Abstract
We examined the co-occurrence of migraine and endometriosis within the largest known collection of families containing multiple women with surgically confirmed endometriosis and in an independent sample of 815 monozygotic and 457 dizygotic female twin pairs. Within the endometriosis families, a significantly increased risk of migrainous headache was observed in women with endometriosis compared to women without endometriosis (odds ratio [OR] 1.57, 95% confidence interval [CI]: 1.12–2.21, P = 0.009). Bivariate heritability analyses indicated no evidence for common environmental factors influencing either migraine or endometriosis but significant genetic components for both traits, with heritability estimates of 69 and 49%, respectively. Importantly, a significant additive genetic correlation (rG = 0.27, 95% CI: 0.06–0.47) and bivariate heritability (h2 = 0.17, 95% CI: 0.08–0.27) was observed between migraine and endometriosis. Controlling for the personality trait neuroticism made little impact on this association. These results confirm the previously reported comorbidity between migraine and endometriosis and indicate common genetic influences completely explain their co-occurrence within individuals. Given pharmacological treatments for endometriosis typically target hormonal pathways and a number of findings provide support for a relationship between hormonal variations and migraine, hormone-related genes and pathways are highly plausible candidates for both migraine and endometriosis. Therefore, taking into account the status of both migraine and endometriosis may provide a novel opportunity to identify the genes underlying them. Finally, we propose that the analysis of such genetically correlated comorbid traits can increase power to detect genetic risk loci through the use of more specific, homogenous and heritable phenotypes.
Keywords: heritability, headache, women, hormones, pain, comorbid
INTRODUCTION
Typical migraine is a frequent, debilitating and painful disorder that normally affects people during their most productive years. Mutually confirmed prevalence data from population-based studies in Denmark [Rasmussen et al., 1991b; Russell and Olesen, 1995], France [Henry et al., 1993; Michel et al., 1996], the USA [Stewart et al., 1992, 1996] and Canada [Edmeads et al., 1993; O’Brien et al., 1994] all using International Headache Society (IHS) diagnostic criteria [Headache Classification Committee of the International Headache Society, 1988] have shown a homogenous picture regarding migraine prevalence. These studies produce a reasonable estimate of the 1-year prevalence of migraine in adults of 10–12%; 6% among men and 15–18% among women. The World Health Organization [2001] recently identified migraine among the world’s top 20 leading causes of disability, with an impact that extends far beyond the suffering individual, to the family and community. Although migraine is highly prevalent in our society, its aetiology remains relatively obscure and there are no laboratory-based diagnostic tests that identify those who suffer from the disorder. Twin studies indicate that migraine has a significant genetic component, with heritability estimates of 33–65% [Gervil et al., 1999; Honkasalo et al., 1995; Larsson et al., 1995; Mulder et al., 2003; Nyholt et al., 2004; Ulrich et al., 1999].
Endometriosis is a gynaecologic disorder affecting up to 10% of women and is defined by the presence of endometrial tissue outside of the uterus, growing, bleeding cyclically and causing adhesions. Common symptoms are severe pelvic pain, painful menstrual periods (dysmenorrhea) and infertility. The main pathological processes associated with the disease are peritoneal inflammation and fibrosis, and the formation of adhesions and ovarian cysts. The disorder often recurs and has a major impact on women’s health, relationships, productivity and life choices. The risk of endometriosis increases with age within the reproductive years, however its aetiology remains obscure. Some risk factors relate to oestrogen levels, including positive smoking history (which reduces ovarian production of oestrogen and reduces risk of endometriosis) and increased peripheral body fat (which increases oestrogen), plus increased exposure to menstruation (i.e., shorter cycle length, longer duration of flow and nulliparity) [Berkley et al., 2005; Eskenazi and Warner, 1997]. Genetic factors also contribute to endometriosis with a heritability of 51% and relative recurrence risk to siblings of 2.34 estimated in our Australian sample of twins [Treloar et al., 1999]; however, the value may be as high as 15 in the sisters of women with more severe disease, based upon imaging studies [Kennedy et al., 1997].
A Finnish clinical study published in 1975 of 125 patients, aged 19–44 who were in hospital for abdominal operation found of the 50 patients with the solitary diagnosis of endometriosis, headache was reported by 42 (84%) compared to only 26 (60%) of the 44 controls with no endometriosis ( = 7.26, P = 0.007). In addition, 14 (28%) of the endometriosis patients described their headache to be of the nature of migraine, compared to only 8 (18%) of controls ( = 5.19, P = 0.023) [Tervila and Marttila, 1975]. Also, a USA clinical trial assessing the effect of leuprolide acetate [a synthetic nonapeptide analogue of naturally occurring gonadotropin releasing hormone (GnRH or LH-RH) which is a very potent blocker of follicle-stimulating hormone (FSH) and luteinizing hormone (LH): lowering levels of oestrogen, progesterone and testosterone] and oophorectomy (surgical removal of an ovary or ovaries) on IHS menstrual migraine in 29 reproductive age female subjects, noted 11 of 29 volunteers experienced dysmenorrhea and 10 of 23 patients (with previous pelvic surgery) had surgically confirmed endometriosis. These symptoms and findings were present equally in those whose migraines responded to leuprolide and those who did not respond [Lichten et al., 1995].
More recently, two studies reported comorbidity of migraine and endometriosis. The first study of 133 Italian women with endometriosis reported a significantly higher (P < 0.001) prevalence of migraine (n = 51, 38.3%, 95% CI: 30.1–47.2%) than in 166 Italian women without endometriosis (controls) (n = 25, 15.1%, 95% CI: 10.0–21.4%) [Ferrero et al., 2004]. Similarly, a USA study reported a higher prevalence of endometriosis in 50 females with migraine than in 52 age-matched women without migraine (30 versus 4%, P < 0.001) [Tietjen et al., 2006]. When migraine and endometriosis are comorbid, the well-being of the patient may be further impaired.
Therefore, to further investigate the comorbidity of migraine and endometriosis we collected migraine symptom data from women in our cohort of 931 Australian families containing at least two sisters with endometriosis (≥1 affected-sister-pair, ASP) [Treloar et al., 2005]. In addition, we used an independent sample of 815 monozygotic (MZ) and 457 dizygotic (DZ) female twin pairs for whom endometriosis status and migraine symptom data were available to assess the aetiology of migraine and endometriosis comorbidity.
Also, several studies have demonstrated a cross-sectional relation between psychiatric disorders and migraine in community samples, and, in particular, subjects with migraine report more affective and anxiety disorders and exhibit elevated rates of neuroticism and somatisation compared to non-migraine subjects [Merikangas et al., 1993]. Indeed, a recent cross-sectional study of female headache outpatients and healthy controls found anxiety was more common in migraine with endometriosis group, than in the cohort with migraine without endometriosis (OR = 2.2, 95% CI: 1.0–4.7) [Tietjen et al., 2007]. Given persons with a high degree of neuroticism are sensitive and vulnerable to various stimuli, including stress and pain, they are more likely to fulfil the disability criterion and thereby the criteria for migraine; we also investigated whether neuroticism [Eysenck and Eysenck, 1974] underlies the co-occurrence of migraine and endometriosis in our twin data.
