Abstract
Discovered and crystallized over sixty years ago, serotonin's important functions in the brain and body were identified over the ensuing years by neurochemical, physiological and pharmacological investigations. This 2008 M. Rapport Memorial Serotonin Review focuses on some of the most recent discoveries in serotonin that are based on genetic methodologies. These include examples of the consequences that result from direct serotonergic gene manipulation (gene deletion or overexpression) in mice and other species; an evaluation of some phenotypes related to functional human serotonergic gene variants, particularly in SLC6A4, the serotonin transporter gene; and finally, a consideration of the pharmacogenomics of serotonergic drugs with respect to both their therapeutic actions and side effects. The serotonin transporter (SERT) has been the most comprehensively studied of the serotonin system molecular components, and will be the primary focus of this review. We provide in-depth examples of gene-based discoveries primarily related to SLC6A4 that have clarified serotonin's many important homeostatic functions in humans, non-human primates, mice and other species.
Introduction
Serotonin and the serotonin transporter modulate many brain and body processes involved in health and disease (Fig. 1). Early vasoactive response assays and pharmacological studies by M. Rapport, V. Erspamer, I. H. Page and others first identified serotonin as an endogenous substance that stimulated smooth muscle responses in the periphery in studies that began over sixty years ago (Erspamer, 1954; Page, 1976; Rapport, 1997; Rapport et al., 1948). However, gene variants that change the components of the serotonergic system so as to alter peripheral and central serotonin functions have only recently been discovered (Chen et al., 2001; Linder et al., 2008; Murphy and Lesch, 2008). Many earlier books, reviews and research papers have explicated the remarkable roles of the 14-plus serotonin receptors and the single mammalian serotonin transporter in the functions that serotonin plays as a neurotransmitter and neuromodulator in neurochemical, pharmacological, physiological and behavioral processes (Baumgarten and Gothert, 2000; Fox et al., 2007a; Hahn and Blakely, 2002; Murphy et al., 2004a; Murphy and Lesch, 2008; Roth, 2006; Vanhoutte et al., 1990).
Figure 1. Serotonin: Modulatory involvement in Complex Quantitative Traits and Complex Disorders.

This Rapport Memorial Review focuses on recent developments based upon gene manipulations in non-human species and gene variant studies in humans that have challenged and changed some of our more classical views and interpretations of the pharmacology and physiology of serotonin's actions. In doing so, it highlights important examples of how gene-based discoveries have been re-writing the history of the central and peripheral serotonergic systems. The emphasis is on examples from experiments focused on one major component of the serotonergic systems, the serotonin transporter and its gene (SLC6A4 in humans; Slc6a4 in mice, otherwise known in some published papers as 5-HTT or SERT).
Why focus on SLC6A4 and SERT?
SERT functions in all serotonergic systems via transport-mediated regulation of the availability of serotonin to its homo- and hetero-receptors in brain, blood and peripheral organs. Changes in SERT, as documented below, alter the expression and/or function of most, if not all, serotonin receptors as well as the synthesis, clearance and metabolism of serotonin. These changes have important clinical implications as SERT inhibitors. These include the drug classes of serotonin reuptake inhibitors (SRIs), the specific serotonin reuptake inhibitors (SSRIs) and also combined serotonin-norepinephrine reuptake inhibitors (SNRIs), which are among the world's most widely-prescribed drugs for the treatment of neuropsychiatric and many other disorders and are also the target of serotonergic recreational drugs such as MDMA (Ecstasy) as well as cocaine. The review perspectives and additional supporting data presented here are from two sources that have provided the most up-to-date recent history of the contributions attributable to genetic alterations in serotonergic system genes, principally involving SLC6A4. The two examples that are examined are primarily from our laboratory and from colleagues in this field: (1) First, there are human gene variants in SLC6A4, many of which have now been documented to produce changes in SERT expression, function and/or regulation. These changes have been shown to be associated with alterations in brain SERT binding site densities, brain-imaged responses to emotional stimuli, personality traits and multiple disorders involving the central nervous system (CNS) and cardiac, pulmonary and other systems [Reviews: (Murphy et al., 2004a; Murphy and Lesch, 2008)]. Additionally, some of these SLC6A4 variants have been found to be associated with therapeutic responses as well as side effects of drugs affecting SERT and the serotonergic systems, especially SRIs and related SERT-altering medications. As human SLC6A4 variants are associated with prominent psychological and behavioral phenotypes in anxiety-related emotional domains, special attention is directed towards studies that have documented these findings in humans, and to relevant anxiety-related behavioral phenotypes in mice. This review will note recent findings in one anxiety disorder, obsessive-compulsive disorder (OCD) and related OCD spectrum disorders, as prime examples of conditions with exceptional serotonergic interest since specific functional SLC6A4 variants have been found to be associated with these disorders in multiple studies (Delorme et al., 2005; Hu et al., 2006; Kilic et al., 2003; Ozaki et al., 2003; Sutcliffe et al., 2005; Wendland et al., 2008a; Wendland et al., 2008b). Of special interest, SRIs are the only drug group found in replicated studies to be therapeutically useful in OCD, an anxiety disorder that does not respond to other anxiolytic or antidepressant agents such as the tricyclic antidepressants (Greist et al., 1995; Pigott et al., 1990).
(2) Secondly, we will discuss genetically engineered SERT-deficient mice (Bengel et al., 1998; Murphy and Lesch, 2008), which have elucidated the roles of changes in extracellular fluid (ECF) and intracellular (whole tissue) serotonin levels with regard to serotonin receptor activation and adaptation, serotonin clearance and synthesis, plus pharmacological, physiological and behavioral phenotypes that emerge in Slc6a4 knockout (-/-) mice, produced by homologous recombination in ES cells, and in single-allele-deficient, heterozygous (+/-) mice (Ansorge et al., 2004; Bengel et al., 1998; Fox et al., 2007a; Li, 2006), or in other mice produced by transgenic techniques that are either SERT-deficient (Thakker et al., 2005; Zhao et al., 2006) or have SERT over-expression (Jennings et al., 2006).
Human SLC6A4 Variants: Their Function And Relevance To Health And Disease
Human SLC6A4 maps to chromosome 17q11.2 and is composed of 15 exons spanning ∼40 kb (Fig. 2). The sequence of the transcript predicts a protein comprised of 630 amino acids with 12 transmembrane domains. Alternative promoters, differential splicing involving exons 1A, B, and C, and 3′-untranslated-region variability result in multiple mRNA species, as well as specifically-evaluated polymorphisms, that are likely to regulate gene expression SLC6A4 in humans. Most well-studied is the promoter region SLC6A4 variant, 5HTTLPR, which together with two intrinsic single nucleotide polymorphisms (SNPs) [rs25531 and rs25532], all located upstream of the transcription start site, modulate the transcriptional activity of SLC6A4 (Hu et al., 2006; Lesch et al., 1996; Wendland et al., 2008b) (Fig. 2). Additional variants at the SLC6A4 locus include a functional variable number of tandem repeats (VNTR) polymorphism in intron 2 (Fig. 2) and a number of other coding region SNPs that change the structure or function of the transporter protein, such as I425V and G56A (Kilic et al., 2003; Lesch et al., 1996; Lovejoy et al., 2003; Prasad et al., 2005; Sutcliffe et al., 2005). Most of these latter SNPs are rare, but the promoter region rs25531 polymorphism has a minor allele frequency of 9-15% in Caucasians and 24% in African-Americans (Hu et al., 2006; Wendland et al., 2006b). Several of the less-common SLC6A4 SNPs and their haplotypes are associated with behavioral phenotypes or disorders, including obsessive-compulsive disorder and autism (Hu et al., 2006; Kilic et al., 2003; Ozaki et al., 2003; Prasad et al., 2005; Sutcliffe et al., 2005; Wendland et al., 2008b).
Figure 2. Human SERT Gene Organization, with Multiple Functional Variants.
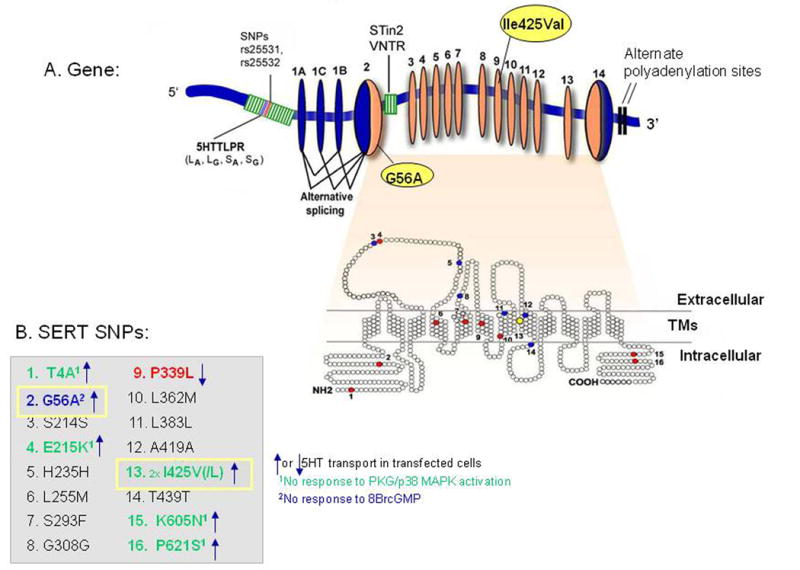
Human SLC6A4 Variants
Many of the phenotypes discovered in SERT-targeted mouse models (Murphy and Lesch, 2008) have been glimpsed in studies examining the association of human diseases or traits with common human SLC6A4 variants such as 5HTTLPR (including rs25531 and rs25532), and the intron 2 VNTR, as well as the less common I425V, I425L, and G56A variants in SLC6A4 coding regions. The short variant of the 5HTTLPR as well as the SNP rs25531 and rs25532 variants (and most likely the shorter STin 2 VNTR 9 and 10 repeat alleles) plus the P339L SNP might confer as much as 50-80% lower expression levels of SERT. In contrast, the higher expressing SERT alleles can confer up to 5-fold greater serotonin uptake capacity (Hu et al., 2006; Prasad et al., 2005; Wendland et al., 2007; Wendland et al., 2008b). Thus, combinations of the frequent SLC6A4 variants, and possibly also the less common variants such as P339L (Prasad et al., 2005), may act together to confer from five to twenty-fold differences in SERT expression and function levels (Fig. 3).
Figure 3. Potential Five-Fold Range of SERT Expression/Function Related to Common Plus Rare Human SLC6A4 Gene Variants.
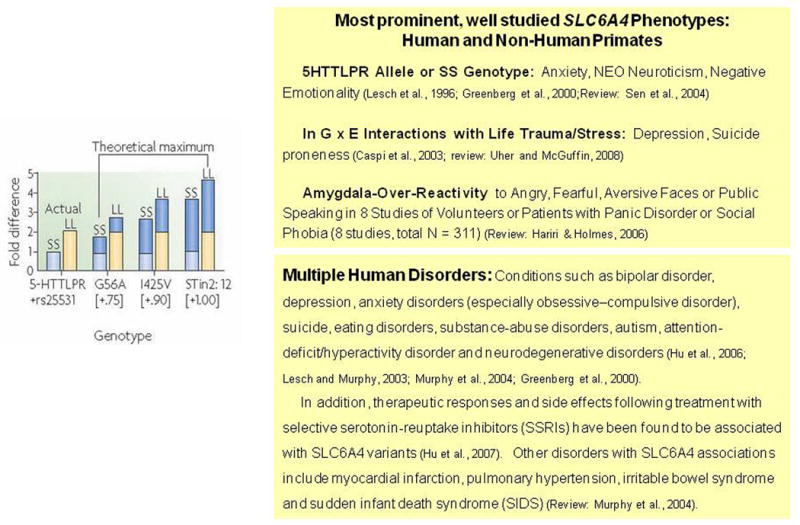
Humans with the 5HTTLPR SS genotype closely resemble heterozygote Slc6a4 +/- mice in regard to levels of SERT expression and function (Fig. 4). These similarities allow predictive appraisals of phenotypes across species (Fox et al., 2007a; Kalueff et al., 2007b; Lesch and Murphy, 2003; Murphy et al., 2004a; Murphy et al., 2001; Murphy et al., 2003a). Thus, it is no surprise to find that anxiety and depression-related personality traits and affective disorders (Lesch et al., 1996; Munafo et al., 2006; Sen et al., 2004), alcohol and other drug dependencies (Lesch, 2005), as well as some sleep and thermoregulatory disorders (Rausch et al., 2003; Wisor et al., 2003), irritable bowel syndrome (IBS) (Chen et al., 2001; Colucci et al., 2008; Van Kerkhoven et al., 2007; Yeo et al., 2004), the serotonin syndrome (Fox et al., 2007b; Fox and Murphy, in press; Isbister and Buckley, 2005), pulmonary hypertension and chronic obstructive pulmonary disease (Eddahibi et al., 2003; Eddahibi et al., 2000), musculoskeletal disorders (Bliziotes et al., 2002; Sample et al., in press; Warden et al., 2005), autism (Sutcliffe et al., 2005) and sudden infant death syndrome (SIDS) (Nonnis Marzano et al., 2008; Weese-Mayer et al., 2007) are associated with SLC6A4 variants in humans and other species, especially when interactions between SLC6A4 and life stress are taken into account (Canli et al., 2006; Caspi et al., 2003; Kalin et al., 2008; Kendler et al., 2005; Uher and McGuffin, 2008) (Fig. 1). Some of the disorders found to be associated with SLC6A4 in humans such as SIDS and myocardial valvulopathy and infarction have not yet been investigated in Slc6a4 -/- and Slc6a4 +/- mice (Fumeron et al., 2002; Nakatani et al., 2005). Likewise, some of the Slc6a4 -/- mouse phenotypes, including obesity and type-2-diabetes, have yet to be studied for SLC6A4 variant frequency distortions in cohorts of human patients; these would seem to be of high priority for investigation.
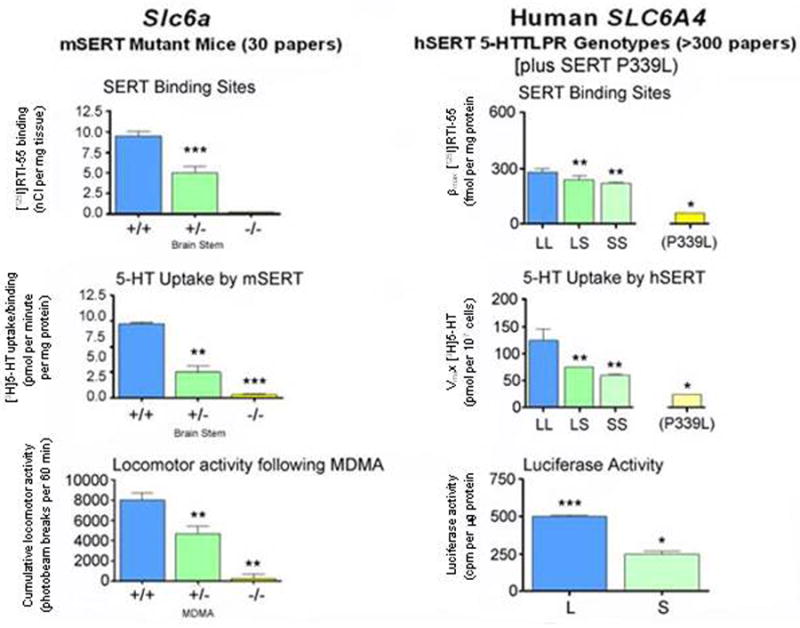
Figure 4. Comparison of serotonin-transporter (SERT) expression and function in Slc6a4-mutant mice and in humans with different SLC6A4 5-HTTLPR genotypes.
A genetic contribution of SLC6A4 to temperament and behavioral traits including anxiety, excess stress responsiveness, dominance relationships in non-human primate species and alcohol and drug preferences have been established in humans and several other species, probably reflecting selective forces among our ancestral and related animal species. Recent research efforts in this area have therefore been focused on mice, rats and non-human primates, especially rhesus macaques, and are currently proceeding towards the elaboration of an interdisciplinary perspective that will blend behavioral genetics and evolutionary psychology, as well as cognitive and social neuroscience (Barr et al., 2003; Homberg et al., 2007; Kalin et al., 2008; Olivier et al., 2008b). In models ranging from non-human primates and to C. elegans and D. melanogaster, environmental influences might be less complex than in humans and thus less likely to confound associations between behavior and genes (Barr et al., 2003; Canli and Lesch, 2007; Murakami and Murakami, 2007; Murphy et al., 2004a; Murphy and Lesch, 2008; Park et al., 2006; Thimgan et al., 2006; Weiger, 1997). Maternal-separation gene-environment studies in rhesus macaques have demonstrated the occurrence of interactions that affect behavioral traits, including stress reactivity and alcohol preference and dependence, based upon differential occurrence of a repeat-length promoter region variant that is orthologous to the human 5HTTLPR (Barr et al., 2003; Bennett et al., 2002). This is consistent with data that the 5HTTLPR, and most likely other SLC6A4 variants, influence the risk for anxiety, affective and other behavioral disorders through gene-environment interactions. However, the molecular and neural mechanisms that underlie the interplay of genes and environmental adversity that constitute disease risk remain incompletely understood (Canli et al., 2005; Canli et al., 2006; Caspi et al., 2003; Kendler et al., 2005; Lazary et al., 2008; Uher and McGuffin, 2008).
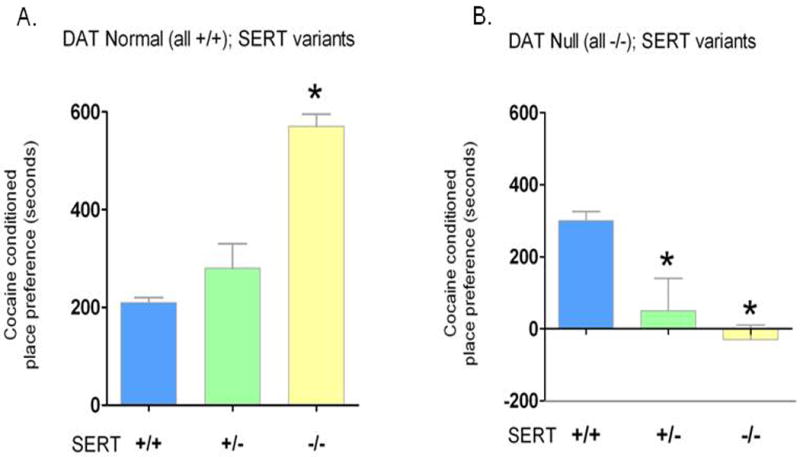
Finally, gene-gene interactions have begun to be studied in combined association studies in humans (Benjamin et al., 2000a; Benjamin et al., 2000b; Pattin and Moore, 2008; Smolka et al., 2007; Strobel et al., 2003). More direct studies have been possible in mouse models: for example, Slc6a4 mutant mice have been interbred with mice that lacked either one or both copies of the dopamine transporter gene (Slc6a3) (Fig. 5), the noradrenaline-transporter gene (Slc6a2), the MAOA gene (Maoa), the 5-HT1b-receptor gene (Htr1b) or the BDNF gene (Bdnf), with consequent amplified, reduced or qualitatively different new phenotypes (Holmes et al., 2003a; Murphy et al., 2003a; Salichon et al., 2001; Shen et al., 2004; Sora et al., 2001; Sora et al., 1998). These studies of ‘experimental epistasis’ complement those in which the Slc6a4 -/- was placed on congenic C57BL/6J, I29S6 or CD-1 background strains to yield more subtle SERT-related phenotype differences (Holmes et al., 2003a; Murphy et al., 2003a).
Figure 5. Increased Cocaine Preference in SERT +/+, +/- and -/- Mice and Consequent Decreased Cocaine Preference in these Mice Cross-Bred with Dopamine Transporter (DAT)-Deficient Mice.
Sert-Deficient and Sert-Over-Expressing Mouse Models
To explore the question of what human genetic disorders might be attributable to life-long SLC6A4 dysfunction, mice with reduced or absent SERT were generated by targeted disruption of Slc6a4 (Bengel et al., 1998). The primary functional basis for the multiple phenotypic changes that occur in SERT-deficient Slc6a4 +/- and Slc6a4 -/- mice is a profound alteration in serotonin neurochemistry and regulatory responses (Bengel et al., 1998; Kim et al., 2005; Mathews et al., 2004). These abnormalities act in somewhat different ways during development (when excess serotonin has neurotrophic and morphogenic effects) than in adulthood (when the continuing excess of extracellular serotonin or deficient tissue serotonin seems most important) (Alexandre et al., 2006; Ansorge et al., 2008; Ansorge et al., 2004; Gaspar et al., 2003; Gobbi et al., 2001; Kim et al., 2005; Lauder, 1990; Murphy et al., 2001; Sodhi and Sanders-Bush, 2004).
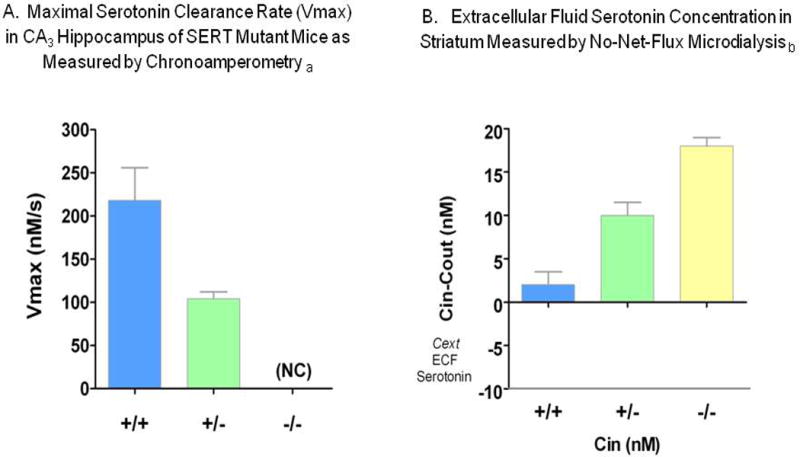
Basal extracellular fluid serotonin concentrations are markedly increased in the brain regions investigated thus far, the striatum and cortex of Slc6a4 +/- and Slc6a4 -/- mice. Likewise, serotonin release following KCl-induced depolarization is markedly differentially changed in these SERT-deficient mice (Fabre et al., 2000; Mathews et al., 2004) (Figs. 6, 7). In addition, serotonin clearance as measured in the hippocampal CA3 region is prolonged in Slc6a4 -/- mice, to the point where its removal cannot be distinguished from diffusion alone, and is prolonged to an intermediate extent in Slc6a4 +/- mice (Montanez et al., 2003) (Fig. 7).
