Abstract
The application of transcranial slow oscillation stimulation (tSOS; 0.75 Hz) was previously shown to enhance widespread endogenous EEG slow oscillatory activity when applied during a sleep period characterized by emerging endogenous slow oscillatory activity. Processes of memory consolidation typically occurring during this state of sleep were also enhanced. Here, we show that the same tSOS applied in the waking brain also induced an increase in endogenous EEG slow oscillations (0.4–1.2 Hz), although in a topographically restricted fashion. Applied during wakefulness tSOS, additionally, resulted in a marked and widespread increase in EEG theta (4–8 Hz) activity. During wake, tSOS did not enhance consolidation of memories when applied after learning, but improved encoding of hippocampus-dependent memories when applied during learning. We conclude that the EEG frequency and related memory processes induced by tSOS critically depend on brain state. In response to tSOS during wakefulness the brain transposes stimulation by responding preferentially with theta oscillations and facilitated encoding.
Keywords: transcranial slow oscillation stimulation, sleep, plasticity, cortex, tDCS (transcranial direct current stimulation)
The formation of long-term memories encompasses the stages of encoding and subsequent consolidation, which are linked to separate brain states. Whereas encoding takes place during wakefulness and, for hippocampus-dependent declarative memories, is associated with increased EEG theta activity (1–7), consolidation of such memories appears to be most effectively established “offline” during slow wave sleep (SWS) (8–10), a state that is closely linked to the appearance of EEG slow oscillations (11–13). Slow oscillations are global up and down states of neocortical activity with a peak frequency of ≈0.75 Hz in humans (11–16). A recent study using anodal transcranial slow oscillation stimulation (tSOS) at 0.75 Hz (17) to induce slow oscillatory activity in underlying neocortical tissue during nonrapid eye movement sleep in healthy humans provided direct evidence for a causative role of slow oscillation potential fields for the consolidation of hippocampus-dependent memories. In contrast, application of anodal transcranial oscillatory stimulation at theta frequency (5 Hz) failed to produce these effects. Thus, the study demonstrated one possible mechanism through which declarative memory consolidation during sleep is coupled to the endogenously generated slow oscillations characterizing SWS (18).
The objective of the present study was to test whether tSOS induced slow oscillations reflect a mechanism for memory consolidation that is specific for SWS, or whether induced slow oscillatory activity may also subserve memory during wakefulness. One hypothesis is that externally imposed neural dynamics resemble endogenous activity during SWS and may activate processes that internally support memory consolidation beyond sleep. Alternatively, the enhancing effect of tSOS and associated EEG changes on memory consolidation may depend on the functional brain state (19). In a series of experiments during wake, we tested whether tSOS would influence the generation of slow oscillations in the waking brain and likewise improve memory consolidation. The associated changes in EEG activity were taken as a measure of the preparedness of the brain to resonate in a certain frequency.
Results
Transcranial SOS During Quiet Wake Produces a Local Increase in Slow Oscillations and Widespread Increase in Theta and Beta EEG Activities.
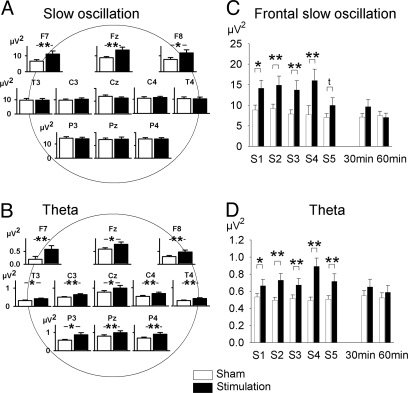
In Exp. 1 (Fig. S1), tSOS (at 0.75 Hz) induced distinct changes in EEG activity (as assessed during the 1-min stimulation-free intervals after each of the 5-min intervals of stimulation). EEG power in the slow oscillation frequency band (0.4–1.2 Hz) was distinctly increased; however, clearly restricted to the electrode sites closest to the location of the stimulating electrodes, i.e., at the frontal leads F7, Fz, and F8 (stimulation × lead: F10,150 = 4.03, P < 0.01; P < 0.05 for post hoc comparisons at respective electrode sites) (Figs. 1A and 3A). Also, the effect seemed to decrease already at the 5th stimulation period (Fig. 1C). Stimulation produced a most pronounced increase in power in the theta frequency band (4–8 Hz; stimulation: F1,15 = 34.45, P < 0.001; Figs. 1B and 3A). Notably, these effects were equally distributed across electrode sites (stimulation × lead: F10,150 = 0.73, P > 0.5). Transcranial SOS also increased beta activity (15–25 Hz, stimulation: F1,15 = 21.47, P < 0.001) (Fig. 3A). For frontal slow oscillation activity, theta and beta frequencies, power was specifically increased during the 1-min stimulation-free intervals after the five stimulation intervals, but not at 30 or 60 min after the stimulation period (stimulation × time: frontal slow oscillation, F7,105 = 1.99, P = 0.11; theta, F7,105 = 10.18, P < 0.001; beta, F7,105 = 4.89, P = 0.005) (Fig. 1 C and D; Fig. S2). All other frequency bands (i.e., delta, slow and fast alpha) were not consistently influenced.