MATERIALS AND METHODS
SAMPLES
The endometriosis ASP families were recruited for our studies aimed at identifying the molecular mechanisms underlying the disorder by looking for genomic regions co-inherited with endometriosis and represent the largest known cohort of women diagnosed (surgically confirmed) with endometriosis and their family members [Treloar et al., 2005].
Given the strong selection bias of the endometriosis ASP families (i.e., there is little variation in endometriosis status due to most women being affected), to determine whether comorbidity of migraine and endometriosis results from (i) coincidence or selection bias, (ii) one condition causing another or (iii) shared environmental or genetic risk factors, we investigated whether genetic influences on endometriosis overlap with genetic influences on migraine, utilising a community sample of twins for whom endometriosis and migraine phenotype data were available.
The twin sample participants were members of the cohort of 1,979 female twin pairs that were identified originally in 1980–1982 from the Australian National Health and Medical Research Council (NHMRC) Twin Register and followed up in 1988–1990 [Heath et al., 1994].
Approval to conduct the research was obtained from the Queensland Institute of Medical Research (QIMR) Human Research Ethics Committee and from the Australian Twin Registry.
ASSESSMENT
Analogous to our previous studies on the genetics of migraine [Nyholt et al., 2004, 2005], migraine symptom data were obtained from 961 women aged 40–70 in our endometriosis ASP families during the course of an extensive, semi-structured telephone interview that included diagnostic assessments of migraine, based on IHS criteria (Table I) and similar to the Finnish Migraine-Specific Questionnaire [Kallela et al., 2001]. The interview performed by lay interviewers trained and experienced in migraine diagnosis, yielded diagnoses for migraine without aura (MO) and migraine with aura (MA), with the use of visual prodromal symptoms as an index of MA.
TABLE I.
Diagnostic criteria for migraine without aura, excerpt from International Headache Society (IHS) classification of headache
| Migraine without aura (MO) |
| A. At least five attacks fulfilling B through D |
| B. Headache lasting 4–72 h (untreated or unsuccessfully treated) |
| C. Headache has at least two of the following characteristics: |
| 1. Unilateral location |
| 2. Pulsating quality |
| 3. Moderate or severe intensity (inhibits or prohibits daily activities) |
| 4. Aggravation by walking stairs or similar routine physical activity |
| D. During headache at least one of the following: |
| 1. Nausea and/or vomiting |
| 2. Photophobia and phonophobia |
In 1993–1994, a questionnaire focused on gynaecologic conditions and hysterectomy was sent to both members of 1,570 female twin pairs, plus a further 158 individual female twins in incomplete pairs (3,298 individuals) who could still be contacted and were willing to participate in twin research [Treloar et al., 1999]. Participants were asked to complete a four-page questionnaire (“Gynaecological Health Study”), which included questions on gynaecologic problems that might predispose to hysterectomy, other surgical interventions, and medical and hormonal treatments. Twins were asked to report whether they had ever had endometriosis, their age at its onset, the nature of its investigation and/or treatment, and whether their cotwin or mother had endometriosis.
In 1993–1995, a telephone interview (by lay interviewers trained and experienced in migraine diagnosis) including a diagnostic assessment of psychiatric disorders, including alcohol use and abuse, anxiety, depression and phobias was administered to the same cohort of twins [Heath et al., 1997]. As before, twins were unselected with regard to personal or family history of alcoholism or other psychiatric or medical disorders. Participants answering “yes” to ever having “migraine or recurrent attacks of headache” (screening positive) then answered a number of questions developed by an experienced migraine researcher (K.R.M.) based on IHS criteria [Nyholt et al., 2004, 2005].
Neuroticism scores were collected via lay telephone interview (by lay interviewers trained and experienced in the assessment of psychiatric disorders) as part of a previous study in 1988 on the same set of twins for whom migraine and endometriosis data were available. The neuroticism sample and data have been described elsewhere [Birley et al., 2006; Kirk et al., 2000], and previous analyses have shown that this population-based twin sample is typical of the Australian population in many respects including the prevalence of psychiatric symptoms [Kendler et al., 1986].
STATISTICAL ANALYSIS
Cross-tabulations and kappa (K) calculations were performed using SPSS 13 (SPSS Inc.). Tetrachoric correlations (rt) and odds ratios (ORs) were calculated using the Mystat program (http://www.qimr.edu.au/davidD/)
Latent class analysis (LCA) is a statistical method for finding subtypes of related cases (latent classes) from multivariate categorical data. LCA model associations (covariation) between observed variables that imperfectly measure a non-observable (latent) variable [McCutcheon, 1987; Rindskopf and Rindskopf, 1986]. By assessing the symptom profile of individual patients, LCA produces mutually exclusive groups (classes) of patients based upon their patterns of symptoms.
As described previously [Nyholt et al., 2004, 2005], latent class cluster models were fitted to 10 trichotomous symptom response variables (i.e., negative to screening question, screening positive but negative for symptom, and screening positive and positive for symptom) based on IHS diagnostic criteria (Table I) using the Latent GOLD 2.0 package (Statistical Innovations Inc.). For each Latent GOLD run, up to 10,000 iterations of the EM algorithm were allowed using a convergence criterion of 1 × 10−10. Each LCA solution was restarted at least 100 times with new starting values to find the maximum likelihood estimates for the parameters.
Classification information was requested for latent class cluster models. For each symptom profile, the classification output contains the associated probabilities of belonging to each cluster. Also, bivariate residuals were obtained, which identify correlations between symptom pairs that have not been adequately explained by the model.
Although estimates of class membership and symptom endorsement probabilities ignored the twin structure of the data, which may result in the likelihood-ratio chi-square (χ2) test overestimating the significance of adding an extra class, as in traditional factor analysis [Neale and Cardon, 1992], estimates of symptom endorsement and class membership probabilities will remain statistically unbiased [Madden et al., 1997]. Subsequently, and as recommended for large sample sizes, the comparative fits of LCA models were assessed by evaluating the Bayes information criterion (BIC) [Schwarz, 1978] where, if the BIC of a more complex model fails to decrease, the simpler model (having the lower BIC) will be selected.