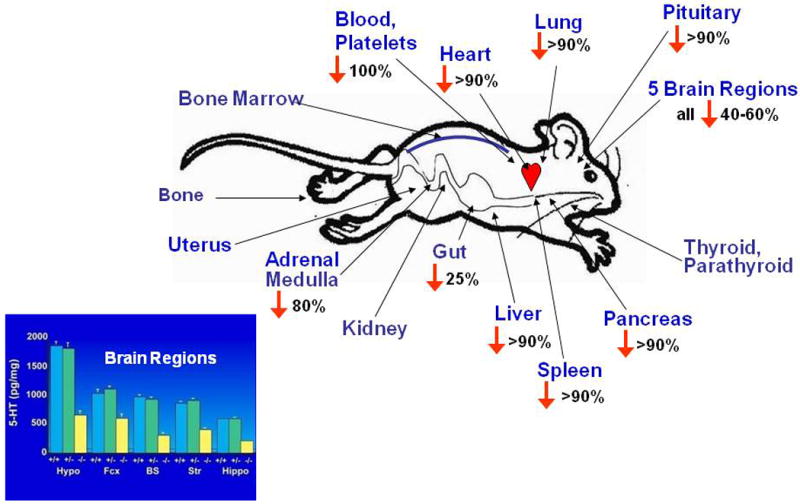
Figure 6. Comparisons of SERT-Deficient vs. SERT Over-Expressing Mice: ECF Serotonin Concentration Differences.
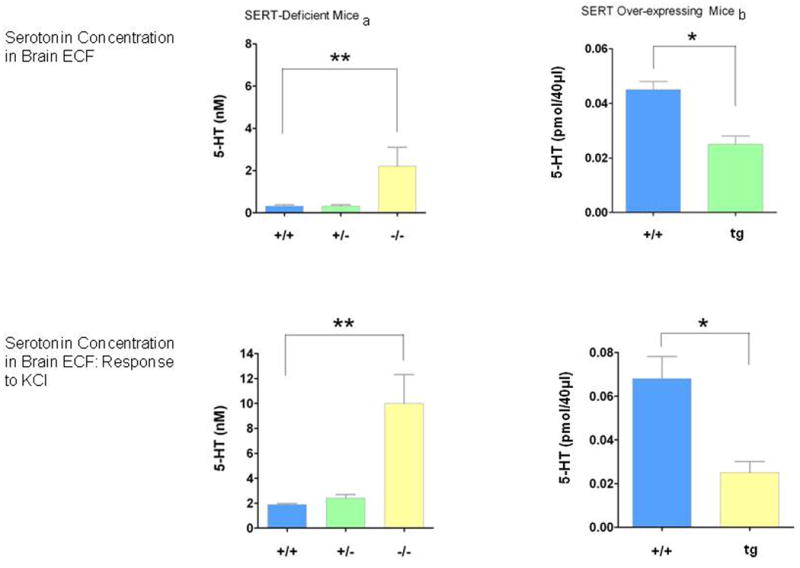
Figure 7. Serotonin Clearance Rates and ECF Serotonin Measurements in SERT-Deficient Mice.
The consequentially increased extracellular serotonin concentrations thus amplify and extend the duration of serotonin signaling at serotonin receptors, significantly altering the homeostatic state of the entire serotonin system (Kim et al., 2005; Mathews et al., 2004) (Fig. 8). In contrast to the increases in extracellular fluid levels, serotonin brain-tissue concentrations are decreased by 40–60% in Slc6a4 -/- mice, a predictable result of the failure to re-accumulate released serotonin due to the SERT-deficient state (Bengel et al., 1998; Fox and Murphy, in press; Kim et al., 2005). Both blood plasma and platelets are devoid of serotonin in Slc6a4 -/- mice (Chen et al., 2001; Eddahibi et al., 2000). Furthermore, most peripheral organs have markedly depleted serotonin levels, confirming that their normal serotonin content is dependent upon SERT (Bengel et al., 1998; Kim et al., 2005; Li et al., 2003; Tjurmina et al., 2002) (Fig. 9), with a lack of or inadequate compensation by other transporters that may normally or under special circumstances become more relevant in SERT-deficient mice (Chen et al., 2001; Schmitt et al., 2003; Zhou et al., 2002).
Figure 8. Comparison of Serotonin Dynamic Homeostasis in SERT +/+ vs. SERT -/- Mice.
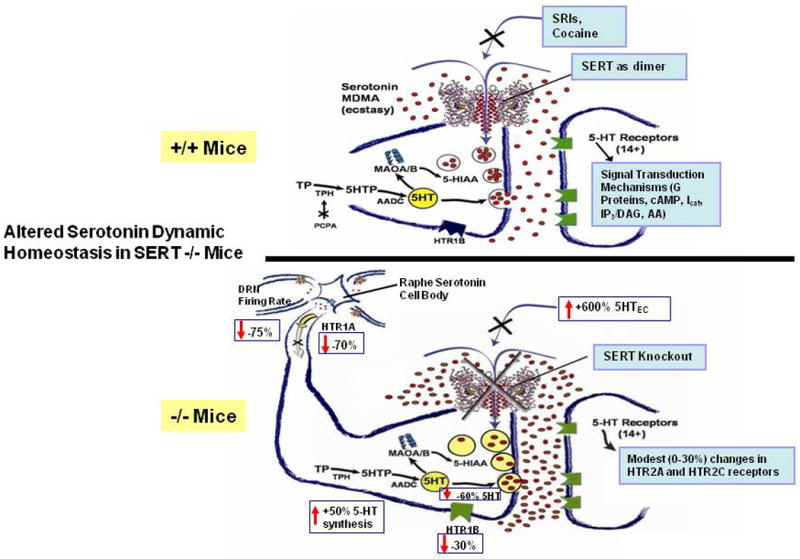
Figure 9. Tissue Serotonin Reductions in the Periphery and in Five Brain Regions of SERT -/- Mice.
As a consequence of the deficient recycling of serotonin by its transporter, serotonin synthesis and turnover are increased across brain regions in Slc6a4 -/- mice, with some evidence suggesting a greater increase in female than male mice (Fox and Murphy, in press; Kim et al., 2005). Dopaminergic neurons in the substantia nigra can accumulate excess serotonin in Slc6a4 -/- mice through the dopamine transporter (DAT) (Zhou et al., 2002). Furthermore, expression of the organic cation transporters (OCTs), OCT1 and OCT3, which are low-affinity transporters of monoamines, is increased (Chen et al., 2001; Lesch and Mossner, 2006; Schmitt et al., 2003; Zhou et al., 2002), suggestive of some partial but inefficient attempt at compensation for SERT-deficient mice via heterologous, non-specific DAT, OCTs and perhaps other monoamine transporters.
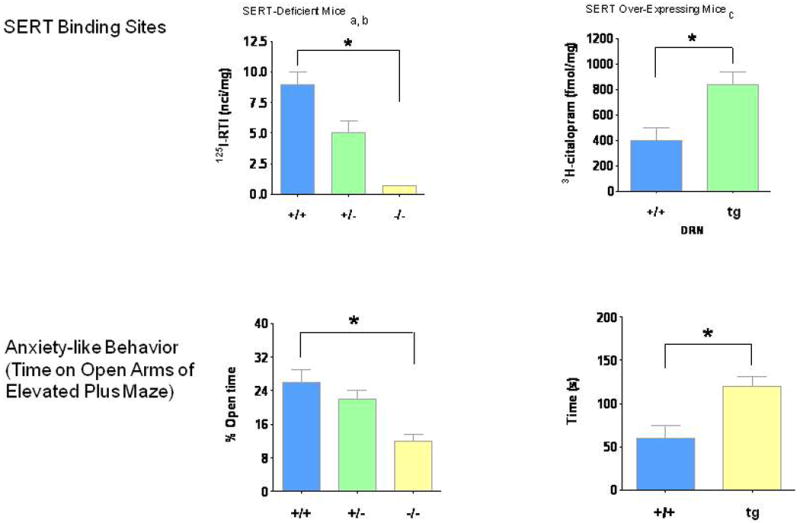
Likewise, Slc6a4 +/- mice have fewer specific SERT binding sites, decreased serotonin clearance (Figs. 7, 10, 11), ∼three-fold elevated extracellular serotonin concentrations in striatum and cortex measured by no-net-flux microdialysis (Fig. 7) and reduced serotonin uptake in synaptosomes studied in vitro (Bengel et al., 1998; Mathews et al., 2004; Montanez et al., 2003; Perez and Andrews, 2005); however, there have unchanged tissue serotonin concentrations in the brain and periphery and have unchanged brain serotonin synthesis and turnover (Bengel et al., 1998; Kim et al., 2005; Mathews et al., 2004). Thus the loss of one Slc6a4 allele leads to a decrease in many transporter functions, but this single copy of a Slc6a4 allele is adequate to maintain most overall tissue serotonin homeostasis.
Figure 10. Differential Expression of SERT in SERT +/+, +/- and -/- Mice and Comparison to mC-DASB Binding in Human Brain.
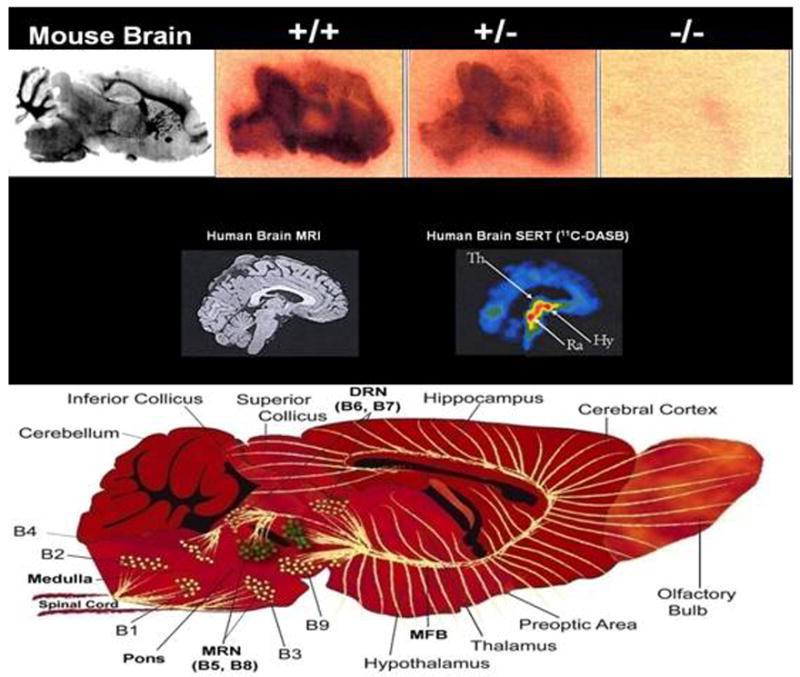
Figure 11. Comparisons of SERT-Deficient vs. SERT Over-Expressing Mice: SERT Binding Sites and Anxiety-Like Behavioral Differences.
Electrophysiological Changes in SERT-Deficient Mice
The spontaneous firing rate of putative serotonergic neurons in the dorsal raphe of anesthesized Slc6a4 -/- mice is ∼35% of that of wild-type control mice, with intermediate values (∼60%) in Slc6a4 +/- mice (Gobbi et al., 2001). In addition, the time required for CA3 hippocampal neurons to return to normal firing rates following microiontophoretic application of serotonin is prolonged (Mannoury la Cour et al., 2001). This most likely represents the failure of the rapid clearance of serotonin, as has been documented in the CA3 hippocampal region (Gobbi et al., 2001; Montanez et al., 2003) (Fig. 7). As expected, the SRI, paroxetine, further increased the serotonin-induced prolongation of hippocampal neuron recovery time in Slc6a4 +/- mice (Gobbi et al., 2001). In brain slice preparations studied in vitro, similar changes were not observed, suggesting that the sustained presence of excess ECF serotonin in vivo is a required determinate of the in vivo alterations (Mannoury la Cour et al., 2001; Mannoury la Cour et al., 2004)
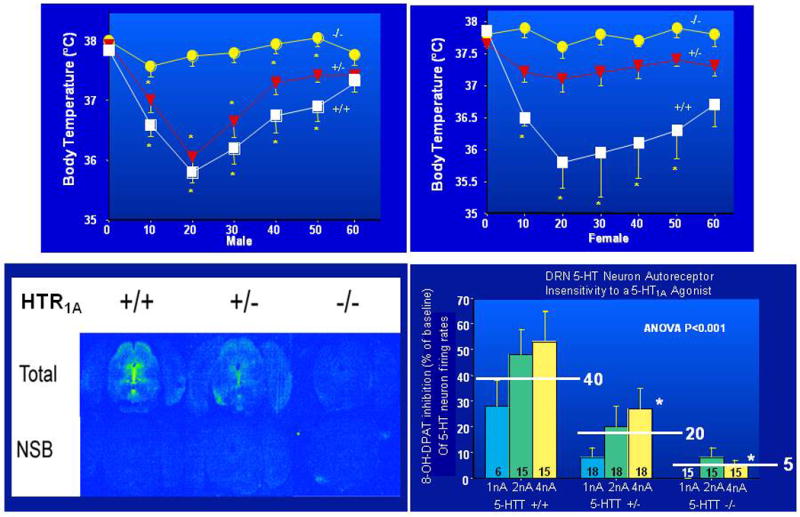
5-HT1A receptors in the brainstem raphe area are substantially decreased in Slc6a4 -/- female mice (>60%), with somewhat smaller reductions in males and intermediate values in Slc6a4 +/- mice (Fig. 12). Female, but not male, Slc6a4 -/- mice also show modest reductions in 5-HT1A receptors in the hypothalamus and in some areas of the amygdala and septum, but show no changes in the cortex or hippocampus; thus, Slc6a4 +/- and Slc6a4 -/- mice display a downregulation of 5-HT1A receptors at presynaptic somatodendritic sites, accompanied by insensitivity of these receptors to 5-HT1A -mediated neuroendocrine responses (Bouali et al., 2003; Fabre et al., 2000; Li et al., 2000; Li et al., 1999). This is similar to the changes observed in rodents chronically treated with SSRIs. In SERT-deficient mice, restoration of 5-HT1A-mediated function in the medial hypothalamus by addition of recombinant 5-HT1A receptors via an adenoviral vector produced a rescue and near-normalization of the abnormal 5-HT1A system neuroendocrine responses and behavioral abnormalities in these mice (Alexandre et al., 2006; Li et al., 2004a).
Figure 12. Absent or Attenuated 5-HT1A - Mediated Raphe 5-HT Neuron Firing Rate and Temperature Responses to the 5-HT1A Agonist, 8-OH-DPAT.
5-HT2A receptor binding sites are decreased in the striatum, claustrum and cortex, but increased in the septum and hypothalamus of Slc6a4 -/- mice (Li et al., 2003; Rioux et al., 1999). In contrast, 5-HT2C receptor binding sites are increased in the amygdala and choroid plexus of Slc6a4 -/- mice, but unchanged in other regions (Li et al., 2003). Chronic SRI treatment likewise usually increases 5-HT2C receptor expression, but does not affect 5-HT2A receptor levels (Laakso et al., 1996).
Pharmacological Changes in SERT-Deficient Mice
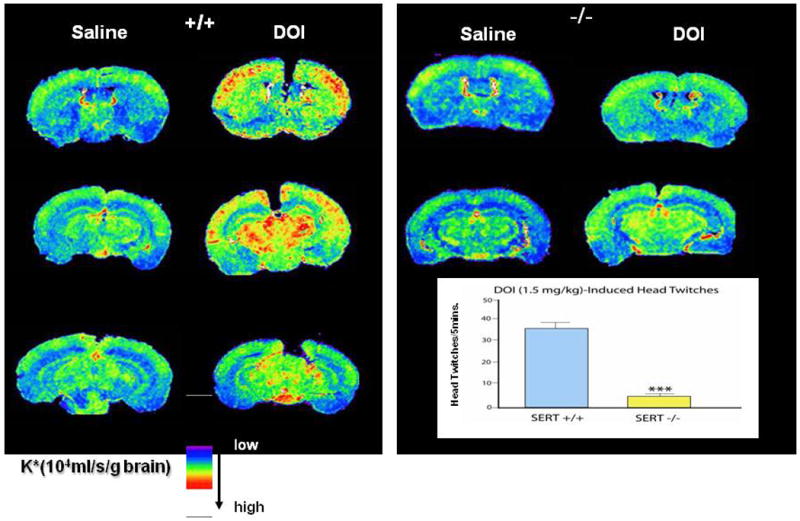
These relatively modest changes in receptor numbers were accompanied by much greater changes in multiple responses to selective serotonin receptor agonists. For example, changes in hippocampal neuron firing rates, temperature and other responses to the 5-HT1A agonists 8-OH-DPAT and ipsapirone (Fig. 12), suppressed locomotor-behavior responses to the 5-HT1B agonist RU 24969, and in particular responses to DOI (a 5-HT2A/2C agonist with hallucinogenic effects) were all essentially absent in Slc6a4 -/- mice (Fig. 13), with intermediate changes in Slc6a4 +/- mice on some measures (Holmes et al., 2002c; Lesch and Mossner, 2006; Ren-Patterson et al., 2006). These diminished receptor-mediated responses do not seem attributable to abnormalities in G-proteins or coupling, but recent evidence indicates that signaling in the 5-HT2A/2C-phospholipase A/arachidonic acid pathway is markedly reduced in the cortex, striatum and substantia nigra of Slc6a4 +/- and Slc6a4 -/- mice (Gobbi et al., 2001; Holmes et al., 2002c; Li et al., 2000; Li et al., 2003; Qu et al., 2005) (Fig. 13).
Figure 13. Attenuation of Post-Receptor Signaling via PLA2 and 3H-Arachidonic Acid Induced by the HTR2A Receptor Agonist DOI, Accompanied by a Reduction in the Head Twitch Response to DOI.
Phenotypical Changes in SERT-Deficient Mice
Various approaches have been used to experimentally alter Slc6a4 expression and SERT function in mice, including the constitutive targeted disruption of Slc6a4 reviewed here in greatest detail. In humans, the 5HTTLPR short (s) variant and SS genotype, which results in lower expression of SLC6A4, strongly resemble the reductions in expression and function of Slc6a4 +/- mice (Fig. 14) associated with anxiety-related, harm-avoidant and negative personality traits (Anguelova et al., 2003; Kendler et al., 2005; Lesch et al., 1996; Lesch and Murphy, 2003; Sen et al., 2004). Slc6a4 +/- and Slc6a4 -/- mice were therefore predicted to exhibit increased anxiety-like behaviors. This was indeed found using multiple measures of anxiety-like behaviors in both male and female Slc6a4 -/- mice with different genetic backgrounds, as well as in SERT-deficient mice produced by other transgenic approaches (Holmes et al., 2003a; Holmes et al., 2003c; Lira et al., 2003; Zhao et al., 2006) (Fig. 11). A few gender differences and an intermediate phenotype in heterozygote mice have also been generally observed (Ansorge et al., 2004; Holmes et al., 2003c; Kalueff et al., 2007b; Lira et al., 2003; Tjurmina et al., 2002; Zhao et al., 2006). After mild postnatal foot-shock stress experiences or exposure to predator odors, anxiety-like behaviors were intensified in Slc6a4 -/- mice but not in +/+ mice (Adamec et al., 2006; Carroll et al., 2007). Latent anxiety-like behaviors have also been reported in Slc6a4 +/- mice that experience poor maternal care. Brain-derived neurotrophic factor (BDNF) has been identified as one molecular substrate of the epigenetic programming that contributes these effects (Carola et al., 2008). Repeated stressful experiences in adult Slc6a4 -/- mice promotes depression-like behavior (Wellman et al., 2007). These mice show deficits in extinction recall following fear conditioning and abnormalities in the dendritic morphology and spine density of pyramidal neurons in their infralimbic cortex and basolateral amygdala (Wellman et al., 2007).
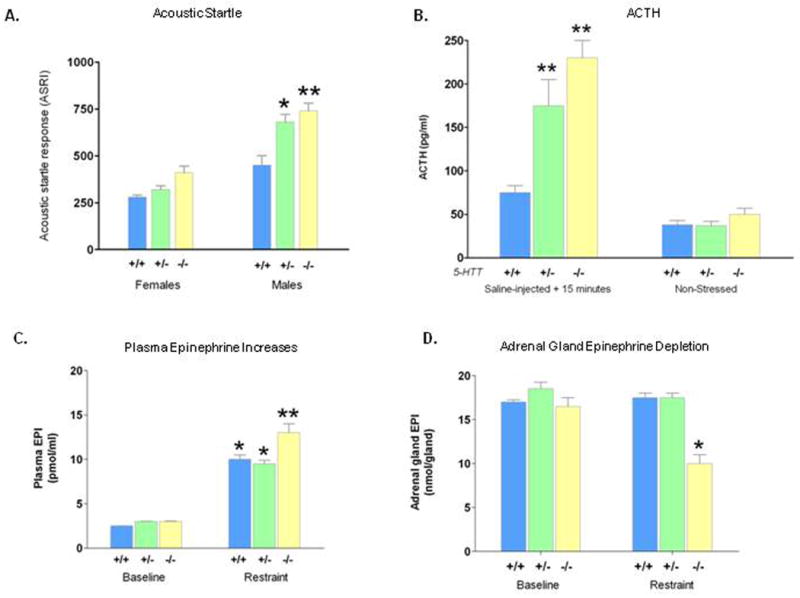
Figure 14. Acoustic Startle, Plasma ACTH and Plasma and Adrenal Gland Epinephrine Responses to Stress in SERT-Deficient Mice.
