related Kaposi sarcoma: Current status and perspectives. Cancer Treat Rev. 2006 doi: 10.1016/j.ctrv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Bower M, Fox P, Fife K, Gill J, Nelson M, Gazzard B. Highly active anti-retroviral therapy (HAART) prolongs time to treatment failure in Kaposi's sarcoma. Aids. 1999;13:2105–2111. doi: 10.1097/00002030-199910220-00014. [DOI] [PubMed] [Google Scholar]
- 4.Tam HK, Zhang ZF, Jacobson LP, Margolick JB, Chmiel JS, Rinaldo C, Detels R. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916–922. doi: 10.1002/ijc.10274. [DOI] [PubMed] [Google Scholar]
- 5.Holkova B, Takeshita K, Cheng DM, Volm M, Wasserheit C, Demopoulos R, Chanan-Khan A. Effect of highly active antiretroviral therapy on survival in patients with AIDS-associated pulmonary Kaposi's sarcoma treated with chemotherapy. J Clin Oncol. 2001;19:3848–3851. doi: 10.1200/JCO.2001.19.18.3848. [DOI] [PubMed] [Google Scholar]
- 6.Paparizos VA, Kyriakis KP, Papastamopoulos V, Hadjivassiliou M, Stavrianeas NG. Response of AIDS-associated Kaposi sarcoma to highly active antiretroviral therapy alone. J Acquir Immune Defic Syndr. 2002;30:257–258. doi: 10.1097/00042560-200206010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Gill J, Bourboulia D, Wilkinson J, Hayes P, Cope A, Marcelin AG, et al. Prospective study of the effects of antiretroviral therapy on Kaposi sarcoma--associated herpesvirus infection in patients with and without Kaposi sarcoma. J Acquir Immune Defic Syndr. 2002;31:384–390. doi: 10.1097/00126334-200212010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Carbonero L, Barrios A, Saballs P, Sirera G, Santos J, Palacios R, et al. Pegylated liposomal doxorubicin plus highly active antiretroviral therapy versus highly active antiretroviral therapy alone in HIV patients with Kaposi's sarcoma. Aids. 2004;18:1737–1740. doi: 10.1097/01.aids.0000131385.60974.b9. [DOI] [PubMed] [Google Scholar]
- 9.Cattelan AM, Calabro ML, Aversa SM, Zanchetta M, Meneghetti F, De Rossi A, Chieco-Bianchi L. Regression of AIDS-related Kaposi's sarcoma following antiretroviral therapy with protease inhibitors: biological correlates of clinical outcome. Eur J Cancer. 1999;35:1809–1815. doi: 10.1016/s0959-8049(99)00161-6. [DOI] [PubMed] [Google Scholar]
- 10.Cattelan AM, Calabro ML, Gasperini P, Aversa SM, Zanchetta M, Meneghetti F, et al. Acquired immunodeficiency syndrome-related Kaposi's sarcoma regression after highly active antiretroviral therapy: biologic correlates of clinical outcome. J Natl Cancer Inst Monogr. 2000:44–49. doi: 10.1093/oxfordjournals.jncimonographs.a024256. [DOI] [PubMed] [Google Scholar]
- 11.Lichterfeld M, Qurishi N, Hoffmann C, Hochdorfer B, Brockmeyer NH, Arasteh K, et al. Treatment of HIV-1-associated Kaposi's sarcoma with pegylated liposomal doxorubicin and HAART simultaneously induces effective tumor remission and CD4+ T cell recovery. Infection. 2005;33:140–147. doi: 10.1007/s15010-005-4099-z. [DOI] [PubMed] [Google Scholar]
- 12.Esdaile B, Davis M, Portsmouth S, Sarker D, Nelson M, Gazzard B, Bower M. The immunological effects of concomitant highly active antiretroviral therapy and liposomal anthracycline treatment of HIV-1-associated Kaposi's sarcoma. Aids. 2002;16:2344–2347. doi: 10.1097/00002030-200211220-00019. [DOI] [PubMed] [Google Scholar]
- 13.Stebbing J, Wildfire A, Portsmouth S, Powles T, Thirlwell C, Hewitt P, et al. Paclitaxel for anthracycline-resistant AIDS-related Kaposi's sarcoma: clinical and angiogenic correlations. Ann Oncol. 2003;14:1660–1666. doi: 10.1093/annonc/mdg461. [DOI] [PubMed] [Google Scholar]
- 14.Nunez M, Saballs P, Valencia ME, Santos J, Ferrer E, Santos I, et al. Response to liposomal doxorubicin and clinical outcome of HIV-1-infected patients with Kaposi's sarcoma receiving highly active antiretroviral therapy. HIV Clin Trials. 2001;2:429–437. doi: 10.1310/700b-9qt3-hgn9-q3fq. [DOI] [PubMed] [Google Scholar]
- 15.Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. Aids. 2006;20:1019–1026. doi: 10.1097/01.aids.0000222074.45372.00. [DOI] [PubMed] [Google Scholar]
- 16.Kitahata MM, Dillingham PW, Chaiyakunapruk N, Buskin SE, Jones JL, Harrington RD, et al. Electronic human immunodeficiency virus (HIV) clinical reminder system improves adherence to practice guidelines among the University of Washington HIV Study Cohort. Clin Infect Dis. 2003;36:803–811. doi: 10.1086/368085. [DOI] [PubMed] [Google Scholar]
- 17.Krown SE, Testa MA, Huang J. AIDS-related Kaposi's sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1997;15:3085–3092. doi: 10.1200/JCO.1997.15.9.3085. [DOI] [PubMed] [Google Scholar]
- 18.Kitahata MM, Reed SD, Dillingham PW, Van Rompaey SE, Young AA, Harrington RD, Holmes KK. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS. 2004;15:803–810. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- 19.Pati S, Pelser CB, Dufraine J, Bryant JL, Reitz MS, Jr, Weichold FF. Antitumorigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood. 2002;99:3771–3779. doi: 10.1182/blood.v99.10.3771. [DOI] [PubMed] [Google Scholar]