Subgroups of migraine/severe headache sufferers were identified from the LCA results. Briefly, LCA identified one asymptomatic class (CL0) and three major symptomatic classes, representing (i) a mild form of recurrent non-migrainous headache (CL1), (ii) a moderately severe form of migrainous headache (CL2) typically without visual aura, loading (i.e., present at least 50% of the time) on all IHS MO symptoms except “unilateral location” and “nausea, vomiting or diarrhoea”, and (iii) a severe form of migraine typically with visual aura, loading on all IHS symptoms (CL3). Of particular importance, although “aura” was often associated with other and more severe neurological symptoms and was predominantly found in latent class 3 (CL3), almost one quarter of class-3 individuals did not report “aura”. Furthermore “aura” was present in 39.3% of individuals in class-2 (CL2). Individuals in LCA groups CL2 and CL3 are considered to suffer migrainous headache. We have previously shown our LCA-derived migraine phenotype provides a stable and accurate correspondence between genetic risk and migraine headache [Nyholt et al., 2004, 2005]. Importantly, genetic-model fitting indicated a greater genetic contribution to migraine using the LCA migrainous headache classification (h2 = 0.41, 95% CI: 0.23–0.49) compared with the IHS MO/MA classification (h2 = 0.33, 95% CI: 0.05–0.44) [Nyholt et al., 2004].
GENETIC-MODEL FITTING
We used genetic-model fitting techniques in Mx software [Neale et al., 2003] to obtain estimates of the genetic and environmental factors. These techniques make optimum use of the information available in the twin-co-twin covariance structure [Neale and Cardon, 1992]. Model fitting is based on the comparison of the observed and expected variance-covariance matrices in MZ and DZ twin pairs.
Genetic-model fitting used structural equation modelling (SEM) to estimate parameters of a model that included additive genetic effects (A), non-additive genetic effects (i.e., dominance and/or epistasis) (D) or shared family environment (C), and random or unique environment (E) [Neale and Cardon, 1992]. For each model, we obtained: a χ2 goodness-of-fit statistic (−2LL) and calculated the sample size adjusted BIC parsimony measure which indexes the fit of the model and its parsimony. Nested models were compared using the likelihood ratio (χ2 difference) test (Δ-2LL), a significant χ2 value indicating a deterioration in model fit, and by examining the change in BIC (better fitting models produce lower values of BIC). Likelihood-based 95% confidence intervals for estimates of genetic and environmental parameters were then computed using Mx [Neale et al., 2003].
RESULTS
A large proportion of the 961 women in our endometriosis ASP families suffer from migraine headache: 509 (53%) were diagnosed with migrainous headache via our LCA approach [Nyholt et al., 2004, 2005] and 345 (35.9%) satisfied the strict criteria for IHS MO and/or MA.
Analysis of the 931 women for whom both migraine and endometriosis status were available (i.e., 30 women had unknown endometriosis status) revealed a significantly increased risk of migrainous headache in women with endometriosis compared to women without endometriosis (Table II) ( = 6.86, P = 0.009), with an OR of 1.57 (95% CI: 1.12–2.21). Similarly, an increased risk of IHS migraine in women with endometriosis compared to women without endometriosis was observed (Table II) (OR = 1.35, 95% CI: 0.94–1.95). As shown in Table II, fewer individuals are considered affected for the strict IHS migraine phenotype compared to the more sensitive LCA-based migrainous headache phenotype [Nyholt et al., 2004] (35.8 versus 52.9%), resulting in a reduction in power to detect a significant relationship between IHS migraine and endometriosis.
TABLE II.
Prevalence of migraine conditional on endometriosis in ASPs
| Migrainous headache |
N | IHS migraine |
N | |
|---|---|---|---|---|
| Non-endometriosis | Unaffected | 92 (0.56) | Unaffected | 114 (0.70) |
| Affected | 72 (0.44) | Affected | 50 (0.30) | |
| Endometriosis | Unaffected | 344 (0.45) | Unaffected | 482 (0.63) |
| Affected | 423 (0.55) | Affected | 285 (0.37) |
It is worth noting that in the endometriosis family dataset, the mean age of women with endometriosis (50.35 yrs) was significantly lower than women without endometriosis (56.88 yrs) (P < 0.001). Similarly, the mean age of women with migraine (50.81 yrs) was significantly lower than women without migraine (52.25 yrs) (P = 0.002). Also, the mean age of women without endometriosis was similar in the non-migraine (57.54 yrs) and migraine subgroup (56.03 yrs), as was the mean age of women with endometriosis in the non-migraine (50.85 yrs) and migraine subgroup (49.94 yrs). Given the prevalence of endometriosis and lifetime migraine both increase with age, age differences between women with and without endometriosis cannot underlie the observed increased prevalence of migraine in endometriosis sufferers (i.e., women with endometriosis would need to be older than women without endometriosis for age to underlie comorbid migraine and endometriosis). Indeed, the observation of women with comorbid migraine and endometriosis being younger than the women without migraine and endometriosis further supports an association between these two traits. As a consequence, in these data it is neither necessary nor sensible to age-adjust ORs relating the risk of migraine in women with endometriosis compared to women without endometriosis.
Migraine symptom data were available on 815 MZ and 457 DZ female twin pairs for whom endometriosis status had previously been determined [Treloar et al., 1999]. Twin-pair cross-tabulations (Table III) show an increased correlation in both migrainous headache and endometriosis for MZ co-twins compared to DZ co-twins, of proband twins presenting with either trait, suggesting the presence of both individual and shared genetic effects.
TABLE III.
Twin-pair cross-tabulationsa
| Zygosity Group | Twin 1 | Twin 2 | rt (95% CI) | ||
|---|---|---|---|---|---|
| Endometriosis |
|||||
| − | + | ||||
| MZ | Endometriosis | − | 713 | 38 | 0.50 (0.34–0.67) |
| + | 47 | 17 | |||
| DZ | Endometriosis | − | 410 | 22 | 0.11 (−0.25–0.47) |
| + | 23 | 2 | |||
|
Migrainous headache |
|||||
| − | + | ||||
| MZ | Endometriosis | − | 444.5 | 311 | 0.14 (−0.01–0.30) |
| + | 28 | 31.5 | |||
| DZ | Endometriosis | − | 236 | 196.5 | −0.06 (−0.29–0.18) |
| + | 14.5 | 10 | |||
| MZ | Migrainous headache | − | 348 | 121 | 0.56 (0.47–0.64) |
| + | 128 | 218 | |||
| DZ | Migrainous headache | − | 155 | 91 | 0.25 (0.11–0.38) |
| + | 100 | 111 | |||
|
Migrainous IHS headache |
|||||
| − | + | ||||
| MZ | Endometriosis | − | 619.5 | 136 | 0.15 (−0.15–0.44) |
| + | 43.5 | 16 | |||
| DZ | Endometriosis | − | 330 | 102.5 | −0.05 (−0.58–0.47) |
| + | 19.5 | 5 | |||
| MZ | Migrainous IHS headache | − | 594 | 67 | 0.69 (0.59–0.79) |
| + | 71 | 83 | |||
| DZ | Migrainous IHS headache | − | 280 | 70 | 0.27 (0.07–0.48) |
| + | 69 | 38 | |||
Counts may not be a whole number for endometriosis × migraine cross-tabulations, as tables were averaged over using either Twin 1 or Twin 2 as proband.