It has occasionally seemed paradoxical to some scientists that SERT-deficient mice have a strong anxiety-like behavioral phenotype, while SRIs are used as drugs to treat anxiety and depression disorders. One likely explanation for this discrepancy is the life-long developmental presence of serotonin deficiency, with likely adaptive and compensatory rewiring of brain circuits and systems in SERT-deficient mice, versus administration of an SRI for the first time to adults who are anxious or depressed. In fact, mice treated with the SRI fluoxetine during very early development also exhibited increased anxiety-like behavior as well as other related behaviors that included avoidance of open-field, center-space exposure (Alexandre et al., 2006; Ansorge et al., 2004; Maciag et al., 2006). Additionally, when SERT-deficient mice were interbred with mice that lack one copy of the BDNF gene, the anxiety-like behaviors and tissue serotonin depletion are substantially increased, in keeping with other data that show that BDNF is required for the development and maintenance of the brain's serotonin system (Mamounas et al., 2000; Ren-Patterson et al., 2006). Thus, strong developmental influences seem especially important in this and perhaps other phenotypes of SERT-deficient mice.
In direct contrast to these results with SERT-deficient mice, a marked increase in SERT binding sites and reduced anxiety-like behaviors was found in transgenic mice generated using a human yeast artificial chromosome (YAC) construct that causes a two- to three-fold overexpression of SERT accompanied by reduced ECF serotonin concentrations (Jennings et al., 2006) (Fig. 6). This appears to confirm a direct, two-way relationship between serotonin availability and anxiety-related behaviors (Jennings et al., 2006). The anxiety-like behavior in the Slc6a4 +/- and Slc6a4 -/- mice can be normalized by the 5-HT1A antagonist WAY 100635, suggesting that these receptors are a participant in their anxiety-like behaviors (Holmes et al., 2003c).
Neuroendocrine and Behavioral Sympathoadrenal Responses to Stress
Perhaps related to the anxiety-like behavioral phenotypes in SERT-deficient mice (and the major anxiety-related phenotypes in humans with lesser SERT expression and function), behavioral startle responses were markedly increased in Slc6a4 +/- and -/- male mice (Fig. 14A). Likewise, Slc6a4 -/- and Slc6a4 +/- mice respond to mild stress with greater than normal levels of adrenocorticotropic hormone (ACTH) (Fig. 14B) and oxytocin release (Li et al., 2000; Li et al., 1999; Li et al., 2003; Murphy et al., 2001), although they have reduced basal plasma corticosterone levels. These exaggerated ACTH responses are further increased in Slc6a4 -/- mice that have been interbred with Bdnf +/- mice (Ren-Patterson et al., 2006). ACTH elevations in response to the placement of mice on the elevated plus maze, which increases stress, are also exaggerated in Slc6a4 -/- mice (Li, 2006). Likewise, elevated plasma corticosterone levels are found in Slc6a4 -/- mice in response to chronic mild stress (Lanfumey et al., 2000).
Hypothalamo-pituitary adrenomedullary responses to restraint stress in conscious mice have also been examined (Tjurmina et al., 2002). At baseline, adrenal and pituitary serotonin concentrations in Slc6a4 -/- mice are markedly lower than in littermate controls. Restraint stress increases plasma levels of catecholamines in all genotypes, but these responses are exaggerated in Slc6a4 -/- mice (Fig. 14C). These responses are associated with significant reductions in levels of epinephrine, norepinephrine and serotonin in the adrenal glands and similarly pituitary tissue ACTH is also significantly reduced (Tjurmina et al., 2002) (Fig. 14D). These differences suggest that one function of SERT is to restrain adrenomedullary activation in response to stress (Tjurmina et al., 2004). The usual increase in tyrosine hydroxylase transcription and adrenomedullary angiotensin-II receptor expression changes that follow adrenal epinephrine and norepinephrine release in response to restraint and other forms of stress is absent in Slc6a4 -/- mice (Armando et al., 2003). Thus, exaggerated adrenomedullary responses seem to be an autonomic correlate of the anxiety-like behaviors and exaggerated hypothalamo-pituitary adrenal responses that occur in Slc6a4 -/- mice (Armando et al., 2003; Tjurmina et al., 2002; Tjurmina et al., 2004).
Somatosensory Cortex involving the Whisker Barrel Pathway
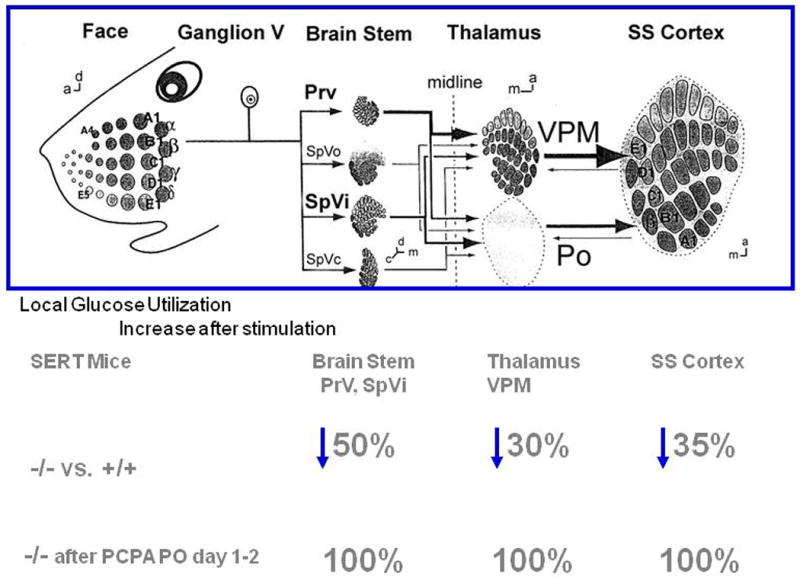
Cytoarchitectonic changes are found in the somatosensory barrelfield layer IV cortex of Slc6a4 -/- mice and to an intermediate extent, in Slc6a4 +/- mice (Persico et al., 2001; Salichon et al., 2001) (Fig. 15). Of interest, similar changes are also found in mice that lack the gene that codes for monoamine oxidase A (Maoa), the major enzyme involved in the metabolism of serotonin (Salichon et al., 2001) (Fig. 15). To evaluate whether these morphological changes were of functional importance, responses to brief whisker stimulation in cortical barrelfields were investigated using cerebral glucose utilization measurements (Esaki et al., 2005). In Slc6a4 +/+ mice, unilateral stimulation of whiskers leads to a significant glucose utilization response in the contralateral somatosensory cortex. The magnitude of this response is significantly reduced in Slc6a4 -/- mice (Esaki et al., 2005) (Fig. 16). Remarkably, this reduction is also observed in other components of the trigeminal nerve as well as spinal and thalamocortical somatosensory pathways, which are components of barrelfield activation pathway (Esaki et al., 2005) (Fig. 16).
Figure 15. Whisker Barrel Disruption in Layer IV Somatosensory Cortex of SERT Mutant Mice, Compared To Findings in Studies of Other Mutant Mice.
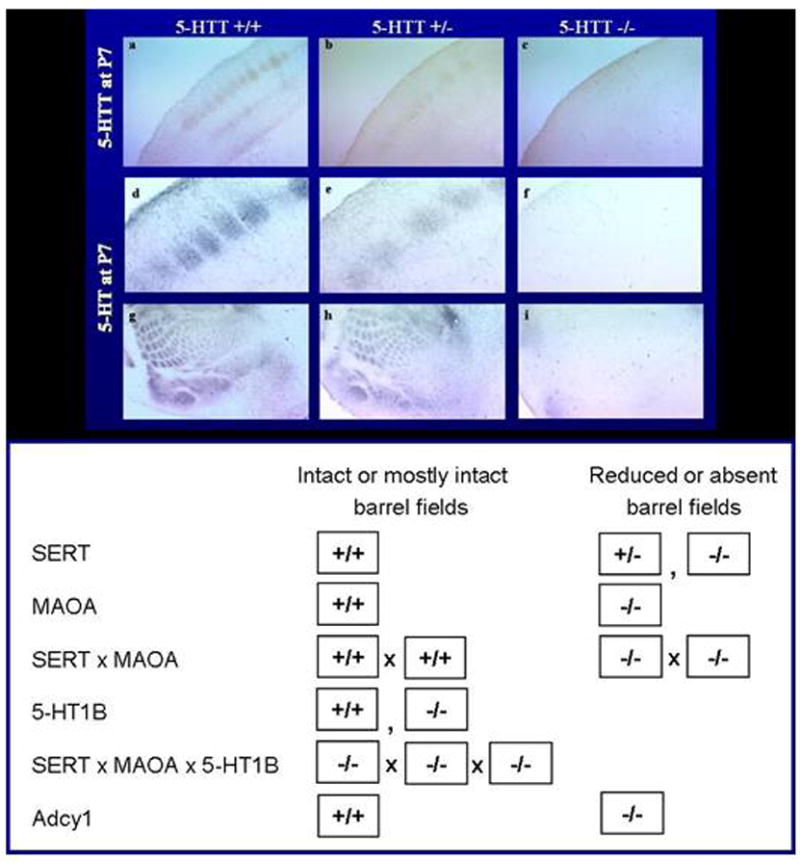
Figure 16. SERT -/- Mice Show Reduced Local Cerebral Glucose Utilization in Whisker-to-Barrel Pathway in Response to Unilateral Vibrissal Stimulation.
Rescue of cortical barrel architecture abnormalities in Slc6a4 -/- and +/- mice can be accomplished by treatment with the serotonin-synthesis inhibitor parachlorophenylalanine (PCPA) in a narrow developmental time window between postnatal days 1 and 2 but markedly less so thereafter (Persico et al., 2001) (Fig. 16). Partial genetic rescue also occurs following inactivation of 5-HT1B in this cortical area (Salichon et al., 2001), confirming the importance of serotonin and its 5-HT1B receptor in barrelfield development (Fig. 15).
Sleep, Brain Excitability, Body Temperature and Gut Motility
Slc6a4 -/- and Slc6a4 +/- mice have substantially increased rapid eye movement (REM) sleep time (Wisor et al., 2003). Furthermore, brain excitability (as reflected in their susceptibility to pentylenetetrazole-induced seizures) is reduced in these mice (Fox et al., 2007a). Baseline body temperature is also increased (Li et al., 2004a). Gut physiological function is abnormal in Slc6a4 -/- mice, which have increased colonic motility and other symptoms that resemble those found in the human irritable bowel syndrome (Chen et al., 2001). These gut function changes have also been found to accompany the serotonin precursor (5-hydroxytryptophan; 5-HTP)-induced serotonin syndrome (Fox et al., 2007b; Fox and Murphy, in press), (Fox and Murphy, unpublished observations).
Sensory, bladder and vascular function
Sensory function is reduced in Slc6a4 -/- mice, as reflected by their reduced spinal reflexes and reduced responses to mildly painful thermal or nerve crush injuries (Ren-Patterson et al., 2005; Vogel et al., 2003). Likewise, bladder responses to stretching are reduced in female Slc6a4 -/- mice (Cornelissen et al., 2005). However, basic neurological and motor testing reveals no overt changes in other reflexes, including the eye blink, whisker or righting reflexes (Holmes et al., 2002a).
The elevated blood pressure that normally develops in response to reduced oxygen availability at simulated high altitudes is reduced or absent in Slc6a4 -/- mice (Eddahibi et al., 2000). This might provide an explanation for the protection against the development of human pulmonary hypertension that is apparently afforded by the lesser-expressing S allele and SS genotype of the SLC6A4 5HTTLPR (Eddahibi et al., 2000; Murphy et al., 2004a). Also, Slc6a4 -/- mice display lower left cardiac ventricular weight relative to body weight and develop cardiac fibrosis as well as valvulopathy (Eddahibi et al., 2000; Mekontso-Dessap et al., 2006) phenomena which may be related to deficient platelet 5-HT availability, including the availability of 5-HT to cardiac 5-HT1B receptors, according to results in 5-HT1B genetically-deficient mice (Jaffre et al., 2004). Thus, serotonin and SERT play a role not only in visceral function but also in cardiovascular and lung function.
Bone and Muscle Strength, and Agility
The ability to cling to a wire mesh screen (a test of strength) and to maintain a grasp on a rotating metal rod is reduced in Slc6a4 -/- mice (Holmes et al., 2002a). Likewise, bone weight, thickness and structural resistance to fracture in vitro are also significantly reduced (Bliziotes et al., 2002; Sample et al., in press; Warden et al., 2005). One plausible basis for these impairments is the lifetime reduction in physical activity and exercise that results from reduced horizontal, vertical and risk-assessment behaviors (including standing and surveying and sniffing the environment) in Slc6a4-deficient mice, since bone changes are most marked in weight-bearing bones and not cranium in these mice (Holmes et al., 2002a; Holmes et al., 2002b). There do not appear to have been any comparable evaluations of the effect of human SLC6A4-variant associations on physical fitness or strength. However, similar results would be predicted from these observations in Slc6a4 -/- and Slc6a4 +/- mice, particularly because reductions in bone mineral density and osteoporosis are found in humans receiving long-term SRI treatment (Diem et al., 2007; Haney et al., 2007).
Aggression
Slc6a4-/- mice are less aggressive than control mice in the isolated-resident/intruder test (Holmes et al., 2002a). Additionally, Slc6a4 -/- and +/- mice failed to shorten the latency time to first attack in a second intruder-encounter experiment, unlike Slc6a4 +/+ mice. This confirms a role for altered SERT levels in aggressive behaviors and also indicates that Slc6a4 -/- and Slc6a4 +/- mice have a reduced learning capacity in this paradigm in comparison with wild-type mice. The findings might also be related to other social interaction deficits in the Slc6a4 -/- and Slc6a4 +/- mice and suggest that they might be a useful model of some aspects of social anxiety and autism (Holmes et al., 2002a; Kalueff et al., 2007c), (Moy S et al., under review). Reports of altered aggression in mice with a deletion of one Bdnf allele suggest that studies of the Slc6a4 × Bdnf double-mutant mice might provide further evidence of specific epistasis that is similar to that observed for anxiety-like behaviors and neuroendocrine responses in these dual gene-deficient mice models (Murphy et al., 2003a; Ren-Patterson et al., 2006; Ren-Patterson et al., 2005).
Pharmacological Challenges in SERT-Deficient Mice
Many pharmacologic agents that act through SERT (including 3,4-methylenedioxymethamphetamine (MDMA, Ecstasy), 2′-NH2-1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (2′-NH2-MPTP) and SRIs), have reduced efficacy in Slc6a4 -/- and Slc6a4 +/- mice reflecting the reduced availability or downregulated receptor function of these drug targets (Bengel et al., 1998; Fox et al., 2007a; Gobbi et al., 2001; Holmes et al., 2002a; Li et al., 1999; Qu et al., 2005). MDMA self-administration is absent in Slc6a4 -/- mice, and alcohol preference and ingestion are reduced (although alcohol's sedative effects are increased) (Boyce-Rustay et al., 2006; Daws et al., 2006; Kelai et al., 2003; Montanez et al., 2003; Trigo et al., 2007). By contrast, cocaine preference is intact or enhanced in Slc6a4 -/- and Slc6a4 +/- mice (Sora et al., 1998) (Fig. 5). However, in Slc6a4-mutant mice that have been interbred with mice that lack the dopamine transporter, cocaine preference is abolished (Sora et al., 2001; Sora et al., 1998) (Fig. 5).
Genetic vulnerability to an exaggerated, ‘serotonin syndrome’ is present in Slc6a4 +/- and Slc6a4 -/- mice with a targeted disruption of SERT and has implications for similar SRI side effects in humans with polymorphisms that reduce SERT gene function. One of the most remarkable group of drug response alterations observed in SERT-deficient mice are serotonin syndrome behaviors and temperature changes produced by serotonin agonists (Fox et al., 2007b; Fox and Murphy, in press). These may prove to provide a model for humans with genetic vulnerabilities to this syndrome (Fox et al., 2007b; Fox and Murphy, in press; Murphy et al., 2004b; Murphy et al., 2003b; Murphy et al., 2000; Pato et al., 1988; Perlis et al., 2003; Popp et al., 2006; Serretti et al., 2006).
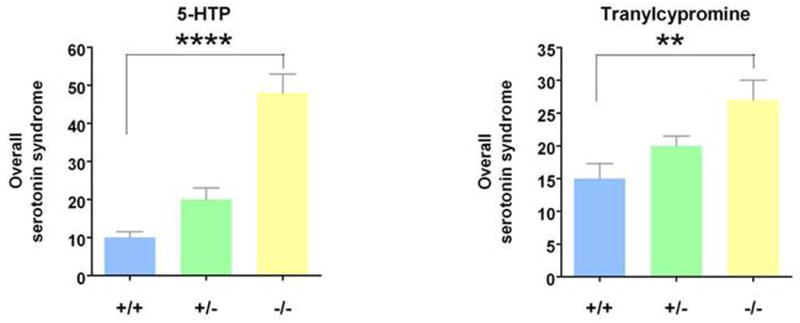
Spontaneous serotonin-syndrome behaviors were first observed in untreated Slc6a4 -/- mice (Fox et al., 2007b; Kalueff et al., 2007a). Follow-up investigations identified serotonin agonists and antagonists that exaggerated or blocked the serotonin syndrome (Fox et al., 2007b). As shown in Fig. 17, markedly exaggerated serotonin syndrome behavioral responses to the amino acid precursor of serotonin, 5-HTP and the MAOI, tranylcypromine occurred in SERT-deficient mice (Fox et al., 2007b). Tranylcypromine is a clinically available antidepressant drug, and 5-HTP is promulgated on more than 100 internet sites and sold over-the-counter as a dietary supplement. 5-HTP is advertised as having antidepressant effects and, like MAO-inhibitors, as a treatment that can enhance and prolong the actions of both these agents as well as therapeutically-used SRIs and illicit drugs of abuse such as MDMA (Silins et al., 2007; Vuori et al., 2003).
Figure 17. Exaggerated Serotonin Syndrome Responses to 5-HTP and to Tranylcypromine In SERT-Deficient Mice.
These findings in this mouse genetic model raise the possibility that humans with lower-expressing SS genotypes or other SERT variants that lead to 50-80% decreases in SERT binding sites or transport function (Lesch et al., 1996; Prasad et al., 2005) may be at higher risk to develop serotonin syndrome neurotoxicity. This is based on highly congruent data from imaging, neuroendocrine, neurophysiologic and other studies that have compared SERT-deficient mice to humans with SERT gene variants that convey lower SERT expression and function (Murphy et al., 2004a; Murphy and Lesch, 2008).
As treatment for the human serotonin syndrome is mostly supportive or targeted towards clinical signs and symptoms using non-specific agents such as benzodiazepines or broad-spectrum serotonin antagonists such as cyproheptidine, we undertook investigations using selective serotonin antagonists and other agents. The selective 5-HT1A antagonist, WAY 100635, essentially blocked all of the abnormal serotonin-syndrome behaviors that followed 5-HTP in Slc6a4 -/- mice, whereas other antagonists were without effect (Fox et al., 2007b). This is notable in that prior studies with higher doses of 5-HTP plus MAOIs in strictly pharmacologic model studies in rats found that 5-HT2A antagonists such as ritanserin or risperidone (an atypical neuroleptic) were effective in preventing temperature change and lethality. In our hands, 5-HT2A antagonists failed to mitigate serotonin syndrome behaviors in SERT-deficient mice (Fox et al., 2007b). These findings may be reflective of a species difference (mouse vs. rat) or model difference (genetic vs. pharmacologic), or may be related to drug dose differences.
The 5-HT1A antagonist WAY 100635 had no effects on Slc6a4 +/+ mice. However, it blocked the occurrence of the small but distinct features of serotonin syndrome behaviors seen in vehicle-treated mice Slc6a4 -/- mice, behaviors we previously reported in untreated Slc6a4 -/- mice (Fox et al., 2007b; Kalueff et al., 2007a). Anxiety-like and/or stress-related phenomena may be involved to some extent in these spontaneous behaviors, as in prior studies WAY 100635 blocked several anxiety-like behaviors in Slc6a4 -/- mice, without any effects in Slc6a4 +/+ mice (Holmes et al., 2003c). Likewise genetic manipulations of hypothalamic 5-HT1A receptors blocked the excess ACTH responses that Slc6a4 -/- mice demonstrate in response to minor stressors (Holmes et al., 2003c; Li et al., 2004a; Li et al., 1999; Murphy et al., 2001).
The results of the present study raise a new consideration of 5-HT1A receptor agents as potential treatments in some human serotonin syndrome cases, such as those with SLC6A4 polymorphism-related vulnerability. Positive results with 5-HT1A agents have not been previously reported in different animal studies of the serotonin syndrome based on drug interaction models alone. Unfortunately, highly selective 5-HT1A antagonists are not yet clinically available, although propranolol, and other partial 5-HT1A antagonists such as buspirone, in addition to adrenergic beta-receptor antagonists, have occasionally been used to treat the serotonin syndrome. Buspirone and related azaspirones have strong 5-HT1A binding capabilities and buspirone is clinically available. However, one of these azapirone compounds, ipsapirone, did not affect 8-OH-DPAT-induced serotonin syndrome behaviors in mice and, thus, the partial agonist properties of these azapirones render them questionable for consideration (Goodwin et al., 1986). Thus, there appears to be a need for selective 5-HT1A antagonist development for use in humans.
As temperature dysregulation is sometimes, although not always, a feature of the human serotonin syndrome, we investigated this particular phenotypic measure. As with behavioral changes, we found gene dose-dependent temperature alterations in SERT-deficient mice, with profound hypothermia (a 7°C change) following 5-HTP, with substantially smaller responses in Slc6a4 +/- and Slc6a4 +/+ mice (Fox and Murphy, in press). This temperature dysregulation may correspond to the diaphoresis and shivering frequently described in case reports of the human serotonin syndrome, where hyperthermia (but not hypothermia) is reported in ∼50% of cases. Hypothermia rather than hyperthermia may also reflect incompletely understood temperature regulation differences between mice, rats and humans, as pro-serotonergic agents may more commonly produce hypothermia in mice and hyperthermia in rats and humans. However, there is considerable variability across drug studies, including differences clearly related to pre-synaptic mechanisms in mice and post-synaptic mechanisms in rats, as well as to effects of ambient temperature differences in some studies that include neurotoxic consequences (Bill et al., 1991; Insel et al., 1982).