Fig. 1.
Topographical distribution and changes over time in EEG spectral power in Exp. 1. (A and B) Topographical distribution of averaged EEG power (±SEM) across the 1-min stimulation-free intervals after the five 5-min intervals of stimulation in the slow oscillation (0.4–1.2 Hz) (A), and theta (4–8 Hz) frequency band (B). (C and D) EEG spectral power across the five 1-min intervals immediately succeeding the stimulation intervals and 30 and 60 min after termination of stimulation in the slow oscillation band at prefrontal locations, i.e., averaged across F7, Fz, and F8 (stimulation × time: F7,105 = 4.82, P < 0.01) (C), and theta band (F7,105 = 7.17, P < 0.001) (D). **, P < 0.01; *, P < 0.05; t, P < 0.1 (t Test) for comparisons between the stimulation and sham condition (n = 16).
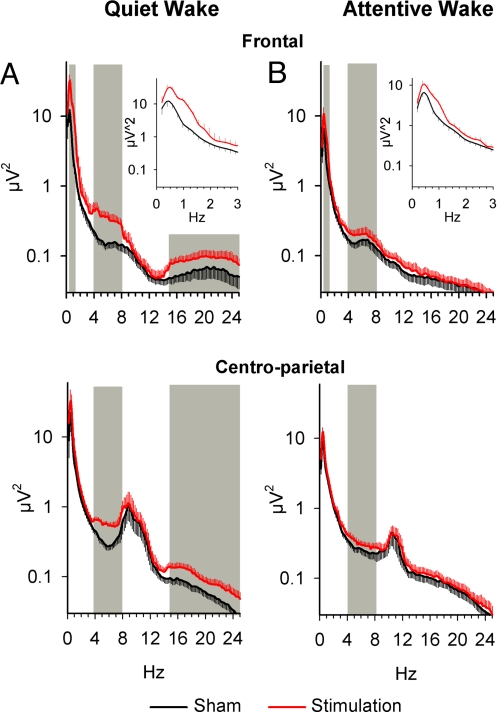
Fig. 3.
Comparative changes in EEG spectral power. tSOS (at 0.75 Hz) induced changes in subsequent EEG spectral power during quiet (A) and attentive (B) wakefulness. EEG power spectra are averages across the five 1-min stimulation-free intervals after the 5-min periods of stimulation and sham stimulation. During quiet wakefulness (Exp. 1, n = 16) and attentive wakefulness (supplementary experiment, n = 13), stimulation enhanced EEG power in the slow oscillation band (0.4–1.2 Hz) at prefrontal electrode sites, i.e., averged across F7, Fz, and F8 (Upper), but not at centro-parietal sites, i.e., across C3,Cz, C4, P3, Pz, and P4 (stimulation × lead: F10,120 = 2.44, P < 0.05, and F10,150 = 4.03, P < 0.01, respectively) (Lower). EEG power in the theta band (4–8 Hz) was enhanced over all recording sites (stimulation: A, F1,12 = 15.44, P < 0.005 and B, F1,15 = 12.85, P < 0.005, respectively). Stimulation during quiet wakefulness was additionally associated with a widespread change in beta band activity (15–25 Hz, stimulation: B, F1,15 = 21.47, P < 0.003). (Insets) Magnified changes in the 0.5–3 Hz range. Hatched areas indicate frequency bands significantly (P < 0.05) modified by tSOS.