Except for a subgroup of women who participated in a later study which collected more detailed symptom data, the majority (84.7%) of the 2,544 female twins were not asked questions relating to having at least five migraine/episodes of headache during their lifetime, whether their average typical migraine/headache lasted between 4 and 72 h, and whether they would describe the pain associated with their headache as moderate or severe. Consequently, although IHS criteria C and D (Table I) status were available on all participants, strict IHS migraine diagnoses could be determined for only a small proportion (15.3%) of the twins and resulted in too small a sample to perform bivariate heritability analyses for IHS migraine. However, we have previously shown there to be a high correlation between our LCA-derived migrainous headache phenotype and migraine diagnosed according to strict IHS criteria [Nyholt et al., 2004, 2005].
In the current twin data for whom IHS migraine status could be determined (n = 389), the tetrachoric correlation between migrainous headache and IHS migraine was estimated at 0.94 (95% CI: 0.87–0.99) with a diagnostic reliability kappa (K) of 0.47 (95% CI: 0.38–0.56, P = 4 × 10−28). The tetrachoric correlation between fulfilling only IHS criteria C1D and IHS migraine was estimated at 0.95 (95% CI: 0.89–1.00), K = 0.52 (95% CI: 0.43–0.60, P = 4 × 10−31), while the tetrachoric correlation between fulfilling IHS criteria C1D and being in the most severe migrainous headache class (CL3) [Nyholt et al., 2004] (IHS criteria C+D+CL3) (termed migrainous IHS headache) and IHS migraine was estimated at 0.90 (95% CI: 0.85–0.95), K = 0.69 (95% CI: 0.61–0.77, P = 4 × 10−42). As suggested by the high correlation between these migraine measures, similar results from bivariate heritability analyses were obtained for migrainous headache, IHS criteria C+D and IHS criteria C+D+CL3 (migrainous IHS headache). Given all individuals diagnosed as ‘affected’ under the strict IHS scheme are also diagnosed with migrainous headache, for simplicity we only report bivariate heritability results based on the latter, more severe and ‘clinically relevant’ migraine phenotype, migrainous IHS headache (see Table III for MZ/DZ cross-tabulations). Individuals classified as migrainous IHS headache suffer a severe form of migraine headache (i.e., suffering a greater number of IHS symptoms) and typically fulfil strict IHS criteria.
Bivariate analyses utilising the correlated liability (triangular decomposition or “Cholesky” factor) model performed using the Mx program [Neale et al., 2003], indicated significant additive genetic components for both migrainous IHS headache and endometriosis, with heritability estimates of 69% (95% CI: 60–77%) and 48.76% (95% CI: 32–63%), respectively. There was no evidence for common environmental or non-additive genetic factors influencing either migraine or endometriosis, with non-shared environmental factors (which also subsumes any errors of measurement) explaining the remaining variance in liability to migraine (31%, 95% CI: 23–40%) and endometriosis (51.24%, 95% CI: 37–68%). Also, there was no significant evidence for age, age2 or age3 effects.
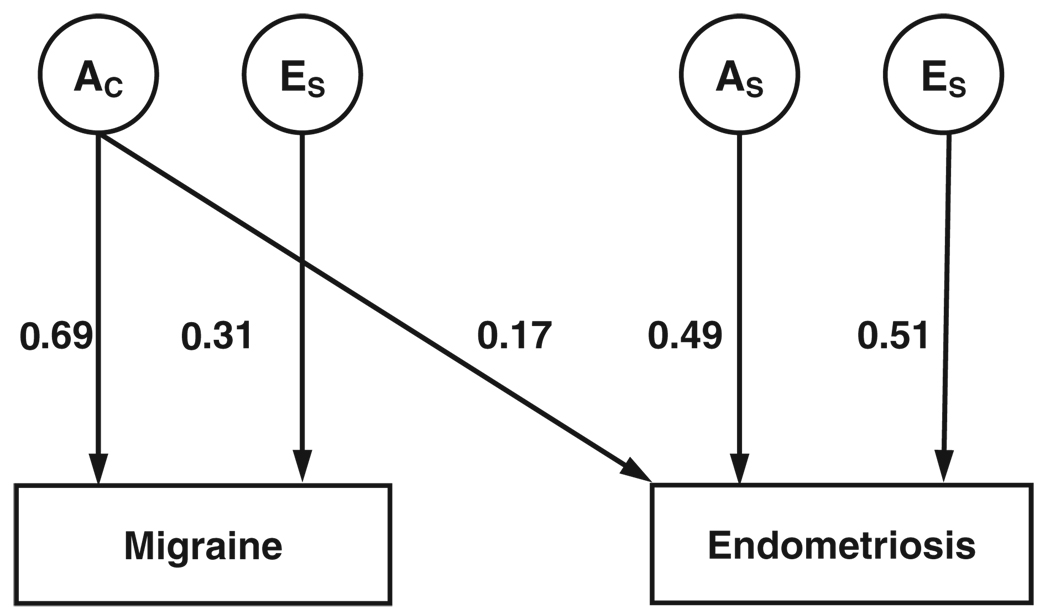
Importantly, a significant additive genetic correlation was observed between migraine and endometriosis (rG = 0.27, 95% CI: 0.06–0.47), while the observed non-shared environmental correlation between migraine and endometriosis (rE = 0.08, 95% CI: 0–0.32) was not significant. Moreover, the significant bivariate heritability estimate of 15.37% (95% CI: 4–27%) (P = 0.010), and non-significant non-shared environmental factors estimate of 2.99% (95% CI: 0–13%) (P = 0.560) indicate common genetic influences wholly underlie the observed covariance (comorbidity) between migraine and endometriosis. Figure 1 portrays the bivariate model best fitting the covariation between migraine and endometriosis (i.e., only the significant (P≤0.05) parameters are included).
Fig. 1.
Path diagram of the best fitting bivariate correlated liability (triangular decomposition or “Cholesky” factor) model. Latent variables are represented by circles and phenotypes are represented by boxes. AC represents additive genetic influences common to migraine and endometriosis; AS and ES are specific additive genetic and non-shared environmental influences. Parameter values represent the standardised proportion of variance in liability accounted for by the latent variables.
The principal genetic features of this model are as follows. First, the common additive genetic factors accounting for 69% (95% CI: 60–77%) of the variance in migraine also account for 17% (95% CI: 8–27%) (P = 0.0005) of the variance in endometriosis and completely explain their covariance (i.e., bivariate heritability of 17%). Secondly, there are specific additive genetic factors accounting for 49% (95% CI: 32–64%) of variance in endometriosis. Individual (non-shared) environmental factors explain the remaining variance in liability to migraine and endometriosis.