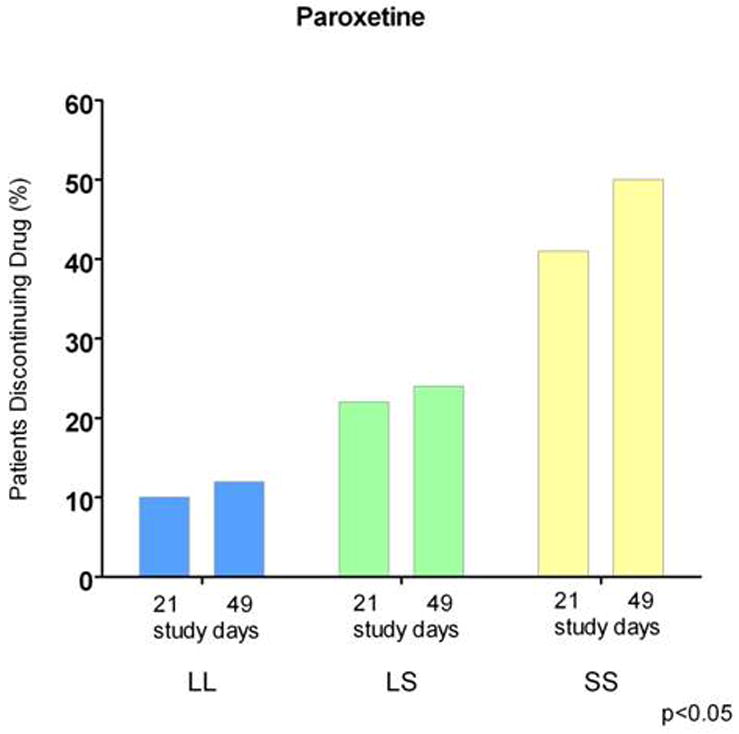
The behavioral and physiological serotonin syndrome was initially characterized in rodents given serotonin agonists (Jacobs et al., 2006). The human serotonin syndrome was first identified by name in 1982 (Insel et al., 1982), although toxic reactions related to MAOI drug interactions had been described earlier, and Sternbach (1990) described criteria for their diagnosis, which has been refined in subsequent reports and reviews (Dunkley et al., 2003; Insel et al., 1982; Isbister and Buckley, 2005; Kalueff et al., 2008). Considerable data indicates that the severity and duration of the serotonin syndrome is a strong function of the amount of excess serotonin produced, usually by combined administration of pro-serotonergic agents such as SRIs and MAOIs. This has been partly based on moderate correlations of amounts of drug ingested in suicide attempts relative to outcomes. There is nonetheless considerable variability within the spectrum of both occurrences and outcomes, including mild, sometimes subclinical states, as well as lethality. Of special note, it is likely that relatively mild serotonin syndrome occurrence may contribute to early discontinuation of SRIs and side effects during SRI treatment that are strongly associated with the SS genotype and S allele of the SERT variant (Murphy et al., 2004b; Murphy et al., 2003b; Pato et al., 1988; Serretti et al., 2006). Two examples from several studies illustrate these phenomena: (1) delayed but essentially universal occurrence of OCD and depressive symptoms re-occur in OCD patients following clomipramine discontinuation (Pato et al., 1988) (Fig 18) and (2) adverse effects and treatment discontinuation in depressed patients following paroxetine discontinuation are highly related to SERT 5HTTLPR genotype (Murphy et al., 2003b) (Fig. 19).
Figure 18. Return of Depression and OCD Symptoms After Treatment Discontinuation In Recovered Patients (Mean Rating Changes in 18 Patients with Obsessive-Compulsive Disorder Before And During Double-Blind Discontinuation of Clomipramine, with Placebo Substitution).
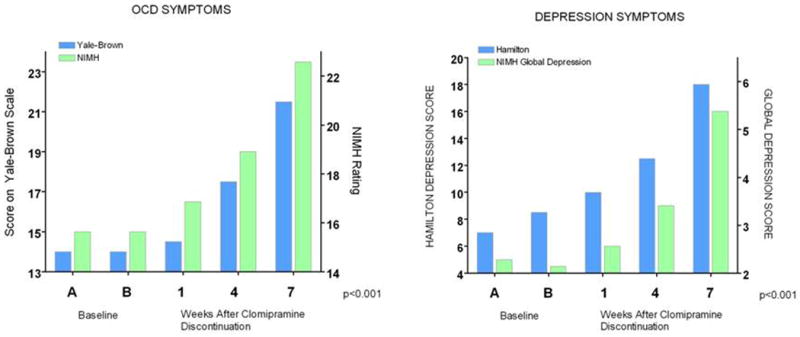
Figure 19. SERT 5HTTLPR Predicts Treatment Discontinuation Due to Adverse Effects Among 246 Depressed Patients, with SS Patients Showing Higher Discontinuation Rates.
These data provide evidence that genetic variation in murine SERT expression and function have direct consequences on the outcome, severity and lethality of the same doses or combinations of drugs that have been associated with the serotonin syndrome or non-tolerability of SRIs in humans. Common human SERT polymorphisms can lead to a four-fold variation in SERT mRNA and transport capacity (Hu et al., 2006), and rare variants can reduce transport by as much as 80%, or raise it an additional five-fold by different mechanisms leading to an overall theoretical 20-fold difference in SERT expression and function (Kilic et al., 2003; Murphy and Lesch, 2008; Prasad et al., 2005) (Fig. 3). Thus, SERT provides an interesting example of likely mouse-human congruence in genetic vulnerability to serotonin syndrome features and likely milder symptomatology that may lead to discontinuation of SRI and related medications in humans. Functional variants exist in additional genes that might be expected to confer vulnerability to serotonin neurotoxicity, including MAOA, serotonin receptor genes and catecholamine-related genes (Murphy et al., 2004b; Murphy et al., 2003b). Additional examples likely exist and might be modeled in gene-targeted mice that have been created for these genes and for some of the gene × gene epistatic double knockout models constructed, as already reported (Berger and Tecot, 2006; Murphy et al., 2003a; Sallinen et al., 1998).
In regard to SERT, similar heterogeneity in responses across individuals might be shown to exist for the serotonin syndrome as has been found for antidepressant efficacy and other side effects of antidepressant treatment (Murphy et al., 2004b; Murphy et al., 2003b; Serretti et al., 2006). Likewise, there may be considerable differences in beneficial treatments for the serotonin syndrome, with some individuals responding better to one class of drugs than others – all examples of the projected, soon-to-come “personalized medicine”.
Relevance To Human Disease
As described above, serotonin functions as both a short-range neurotransmitter, a paracrine neuronal modulator along axons and relatively large brain regions at heterologous receptors and also as a long-range signaling modulator, with multiple effects on whole-organism functions via plasma, platelet and neuroendocrine, gut, adrenal and other peripheral systems across many species (Barnes and Sharp, 1999; Lucki, 1998; Murphy et al., 2004a; Weiger, 1997). This extensive pleiotropy is clearly demonstrated in this review of the multiple mouse phenotypes that result from the single gene alterations of Slc6a4. Additional sources of alterations related to human SLC6A4 variants and to differences in the expression and function of SERT in other species may relate to the existence of multiple central and peripheral serotonergic systems and differential changes in these systems.
One perspective suggests that the anatomy of the serotonin system may be considered as four-interacting subsystems (Fig. 20). Of these, the serotonin subsystem in the CNS has been the most comprehensively studied (Fig. 10). Cell bodies in the dorsal, medial and other raphe nuclei provide modulatory serotonergic input to multiple neuronal circuits in the brain through the midbrain and upper pons cell groups, plus input to the spinal cord from its brainstem caudal raphe cell groups (Figs. 10, 20). The genetic lineage of these different brain raphe serotonin cell groups has recently been described (Jensen et al., 2008).
Figure 20. Central and Peripheral Serotonergic Systems.
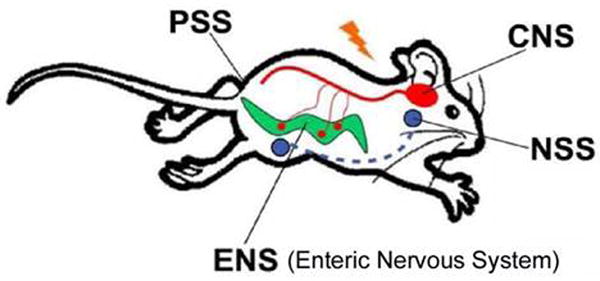
Serotonin also functions in the enteric nervous system (ENS), the hypothalamo-pituitary-adrenocortical (HPA) system, the adrenomedullary neuroendocrine serotonin system (NSS) and the peripheral serotonin system (PSS), which includes the lungs, the heart, the blood vessels, the pancreas and platelets DRN, dorsal raphe nucleus; MFB, medial frontal bundle; MRN, median raphe nucleus.
In the periphery, a partially separate serotonergic enteric neural system (ENS) exists within the gut (Gershon et al., 1993; Gershon et al., 1983) and interacts with other CNS and peripheral serotonergic mechanisms (Fig. 20). Furthermore, several other tissues without any prominent serotonergic innervations (such as the lung, heart, blood vessel and pancreatic tissue, as well as platelets) contain functional SERT (Kim et al., 2005). These tissues normally take up and store serotonin in vesicles, and can release it in response to local stimuli. Thus, they may be considered to be a peripheral serotonergic system (PNS) (Fig. 20). A neuroendocrine serotonergic system responds to serotonin release with diverse signaling through hormones such as prolactin, CRH, ACTH, corticosterone and oxytocin. This system has frequently been used as an accessible way to evaluate serotonergic system function in humans through the so-called serotonergic pharmacologic challenge strategy (Power and Cowen, 1992).
In human studies, mRNA localization and brain imaging of SERT using PET and SPECT methodology has become increasingly utilized, although precise localization of specific structures is quite modest (Fig. 10). In rodents, SERT mRNA in brain closely parallels the distribution of tryptophan hydroxylase in serotonin-containing cell bodies, with concentrations highest in the dorsal raphe, lower concentrations in other raphe nuclei and still lower concentrations in a few other areas such as the dorsomedial nucleus of the hypothalamus and cortex (Hoffman et al., 1998). Likewise, SERT protein expression is highest overall in raphe areas. During development, it extends in spatiotemporal congruence with axons of raphe neurons prior to synapse formation. An exception is the transient expression of SERT protein and mRNA during early postnatal development in two patterns: a) together with vesicular transporter (VMAT2) mRNA in trigeminal, cochlear and solitary nuclei of the brainstem and sensory nuclei of the thalamus (ventrobasal, lateral and medial geniculate nuclei); and b) without VMAT2 mRNA in some limbic areas including septum, olfactory areas, and hippocampus (Hansson et al., 1998).
Attempts to evaluate localization and density of SERT in humans and rodents during the past decade used [123I]-βCIT [(I-2β-carbomethoxy-3β-(4-iodophenyl)tropane] (RTI-55) in SPECT studies, although βCIT's equal affinity for DAT results in visualization of SERT verified by SRI antagonism only in a single midbrain region. There is evident need for replication of the results found with βCIT using positron emission tomography (PET) methodology. In fact, more selective SERT ligands have become available recently for human PET studies including McN 5652 ([+]-6β-(4-methylthiophenyl)-1, 2, 3, 5, 6α, 10β-hexahydropyrrolo[2,1-a]isoquinoline), and a group of diarylsulfides, particularly DASB (3-amino-4[2-(dimethyl-aminomethylphenylthio)] benzonitrile) and AFM (2-[2-(dimethyl-aminomethylphenylthio)]-5-fluoromethylphenylamine) (Ginovart et al., 2001; Houle et al., 2000; Huang et al., 2002; Ichise et al., 2003; Li et al., 2004b; Oquendo et al., 2007; Reimold et al., 2007). These compounds are in early stages of evaluation in humans, where DASB has promising properties (Fig. 10).
[11C]-DASB is highly selective for cloned hSERT, with nanomolar affinity for SERT (Ki = 1nM) and much lower, micromolar affinity for the dopamine transporter (DAT), (Ki = 1420 nM), and norepinephrine transporter (NET) (Ki = 1350 nM) (62). DASB penetrates the blood-brain barrier well and is rapidly metabolized, such that approximately 50% of the parent compound is cleared from plasma by twenty minutes post-injection (Ginovart et al., 2001; Ichise et al., 2003). The metabolites of DASB are mainly polar, hydrophilic species, which are unlikely to pass the blood-brain barrier. Scanning time required for DASB is the shortest of all for the newly available ligands (Huang et al., 2002).
PET studies in humans show that [11C]-DASB binds reversibly to SERT throughout the brain. The highest uptake is in hypothalamus; intermediate levels are seen in the midbrain, thalamus, and striatum with lower levels observed in cortical regions and white matter and with no apparent binding in the cerebellum (Ginovart et al., 2001; Houle et al., 2000; Ichise et al., 2003). These data agree with SERT densities found in post-mortem human brain (Cortes et al., 1988; Laruelle et al., 1988) and in rodent brain regions (Bengel et al., 1997; Ginovart et al., 2001). A simplified method of [11C]-DASB tissue data analysis using a one-compartment model has been proposed for routine use where the cerebellum, because of its negligible levels of SERT binding, is used as a reference tissue to estimate SERT binding potential instead of an arterial input function (Ginovart et al., 2001; Ichise et al., 2003). [11C]-DASB displays quite high signal-to-noise ratios in vivo (Ginovart et al., 2001; Houle et al., 2000).
[11C]-DASB's specificity for SERT in vivo in humans was demonstrated by an 80% decrease in brain radioactivity after blockade of SERT sites with a single 40 mg oral dose of the highly selective SRI, citalopram (Houle et al., 2000). In addition, treatment of patients with depression using lower doses (20 mg/day) of either paroxetine or citalopram for four weeks produced a mean decrease of 80% in striatal [11C]-DASB-labeled SERT sites (Meyer et al., 2001). A number of studies have documented associations between DASB-labeled SERT binding sites and SLC6A4 5HTTLPR genotypes (Praschak-Rieder et al., 2007; Reimold et al., 2007), but others did not find a consistent association, although ethnicity and clinical state differences, including levels of anxiety, were found to influence these results (Oquendo et al., 2007). In mice with a targeted disruption of SERT studied autoradiographically with [11C]-DASB, Slc6a4 -/- mice had no specific binding, whereas Slc6a4 +/- mice had a ∼50% decrease in SERT sites in all brain regions, consistent with prior studies using the ligands RTI-55 and cyanoimipramine in these mice, and thus validating DASB as a highly specific SERT ligand (Bengel et al., 1997; Li et al., 2004b; Montanez et al., 2003).
Disorders with Implicated SERT Involvement
SERT may contribute to several human disorders, based on the genetic association studies focused primarily on the SLC6A4 5HTTLPR polymorphism, and to a lesser degree on the intronic VNTR near exon 2 plus additional studies of newly discovered rarer SLC6A4 variants. Recent studies have begun to incorporate SERT SNPs and haplotypes (Conroy et al., 2004; Hu et al., 2006; Mynett-Johnson et al., 2000; Weese-Mayer et al., 2003; Wendland et al., 2008a; Wendland et al., 2007; Wendland et al., 2006a; Wendland et al., 2006b; Wendland et al., 2008b). Simple case-control comparisons have been most common in all of these studies, and most reports (>400 total) comprise investigations of neuropsychiatric diagnostic phenotypes, some of which have been partially catalogued and reviewed elsewhere (Anguelova et al., 2003; Glatt et al., 2001; Hahn and Blakely, 2002). Many of these studies acknowledge limitations, which often include small sample sizes, recognized or suspected variability in the clinical phenotype, and coexisting disorders. Nonetheless, a few examples of special interest warrant discussion, including several examples of apparent SERT-related dysfunction in the periphery, a somewhat neglected area of interest (Blakely, 2001), as well as OCD.
Obsessive-Compulsive Disorder
OCD is frequently familial, with a five -to eight-fold increase in lifetime risk found for sibs or other first degree relatives of OCD probands compared to control probands (Hanna et al., 2002; Shugart et al., 2006). Even higher relative recurrence risks are found in early childhood-onset OCD, which is frequently distinguished by high comorbidity with Tourette disorder and chronic tics (Hemmings and Stein, 2006). Other than the reported relationship of streptococcal infection in a subset of ‘PANDAS’ (Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection) OCD cases (Snider and Swedo, 2004), there are no current environmental hypotheses with strong empirical support. Treatments for this chronic, disabling disorder, while helpful, generally bring only partial relief. No cure exists and thus, the discovery of genes seems important for elucidation of pathogenic mechanisms and development of rational treatments. The main features of OCD are persistent, intrusive, senseless thoughts and impulses (obsessions) and repetitive, intentional behaviors (compulsions). Individuals with the disorder recognize that their thoughts and behaviors are excessive and unreasonable, and they struggle to resist them. The lifetime prevalence of OCD is estimated to be 1-3%, based on population-based surveys conducted in many communities nationally and internationally. Although the disorder affects individuals of all ages, the period of greatest risk is from childhood to early adulthood (Neale and Sham, 2004; Nestadt et al., 2000). Patients experience a chronic or episodic course with exacerbations that can substantially impair social, occupational, and academic functioning. According to the World Health Organization, OCD is among the ten most disabling medical conditions worldwide (Murray and Lopez, 1996).
Because SERT inhibitors are the single most effective OCD medication, serotonin system genes, especially SCL6A4 and HTR1Dβ, HTR2A and HTR2C serotonin receptors and tryptophan hydroxylase genes have been most thoroughly investigated (Cavallini et al., 1998; Chabane et al., 2004; Enoch et al., 2001; Hemmings et al., 2003; Hu et al., 2006; Mossner et al., 2005; Mundo et al., 2002; Walitza et al., 2002). Most of these reports are case-control comparisons of one single common gene variant and SNP-based haplotypes within these genes; a few are within-family investigations of trios. In addition, a small number of studies have identified hints of within-family associations of uncommon or rare gene variants or chromosomal anomalies with an OCD phenotype and related comorbid disorders.
One example of a rare variant associated with OCD is SLC6A4 Ileu425Val, a missense SNP producing a gain-of-function and loss of normal regulation of the serotonin transporter (Kilic et al., 2003; Ozaki et al., 2003). This was found to segregate with a primarily obsessive-compulsive disorder but complexly-comorbid phenotype in two studies that showed SLC6A4 I425V as having a 1.5% prevalence in 530 individuals with OCD from five unrelated families and a 0.23% frequency in four control populations totaling 1300 individuals, yielding a significant OCD-control difference (Fisher's exact test corrected for family coefficient of identity p = 0.004, odds ratio = 6.54) (Delorme et al., 2005; Kilic et al., 2003; Ozaki et al., 2003; Wendland et al., 2008a). SLC6A4 I425V occurred relatively recently in evolution, as Ileu425 is conserved across at least nine other species including some as distant from H. sapiens as the fly, D. melanogaster, and the worm, C. elegans (Murphy et al., 2004a; Sutcliffe et al., 2005) (Fig. 21). Of interest, another SLC6A4 I425 variant, Ileu425Leu, was recently reported in male sib pairs, both of whom had autism (Sutcliffe et al., 2005). SLC6A4 I425V was the first SERT coding region variant demonstrated to be functional and proposed to be associated with an OCD spectrum phenotype (Delorme et al., 2005; Kilic et al., 2003; Ozaki et al., 2003). SLC6A4 I425V has been designated OCD1 in Online Mendelian Inheritance in Man (OMIM). It is located in transmembrane domain 8 of the transporter, where it may modify the α-helical secondary structure of the SERT protein and thereby transport function (Murphy et al., 2004a) or where it may more directly affect the 5-HT translocation channel (Reimold et al., 2007; Yamashita et al., 2005). Expression studies of SLC6A4 I425V cDNA in COS7 and HeLa cells documented a transporter gain-of-function via constitutive activation of serotonin transport in a nitric oxide-dependent pathway that resulted in an approximate two-fold increase in serotonin uptake accompanied by an increase in serotonin affinity (Kilic et al., 2003; Zai et al., 2004; Zhang and Rudnick, 2005). This SLC6A4 I425V variant may also lead to other differences, for example changes in SERT trafficking to the membrane surface (Prasad et al., 2005) or changes in phosphorylation status producing alterations in substrate or ion transport or in the ability to interact with regulatory proteins. The high resolution structure of a bacterial SERT homologue shows that transmembrane domain 8 contributes to the substrate binding site and that the position corresponding to SLC6A4 I425 interacts with residues (Ser-190 and Leu-191) in transmembrane domain 3, which also contribute to the binding site (Rudnick, 2006; Yamashita et al., 2005) (Fig. 22). This may be responsible for the recently reported differential ability of SRIs to interact with SERT function: SLC6A4 I425V was associated with decreased potency of sertraline and citalopram to inhibit serotonin transport; furthermore, sertraline's potency to inhibit binding of 125I-β-CIT was reduced by SLC6A4 I425V in transfected HeLa cell lines (Prasad et al., 2005; Zhang and Rudnick, 2005).
Figure 21. Evolutionary Relatedness and Sequence Comparison of SERT from Several Species: lle425 and Gly56 are Highly Conserved Across >8 of 10 Species.
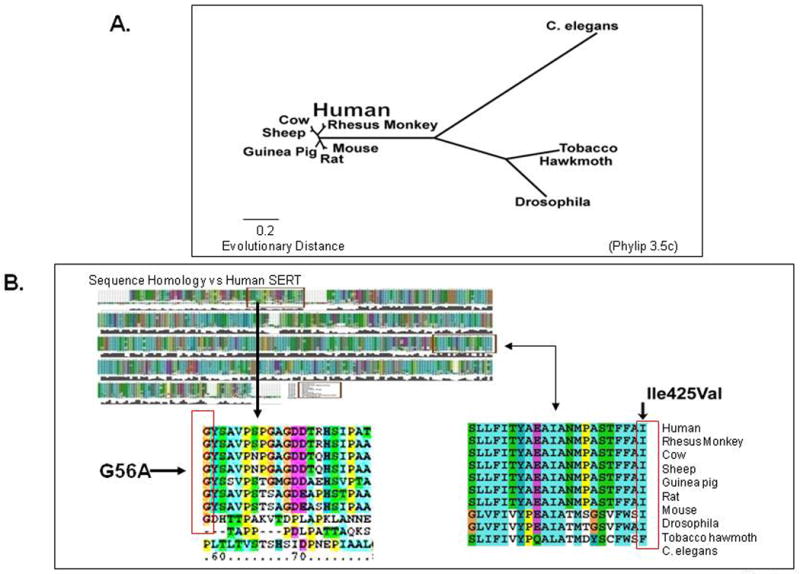
Figure 22. Bacterial LeuT Homology Model for hSERT.