- 20.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Dupont C, Vasseur E, Beauchet A, Aegerter P, Berthe H, de Truchis P, et al. Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92. Centre d'information et de soins de l'immunodeficience humaine. Aids. 2000;14:987–993. doi: 10.1097/00002030-200005260-00010. [DOI] [PubMed] [Google Scholar]
- 22.Dupin N, Rubin De Cervens V, Gorin I, Calvez V, Pessis E, Grandadam M, et al. The influence of highly active antiretroviral therapy on AIDS-associated Kaposi's sarcoma. Br J Dermatol. 1999;140:875–881. doi: 10.1046/j.1365-2133.1999.02818.x. [DOI] [PubMed] [Google Scholar]
- 23.Lebbe C, Blum L, Pellet C, Blanchard G, Verola O, Morel P, et al. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi's sarcoma. Aids. 1998;12:F45–49. doi: 10.1097/00002030-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Bihl F, Mosam A, Henry LN, Chisholm JV, 3rd, Dollard S, Gumbi P, et al. Kaposi's sarcoma-associated herpesvirus-specific immune reconstitution and antiviral effect of combined HAART/chemotherapy in HIV clade C-infected individuals with Kaposi's sarcoma. Aids. 2007;21:1245–1252. doi: 10.1097/QAD.0b013e328182df03. [DOI] [PubMed] [Google Scholar]
- 25.Cattelan AM, Calabro ML, De Rossi A, Aversa SM, Barbierato M, Trevenzoli M, et al. Long-term clinical outcome of AIDS-related Kaposi's sarcoma during highly active antiretroviral therapy. Int J Oncol. 2005;27:779–785. [PubMed] [Google Scholar]
- 26.Martinez V, Caumes E, Gambotti L, Ittah H, Morini JP, Deleuze J, et al. Remission from Kaposi's sarcoma on HAART is associated with suppression of HIV replication and is independent of protease inhibitor therapy. Br J Cancer. 2006;94:1000–1006. doi: 10.1038/sj.bjc.6603056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasti G, Talamini R, Antinori A, Martellotta F, Jacchetti G, Chiodo F, et al. AIDS-related Kaposi's Sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS Clinical Trial Group Staging System in the Haart Era--the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive From Antiretrovirals. J Clin Oncol. 2003;21:2876–2882. doi: 10.1200/JCO.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 28.Kestens L, Melbye M, Biggar RJ, Stevens WJ, Piot P, De Muynck A, et al. Endemic African Kaposi's sarcoma is not associated with immunodeficiency. Int J Cancer. 1985;36:49–54. doi: 10.1002/ijc.2910360109. [DOI] [PubMed] [Google Scholar]
- 29.Aoki Y, Tosato G. Interactions between HIV-1 Tat and KSHV. Curr Top Microbiol Immunol. 2007;312:309–326. doi: 10.1007/978-3-540-34344-8_12. [DOI] [PubMed] [Google Scholar]
- 30.Tulpule A, Groopman J, Saville MW, Harrington W, Jr, Friedman-Kien A, Espina BM, et al. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer. 2002;95:147–154. doi: 10.1002/cncr.10634. [DOI] [PubMed] [Google Scholar]
- 31.Stebbing J, Portsmouth S, Nelson M, Mandalia S, Kandil H, Alexander N, et al. The efficacy of ritonavir in the prevention of AIDS-related Kaposi's sarcoma. Int J Cancer. 2004;108:631–633. doi: 10.1002/ijc.11648. [DOI] [PubMed] [Google Scholar]
- 32.Portsmouth S, Stebbing J, Gill J, Mandalia S, Bower M, Nelson M, Gazzard B. A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi's sarcoma. Aids. 2003;17:F17–22. doi: 10.1097/00002030-200307250-00001. [DOI] [PubMed] [Google Scholar]
- 33.Grundhoff A, Ganem D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J Clin Invest. 2004;113:124–136. doi: 10.1172/JCI200417803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustafson EA, Schinazi RF, Fingeroth JD. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. J Virol. 2000;74:684–692. doi: 10.1128/jvi.74.2.684-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lock MJ, Thorley N, Teo J, Emery VC. Azidodeoxythymidine and didehydrodeoxythymidine as inhibitors and substrates of the human herpesvirus 8 thymidine kinase. J Antimicrob Chemother. 2002;49:359–366. doi: 10.1093/jac/49.2.359. [DOI] [PubMed] [Google Scholar]
- 36.Huang LM, Chao MF, Chen MY, Shih H, Chiang YP, Chuang CY, Lee CY. Reciprocal regulatory interaction between human herpesvirus 8 and human immunodeficiency virus type 1. J Biol Chem. 2001;276:13427–13432. doi: 10.1074/jbc.M011314200. [DOI] [PubMed] [Google Scholar]
- 37.Sgadari C, Barillari G, Toschi E, Carlei D, Bacigalupo I, Baccarini S, et al. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8:225–232. doi: 10.1038/nm0302-225. [DOI] [PubMed] [Google Scholar]
- 38.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 39.Chokunonga E, Levy LM, Bassett MT, Mauchaza BG, Thomas DB, Parkin DM. Cancer incidence in the African population of Harare, Zimbabwe: second results from the cancer registry 1993-1995. Int J Cancer. 2000;85:54–59. doi: 10.1002/(sici)1097-0215(20000101)85:1<54::aid-ijc10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 40.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997. Br J Cancer. 2000;82:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]