Furthermore, bivariate heritability analyses utilising direction-of-causation models [Duffy and Martin, 1994; Neale and Cardon, 1992] did not support migraine as the cause of endometriosis or vice versa, with both models providing a similar fit to the data (sample size adjusted BIC parsimony measures of −8,280.13 and −8,279.44, respectively).
Finally, although results from trivariate heritability analyses indicated that neuroticism and migraine share common additive genetic factors, neuroticism and endometriosis do not. Moreover, inclusion of neuroticism in the model caused no significant change in the variance component estimates of genetic and environmental covariance between migraine and endometriosis (data not shown).
DISCUSSION
Our results represent the two largest studies to date on the comorbidity of migraine and endometriosis and the first study on bivariate heritability of these two disorders. The results do not support migraine and endometriosis co-occurrence being due to coincidence or selection bias, shared environmental effects or one condition causing another. Instead, the results confirm migraine and endometriosis comorbidity and indicate that additive genetic effects completely explain their co-occurrence within individuals and which are unrelated to personality.
Although migraine diagnosis is preferably performed via direct communication between physician and patient (which is impractical for thousands of participants spread throughout Australia), our study obtained symptom information and level of detail similar to other migraine studies, which were repeatedly found to be a valid diagnostic tool for migraine [Gervil et al., 1998; Rasmussen et al., 1991a; Russell et al., 1995]. Compared to their sole use, our combined use of both questionnaire and interview increases the reliability of symptom data. For example, using a similar screening approach, Stewart et al. (1997) obtained a 92.6% positive predictive value of their telephone interview diagnosis compared with their clinical examination [Stewart et al., 1997]. Furthermore, any misclassification resulting from diagnosis based on questionnaire and/or trained lay interview is expected to result in underestimation of migraine prevalence compared to expert interview [Tzourio et al., 2003], will be minimised via our more sensitive LCA approach and be consistent across the endometriosis and non-endometriosis individuals, and thus have negligible effect on the results of our comorbidity analyses.
The agreement (Cohen’s kappa) between LCA-derived migrainous headache and strict IHS migraine was 0.66 in the endometriosis family data and 0.69 between LCA-derived migrainous IHS headache and strict IHS migraine in the twin data. These kappas represent substantial agreement [Landis and Koch, 1977] and are very good in light of the uncertainty associated with the clinical diagnosis of migraine. For example, kappas range from 0.55 to 0.81 among different neurologists assigning headache diagnosis based on review of videotaped patient interviews [Granella et al., 1994]. These results support the validity and reliability of our LCA-based migraine phenotype; indeed the utility of our LCA approach to facilitate migraine research was highlighted in two recent reviews on migraine genetics [van den Maagdenberg et al., 2007; Wessman et al., 2007].
Similarly, although surgery is required for definitive diagnosis of endometriosis (which is obviously not possible for large population-based samples), we have previously shown our endometriosis self-report data (supplemented with information from medical, surgical and/or pathology reports where available) to have high agreement with medical report (K = 0.52, 95% CI: 0.42–0.61) and self-reported endometriosis adjusted by available medical and pathology data (K = 0.82, 95% CI: 0.78–0.86) [Treloar et al., 1999].
While twin studies indicate that migraine has a significant genetic component, importantly, these studies do not support the presence of genetic sex-specific effects (i.e., effects expressed in one sex but not the other), although this may be due to a lack of power to detect such effects [Martin et al., 1978]. In the absence of sex-specific genetic effects, it is likely that sex hormones play a pivotal role in the increased vulnerability to migraine among women. A number of findings provide support for a relationship between hormonal variations and migraine. These include evidence indicating that many women with migraine report worsening of their headaches around the time of menstruation [Edelson, 1985; Greene, 1967]. In fact, menstrual migraine (i.e., 90% of attacks of MO occur in association with menstruation) is experienced by 24–25% of females with MO [Rasmussen, 1993].
Further support for a relationship between hormonal variations and migraine include reports of the oral contraceptive pill precipitating a first attack of migraine or worsening or improving the frequency and severity of existing attacks [Dalton, 1976; Kudrow, 1975; Ryan, 1978]. In addition, many women with migraine have fewer attacks during pregnancy [Callaghan, 1968; Nattero, 1982; Somerville, 1972], although the condition may be exacerbated in the post-partum period [Stein, 1981]. Also, some women have fewer attacks or cease to have attacks after menopause [Martin et al., 1971].
Finally, the prevalence of migraine has generally been shown to be equal in boys and girls before puberty but increases at a greater rate in girls as adolescence approaches, so that by adulthood the female to male ratio has increased to 3:1 [Lance, 1982; Welch et al., 1984]. It has been postulated that this age-dependent increase in female prevalence could be the result of hormonal fluctuations triggering a primary predisposition [Dennerstein et al., 1978; Greene, 1969].
An association has been reported between the oestrogen receptor 1 (ESR1) gene exon 8 G594A (rs2228480) polymorphism and migraine susceptibility in two independent Australian case-control (CC) cohorts (224CC and 260 CC) [Colson et al., 2004], while an exon 4 C325G (rs1801132) polymorphism was reported to be associated with migraine in women in a Spanish cohort (367 cases, 232 controls) [Oterino et al., 2006]. Interestingly, rs2228480 was not associated in the Spanish cohort and rs1801132 was not found to be associated in the Australian cohort [Colson et al., 2006]. More recently, a large Finnish study of 898 MA cases and 900 controls reported nominal association for 5 out of 26 ESR1 SNPs tested (rs6557170, rs2347867, rs6557171, rs4870062, rs1801132; uncorrected P-values 0.007–0.034), but these results did not remain significant after taking multiple testing into account [Kaunisto et al., 2006]. Although these results indicate that neither rs2228480 nor rs1801132 are likely to be the causal variant(s), they nonetheless provide support for a role of ERS1 in migraine susceptibility and highlight the need for more detailed analysis of variants within ESR1.
Given pharmacological treatments for endometriosis typically target hormonal pathways and ESR1 gene variants have also been reported to be associated with endometriosis [Georgiou et al., 1999; Hsieh et al., 2007; Kim et al., 2005], hormone-related genes and pathways are highly plausible candidates for both migraine and en-dometriosis. Hence, taking into account the status of both migraine and endometriosis may provide a novel opportunity to identify the genes underlying them. The identification of such genes will provide clues to the further elucidation of the complex molecular pathways of migraine and endometriosis and will help in the development of diagnostic tests and rational treatment strategies.