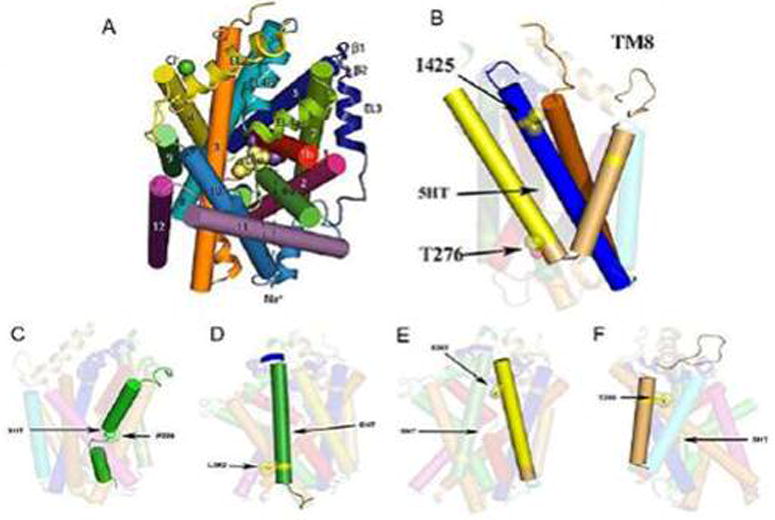
(A) The first high resolution transporter structure resolved: bacterial LeuT (20); (B) homology model of hSERT, with lleu425 on TM8. (C, D, E, F) location of other hSERT variants described recently. TM4 (Leu255Met, TM5 (Ser293Phe); TM6 Pro339Leu); TM7 (Leu362Met) 99 (Sing S, personal communication, March, 2007).
SLC6A4 I425V and other rare gene variants associated with OCD phenotypes are represented in less than 2% of OCD cases. Investigation of rare candidate genes and chromosomal regions is an alternative to the broader scale genome-wide evaluations of the dominant hypothesis of common variants accounting for common diseases. It seems possible that a relatively common disease like OCD might well have uncommon, more highly penetrant functional variants that might more directly contribute to the common OCD phenotype – a scenario that has recently been shown to be relevant in autism (Sutcliffe et al., 2005), whereas other OCD spectrum disorders may be contributed to by other genetic variants (Samuels et al., 2007).
Nonetheless, two recent studies that exploited a more thorough investigation of SLC6A4 for additional variants have provided evidence for more common gene contributions to OCD. Hu and colleagues (Hu et al., 2006) demonstrated that a possible explanation for the lack of consistent association between SLC6A4 and OCD was the previously unappreciated presence of a single nucleotide polymorphism (SNP), rs25531, within the repetitive region that comprises the 5HTTLPR. The minor allele (G) of rs25531 is almost always in phase with the long allele of the 5HTTLPR and attenuates the initially reported gain-of-function relative to the short allele (Heils et al., 1996). Thus, modulation of 5HTTLPR by rs25531 results in three common alleles: LA (highest-expressing), LG and S (both lower-expressing). With this refined understanding of 5HTTLPR functionality, we and our colleagues found an association in two independent samples, one case–control and the other family-based, of the higher-expressing LA allele and LALA genotype with OCD (Hu et al., 2006). A replication attempt (Wendland et al., 2007) using a large case–control design did not corroborate this finding after correction for multiple testing, although an increased frequency of the LA allele and LALA genotype was also observed in OCD probands (Hu et al., 2006; Wendland et al., 2007). However, when the entire 5HTTLPR was sequenced to identify for additional common and possibly functional variants, our group observed that a common SNP, rs25532, modulates 5HTTLPR-conferred SERT expression about as strongly as rs25531(Wendland et al., 2008b). When, in addition to 5HTTLPR and rs25532 and rs25531, two recently described SNPs associated with allelic expression imbalance of SLC6A4, rs2020933 and rs16965628 (Martin et al., 2007), were included in haplotype analyses, our group further discovered a highly significant omnibus association in this large case-control sample (Wendland et al., 2008b). Remarkably, and in keeping with the hypothesis of increased SERT functioning in OCD, a haplotype containing the higher-expressing variant at each locus was significantly more common in OCD probands than in controls. These results illustrate the usefulness of integrating newly-discovered, multiple functional SLC6A4 variants by haplotypic analyses. While it may seem less straightforward to determine functional interactions at the molecular level for several variants spanning many kbp within SLC6A4, haplotypic analyses in additional OCD collections and in other, also presumably serotonin-linked disorders, now seemed warranted. These results may also help to stimulate further study of recently developed mouse and rat models with altered Slc6a4 expression and function (Homberg et al., 2007; Olivier et al., 2008a; Olivier et al., 2008b).
Affective Disorders: Major Depressive Disorder, Recurrent Depression, Bipolar Disorder, Suicide
The different affective disorders, plus a separate complex behavior, suicide, have been the object of many association studies with SLC6A4, as well as several genome-wide scans - that is, testing the chosen trait for linkage to polymorphic markers spread throughout the genome. The latter have been principally focused on bipolar disorder and recurrent affective disorder (Anguelova et al., 2003). Although reliable diagnoses can be made of these disorders based on strong rater agreement, boundary problems arise because of overlapping phenotypes, including some extending towards psychosis (e.g., schizoaffective disorder, psychotic depression) as well as with other “primary” neuropsychiatric disorders such as OCD. For example, probands ascertained as bipolar have ∼20% coexisting OCD, whereas studies of individuals with a primary diagnosis of OCD reveal a ∼10% incidence of comorbid bipolar disorder (LaSalle et al., 2004).
In addition to phenotypic diversity, evidence of genetic heterogeneity has been commented on in several studies of bipolar disorder (Anguelova et al., 2003). Gene-environment interactions are another source of complexity. A landmark study - one of the first to tackle this question of gene-environment complexity epidemiologically in humans - evaluated >800 individuals who had been followed since age three. The hypothesized interaction of 5HTTLPR genotypes and of recent traumatic life events upon depression diagnosis and other depression-related problems like suicide was confirmed: more depressive events occurred in individuals who had the SS genotype and who had four or more recent life traumas, whereas those with the LL genotype were protected (Caspi et al., 2003). One interpretation discussed in several subsequent studies and reviews suggested the possibility that 5HTTLPR may not be directly associated with depression or suicide, but may rather modulate the serotonergic response to life stress (Grabe et al., 2005; Kutcher and Gardner, 2008; Roy et al., 2007; Uher and McGuffin, 2008); this interpretation was derived from (1) recent brain imaging findings of exaggerated amygdala and startle responses to fearful stimuli in individuals with the S 5HTTLPR allele and in SERT-deficient mice (Bertolino et al., 2005; Brocke et al., 2006; Hariri et al., 2002; Holmes, in press; Pezawas et al., 2005; Rhodes et al., 2007; Wellman et al., 2007); and (2) exaggerated ACTH and epinephrine and other neuroendocrine responses to handling or restraint in Slc6a4 +/- or Slc6a4 -/- mice (Caspi et al., 2003; Hariri et al., 2002; Murphy et al., 2001; Tjurmina et al., 2002).
The dependence of a strong association of depression with SLC6A4 upon the environmental influence of severe life-events illustrates a principle known from murine genetic models, including specific studies of SERT-stress interactions (Murphy et al., 2001; Tjurmina et al., 2002), namely that gene effects in complex human disorders may be identifiable and prove etiologically important only when coupled with additional factors (Murphy and Lesch, 2008; Murphy et al., 2003a). As mentioned above and depicted in Figs. 5 and 15, interactions among two or more SERT and related gene variants such as those in BDNF may also yield greater SERT expression and function changes as well as more extreme clinical phenotypes (Deltheil et al., 2008; Kilic et al., 2003; Ozaki et al., 2003; Pezawas et al., 2008).
Primary Pulmonary Hypertension
The progressive and frequently fatal disorder primary pulmonary hypertension (PPH) is characterized by local blood pressure increases associated with abnormal vascular proliferation in the pulmonary artery bed. Serotonin is a potent inducer of smooth muscle cell proliferation in lung vessels, an effect that is attributable to serotonin's cellular internalization via SERT, as it is dose-dependently inhibited by SRIs but not by serotonin receptor antagonists (Eddahibi et al., 2001). Iatrogenic pulmonary hypertension has been identified during the last decade as the result of treatment of obesity by fenfluramine alone or together with phentermine (Abenhaim et al., 1996; Connolly et al., 1997). Fenfluramine is a SERT substrate that also releases serotonin from vesicles once it enters cells via SERT (Schuldiner et al., 1993). Individuals with spontaneous PPH more frequently have the 5HTTLPR LL genotype together with greater SERT mRNA and immunoreactivity in lung tissue as well as greater uptake of serotonin in platelets (Eddahibi et al., 2001). Hypoxia can lead to smooth muscle hyperplasia. Hypoxia leads to increased expression of SERT in lung vessels; a causative role for hypoxia in pulmonary hypertension has been inferred from evidence that Slc6a4 -/- mice are protected against hypoxia-induced pulmonary hypertension (Eddahibi et al., 1999; Eddahibi et al., 2000). Unlike PPH patients, hypoxic patients with chronic obstructive pulmonary disease (COPD) do not differ from controls in 5HTTLPR genotype frequencies; however, the LL variant of 5HTTLPR in COPD patients, who have higher SERT expression in pulmonary artery cells, had significantly more severe pulmonary hypertension than did LS or SS patients, a finding which is in accord with findings in rodent models (Eddahibi et al., 2003; Eddahibi et al., 2000). In addition, a complementary, epistatic interaction has been proposed between this 5HTTLPR genotype and a common bone morphogenetic protein 2 receptor (BMPR2) variant, which is also involved in smooth muscle cell proliferation and has additionally been implicated in familial PPH (Rabinovitch, 2001). These results from both humans and rodents raise the question of whether SRIs might constitute an experimental treatment for PPH.
Myocardial Infarction
Two studies, which together enrolled 976 individuals with myocardial infarction (MI) and 926 controls, found significantly greater risk for MI in individuals with the 5HTTLPR LL genotype (Coto et al., 2003; Fumeron et al., 2002). Remarkable complementary evidence from a study of over 5,000 individuals with MIs receiving antidepressants with high SERT affinity revealed a protective effect of these drugs (a highly significant odds ratio of 0.59) (Sauer et al., 2003). Furthermore, increased SERT affinity among the different SRIs was correlated with reduced risk of MI in these SRI-treated patients (Sauer et al., 2003). Potential interactions with smoking history, platelet aggregation, plasma lipids including cholesterol, depression, anxiety and other variables like cardiovascular reactivity may also be involved (Comings et al., 1999; Coto et al., 2003; Fumeron et al., 2002; McCaffery et al., 2003; Williams et al., 2001).
Irritable Bowel Syndrome (IBS) and its Treatment
Gut tissue has, by far, the greatest (95%) amount of serotonin in the body. Serotonin participates in the local regulation of gut function. Its release from mucosal enterocytes initiates peristaltic and secretory reflexes, whereas its release from enteric neurons mediates fast and slow excitatory neurotransmission. SERT helps to regulate the activity of serotonin in the bowel via SERT's presence in several cell types, including crypt and villus epithelial cells (enterocytes) and enteric serotonergic neurons. Both in the mucosa and in the enteric nervous system, SERT terminates serotonin's actions (Gershon, 1999).
In three case-control studies of IBS, the 5HTTLPR SS genotype was significantly more frequent in diarrhea-predominant subgroups, although a large metanalysis of associations between the originally-described SLC6A4 variant and all types of IBS in different ethnicities was negative (Van Kerkhoven et al., 2007). A subsequent, in-depth review of IBS subtypes and genetic as well as environmental contributions to the disorder pointed out the many complexities in examining relationships between the multiple genetic and other variables that may contribute to IBS and similar GI diseases (Colucci et al., 2008). IBS treatment responses (reductions in colonic transit time) to the 5-HT3 receptor antagonist, alosetron, were significantly more prevalent in diarrhea-predominant patients with the LL genotype (Camilleri et al., 2002). Of related interest, Slc6a4 -/- mice have increased gut motility together with frequent diarrhea alternating with occasional constipation in an IBS-like clinical syndrome (Chen et al., 2001). Adaptive changes occur in the subunit composition of enteric 5-HT3 receptors of Slc6a4 -/- mice. Expression of the 5-HT3B subunit is decreased and this is accompanied by a decrease in the affinity of 5-HT3 receptors for 5-HT and a greater tendency for 5-HT3 receptors to be desensitized. These changes are protective against an environment of excessive 5-HT availability (Liu et al., 2002).
Other Studies
Linkage has been observed to 17q regions containing SLC6A4, the SERT locus in genome-wide scans of DNA collected from individuals with various disorders including bipolar disorder (Dick et al., 2003; Liu et al., 2003; Murphy et al., 2000); ADHD (Fisher et al., 2002; Kent et al., 2002); Tourette's syndrome (Zhang et al., 2002), and autism (IMGSAC, 2001; Yonan et al., 2003). In addition, highly relevant investigations to SLC6A4 are being conducted of potential disease-related quantitative traits – also termed endophenotypes – which include biochemical, endocrinological, or other physiological and psychological measurements that are studied in human populations. These endophenotypes may interact with other vulnerability factors, sometimes with gender and ethnicity differences (Weese-Mayer et al., 2003) that contribute to specific disorders. Some examples include personality characteristics such as anxiety, neuroticism, and harm avoidance (Lesch et al., 1996; Mazzanti et al., 1998), fear conditioning (Garpenstrand et al., 2001), and exaggerated amygdala or cardiovascular responsivity to stressful and other stimuli (Hariri et al., 2002; Schmidt et al., 2000; Williams et al., 2001).
Furthermore, results from investigations of Slc6a4 +/- and -/- mice suggest that SERT differences may contribute to additional genetically complex disorders including polysubstance abuse (Bengel et al., 1998; Lesch and Murphy, 2003; Sora et al., 2001; Sora et al., 1999), REM sleep disorders (Wisor et al., 2003), obesity (Holmes et al., 2003d), gastro-intestinal disorders in addition to IBS (Chen et al., 2001), pain-related disorders (Vogel et al., 2003), and anxiety disorders (Gingrich and Hen, 2001; Holmes et al., 2003b; Holmes et al., 2003c; Jacob et al., 2004; Murphy et al., 2001; Murphy et al., 2003a). A general principle underlying all of these proposals of SLC6A4 as a genetically vulnerability factor for multiple CNS and peripheral disorders is that genetic variants of SERT may act both in the brain and in specific organs like heart, lung, gut, and adrenal gland plus other endocrine and immune systems during development in particular and in some instances, continuing during adulthood (Meredith et al., 2005a; Meredith et al., 2005b; Persico et al., 2001; Yavarone et al., 1993).
A Role for SERT in SRI Pharmacogenetics, Therapeutic Responses, and Major Side Effects
The serotonin reuptake inhibitors (SRIs) include fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram and escitalopram, all of which are highly potent (Ki ∼1 nM) and selective inhibitors of SERT's function at presynaptic terminals, axons, dendrites, neuronal cell bodies and other cells that express SERT. Blockade of SERT by SRIs under normal circumstances has minimal immediate consequences for physiological or behavioral endpoints in humans. Repeated, long-term administration for three to six weeks is required for antidepressant actions and most other beneficial effects, while treatment for ten-to-twelve weeks or longer is required for efficacy in obsessive-compulsive disorder. It appears that slowly-developing adaptations in receptor and post-receptor neuronal signaling, responses to multiple neurotrophins as well as plastic remodeling of brain regions such as the hippocampus via neurogenesis appear to be required for therapeutic efficacy in OCD, as in depression (Manji et al., 2003; Schmitt et al., 2007).
Among the other large class of less-selective reuptake-inhibiting tricyclic antidepressants, clomipramine has relatively greater efficacy at SERT than does desipramine or other tricyclics. Although depression and most anxiety disorders respond equally well to all tricyclics, SRIs, SSRIs and SNRIs, treatment efficacy in OCD and autism appears restricted to the SRIs or to clomipramine (Blier and de Montigny, 1998; McDougle et al., 1998).
SRI Drug Metabolism
Most investigations of the role of genes in individual differences in drug response have focused on pathways involved in drug metabolism (Weinshilboum, 2003; Weinshilboum and Wang, 2004). All tricyclic antidepressants and most SRIs (fluoxetine, fluvoxamine, paroxetine, and desmethylcitalopram, but not citalopram) are substrates of the drug-metabolizing enzyme, CYP2D6; some of these and other SRIs are metabolized by CYP3A4 (e.g., sertraline and norfluoxetine) or CYP1A2 (fluvoxamine). Variants in the genes that encode these enzymes occur and can result in ultrarapid or slow metabolism of these drugs in 7–10% of Caucasians, and in different proportions in other ethnic groups, with resultant drug plasma level differences in patients (Steimer et al., 2001). Microarray-based gene chips are now available to evaluate genotypes but have not yet been exploited in clinical trials (de Leon et al., 2006).
Drug-metabolizing variability among patients is of special clinical concern because tricyclic antidepressants have a relatively narrow therapeutic index and exhibit significantly increased toxicity at elevated drug concentrations. In contrast, SRIs have a flat dose-response curve and wide therapeutic index, and hence toxicity due to excessive drug concentrations seems to be uncommon. However, the 1-10% of Caucasians who are CYP2D6 specific ultra-rapid metabolizers would seem to be at some risk for SRI under-dosing (Dahl et al., 1995; Steimer et al., 2001). In addition, both SRI and tricyclic drug metabolism may be affected by drug-drug interactions due to the large number of agents that are substrates of these hepatic enzymes, as recently documented in an epidemiologic study (Preskorn et al., 2007). Of course, many independent non-gene factors including gender, age, body fat, disease, and time of administration as well as ethnic differences may affect drug concentrations in plasma and tissues, including brain (Fig. 23).
Figure 23. SERT and Other Putative Serotonin-Related Pharmacogenetic Interactions in Humans and Mice.
Genes studied include SLC6A4 (SERT), Environmenral Factors include traumetic life events (TLE). Other medications such as Monoamine Oxidase Inhibitor (MAOI), Tryptophan (TP), 5-hydroxytryptophan (5-HTP), Lysergic Acid (LSD), Methylenedioxymethamphetamine (MDMA), which can lead to the serotonin syndrome (S/S).
SERT and SRI Pharmacogenetics
Associations between the SERT 5HTTLPR polymorphism and both therapeutic responses and adverse reactions to SRIs have been reported (Binder and Holsboer, 2006). Thirteen out of eighteen investigations, which enrolled a total of ∼2100 individuals, found the 5HTTLPR SS genotype or S allele predictive of reduced antidepressant efficacy; other studies found greater numbers and a greater variety of side effects during SRI treatment in S allele or SS genotype individuals (Murphy et al., 2004b; Perlis et al., 2003; Popp et al., 2006; Putzhammer et al., 2005; Smits et al., 2007). Two meta-analyses evaluated possible relationships between antidepressant responses to SRIs and the SERT allelic 5HTTLPR. The first one collected nineteen reports in the literature as of 2006 (Murphy et al., 2006). When inclusion criteria such as sufficient statistical information, primary diagnoses of major depressive disorder (MDD, DSM criteria), and a treatment response of >50% reduction from basal MDD symptoms, were applied, ten studies with a total of 719 patients were included in the meta-analysis. An odds ratio was calculated comparing responding versus non-responding patients with the LL genotype versus those with an S allele (SS and SL genotypes), based on our prior data available at the time that S was the dominant allele (Lesch et al., 1996). A fixed effects model, with effect sizes weighted by sample size (Cooper and Hedges, 1994), yielded a weighted effect size of 2.09 ± 1.02 (p = 0.002), indicating that individuals with the LL genotype were predominantly in the responder groups across these studies (Fig. 24). A secondary analysis revealed that all of the studies with an odds ratio <1 included mostly Asian subjects, and that in these three studies (Kato et al., 2005; Kim et al., 2000; Yoshida et al., 2002) the percentage of individuals with LL genotypes averaged 7% (range 4-11%). In the investigations that utilized predominantly Caucasian samples (Arias et al., 2003; Durham et al., 2004; Joyce et al., 2003; Pollock et al., 2000), the percentage of individuals with LL genotypes was 26% (range 16-37%). This suggests that a biasing effect related to ethnicity may have influenced the results of this meta-analysis (Murphy et al., 2006).
Figure 24. Metanalysis of Ten Studies Examining Antidepressant Responses to SRIs and SERT 5HTTLPR Polymorphism.
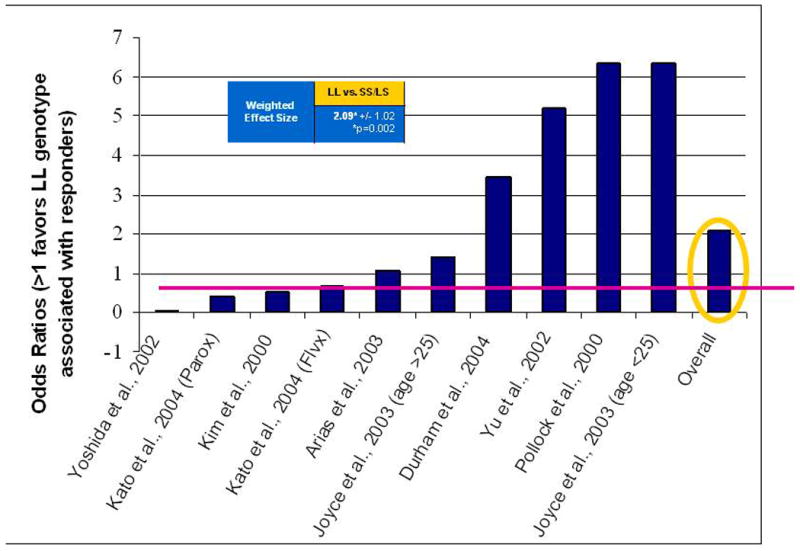
The second meta-analysis, utilized the same investigations as those reported on in the initial meta-analysis, but added additional subjects and data not available for the first meta-analysis (Serretti et al., 2006). These data came from two sources: primarily this group's own treatment studies (n = 4) and a few reports that did not meet inclusion criteria for the previous meta-analysis conducted by our group (n = 3) (Murphy et al., 2006). In this additional, larger report, 15 different sub-analyses were conducted based on “remission”, “response” or “responses within 4 weeks” for different combinations of genotypes and ethnicities (Asian or Caucasians) (Serretti et al., 2006). “Response” rate was most robust for LL genotype patients (OR = 2.01); this comparison was based on the largest number of studies (N = 10) included in the different sub-analyses. Thus both meta-analyses concur in finding the S allele associated with poorer antidepressant responses and best responses found in those with the LL genotype (Murphy et al., 2006; Serretti et al., 2006).