Most recently, a retrospective study of 258 Taiwanese women with menstrual migraine who underwent laparoscopic uterosacral nerve ablation (LUNA) for the treatment of dysmenorrhea found a significant amelioration of migraine severity after LUNA in women who had at least one grade improvement in the multidimensional scoring of dysmenorrhea at the 12 month follow-up. The results suggest LUNA may offer an approximate 25% chance of lessening migraine severity in certain women [Juang et al., 2006].
Furthermore, women with comorbid migraine and endometriosis may particularly benefit from the use of nonsteroidal anti-inflammatory drugs (NSAIDs) commonly used to treat migraine, as NSAIDs are one of the recommended therapies in women with menorrhagia [Kirtava et al., 2003; MacGregor, 2000]. Menorrhagia (excessive and/or prolonged menstrual bleeding) is a risk factor for endometriosis [James, 2005] and was also recently associated with migraine [Tietjen et al., 2006]. Similarly, given the strong oestrogen link to both migraine and endometriosis, women comorbid for these conditions may especially benefit from therapies which suppress and/or stabilise oestrogen production. In contrast, treatment of migraine with ergotamine may exacerbate endometriosis, as ergot derivatives are known to induce fibrosis [Robert et al., 1984].
In summary, these results confirm the previously reported comorbidity between migraine and endometriosis and indicate common genetic influences completely underlie their co-occurrence within individuals. Therefore, to improve the physical health and emotional well-being of many women—via improved treatment and pain relief—we suggest the presence of migraine be investigated in women with endometriosis and vice versa. Additionally, our data indicate comorbid migraine and endometriosis represents a subset of individuals with unique genetic effects and may therefore provide a novel opportunity to identify the genes underlying them. Consequently, we propose that the analysis of such genetically correlated comorbid traits can increase power to detect genetic risk loci through the use of more specific, homogenous and heritable phenotypes. Also, the identification of genetically correlated comorbid traits has particular relevance to the currently popular genome-wide association (GWA) paradigm by providing a tangible means to substantially improve the efficient use of both existing and future GWA data. For example, in addition to the primary association analysis of GWA data to the phenotype for which it was ascertained, secondary analyses may be performed for genetically correlated comorbid traits. Furthermore, examination of comorbid/non-comorbid cases (and controls) provides a unique opportunity to knowingly stratify according to genetic homogeneity.
ACKNOWLEDGMENTS
We thank Dr Daniel T. O’Connor for assistance with recruitment and confirmation of endometriosis diagnoses, Barbara Haddon for coordinating the endometriosis study recruitment and Clare Redfern for coordinating the collection of migraine data in endometriosis families. DRN is supported by an NHMRC RD Wright Fellowship (#339462). This research was supported in part by NHMRC Project Grant (#339430). The 1993–1994 Gynaecological Health Study was funded by the Mayne Bequest Fund (The University of Queensland, Brisbane, Australia) and the Australian Gynaecological Endoscopy Society. The Australian endometriosis study recruitment was funded by Cooperative Research Centre for Discovery of Genes for Common Human Diseases (CRC), by Cerylid Biosciences (Melbourne), and by donations from Neville and Shirley Hawkins, the Western Australian Endometriosis Support Association and the Endometriosis Association.
REFERENCES
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–1589. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- Birley AJ, Gillespie NA, Heath AC, Sullivan PF, Boomsma DI, Martin NG. Heritability and nineteen-year stability of long and short EPQ-R Neuroticism scales. Pers Individ Dif. 2006;40:737–748. [Google Scholar]
- Callaghan N. The migraine syndrome in pregnancy. Neurology. 1968;18:197–199. doi: 10.1212/wnl.18.2.197. [DOI] [PubMed] [Google Scholar]
- Colson NJ, Lea RA, Quinlan S, MacMillan J, Griffiths LR. The estrogen receptor 1 G594A polymorphism is associated with migraine susceptibility in two independent case/control groups. Neurogenetics. 2004;5:129–133. doi: 10.1007/s10048-004-0181-4. [DOI] [PubMed] [Google Scholar]
- Colson NJ, Lea RA, Quinlan S, Griffiths LR. No role for estrogen receptor 1 gene intron 1 Pvu II and exon 4 C325G polymorphisms in migraine susceptibility. BMC Med Genet. 2006;7:12. doi: 10.1186/1471-2350-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton K. Migraine and oral contraceptives. Headache. 1976;15:247–251. doi: 10.1111/j.1526-4610.1976.he1504247.x. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Laby B, Burrows GD, Hyman GJ. Headache and sex hormone therapy. Headache. 1978;18:146–153. doi: 10.1111/j.1526-4610.1978.hed1803146.x. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Martin NG. Inferring the direction of causation in cross-sectional twin data: Theoretical and empirical considerations. Genet Epidemiol. 1994;11:483–502. doi: 10.1002/gepi.1370110606. [DOI] [PubMed] [Google Scholar]
- Edelson RN. Menstrual migraine and other hormonal aspects of migraine. Headache. 1985;25:376–379. doi: 10.1111/j.1526-4610.1985.hed2507376.x. [DOI] [PubMed] [Google Scholar]
- Edmeads J, Findlay H, Tugwell P, Pryse-Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension-type headache on lifestyle, consulting behaviour, and medication use: A Canadian population survey. Can J Neurol Sci. 1993;20:131–137. doi: 10.1017/s0317167100047697. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder and Stoughton; 1974. [Google Scholar]
- Ferrero S, Pretta S, Bertoldi S, Anserini P, Remorgida V, Del Sette M, Gandolfo C, Ragni N. Increased frequency of migraine among women with endometriosis. Hum Reprod. 2004;19:2927–2932. doi: 10.1093/humrep/deh537. [DOI] [PubMed] [Google Scholar]
- Georgiou I, Syrrou M, Bouba I, Dalkalitsis N, Paschopoulos M, Navrozoglou I, Lolis D. Association of estrogen receptor gene polymorphisms with endometriosis. Fertil Steril. 1999;72:164–166. doi: 10.1016/s0015-0282(99)00198-3. [DOI] [PubMed] [Google Scholar]
- Gervil M, Ulrich V, Olesen J, Russell MB. Screening for migraine in the general population: Validation of a simple questionnaire. Cephalalgia. 1998;18:342–348. doi: 10.1046/j.1468-2982.1998.1806342.x. [DOI] [PubMed] [Google Scholar]
- Gervil M, Ulrich V, Kyvik KO, Olesen J, Russell MB. Migraine without aura: A population-based twin study. Ann Neurol. 1999;46:606–611. doi: 10.1002/1531-8249(199910)46:4<606::aid-ana8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Granella F, D’Alessandro R, Manzoni GC, Cerbo R, Colucci D’Amato C, Pini LA, Savi L, Zanferrari C, Nappi G. International Headache Society classification: Interobserver reliability in the diagnosis of primary headaches. Cephalalgia. 1994;14:16–20. doi: 10.1046/j.1468-2982.1994.1401016.x. [DOI] [PubMed] [Google Scholar]
- Greene R. Menstrual headache. In: Friedman AP, editor. Research and Clinical Studies in Headache. vol. 1. Basel: Karger; 1967. pp. 62–73. [Google Scholar]
- Greene R. Hormonal factors; Proceedings of Migraine and Manipulation Symposium; British Association of Manipulative Medicine; 1969. [Google Scholar]
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8:1–96. [PubMed] [Google Scholar]
- Heath AC, Cloninger CR, Martin NG. Testing a model for the genetic structure of personality: A comparison of the personality systems of Cloninger and Eysenck. J Pers Soc Psychol. 1994;66:762–775. doi: 10.1037//0022-3514.66.4.762. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Henry P, Michel P, Brochet B, Dartigues JF, Tison S, Salamon R. A nationwide survey of migraine in France: Prevalence and clinical features in adults. GRIM. Cephalalgia. 1993;12:229–237. doi: 10.1046/j.1468-2982.1992.1204229.x. [DOI] [PubMed] [Google Scholar]
- Honkasalo ML, Kaprio J, Winter T, Heikkila K, Sillanpaa M, Koskenvuo M. Migraine and concomitant symptoms among 8167 adult twin pairs. Headache. 1995;35:70–78. doi: 10.1111/j.1526-4610.1995.hed3502070.x. [DOI] [PubMed] [Google Scholar]
- Hsieh YY, Wang YK, Chang CC, Lin CS. Estrogen receptor α-351 XbaIG and -397 PvuIIC-related genotypes and alleles are associated with higher susceptibilities of endometriosis and leiomyoma. Mol Hum Reprod. 2007;13:117–122. doi: 10.1093/molehr/gal099. [DOI] [PubMed] [Google Scholar]
- James AH. More than menorrhagia: a review of the obstetric and gynaecological manifestations of bleeding disorders. Haemophilia. 2005;11:295–307. doi: 10.1111/j.1365-2516.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- Juang C-M, Yen M-S, Twu N-F, Horng H-C, Yu H-C, Hsu W-L. Impact of Laparoscopic Uterosacral Nerve Ablation on Menstrual Migraine. J Gynecol Surg. 2006;22:97–103. [Google Scholar]
- Kallela M, Wessman M, Fa¨rkkila¨ M. Validation of a migraine-specific questionnaire for use in family studies. Eur J Neurol. 2001;8:61–66. doi: 10.1046/j.1468-1331.2001.00165.x. [DOI] [PubMed] [Google Scholar]
- Kaunisto MA, Kallela M, Hamalainen E, Kilpikari R, Havanka H, Harno H, Nissila M, Sako E, Ilmavirta M, Liukkonen J, Teirmaa H, Tornwall O, Jussila M, Terwilliger J, Farkkila M, Kaprio J, Palotie A, Wessman M. Testing of variants of the MTHFR and ESR1 genes in 1798 Finnish individuals fails to confirm the association with migraine with aura. Cephalalgia. 2006;26:1462–1472. doi: 10.1111/j.1468-2982.2006.01228.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath A, Martin NG, Eaves LJ. Symptoms of anxiety and depression in a volunteer twin population. The etiologic role of genetic and environmental factors. Arch Gen Psychiatry. 1986;43:213–221. doi: 10.1001/archpsyc.1986.01800030023002. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Hadfield R, Barlow D, Weeks DE, Laird E, Golding S. Use of MRI in genetic studies of endometriosis. Am J Med Genet. 1997;71:371–372. [PubMed] [Google Scholar]
- Kim SH, Choi YM, Jun JK, Kim SH, Kim JG, Moon SY. Estrogen receptor dinucleotide repeat polymorphism is associated with minimal or mild endometriosis. Fertil Steril. 2005;84:774–777. doi: 10.1016/j.fertnstert.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Kirk KM, Birley AJ, Statham DJ, Haddon B, Lake RI, Andrews JG, Martin NG. Anxiety and depression in twin and sib pairs extremely discordant and concordant for neuroticism: Prodromus to a linkage study. Twin Res. 2000;3:299–309. doi: 10.1375/136905200320565274. [DOI] [PubMed] [Google Scholar]
- Kirtava A, Drews C, Lally C, Dilley A, Evatt B. Medical, reproductive and psychosocial experiences of women diagnosed with von Willebrand’s disease receiving care in haemophilia treatment centres: A case-control study. Haemophilia. 2003;9:292–297. doi: 10.1046/j.1365-2516.2003.00756.x. [DOI] [PubMed] [Google Scholar]
- Kudrow L. The relationship of headache frequency to hormone use in migraine. Headache. 1975;15:36–40. doi: 10.1111/j.1526-4610.1975.hed1501036.x. [DOI] [PubMed] [Google Scholar]
- Lance JW. Mechanism and Management of Headache. 4th edition. London: Butterworth Scientific; 1982. [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Larsson B, Bille B, Pederson NL. Genetic influence in headaches: A Swedish twin study. Headache. 1995;35:513–519. doi: 10.1111/j.1526-4610.1995.hed3509513.x. [DOI] [PubMed] [Google Scholar]
- Lichten EM, Lichten JB, Whitty A, Pieper D. The use of leuprolide acetate in the diagnosis and treatment of menstrual migraine: The role of artificially induced menopause. Headache Quarterly. 1995;6:313–317. [Google Scholar]
- MacGregor A. Migraine is associated with menstruation. Funct Neurol. 2000;15:143–153. [PubMed] [Google Scholar]
- Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Martin NG, Heath AC. Nicotine withdrawal in women. Addiction. 1997;92:889–902. [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Kearsey MJ, Davies P. The power of the classical twin study. Heredity. 1978;40:97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- Martin PL, Burnier AM, Segre EJ, Huix FJ. Graded sequential therapy in the menopause: A double blind study. Am J Obstet Gynecol. 1971;111:178–186. doi: 10.1016/0002-9378(71)90887-8. [DOI] [PubMed] [Google Scholar]
- McCutcheon AL. Latent Class Analysis. Beverly Hills, CA: Sage Publications; 1987. [Google Scholar]
- Merikangas KR, Merikangas JR, Angst J. Headache syndromes and psychiatric disorders: Association and familial transmission. J Psychiatry Res. 1993;27:197–210. doi: 10.1016/0022-3956(93)90008-p. [DOI] [PubMed] [Google Scholar]