Curiously, in contrast to these results in depressed patients, two studies of responses to SRIs in patients with OCD did not observe associations between the SERT 5HTTLPR genotypes and therapeutic responses (Billett et al., 1997; Di Bella et al., 2002). Whether or how this might relate to reports of more frequent LL and other higher-expressing genotypes in OCD or to the fact that patients with OCD only respond to SRI antidepressants, whereas depressed patients respond to all types of antidepressants, including norepinephrine reuptake inhibitors and MAO inhibitors, remains to be evaluated (Bengel et al., 1999; McDougle et al., 1998; Wendland et al., 2008b). Of interest, Slc6a4 +/- and -/- mice failed to reliably demonstrate the usual “antidepressant responses” in helplessness-related behaviors (e.g., immobility in a swim test), although responses to other non-SRI antidepressants were unchanged (Holmes et al., 2002c) (Fig. 25). One explanation about why humans with the SS genotype and mice with lesser-expressing SERT have deficient responses to antidepressants is that a true “floor” effect may be responsible - that is, further SRI-dependent inhibition of SERT activity might not be biologically meaningful or adequate.
Figure 25. Lack of Behavioral Immobility Response to Fluoxetine in SERT -/- Mice (C57BL/6J background), but Intact or Exaggerated Responses to Tricyclic Antidepressants.
Regarding adverse reactions observed during SRI treatment of depression, a 3.5-fold greater incidence of insomnia and a 9.5-fold greater incidence of agitation but not other side effects including nausea or headache were found during treatment with fluoxetine in individuals with the SS 5HTTLPR genotype compared to SL or LL individuals (Perlis et al., 2003) (Fig. 26). Similarly, a 5.3-fold higher incidence of hypomania as a side effect occurred in bipolar patients receiving “serotonergic” (mostly SRI) antidepressants who possessed the SS genotype versus the other genotypes (Mundo et al., 2001) (Fig. 26). A subsequent study found an association between the SS genotype and a rapid cycling bipolar course of illness (four or more episodes per year) in individuals in an SRI study, but not a direct association between the SS genotype and hypomania, raising the question as to whether the original finding was reflective of a clinical subgroup rather than a pharmacogenetic phenotype (Rousseva et al., 2003). The largest study to date found that intolerance to antidepressant treatment with citalopram was strongly associated with the 5HTTLPR S allele and SS genotype; therapeutic responses were not different in those with the different 5HTTLPR alleles, including rs25531 (Hu et al., 2007). Suicide attempts, which represent a controversial possible side effect of SRIs, were found to be significantly associated with the 5HTTLPR SS genotype and S allele as well as the SLC6A4 STin2 shorter [‘10’] allele in a recent large review and several subsequent studies, including some showing interactive effects with prior traumatic life events (Anguelova et al., 2003; Caspi et al., 2003; Kalin et al., 2008; Lin and Tsai, 2004; Lopez de Lara et al., 2006; Roy et al., 2007). If further studies continue to support these treatment and side effect associations, 5HTTLPR genotyping may prove to be clinically important in avoiding lengthy treatment trials with one and then a second SRI (as some current guidelines for antidepressant treatment with SRIs suggest) before considering another drug class or treatment modality (Trivedi et al., 2006). One conjecture about mechanisms underlying both the possible enhanced susceptibility to hypomania and to impulsive, violent suicide is that serotonergic modulation is diminished in SS individuals, leading to unrestrained activation, perhaps via catecholamine influences, as suggested some years ago for “switches” into mania (Bunney et al., 1972; Kamali et al., 2001; Murphy et al., 1971).
Figure 26. Antidepressant Response and Side Effects: Associations with SERT 5HTTLPR.
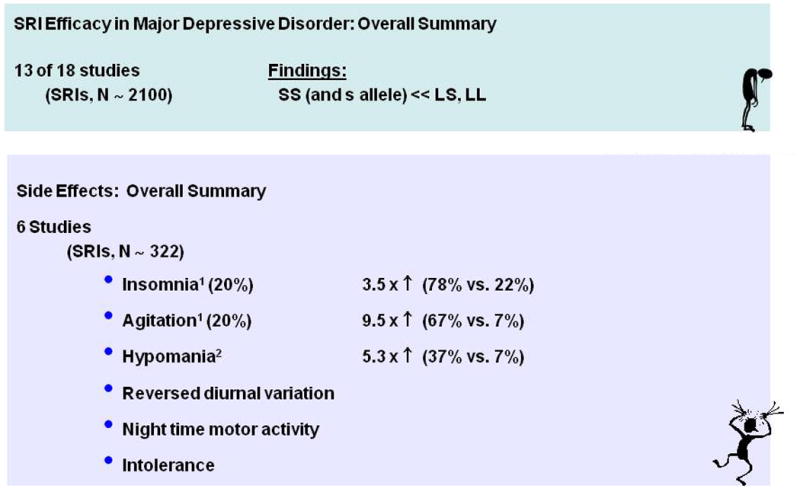
In addition to the SLC6A4 polymorphism-SRI interactions, a single nucleotide polymorphism in the 5-HT2A receptor (T102C variant), one of the 14-plus post-synaptic serotonin receptors at which increased serotonin concentrations produced by SRI administration acts, has also been found to be strongly associated with intolerance to paroxetine (Murphy et al., 2003b). Another very large study also implicated the 5-HT2A receptor in response to SRIs (McMahon et al., 2006). Subjects with the T102C 5-HT2A variant discontinued paroxetine early during the double-blind study and exhibited significantly greater side effect severity (Murphy et al., 2003b). SLC6A4 genotyping was not reported in these studies, and other possible interactions between SLC6A4 variants and the above-mentioned 5-HT2A receptor gene variant have not yet been published.
Additional considerations are that in humans, as has been shown in mice, one or more genes interact with SERT alleles to yield altered neurochemistry and behavior. Studies of such epistatic involvement have only recently begun and include investigations of genes that encode DAT, NET, MAOA and BDNF, all of which have functional variants in humans (Murphy et al., 2003a). For example, it is plausible that uncommon side effects such as the serotonin syndrome - which includes dilated pupils, facial flushing, diaphoresis, shivering, myoclonic jerks, and tremors - or reactions to tyramine-containing products, or even other toxically mediated behaviors observed in SRI or MAOI-treated individuals might represent specific consequences of individual SLC6A4 × functional MAOA gene interactions (Insel et al., 1982; Murphy, 1977).
Conclusions
Elevations in extracellular serotonin occur in gene dose-proportionate ratios in SERT-deficient mice and these provide the basis for virtually all of the extensive pleiotropic phenotypic changes discovered in these mice. Secondary changes in central and peripheral serotonin system homeostasis as evaluated in brain, gut, and neuroendocrine functions are all in keeping with predictions from prior pharmacologic investigations and from some studies of gene-targeted serotonin receptor mice (Berger and Tecot, 2006).
In summary, SERT-deficient mice constitute a behaviorally inhibited mouse, underexploratory, underactive, underaggressive, harm avoidant and “anxious”. At the same time, they are over-sensitive to mild to moderate stimulation, with exaggerated neuroendocrine (ACTH, catecholamine) and behavioral (startle) responses and they are liable to gut dysfunction (IBS), bone weakness and substance abuse, including being alcohol and cocaine-preferring (Fig. 27). Thus, they represent a model for not just any one human genetic disease, but rather a single gene pleiotropy model for multiply-comorbid, complex disorders of modern western civilization, all related to serotonin.
Figure 27. Phenotypical Consequences of the Targeted Disruption of SERT in the Mouse.
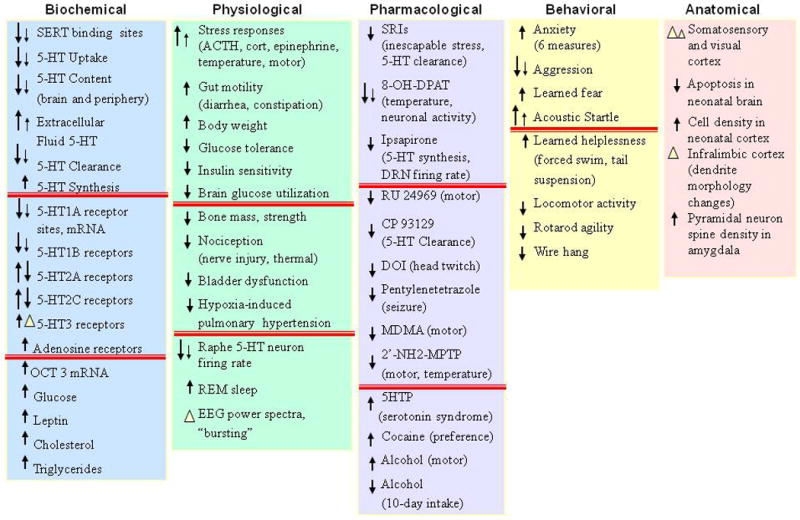
Likewise, secondary changes in serotonin receptor regulation might well have been anticipated from pharmacologic and gene-targeting studies, such as the major 5-HT1A alterations and their associations with an anxiety-like behavioral phenotype found in mice with deleted 5-HT1A receptors (Gross et al., 2000). Others, such as the 5-HT2A/2C signaling and behavioral changes associated with the arachidonic acid pathway were unanticipated.
Similarly, the phenotypic abnormalities associated with human SLC6A4 have been partially anticipated from prior knowledge of the multiple roles for serotonin found in earlier physiologic and pharmacologic studies, but the extent of these associations now documented in over four hundred papers (mostly investigating the originally-reported 5HTTLPR polymorphism (Lesch et al., 1996) was unanticipated. The strong relationship of this polymorphism to treatment responses to serotonin reuptake inhibitors in humans and the related susceptibility of Slc6a4-deficient mice to fail to respond to SRIs and to develop serotonin syndrome abnormal behaviors represent additional new illustrations of gene-based influences on serotonergic pharmacologic responses – a new SERT pharmacogenomics.
In the future, more extensive genotyping of SLC6A4 taking into account the newly discovered variants within the gene and its 5′, 3′ and intronic regions as well as the use of gene chips beyond the already available chips targeted exclusively to the CYP450 system may become possible (de Leon et al., 2006; Kaddurah-Daouk et al., 2008; Perlis, 2007; Sjoqvist and Eliasson, 2007). More extensive ascertainment of life stresses and other environmental factors that may contribute increased vulnerability to adverse events in susceptible individuals or subgroups may assist difficult decision-making in clinical practice, such as that found in the currently much-debated treatment of pediatric and adolescent patients with SRIs (Caspi et al., 2003; Wooltorton, 2003) (Fig. 23). It is also possible that correct evaluation of the association between side effects including suicide attempts in SRI-treated subgroups may depend upon knowing the frequency of especially vulnerable individuals, such as those with certain SLC6A4 genotype variants, in each study. SLC6A4 and MAOA variants have already begun to appear in the statements provided for defendants in legal court-oriented manuscripts (Bernet et al., 2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Begaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol Psychiatry. 2003;8:646–653. doi: 10.1038/sj.mp.4001336. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28:199–207. doi: 10.1523/JNEUROSCI.3973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Arias B, Catalan R, Gasto C, Gutierrez B, Fananas L. 5-HTTLPR polymorphism of the serotonin transporter gene predicts non-remission in major depression patients treated with citalopram in a 12-weeks follow up study. J Clin Psychopharmacol. 2003;23:563–567. doi: 10.1097/01.jcp.0000095350.32154.73. [DOI] [PubMed] [Google Scholar]
- Armando I, Tjurmina OA, Li Q, Murphy DL, Saavedra JM. The serotonin transporter is required for stress-evoked increases in adrenal catecholamine synthesis and angiotensin II AT(2) receptor expression. Neuroendocrinology. 2003;78:217–225. doi: 10.1159/000073705. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Gothert M. Serotoninergic Neurons and 5-HT Receptors in the CNS. Springer; 2000. [Google Scholar]
- Bengel D, Greenberg BD, Cora-Locatelli G, Altemus M, Heils A, Li Q, Murphy DL. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Mol Psychiatry. 1999;4:463–466. doi: 10.1038/sj.mp.4000550. [DOI] [PubMed] [Google Scholar]
- Bengel D, Johren O, Andrews AM, Heils A, Mossner R, Sanvitto GL, Saavedra JM, Lesch KP, Murphy DL. Cellular localization and expression of the serotonin transporter in mouse brain. Brain Res. 1997;778:338–345. doi: 10.1016/s0006-8993(97)01080-9. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Osher Y, Kotler M, Gritsenko I, Nemanov L, Belmaker RH, Ebstein RP. Association between tridimensional personality questionnaire (TPQ) traits and three functional polymorphisms: dopamine receptor D4 (DRD4), serotonin transporter promoter region (5-HTTLPR) and catechol O-methyltransferase (COMT) Mol Psychiatry. 2000a;5:96–100. doi: 10.1038/sj.mp.4000640. [DOI] [PubMed] [Google Scholar]
- Benjamin J, Osher Y, Lichtenberg P, Bachner-Melman R, Gritsenko I, Kotler M, Belmaker RH, Valsky V, Drendel M, Ebstein RP. An interaction between the catechol O-methyltransferase and serotonin transporter promoter region polymorphisms contributes to tridimensional personality questionnaire persistence scores in normal subjects. Neuropsychobiology. 2000b;41:48–53. doi: 10.1159/000026632. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Berger M, Tecot LH. Serotonin System Gene Knockouts. A story of mice with implications for man. In: Roth BL, editor. The Receptors: The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics. Humana Press, Inc.; Totowa, NJ: 2006. pp. 537–561. [Google Scholar]
- Bernet W, Vnencak-Jones CL, Farahany N, Montgomery SA. Bad nature, bad nurture, and testimony regarding MAOA and SLC6A4 genotyping at murder trials. J Forensic Sci. 2007;52:1362–1371. doi: 10.1111/j.1556-4029.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri A, Kolachana B, Nardini M, Weinberger DR, Scarabino T. Variation of human amygdala response during threatening stimuli as a function of 5′HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Bill DJ, Knight M, Forster EA, Fletcher A. Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br J Pharmacol. 1991;103:1857–1864. doi: 10.1111/j.1476-5381.1991.tb12342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billett EA, Richter MA, King N, Heils A, Lesch KP, Kennedy JL. Obsessive compulsive disorder, response to serotonin reuptake inhibitors and the serotonin transporter gene. Mol Psychiatry. 1997;2:403–406. doi: 10.1038/sj.mp.4000257. [DOI] [PubMed] [Google Scholar]
- Binder EB, Holsboer F. Pharmacogenomics and antidepressant drugs. Ann Med. 2006;38:82–94. doi: 10.1080/07853890600551045. [DOI] [PubMed] [Google Scholar]
- Blakely RD. Physiological genomics of antidepressant targets: keeping the periphery in mind. J Neurosci. 2001;21:8319–8323. doi: 10.1523/JNEUROSCI.21-21-08319.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. Biol Psychiatry. 1998;44:313–323. doi: 10.1016/s0006-3223(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Bliziotes M, Gunness M, Eshleman A, Wiren K. The role of dopamine and serotonin in regulating bone mass and strength: studies on dopamine and serotonin transporter null mice. J Musculoskelet Neuronal Interact. 2002;2:291–295. [PubMed] [Google Scholar]
- Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–2212. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–1965. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Brocke B, Armbruster D, Muller J, Hensch T, Jacob CP, Lesch KP, Kirschbaum C, Strobel A. Serotonin transporter gene variation impacts innate fear processing: Acoustic startle response and emotional startle. Mol Psychiatry. 2006;11:1106–1112. doi: 10.1038/sj.mp.4001908. [DOI] [PubMed] [Google Scholar]
- Bunney WE, Jr, Goodwin FK, Murphy DL. The “switch process” in manic-depressive illness. 3. Theoretical implications. Arch Gen Psychiatry. 1972;27:312–317. doi: 10.1001/archpsyc.1972.01750270022003. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, McKinzie S, Urrutia R. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, Frazzetto G, Pascucci T, Audero E, Puglisi-Allegra S, Cabib S, Lesch KP, Gross C. Identifying molecular substrates in a mouse model of the serotonin transporter × environment risk factor for anxiety and depression. Biol Psychiatry. 2008;63:840–846. doi: 10.1016/j.biopsych.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A. Effects of Mild Early Life Stress on Abnormal Emotion-related Behaviors in 5-HTT Knockout Mice. Behav Genet. 2007;37:214–222. doi: 10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cavallini MC, Di Bella D, Pasquale L, Henin M, Bellodi L. 5HT2C CYS23/SER23 polymorphism is not associated with obsessive-compulsive disorder. Psychiatry Res. 1998;77:97–104. doi: 10.1016/s0165-1781(97)00151-0. [DOI] [PubMed] [Google Scholar]
- Chabane N, Millet B, Delorme R, Lichtermann D, Mathieu F, Laplanche JL, Roy I, Mouren MC, Hankard R, Maier W, Launay JM, Leboyer M. Lack of evidence for association between serotonin transporter gene (5-HTTLPR) and obsessive-compulsive disorder by case control and family association study in humans. Neurosci Lett. 2004;363:154–156. doi: 10.1016/j.neulet.2004.03.065. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci R, Blandizzi C, Bellini M, Ghisu N, Tonini M, Del Tacca M. The genetics of the serotonin transporter and irritable bowel syndrome. Trends Mol Med. 2008;14:295–304. doi: 10.1016/j.molmed.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Comings DE, MacMurray JP, Gonzalez N, Ferry L, Peters WR. Association of the serotonin transporter gene with serum cholesterol levels and heart disease. Mol Genet Metab. 1999;67:248–253. doi: 10.1006/mgme.1999.2870. [DOI] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Conroy J, Meally E, Kearney G, Fitzgerald M, Gill M, Gallagher L. Serotonin transporter gene and autism: a haplotype analysis in an Irish autistic population. Mol Psychiatry. 2004 doi: 10.1038/sj.mp.4001459. [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges LV. The Handbook of Research Synthesis. Russell Sage Foundation; New York: 1994. [Google Scholar]
- Cornelissen LL, Brooks DP, Wibberley A. Female, but not male, serotonin reuptake transporter (5-HTT) knockout mice exhibit bladder instability. Auton Neurosci. 2005;122:107–110. doi: 10.1016/j.autneu.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cortes R, Soriano E, Pazos A, Probst A, Palacios JM. Autoradiography of antidepressant binding sites in the human brain: localization using [3H]imipramine and [3H]paroxetine. Neuroscience. 1988;27:473–496. doi: 10.1016/0306-4522(88)90282-5. [DOI] [PubMed] [Google Scholar]
- Coto E, Reguero JR, Alvarez V, Morales B, Batalla A, Gonzalez P, Martin M, Garcia-Castro M, Iglesias-Cubero G, Cortina A. 5-Hydroxytryptamine 5-HT2A receptor and 5-hydroxytryptamine transporter polymorphisms in acute myocardial infarction. Clin Sci (Lond) 2003;104:241–245. doi: 10.1042/CS20020246. [DOI] [PubMed] [Google Scholar]
- Dahl ML, Johansson I, Bertilsson L, Ingelman-Sundberg M, Sjoqvist F. Ultrarapid hydroxylation of debrisoquine in a Swedish population. Analysis of the molecular genetic basis. J Pharmacol Exp Ther. 1995;274:516–520. [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Susce MT, Murray-Carmichael E. The AmpliChip CYP450 genotyping test: Integrating a new clinical tool. Mol Diagn Ther. 2006;10:135–151. doi: 10.1007/BF03256453. [DOI] [PubMed] [Google Scholar]
- Delorme R, Betancur C, Wagner M, Krebs MO, Gorwood P, Pearl P, Nygren G, Durand CM, Buhtz F, Pickering P, Melke J, Ruhrmann S, Anckarsater H, Chabane N, Kipman A, Reck C, Millet B, Roy I, Mouren-Simeoni MC, Maier W, Rastam M, Gillberg C, Leboyer M, Bourgeron T. Support for the association between the rare functional variant I425V of the serotonin transporter gene and susceptibility to obsessive compulsive disorder. Mol Psychiatry. 2005 doi: 10.1038/sj.mp.4001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Guilloux JP, Nicolas L, Delomenie C, Reperant C, Le Maitre E, Leroux-Nicollet I, Benmansour S, Coudore F, David DJ, Gardier AM. Consequences of changes in BDNF levels on serotonin neurotransmission, 5-HT transporter expression and function: studies in adult mice hippocampus. Pharmacol Biochem Behav. 2008;90:174–183. doi: 10.1016/j.pbb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Di Bella D, Erzegovesi S, Cavallini MC, Bellodi L. Obsessive-Compulsive Disorder, 5-HTTLPR polymorphism and treatment response. Pharmacogenomics J. 2002;2:176–181. doi: 10.1038/sj.tpj.6500090. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T, Flury L, Bowman ES, Miller MJ, Rau NL, Moe PR, Samavedy N, El-Mallakh R, Manji H, Glitz DA, Meyer ET, Smiley C, Hahn R, Widmark C, McKinney R, Sutton L, Ballas C, Grice D, Berrettini W, Byerley W, Coryell W, DePaulo R, MacKinnon DF, Gershon ES, Kelsoe JR, McMahon FJ, McInnis M, Murphy DL, Reich T, Scheftner W, Nurnberger JI., Jr Genomewide linkage analyses of bipolar disorder: a new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am J Hum Genet. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem SJ, Blackwell TL, Stone KL, Yaffe K, Haney EM, Bliziotes MM, Ensrud KE. Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167:1240–1245. doi: 10.1001/archinte.167.12.1240. [DOI] [PubMed] [Google Scholar]
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96:635–642. doi: 10.1093/qjmed/hcg109. [DOI] [PubMed] [Google Scholar]
- Durham LK, Webb SM, Milos PM, Clary CM, Seymour AB. The serotonin transporter polymorphism, 5HTTLPR, is associated with a faster response time to sertraline in an elderly population with major depressive disorder. Psychopharmacology (Berl) 2004;174:525–529. doi: 10.1007/s00213-003-1562-3. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet AS, Dartevelle P, Housset B, Hamon M, Weitzenblum E, Adnot S. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003;108:1839–1844. doi: 10.1161/01.CIR.0000091409.53101.E8. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, Adnot S. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ Res. 1999;84:329–336. doi: 10.1161/01.res.84.3.329. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest. 2000;105:1555–1562. doi: 10.1172/JCI8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Greenberg BD, Murphy DL, Goldman D. Sexually dimorphic relationship of a 5-HT2A promoter polymorphism with obsessive-compulsive disorder. Biol Psychiatry. 2001;49:385–388. doi: 10.1016/s0006-3223(00)01040-4. [DOI] [PubMed] [Google Scholar]
- Erspamer V. Pharmacology of Indolealkeylamines. Pharmacol Revs. 1954;6:425–487. [PubMed] [Google Scholar]
- Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci U S A. 2005;102:5582–5587. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Francks C, McCracken JT, McGough JJ, Marlow AJ, MacPhie IL, Newbury DF, Crawford LR, Palmer CG, Woodward JA, Del'Homme M, Cantwell DP, Nelson SF, Monaco AP, Smalley SL. A genomewide scan for loci involved in attention-deficit/hyperactivity disorder. Am J Hum Genet. 2002;70:1183–1196. doi: 10.1086/340112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. Psychopharmacology (Berl) 2007a;195:147–166. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- Fox MA, Jensen CL, Gallagher PS, Murphy DL. Receptor mediation of exaggerated responses to serotonin-enhancing drugs in serotonin transporter (SERT)-deficient mice. Neuropharmacology. 2007b;53:643–656. doi: 10.1016/j.neuropharm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fox MA, Murphy DL. Neurochemical, behavioral and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology. doi: 10.1007/s00213-008-1268-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumeron F, Betoulle D, Nicaud V, Evans A, Kee F, Ruidavets JB, Arveiler D, Luc G, Cambien F. Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Temoins de l'Infarctus du Myocarde (ECTIM) Circulation. 2002;105:2943–2945. doi: 10.1161/01.cir.0000022603.92986.99. [DOI] [PubMed] [Google Scholar]
- Garpenstrand H, Annas P, Ekblom J, Oreland L, Fredrikson M. Human fear conditioning is related to dopaminergic and serotonergic biological markers. Behav Neurosci. 2001;115:358–364. [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13 Suppl 2:15–30. [PubMed] [Google Scholar]
- Gershon MD, Chalazonitis A, Rothman TP. From neural crest to bowel: development of the enteric nervous system. J Neurobiol. 1993;24:199–214. doi: 10.1002/neu.480240207. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Payette RF, Rothman TP. Development of the enteric nervous system. Fed Proc. 1983;42:1620–1625. [PubMed] [Google Scholar]
- Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. Positron emission tomography quantification of [(11)C]-DASB binding to the human serotonin transporter: modeling strategies. J Cereb Blood Flow Metab. 2001;21:1342–1353. doi: 10.1097/00004647-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, Risch N, Freimer NB. Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes. Nat Genet. 2001;27:435–438. doi: 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Murphy DL, Lesch KP, Blier P. Modifications of the serotoninergic system in mice lacking serotonin transporters. An in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–995. [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Wood AJ, Green AR. The enhancement by lithium of the 5-HT1A mediated serotonin syndrome produced by 8-OH-DPAT in the rat: evidence for a post-synaptic mechanism. Psychopharmacology (Berl) 1986;90:488–493. doi: 10.1007/BF00174066. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, John U, Cascorbi I. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Greist JH, Jefferson JW, Kobak KA, Katzelnick DJ, Serlin RC. Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis. Arch Gen Psychiatry. 1995;52:53–60. doi: 10.1001/archpsyc.1995.03950130053006. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD. Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex disorders. Pharmacogenomics J. 2002;2:217–235. doi: 10.1038/sj.tpj.6500106. [DOI] [PubMed] [Google Scholar]
- Haney EM, Chan BK, Diem SJ, Ensrud KE, Cauley JA, Barrett-Connor E, Orwoll E, Bliziotes MM. Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med. 2007;167:1246–1251. doi: 10.1001/archinte.167.12.1246. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, Leventhal BL, Cook EH., Jr Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. Am J Med Genet. 2002;114:541–552. doi: 10.1002/ajmg.10519. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998;83:1185–1201. doi: 10.1016/s0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hemmings SM, Kinnear CJ, Niehaus DJ, Moolman-Smook JC, Lochner C, Knowles JA, Corfield VA, Stein DJ. Investigating the role of dopaminergic and serotonergic candidate genes in obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2003;13:93–98. doi: 10.1016/s0924-977x(02)00129-3. [DOI] [PubMed] [Google Scholar]
- Hemmings SM, Stein DJ. The current status of association studies in obsessive-compulsive disorder. Psychiatr Clin North Am. 2006;29:411–444. doi: 10.1016/j.psc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Bio Rev. doi: 10.1016/j.neubiorev.2008.03.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003a;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 2002a;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003b;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Tjurmina O, Li Q, Tolliver T, Huang SJ, Cromer KR, Ren-Patterson RF, Crawley JN, Murphy DL. Adult-onset obesity and neurovegetative abnormalities in serotonin transporter null mutant mice. Abstr, American College of Neuropsychopharmacolgy. 2002b:29904. [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003c;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002c;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Abstract, Society of Neuroscience. New Orleans, LA: 2003d. Age-related obesity in 5-HT transporter deficient mice; p. 4071. [Google Scholar]
- Homberg JR, Olivier JD, Smits BM, Mul JD, Mudde J, Verheul M, Nieuwenhuizen OF, Cools AR, Ronken E, Cremers T, Schoffelmeer AN, Ellenbroek BA, Cuppen E. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146:1662–1676. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000;27:1719–1722. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Rush AJ, Charney D, Wilson AF, Sorant AJ, Papanicolaou GJ, Fava M, Trivedi MH, Wisniewski SR, Laje G, Paddock S, McMahon FJ, Manji H, Lipsky RH. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64:783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hwang DR, Narendran R, Sudo Y, Chatterjee R, Bae SA, Mawlawi O, Kegeles LS, Wilson AA, Kung HF, Laruelle M. Comparative evaluation in nonhuman primates of five PET radiotracers for imaging the serotonin transporters: [11C]McN 5652, [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM. J Cereb Blood Flow Metab. 2002;22:1377–1398. doi: 10.1097/01.WCB.0000040948.67415.05. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- IMGSAC. A genomewide screen for autism: strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet. 2001;69:570–581. doi: 10.1086/323264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Roy BF, Cohen RM, Murphy DL. Possible development of the serotonin syndrome in man. Am J Psychiatry. 1982;139:954–955. doi: 10.1176/ajp.139.7.954. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol. 2005;28:205–214. doi: 10.1097/01.wnf.0000177642.89888.85. [DOI] [PubMed] [Google Scholar]
- Jacob CP, Strobel A, Hohenberger K, Ringel T, Gutknecht L, Reif A, Brocke B, Lesch KP. Association between allelic variation of serotonin transporter function and neuroticism in anxious cluster C personality disorders. Am J Psychiatry. 2004;161:569–522. doi: 10.1176/appi.ajp.161.3.569. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Kenis G, Peeters F, Derom C, Vlietinck R, van Os J. Stress-related negative affectivity and genetically altered serotonin transporter function: evidence of synergism in shaping risk of depression. Arch Gen Psychiatry. 2006;63:989–996. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- Jaffre F, Callebert J, Sarre A, Etienne N, Nebigil CG, Launay JM, Maroteaux L, Monassier L. Involvement of the serotonin 5-HT2B receptor in cardiac hypertrophy linked to sympathetic stimulation: control of interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha cytokine production by ventricular fibroblasts. Circulation. 2004;110:969–974. doi: 10.1161/01.CIR.0000139856.20505.57. [DOI] [PubMed] [Google Scholar]
- Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA, Olverman HJ, Hastie ND, Harmar AJ, Shen S, Sharp T. Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci. 2006;26:8955–8964. doi: 10.1523/JNEUROSCI.5356-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce PR, Mulder RT, Luty SE, McKenzie JM, Miller AL, Rogers GR, Kennedy MA. Age-dependent antidepressant pharmacogenomics: polymorphisms of the serotonin transporter and G protein beta3 subunit as predictors of response to fluoxetine and nortriptyline. Int J Neuropsychopharmacol. 2003;6:339–346. doi: 10.1017/S1461145703003663. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Kristal BS, Weinshilboum RM. Metabolomics: a global biochemical approach to drug response and disease. Annu Rev Pharmacol Toxicol. 2008;48:653–683. doi: 10.1146/annurev.pharmtox.48.113006.094715. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007a;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Laporte JL, Murphy DL. Perspectives on genetic animal models of serotonin toxicity. Neurochem Int. 2008;52:649–658. doi: 10.1016/j.neuint.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Ren-Patterson RF, Murphy DL. The developing use of heterozygous mutant mouse models in brain monoamine transporter research. Trends Pharmacol Sci. 2007b doi: 10.1016/j.tips.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Wheaton M, Murphy DL. What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007c doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Kamali M, Oquendo MA, Mann JJ. Understanding the neurobiology of suicidal behavior. Depress Anxiety. 2001;14:164–176. doi: 10.1002/da.1062. [DOI] [PubMed] [Google Scholar]
- Kato M, Ikenaga Y, Wakeno M, Okugawa G, Nobuhara K, Fukuda T, Fukuda K, Azuma J, Kinoshita T. Controlled clinical comparison of paroxetine and fluvoxamine considering the serotonin transporter promoter polymorphism. Int Clin Psychopharmacol. 2005;20:151–156. doi: 10.1097/00004850-200505000-00005. [DOI] [PubMed] [Google Scholar]
- Kelai S, Aissi F, Lesch KP, Cohen-Salmon C, Hamon M, Lanfumey L. Alcohol intake after serotonin transporter inactivation in mice. Alcohol Alcohol. 2003;38:386–389. doi: 10.1093/alcalc/agg095. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kent L, Doerry U, Hardy E, Parmar R, Gingell K, Hawi Z, Kirley A, Lowe N, Fitzgerald M, Gill M, Craddock N. Evidence that variation at the serotonin transporter gene influences susceptibility to attention deficit hyperactivity disorder (ADHD): analysis and pooled analysis. Mol Psychiatry. 2002;7:908–912. doi: 10.1038/sj.mp.4001100. [DOI] [PubMed] [Google Scholar]
- Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol. 2003;64:440–446. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- Kim DK, Lim SW, Lee S, Sohn SE, Kim S, Hahn CG, Carroll BJ. Serotonin transporter gene polymorphism and antidepressant response. Neuroreport. 2000;11:215–219. doi: 10.1097/00001756-200001170-00042. [DOI] [PubMed] [Google Scholar]
- Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C, Holmes A, Lesch KP, Murphy DL. Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology. 2005;49:798–810. doi: 10.1016/j.neuropharm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kutcher S, Gardner DM. Use of selective serotonin reuptake inhibitors and youth suicide: making sense from a confusing story. Curr Opin Psychiatry. 2008;21:65–69. doi: 10.1097/YCO.0b013e3282f29853. [DOI] [PubMed] [Google Scholar]
- Laakso A, Palvimaki EP, Kuoppamaki M, Syvalahti E, Hietala J. Chronic citalopram and fluoxetine treatments upregulate 5-HT2c receptors in the rat choroid plexus. Neuropsychopharmacology. 1996;15:143–151. doi: 10.1016/0893-133X(95)00176-E. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Mannoury La Cour C, Froger N, Hamon M. 5-HT-HPA interactions in two models of transgenic mice relevant to major depression. Neurochem Res. 2000;25:1199–1206. doi: 10.1023/a:1007683810230. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Vanisberg MA, Maloteaux JM. Regional and subcellular localization in human brain of [3H]paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry. 1988;24:299–309. doi: 10.1016/0006-3223(88)90198-9. [DOI] [PubMed] [Google Scholar]
- LaSalle VH, Cromer KR, Nelson KN, Kazuba D, Justement L, Murphy DL. Diagnostic interview assessed neuropsychiatric disorder comorbidity in 334 individuals with obsessive-compulsive disorder. Depress Anxiety. 2004;19:163–173. doi: 10.1002/da.20009. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontegeny of the serotonergic system in the rat: Serotonin as a developmental signal. Annals New York Academy of Sciences. 1990:297–314. doi: 10.1111/j.1749-6632.1990.tb16891.x. [DOI] [PubMed] [Google Scholar]
- Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Juhasz G, Bagdy G. New Evidence for the Association of the Serotonin Transporter Gene (SLC6A4) Haplotypes, Threatening Life Events, and Depressive Phenotype. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Alcohol dependence and gene × environment interaction in emotion regulation: Is serotonin the link? Eur J Pharmacol. 2005;526:113–124. doi: 10.1016/j.ejphar.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Mossner R. Inactivation of 5HT transport in mice: modeling altered 5HT homeostasis implicated in emotional dysfunction, affective disorders, and somatic syndromes. Handb Exp Pharmacol. 2006:417–456. doi: 10.1007/3-540-29784-7_18. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Murphy DL. Molecular genetics of transporters for norepinephrine, dopamine, and serotonin in behavioral traits and complex diseases. In: Broer S, Wagner CA, editors. Membrane Transport Diseases: Molecular Basis of Inherited Transport Defects. Kluwer Academic/Plenum; New York: 2003. pp. 349–364. [Google Scholar]
- Li Q. Cellular and molecular alterations in mice with deficient and reduced serotonin transporters. Mol Neurobiol. 2006;34:51–66. doi: 10.1385/mn:34:1:51. [DOI] [PubMed] [Google Scholar]
- Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci. 2004a;24:10868–10877. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ma L, Innis RB, Seneca N, Ichise M, Huang H, Laruelle M, Murphy DL. Pharmacologic and Genetic Characterization of Two Selective Serotonin Transporter Ligands: 2-[2-(dimethylaminomethylphenylthio)]-5-fluoromethylphenylamine, (AFM) and (3-amino-4[2-(dimethylaminomethylphenylthio)] benzonitrile), (DASB) J Pharmacol Exp Ther. 2004b;308:481–486. doi: 10.1124/jpet.103.058636. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- Li Q, Wichems CH, Ma L, Van de Kar LD, Garcia F, Murphy DL. Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J Neurochem. 2003;84:1256–1265. doi: 10.1046/j.1471-4159.2003.01607.x. [DOI] [PubMed] [Google Scholar]
- Lin PY, Tsai G. Association between serotonin transporter gene promoter polymorphism and suicide: results of a meta-analysis. Biol Psychiatry. 2004;55:1023–1030. doi: 10.1016/j.biopsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Linder AE, Diaz J, Ni W, Szasz T, Burnett R, Watts SW. Vascular reactivity, 5-HT uptake, and blood pressure in the serotonin transporter knockout rat. Am J Physiol Heart Circ Physiol. 2008;294:H1745–1752. doi: 10.1152/ajpheart.91415.2007. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Liu J, Juo SH, Dewan A, Grunn A, Tong X, Brito M, Park N, Loth JE, Kanyas K, Lerer B, Endicott J, Penchaszadeh G, Knowles JA, Ott J, Gilliam TC, Baron M. Evidence for a putative bipolar disorder locus on 2p13-16 and other potential loci on 4q31, 7q34, 8q13, 9q31, 10q21-24, 13q32, 14q21 and 17q11-12. Mol Psychiatry. 2003;8:333–342. doi: 10.1038/sj.mp.4001254. [DOI] [PubMed] [Google Scholar]
- Liu MT, Rayport S, Jiang Y, Murphy DL, Gershon MD. Expression and function of 5-HT3 receptors in the enteric neurons of mice lacking the serotonin transporter. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1398–1411. doi: 10.1152/ajpgi.00203.2002. [DOI] [PubMed] [Google Scholar]
- Lopez de Lara C, Dumais A, Rouleau G, Lesage A, Dumont M, Chawky N, Alda M, Benkelfat C, Turecki G. STin2 variant and family history of suicide as significant predictors of suicide completion in major depression. Biol Psychiatry. 2006;59:114–120. doi: 10.1016/j.biopsych.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Lovejoy EA, Scott AC, Fiskerstrand CE, Bubb VJ, Quinn JP. The serotonin transporter intronic VNTR enhancer correlated with a predisposition to affective disorders has distinct regulatory elements within the domain based on the primary DNA sequence of the repeat unit. Eur J Neurosci. 2003;17:417–420. doi: 10.1046/j.1460-9568.2003.02446.x. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, N AG, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, Boni C, Hanoun N, Lesch KP, Hamon M, Lanfumey L. Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity. J Neurosci. 2001;21:2178–2185. doi: 10.1523/JNEUROSCI.21-06-02178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannoury la Cour C, Hanoun N, Melfort M, Hen R, Lesch KP, Hamon M, Lanfumey L. GABA(B) receptors in 5-HT transporter- and 5-HT1A receptor-knock-out mice: further evidence of a transduction pathway shared with 5-HT1A receptors. J Neurochem. 2004;89:886–896. doi: 10.1111/j.1471-4159.2004.02367.x. [DOI] [PubMed] [Google Scholar]
- Martin J, Cleak J, Willis-Owen SA, Flint J, Shifman S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Mol Psychiatry. 2007;12:421–422. doi: 10.1038/sj.mp.4001952. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, Virkkunen M, Linnoila M, Goldman D. Role of the serotonin transporter promoter polymorphism in anxiety-related traits. Arch Gen Psychiatry. 1998;55:936–940. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Bleil M, Pogue-Geile MF, Ferrell RE, Manuck SB. Allelic variation in the serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cardiovascular reactivity in young adult male and female twins of European-American descent. Psychosom Med. 2003;65:721–728. doi: 10.1097/01.psy.0000088585.67365.1d. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Epperson CN, Price LH, Gelernter J. Evidence for linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and obsessive-compulsive disorder. Mol Psychiatry. 1998;3:270–273. doi: 10.1038/sj.mp.4000391. [DOI] [PubMed] [Google Scholar]
- McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, Sorant AJ, Papanicolaou GJ, Laje G, Fava M, Trivedi MH, Wisniewski SR, Manji H. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, Seif I, Benhaiem-Sigaux N, Kirsch M, Hamon M, Adnot S, Eddahibi S. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation. 2006;113:81–89. doi: 10.1161/CIRCULATIONAHA.105.554667. [DOI] [PubMed] [Google Scholar]
- Meredith EJ, Chamba A, Holder MJ, Barnes NM, Gordon J. Close encounters of the monoamine kind: immune cells betray their nervous disposition. Immunology. 2005a;115:289–295. doi: 10.1111/j.1365-2567.2005.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith EJ, Holder MJ, Chamba A, Challa A, Drake-Lee A, Bunce CM, Drayson MT, Pilkington G, Blakely RD, Dyer MJ, Barnes NM, Gordon J. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: probing a potential anti-tumor target for psychotropics. FASEB J. 2005b;19:1187–1189. doi: 10.1096/fj.04-3477fje. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11C)]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- Montanez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J Neurochem. 2003;86:210–219. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Mossner R, Walitza S, Geller F, Scherag A, Gutknecht L, Jacob C, Bogusch L, Remschmidt H, Simons M, Herpertz-Dahlmann B, Fleischhaker C, Schulz E, Warnke A, Hinney A, Wewetzer C, Lesch KP. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in children and adolescents with obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2005:1–6. doi: 10.1017/S1461145705005997. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Clark TG, Roberts KH, Johnstone EC. Neuroticism mediates the association of the serotonin transporter gene with lifetime major depression. Neuropsychobiology. 2006;53:1–8. doi: 10.1159/000089915. [DOI] [PubMed] [Google Scholar]
- Mundo E, Richter MA, Zai G, Sam F, McBride J, Macciardi F, Kennedy JL. 5HT1Dbeta Receptor gene implicated in the pathogenesis of Obsessive-Compulsive Disorder: further evidence from a family-based association study. Mol Psychiatry. 2002;7:805–809. doi: 10.1038/sj.mp.4001059. [DOI] [PubMed] [Google Scholar]
- Mundo E, Walker M, Cate T, Macciardi F, Kennedy JL. The role of serotonin transporter protein gene in antidepressant-induced mania in bipolar disorder: preliminary findings. Arch Gen Psychiatry. 2001;58:539–544. doi: 10.1001/archpsyc.58.6.539. [DOI] [PubMed] [Google Scholar]
- Murakami H, Murakami S. Serotonin receptors antagonistically modulate Caenorhabditis elegans longevity. Aging Cell. 2007;6:483–488. doi: 10.1111/j.1474-9726.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- Murphy DL. The behavioral toxicity of monoamine oxidase-inhibiting antidepressants. Adv Pharmacol Chemother. 1977;14:71–105. doi: 10.1016/s1054-3589(08)60185-4. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Brodie HK, Goodwin FK, Bunney WE., Jr Regular induction of hypomania by L-dopa in “bipolar” manic-depressive patients. Nature. 1971;229:135–136. doi: 10.1038/229135a0. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Fox MA, Kalueff A, White C, Cromer K, Wendland JR. Genetic Perspectives on SRI Actions. IUPHAR; Beijing, China: 2006. [Google Scholar]
- Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004a;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nature Reviews Neuroscience. 2008;9:85–94. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Li Q, Engel S, Wichems C, Andrews A, Lesch KP, Uhl G. Genetic perspectives on the serotonin transporter. Brain Res Bull. 2001;56:487–494. doi: 10.1016/s0361-9230(01)00622-0. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Uhl GR, Holmes A, Ren-Patterson R, Hall FS, Sora I, Detera-Wadleigh S, Lesch KP. Experimental gene interaction studies with SERT mutant mice as models for human polygenic and epistatic traits and disorders. Genes Brain Behav. 2003a;2:350–364. doi: 10.1046/j.1601-1848.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr, Hollander SB, Rodrigues HE, Kremer C, Schatzberg AF. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry. 2004b;61:1163–1169. doi: 10.1001/archpsyc.61.11.1163. [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry. 2003b;160:1830–1835. doi: 10.1176/appi.ajp.160.10.1830. [DOI] [PubMed] [Google Scholar]