- Michel P, Pariente P, Duru G, Dreyfus JP, Chabriat H, Henry P, Dreyfuss JP. MIG ACCESS: A population-based, nationwide, comparative survey of access to care in migraine in France. Cephalalgia. 1996;16:50–55. doi: 10.1046/j.1468-2982.1996.1601050.x. [DOI] [PubMed] [Google Scholar]
- Mulder E, van Baal C, Gaist D, Kallela M, Kaprio J, Svensson DA, Nyholt DR, Martin NG, MacGregor AJ, Cherkas LF, Boomsma DI, Palotie A. Genetic and environmental influences on migraine: A twin study across six countries. Twin Res. 2003;6:422–431. doi: 10.1375/136905203770326420. [DOI] [PubMed] [Google Scholar]
- Nattero G. Menstrual headache. Adv Neurol. 1982;33:215–226. [PubMed] [Google Scholar]
- Neale M, Cardon L. Methodology for Genetic Studies in Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling: Box 126 MCV. Richmond, VA: Department of Psychiatry; 2003. [Google Scholar]
- Nyholt DR, Gillespie NA, Heath AC, Merikangas KR, Duffy DL, Martin NG. Latent class analysis does not support migraine with aura and migraine without aura as separate entities. Genet Epidemiol. 2004;26:231–244. doi: 10.1002/gepi.10311. [DOI] [PubMed] [Google Scholar]
- Nyholt DR, Morley KI, Ferreira MAR, Medland SE, Boomsma DI, Heath AC, Merikangas KR, Montgomery GW, Martin NG. Genomewide significant linkage to migrainous headache on chromosome 5q21. Am J Hum Genet. 2005;77:500–512. doi: 10.1086/444510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien B, Goeree R, Streiner D. Prevalence of migraine headache in Canada: A population-based survey. Int J Epidemiol. 1994;23:1020–1026. doi: 10.1093/ije/23.5.1020. [DOI] [PubMed] [Google Scholar]
- Oterino A, Pascual J, Ruiz de Alegria C, Valle N, Castillo J, Bravo Y, Gonzalez F, Sanchez-Velasco P, Cayon A, Leyva-Cobian F, Alonso-Arranz A, Munoz P, Hall JM, McDonnell DP. Association of migraine and ESR1 G325C polymorphism. Neuroreport. 2006;17:61–64. doi: 10.1097/01.wnr.0000192735.85287.f4. [DOI] [PubMed] [Google Scholar]
- Rasmussen BK. Migraine and tension-type headache in a general population: Precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53:65–72. doi: 10.1016/0304-3959(93)90057-V. [DOI] [PubMed] [Google Scholar]
- Rasmussen BK, Jensen R, Olesen J. Questionnaire versus clinical interview in the diagnosis of headache. Headache. 1991a;31:290–295. doi: 10.1111/j.1526-4610.1991.hed3105290.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population—A prevalence study. J Clin Epidemiol. 1991b;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- Rindskopf D, Rindskopf W. The value of latent class analysis in medical diagnosis. Stat Med. 1986;5:21–27. doi: 10.1002/sim.4780050105. [DOI] [PubMed] [Google Scholar]
- Robert M, Derbaudrenghien JP, Blampain JP, Lamy F, Meyer P. Fibrotic processes associated with long-term ergotamine therapy. N Engl J Med. 1984;311:601–602. doi: 10.1056/nejm198408303110916. [DOI] [PubMed] [Google Scholar]
- Russell MB, Olesen J. Increased familial risk and evidence of a genetic factor in migraine. BMJ. 1995;311:541–544. doi: 10.1136/bmj.311.7004.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol. 1995;24:612–618. doi: 10.1093/ije/24.3.612. [DOI] [PubMed] [Google Scholar]
- Ryan RES. A controlled study of the effect of oral contraceptives on migraine. Headache. 1978;17:250–252. doi: 10.1111/j.1526-4610.1978.hed1706250.x. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- Somerville BW. A study of migraine in pregnancy. Neurology. 1972;22:824–828. doi: 10.1212/wnl.22.8.824. [DOI] [PubMed] [Google Scholar]
- Stein GS. Headaches in the first post partum week and their relationship to migraine. Headache. 1981;21:201–205. doi: 10.1111/j.1526-4610.1981.hed2105201.x. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–69. [PubMed] [Google Scholar]
- Stewart WF, Lipton RB, Liberman J. Variation in migraine prevalence by race. Neurology. 1996;47:52–59. doi: 10.1212/wnl.47.1.52. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Staffa J, Lipton RB, Ottman R. Familial risk of migraine: A population-based study. Ann Neurol. 1997;41:166–172. doi: 10.1002/ana.410410207. [DOI] [PubMed] [Google Scholar]
- Tervila L, Marttila P. Headache as a symptom of endometriosis externa. Ann Chir Gynaecol Fenn. 1975;64:239–241. [PubMed] [Google Scholar]
- Tietjen GE, Conway A, Utley C, Gunning WT, Herial NA. Migraine is associated with menorrhagia and endometriosis. Headache. 2006;46:422–428. doi: 10.1111/j.1526-4610.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- Tietjen GE, Bushnell CD, Herial NA, Utley C, White L, Hafeez F. Endometriosis is associated with prevalence of comorbid conditions in migraine. Headache. 2007;47:1069–1078. doi: 10.1111/j.1526-4610.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- Treloar SA, O’Connor DT, O’Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril. 1999;71:701–710. doi: 10.1016/s0015-0282(98)00540-8. [DOI] [PubMed] [Google Scholar]
- Treloar SA, Wicks J, Nyholt DR, Montgomery GW, Bahlo M, Smith V, Dawson G, Mackay IJ, Weeks DE, Bennett ST, Carey A, Ewen-White KR, Duffy DL, O’Connor DT, Barlow DH, Martin NG, Kennedy SH. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am J Hum Genet. 2005;77:365–376. doi: 10.1086/432960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio C, Gagnière B, El Amrani M, Bousser MG, Alpérovitch A. Lay versus expert interviewers for the diagnosis of migraine in a large sample of elderly people. J Neurol Neurosurg Psychiatry. 2003;74:238–241. doi: 10.1136/jnnp.74.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich V, Gervil M, Kyvik KO, Olesen J, Russell MB. The inheritance of migraine with aura estimated by means of structural equation modelling. J Med Genet. 1999;36:225–227. [PMC free article] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Haan J, Terwindt GM, Ferrari MD. Migraine: Gene mutations and functional consequences. Curr Opin Neurol. 2007;20:299–305. doi: 10.1097/WCO.0b013e3281338d1f. [DOI] [PubMed] [Google Scholar]
- Welch KMA, Darnely D, Simkins RT. The role of estrogen in migraine: A review and hypothesis. Cephalalgia. 1984;4:227–236. doi: 10.1046/j.1468-2982.1984.0404227.x. [DOI] [PubMed] [Google Scholar]
- Wessman M, Terwindt GM, Kaunisto MA, Palotie A, Ophoff RA. Migraine: A complex genetic disorder. Lancet Neurol. 2007;6:521–532. doi: 10.1016/S1474-4422(07)70126-6. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva: World Health Organization; The World Health Report 2001—Mental Health: New Understanding, New Hope. 2001