- Murphy VE, Mynett-Johnson LA, Claffey E, Bergin P, McAuliffe M, Kealey C, McKeon P. Search for bipolar disorder susceptibility loci: the application of a modified genome scan concentrating on gene-rich regions. Am J Med Genet. 2000;96:728–732. doi: 10.1002/1096-8628(20001204)96:6<728::aid-ajmg6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Murray CL, Lopez AD. The Global Burden of Disease. Harvard University Press; Cambridge: 1996. [Google Scholar]
- Mynett-Johnson L, Kealey C, Claffey E, Curtis D, Bouchier-Hayes L, Powell C, McKeon P. Multimarkerhaplotypes within the serotonin transporter gene suggest evidence of an association with bipolar disorder. Am J Med Genet. 2000;96:845–849. doi: 10.1002/1096-8628(20001204)96:6<845::aid-ajmg30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Nakatani D, Sato H, Sakata Y, Shiotani I, Kinjo K, Mizuno H, Shimizu M, Ito H, Koretsune Y, Hirayama A, Hori M. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am Heart J. 2005;150:652–658. doi: 10.1016/j.ahj.2005.03.062. [DOI] [PubMed] [Google Scholar]
- Neale BM, Sham PC. The future of association studies: gene-based analysis and replication. Am J Hum Genet. 2004;75:353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ, 3rd, Liang KY, LaBuda M, Walkup J, Grados M, Hoehn-Saric R. A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57:358–363. doi: 10.1001/archpsyc.57.4.358. [DOI] [PubMed] [Google Scholar]
- Nonnis Marzano F, Maldini M, Filonzi L, Lavezzi AM, Parmigiani S, Magnani C, Bevilacqua G, Matturri L. Genes regulating the serotonin metabolic pathway in the brain stem and their role in the etiopathogenesis of the sudden infant death syndrome. Genomics. 2008 doi: 10.1016/j.ygeno.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Cools AR, Olivier B, Homberg JR, Cuppen E, Ellenbroek BA. Stress-induced hyperthermia and basal body temperature are mediated by different 5-HT(1A) receptor populations: A study in SERT knockout rats. Eur J Pharmacol. 2008a;590:190–197. doi: 10.1016/j.ejphar.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Olivier JD, Van Der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, Deen PM, Cuppen E, Cools AR, Ellenbroek BA. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008b;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ, Goldman D, Arango V, Van Heertum RL, Mann JJ, Parsey RV. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 2007;64:201–208. doi: 10.1001/archpsyc.64.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:895, 933–896. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Page IH. The discovery of serotonin. Perspect Biol Med. 1976;20:1–8. doi: 10.1353/pbm.1976.0058. [DOI] [PubMed] [Google Scholar]
- Park SK, George R, Cai Y, Chang HY, Krantz DE, Friggi-Grelin F, Birman S, Hirsh J. Cell-type-specific limitation on in vivo serotonin storage following ectopic expression of the Drosophila serotonin transporter, dSERT. J Neurobiol. 2006;66:452–462. doi: 10.1002/neu.20222. [DOI] [PubMed] [Google Scholar]
- Pato MT, Zohar-Kadouch R, Zohar J, Murphy DL. Return of symptoms after discontinuation of clomipramine in patients with obsessive-compulsive disorder. Am J Psychiatry. 1988;145:1521–1525. doi: 10.1176/ajp.145.12.1521. [DOI] [PubMed] [Google Scholar]
- Pattin KA, Moore JH. Exploiting the proteome to improve the genome-wide genetic analysis of epistasis in common human diseases. Hum Genet. 2008;124:19–29. doi: 10.1007/s00439-008-0522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Andrews AM. Chronoamperometry to determine differential reductions in uptake in brain synaptosomes from serotonin transporter knockout mice. Anal Chem. 2005;77:818–826. doi: 10.1021/ac049103g. [DOI] [PubMed] [Google Scholar]
- Perlis RH. Pharmacogenetic studies of antidepressant response: how far from the clinic? Psychiatr Clin North Am. 2007;30:125–138. doi: 10.1016/j.psc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Mischoulon D, Smoller JW, Wan YJ, Lamon-Fava S, Lin KM, Rosenbaum JF, Fava M. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54:879–883. doi: 10.1016/s0006-3223(03)00424-4. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol Psychiatry. 2008;13:654, 709–616. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Pigott TA, Pato MT, Bernstein SE, Grover GN, Hill JL, Tolliver TJ, Murphy DL. Controlled comparisons of clomipramine and fluoxetine in the treatment of obsessive-compulsive disorder. Behavioral and biological results. Arch Gen Psychiatry. 1990;47:926–932. doi: 10.1001/archpsyc.1990.01810220042005. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA, Davis S, Kirshner MA, Houck PR, Stack JA, Reynolds CF, Kupfer DJ. Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology. 2000;23:587–590. doi: 10.1016/S0893-133X(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Popp J, Leucht S, Heres S, Steimer W. Serotonin transporter polymorphisms and side effects in antidepressant therapy--a pilot study. Pharmacogenomics. 2006;7:159–166. doi: 10.2217/14622416.7.2.159. [DOI] [PubMed] [Google Scholar]
- Power AC, Cowen PJ. Neuroendocrine challenge tests: assessment of 5-HT function in anxiety and depression. Mol Aspects Med. 1992;13:205–220. doi: 10.1016/0098-2997(92)90010-w. [DOI] [PubMed] [Google Scholar]
- Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, Hewlett WA, Sutcliffe JS, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S, Meyer JH. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biol Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Shah R, Neff M, Golbeck AL, Choi J. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13:5–12. doi: 10.1097/00131746-200701000-00002. [DOI] [PubMed] [Google Scholar]
- Putzhammer A, Schoeler A, Rohrmeier T, Sand P, Hajak G, Eichhammer P. Evidence of a role for the 5-HTTLPR genotype in the modulation of motor response to antidepressant treatment. Psychopharmacology (Berl) 2005;178:303–308. doi: 10.1007/s00213-004-1995-3. [DOI] [PubMed] [Google Scholar]
- Qu Y, Villacreses N, Murphy DL, Rapoport SI. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology (Berl) 2005;180:12–20. doi: 10.1007/s00213-005-2231-5. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. Linking a serotonin transporter polymorphism to vascular smooth muscle proliferation in patients with primary pulmonary hypertension. J Clin Invest. 2001;108:1109–1111. doi: 10.1172/JCI14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport MM. The discovery of serotonin. Perspect Biol Med. 1997;40:260–273. doi: 10.1353/pbm.1997.0040. [DOI] [PubMed] [Google Scholar]
- Rapport MM, Green AR, Page IH. Crystalline Serotonin. Science. 1948;108:329–330. doi: 10.1126/science.108.2804.329. [DOI] [PubMed] [Google Scholar]
- Rausch JL, Johnson ME, Corley KM, Hobby HM, Shendarkar N, Fei Y, Ganapathy V, Leibach FH. Depressed patients have higher body temperature: 5-HT transporter long promoter region effects. Neuropsychobiology. 2003;47:120–127. doi: 10.1159/000070579. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, Hu XZ, Goldman D, Reischl G, Solbach C, Machulla HJ, Bares R, Heinz A. Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. J Neural Transm. 2007;114:635–639. doi: 10.1007/s00702-006-0609-0. [DOI] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Lesch KP, Lu B, Murphy DL. Gender-Dependent Modulation of Brain Monoamines and Anxiety-like Behaviors in Mice with Genetic Serotonin Transporter and BDNF Deficiencies. Cellular and Molecular Neurobiology. 2006;26:775–780. doi: 10.1007/s10571-006-9048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Sherrill S, Huang SJ, Tolliver T, Lesch KP, Lu B, Murphy DL. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. J Neurosci Res. 2005;79:756–771. doi: 10.1002/jnr.20410. [DOI] [PubMed] [Google Scholar]
- Rhodes RA, Murthy NV, Dresner MA, Selvaraj S, Stavrakakis N, Babar S, Cowen PJ, Grasby PM. Human 5-HT transporter availability predicts amygdala reactivity in vivo. J Neurosci. 2007;27:9233–9237. doi: 10.1523/JNEUROSCI.1175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux A, Fabre V, Lesch KP, Moessner R, Murphy DL, Lanfumey L, Hamon M, Martres MP. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci Lett. 1999;262:113–116. doi: 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
- Roth BL. The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics. Humana Press; 2006. [Google Scholar]
- Rousseva A, Henry C, van den Bulke D, Fournier G, Laplanche JL, Leboyer M, Bellivier F, Aubry JM, Baud P, Boucherie M, Buresi C, Ferrero F, Malafosse A. Antidepressant-induced mania, rapid cycling and the serotonin transporter gene polymorphism. Pharmacogenomics J. 2003;3:101–104. doi: 10.1038/sj.tpj.6500156. [DOI] [PubMed] [Google Scholar]
- Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Structure/function relationships in serotonin transporter: new insights from the structure of a bacterial transporter. Handb Exp Pharmacol. 2006:59–73. doi: 10.1007/3-540-29784-7_3. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M. D-amphetamine and L-5-hydroxytryptophan-induced behaviours in mice with genetically-altered expression of the alpha2C-adrenergic receptor subtype. Neuroscience. 1998;86:959–965. doi: 10.1016/s0306-4522(98)00100-6. [DOI] [PubMed] [Google Scholar]
- Sample SJ, Behan M, Smith L, Oldenhoff WE, Markel MD, Kalscheur VL, Hao Z, Miletic V, Muir P. Functional adaptation to loading of a single bone is neuronally regulated and involves multiple bones. J Bone Min Res. 23 doi: 10.1359/jbmr.080407. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels J, Shugart YY, Grados MA, Willour VL, Bienvenu OJ, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Wang Y, Pinto A, Fyer AJ, Piacentini J, Pauls DL, Cullen B, Rasmussen SA, Hoehn-Saric R, Valle D, Liang KY, Riddle MA, Nestadt G. Significant linkage to compulsive hoarding on chromosome 14 in families with obsessive-compulsive disorder: results from the OCD Collaborative Genetics Study. Am J Psychiatry. 2007;164:493–499. doi: 10.1176/ajp.2007.164.3.493. [DOI] [PubMed] [Google Scholar]
- Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation. 2003;108:32–36. doi: 10.1161/01.CIR.0000079172.43229.CD. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Storey J, Greenberg BD, Santiago HT, Li Q, Murphy DL. Evaluating gene × psychological risk factor effects in the pathogenesis of anxiety: a new model approach. J Abnorm Psychol. 2000;109:308–320. [PubMed] [Google Scholar]
- Schmitt A, Benninghoff J, Moessner R, Rizzi M, Paizanis E, Doenitz C, Gross S, Hermann M, Gritti A, Lanfumey L, Fritzen S, Reif A, Hamon M, Murphy DL, Vescovi A, Lesch KP. Adult neurogenesis in serotonin transporter deficient mice. J Neural Transm. 2007;114:1107–1119. doi: 10.1007/s00702-007-0724-6. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Mossner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res. 2003;71:701–709. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Steiner-Mordoch S, Yelin R, Wall SC, Rudnick G. Amphetamine derivatives interact with both plasma membrane and secretory vesicle biogenic amine transporters. Mol Pharmacol. 1993;44:1227–1231. [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet. 2004;127:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Serretti A, Calati R, Mandelli L, De Ronchi D. Serotonin transporter gene variants and behavior: a comprehensive review. Curr Drug Targets. 2006;7:1659–1669. doi: 10.2174/138945006779025419. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Shugart YY, Samuels J, Willour VL, Grados MA, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Wang Y, Pinto A, Fyer AJ, Piacentini J, Pauls DL, Cullen B, Page J, Rasmussen SA, Bienvenu OJ, Hoehn-Saric R, Valle D, Liang KY, Riddle MA, Nestadt G. Genomewide linkage scan for obsessive-compulsive disorder: evidence for susceptibility loci on chromosomes 3q, 7p, 1q, 15q, and 6q. Mol Psychiatry. 2006;11:763–770. doi: 10.1038/sj.mp.4001847. [DOI] [PubMed] [Google Scholar]
- Silins E, Copeland J, Dillon P. Qualitative review of serotonin syndrome, ecstasy (MDMA) and the use of other serotonergic substances: hierarchy of risk. Aust N Z J Psychiatry. 2007;41:649–655. doi: 10.1080/00048670701449237. [DOI] [PubMed] [Google Scholar]
- Sjoqvist F, Eliasson E. The convergence of conventional therapeutic drug monitoring and pharmacogenetic testing in personalized medicine: focus on antidepressants. Clin Pharmacol Ther. 2007;81:899–902. doi: 10.1038/sj.clpt.6100188. [DOI] [PubMed] [Google Scholar]
- Smits K, Smits L, Peeters F, Schouten J, Janssen R, Smeets H, van Os J, Prins M. Serotonin transporter polymorphisms and the occurrence of adverse events during treatment with selective serotonin reuptake inhibitors. Int Clin Psychopharmacol. 2007;22:137–143. doi: 10.1097/YIC.0b013e328014822a. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Schumann G, Klein S, Hu XZ, Moayer M, Zimmer A, Wrase J, Flor H, Mann K, Braus DF, Goldman D, Heinz A. Gene-gene effects on central processing of aversive stimuli. Mol Psychiatry. 2007;12:307–317. doi: 10.1038/sj.mp.4001946. [DOI] [PubMed] [Google Scholar]
- Snider LA, Swedo SE. PANDAS: current status and directions for research. Mol Psychiatry. 2004;9:900–907. doi: 10.1038/sj.mp.4001542. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. PNAS. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Li XF, Funada M, Kinsey S, Uhl GR. Visceral chemical nociception in mice lacking mu-opioid receptors: effects of morphine, SNC80 and U-50,488. Eur J Pharmacol. 1999;366:R3–5. doi: 10.1016/s0014-2999(98)00933-9. [DOI] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci U S A. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer W, Muller B, Leucht S, Kissling W. Pharmacogenetics: a new diagnostic tool in the management of antidepressive drug therapy. Clin Chim Acta. 2001;308:33–41. doi: 10.1016/s0009-8981(01)00423-5. [DOI] [PubMed] [Google Scholar]
- Strobel A, Lesch KP, Jatzke S, Paetzold F, Brocke B. Further evidence for a modulation of Novelty Seeking by DRD4 exon III, 5-HTTLPR, and COMT val/met variants. Mol Psychiatry. 2003;8:371–372. doi: 10.1038/sj.mp.4001253. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker DR, Natt F, Husken D, van der Putten H, Maier R, Hoyer D, Cryan JF. siRNA-mediated knockdown of the serotonin transporter in the adult mouse brain. Mol Psychiatry. 2005;10:782–789. 714. doi: 10.1038/sj.mp.4001687. [DOI] [PubMed] [Google Scholar]
- Thimgan MS, Berg JS, Stuart AE. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J Exp Biol. 2006;209:3383–3404. doi: 10.1242/jeb.02328. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143:4520–4526. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Li Q, Murphy DL. Life-long serotonin reuptake deficiency results in complex alterations in adrenomedullary responses to stress. Ann N Y Acad Sci. 2004;1018:99–104. doi: 10.1196/annals.1296.011. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Renoir T, Lanfumey L, Hamon M, Lesch KP, Robledo P, Maldonado R. 3,4-Methylenedioxymethamphetamine Self-Administration is Abolished in Serotonin Transporter Knockout Mice. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:979–986. doi: 10.1111/j.1365-2036.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Paoletti R, Brunello N, Maggi FM. Serotonin: From Cell Biology to Pharmacology and Therapeutics. Springer; 1990. [Google Scholar]
- Vogel C, Mossner R, Gerlach M, Heinemann T, Murphy DL, Riederer P, Lesch KP, Sommer C. Absence of thermal hyperalgesia in serotonin transporter-deficient mice. J Neurosci. 2003;23:708–715. doi: 10.1523/JNEUROSCI.23-02-00708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori E, Henry JA, Ojanpera I, Nieminen R, Savolainen T, Wahlsten P, Jantti M. Death following ingestion of MDMA (ecstasy) and moclobemide. Addiction. 2003;98:365–368. doi: 10.1046/j.1360-0443.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- Walitza S, Wewetzer C, Warnke A, Gerlach M, Geller F, Gerber G, Gorg T, Herpertz-Dahlmann B, Schulz E, Remschmidt H, Hebebrand J, Hinney A. 5-HT2A promoter polymorphism -1438G/A in children and adolescents with obsessive-compulsive disorders. Mol Psychiatry. 2002;7:1054–1057. doi: 10.1038/sj.mp.4001105. [DOI] [PubMed] [Google Scholar]
- Warden SJ, Bliziotes MM, Wiren KM, Eshleman AJ, Turner CH. Neural regulation of bone and the skeletal effects of serotonin (5-hydroxytryptamine) Mol Cell Endocrinol. 2005;242:1–9. doi: 10.1016/j.mce.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Ackerman MJ, Marazita ML, Berry-Kravis EM. Sudden Infant Death Syndrome: review of implicated genetic factors. Am J Med Genet A. 2007;143:771–788. doi: 10.1002/ajmg.a.31722. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Zhou L, Berry-Kravis EM, Maher BS, Silvestri JM, Marazita ML. Association of the serotonin transporter gene with sudden infant death syndrome: a haplotype analysis. Am J Med Genet. 2003;122A:238–245. doi: 10.1002/ajmg.a.20427. [DOI] [PubMed] [Google Scholar]
- Weiger WA. Serotonergic modulation of behaviour: a phylogenetic overview. Biol Rev Camb Philos Soc. 1997;72:61–95. doi: 10.1017/s0006323196004975. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. Inheritance and drug response. N Engl J Med. 2003;348:529–537. doi: 10.1056/NEJMra020021. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R, Wang L. Pharmacogenomics: bench to bedside. Nat Rev Drug Discov. 2004;3:739–748. doi: 10.1038/nrd1497. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, DeGuzman TB, McMahon F, Rudnick G, Detera-Wadleigh SD, Murphy DL. SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive disorder. Psychiatr Genet. 2008a;18:31–39. doi: 10.1097/YPG.0b013e3282f08a06. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Kruse MR, Cromer KC, Murphy DL. A Large Case-Control Study of Common Functional SLC6A4 and BDNF Variants in Obsessive-Compulsive Disorder. Neuropsychopharmacology. 2007;32:2543–2551. doi: 10.1038/sj.npp.1301394. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Kruse MR, Murphy DL. Functional SLITRK1 var321, varCDfs and SLC6A4 G56A variants and susceptibility to obsessive-compulsive disorder. Mol Psychiatry. 2006a;11:802–804. doi: 10.1038/sj.mp.4001848. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006b;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, Murphy DL. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008b;17:717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Central nervous system serotonin function and cardiovascular responses to stress. Psychosom Med. 2001;63:300–305. doi: 10.1097/00006842-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Wurts SW, Hall FS, Lesch KP, Murphy DL, Uhl GR, Edgar DM. Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport. 2003;14:233–238. doi: 10.1097/00001756-200302100-00015. [DOI] [PubMed] [Google Scholar]
- Wooltorton E. Paroxetine (Paxil, Seroxat): increased risk of suicide in pediatric patients. Cmaj. 2003;169:446. [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yavarone MS, Shuey DL, Tamir H, Sadler TW, Lauder JM. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology. 1993;47:573–584. doi: 10.1002/tera.1420470609. [DOI] [PubMed] [Google Scholar]
- Yeo A, Boyd P, Lumsden S, Saunders T, Handley A, Stubbins M, Knaggs A, Asquith S, Taylor I, Bahari B, Crocker N, Rallan R, Varsani S, Montgomery D, Alpers DH, Dukes GE, Purvis I, Hicks GA. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonan AL, Alarcon M, Cheng R, Magnusson PK, Spence SJ, Palmer AA, Grunn A, Juo SH, Terwilliger JD, Liu J, Cantor RM, Geschwind DH, Gilliam TC. A genomewide screen of 345 families for autism-susceptibility loci. Am J Hum Genet. 2003;73:886–897. doi: 10.1086/378778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Ito K, Takahashi H, Kamata M, Higuchi H, T S, Ito K, Inoue K, Tezuka T, Suzuki T, Ohkubo T, Sugawara K, Otani K. Influence of the serotonin transporter gene-linked polymorphic region on the antidepressant response to fluovoxamine in Japanese depressed patient. Prog Neuropsychopharmacol Biol Psychiatry. 2002;158:383–386. doi: 10.1016/s0278-5846(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Zai G, Bezchlibnyk YB, Richter MA, Arnold P, Burroughs E, Barr CL, Kennedy JL. Myelin oligodendrocyte glycoprotein (MOG) gene is associated with obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2004;129:64–68. doi: 10.1002/ajmg.b.30077. [DOI] [PubMed] [Google Scholar]
- Zhang H, Leckman JF, Pauls DL, Tsai CP, Kidd KK, Campos MR. Genomewide scan of hoarding in sib pairs in which both sibs have Gilles de la Tourette syndrome. Am J Hum Genet. 2002;70:896–904. doi: 10.1086/339520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Rudnick G. Serotonin transporter mutations associated with obsessive-compulsive disorder and phosphorylation alter binding affinity for inhibitors. Neuropharmacology. 2005;49:791–797. doi: 10.1016/j.neuropharm.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jaing C, Murphy DL, Lanthorn TH, Holmes A. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006;140:321–334. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Lesch KP, Murphy DL. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 2002;942:109–119. doi: 10.1016/s0006-8993(02)02709-9. [DOI] [PubMed] [Google Scholar]