Transcranial SOS During Quiet Wake Does Not Consolidate Memories.
Performance at learning was comparable between conditions for the four memory tasks [verbal paired-associate learning, nonverbal paired-associate learning, mirror tracing, and finger sequence tapping (P > 0.4; Table 1)]. Retention of these memories across the 7-h wake interval was not affected by tSOS, as expressed by the differences in performance at retrieval testing and learning (P > 0.3), except for a trend toward enhanced mirror tracing speed after stimulation (stimulation × time: F1,15 = 4.17, P < 0.06). Performance changes independent of stimulation are reported in SI Results. Control measures of working memory and retrieval function (digit span, word fluency tests), self- reported mood, and activation also did not differ between conditions (P > 0.1; Table S1).
Table 1.
Performance during learning and retention of memories during sham and stimulation sessions (n = 16; mean ± SEM)
| Memory task | Learning |
Retention |
||
|---|---|---|---|---|
| Sham | Stimulation | Sham | Stimulation | |
| Verbal paired-associates (recalled word-pairs) | 41.31 ± 0.76 | 42.18 ± 0.81 | −0.5 ± 0.82 | −0.56 ± 0.92 |
| Non-verbal paired-associates (recalled drawing-pairs) | 11.13 ± 0.27 | 11.44 ± 0.42 | −0.75 ± 0.53 | −1.44 ± 0.56 |
| Sequence tapping-speed (no. of sequences) | 18.79 ± 1.53 | 18.21 ± 1.18 | 1.52 ± 0.38 | 2.10 ± 1.08 |
| Sequence tapping-accuracy (no. of errors) | 0.81 ± 0.16 | 1.02 ± 0.22 | 0.4 ± 0.38 | 0.01 ± 0.34 |
| Mirror tracing-speed (s) | 78.37 ± 4.53 | 80.80 ± 4.80 | −9.86 ± 2.16 | −16.81 ± 2.51 |
| Mirror tracing-accuracy (no. of errors) | 22.78 ± 3.06 | 23.56 ± 2.71 | 5.69 ± 1.67 | 6.84 ± 3.36 |
Retention is defined by the difference in performance at retrieval testing minus performance at learning. There were no significant differences in retention between the stimulation and sham condition.
Improved Encoding of Verbal Memory by tSOS Applied During Learning.
Experiment 2 (Fig. 1B) was performed to explore the functional significance of changes in EEG activity after electrical tSOS, which revealed a particularly prominent increase in theta and beta EEG activity. These EEG frequencies are closely linked to explicit encoding of hippocampus-dependent memories (2, 3, 6, 7). We tested effects of tSOS applied as in Exp. 1, but this time while subjects engaged in encoding of declarative tasks.
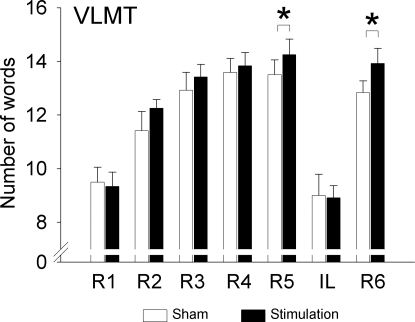
Encoding performance on the Verbal Learning and Memory Test (VLMT) was increased significantly by tSOS, mainly during later presentation of the list and during free recall after the interference list (IL) (stimulation: F1,11 = 5.49, P < 0.05) (Fig. 2). In particular, immediate free recalls of the standard list on the 5th and on the 6th trials (the latter after presentation of the IL) were significantly improved by tSOS compared with sham (F1,11 > 6.22, P < 0.03). In light of recent findings (20, 21) of effects of transcranial stimulation on the rate of false memories, we analyzed aside from total errors also the number of falsely recalled words (i.e., words not in the standard list), preservation type (repetitions) errors, and accuracy (ratio of total correct words by total cited words), but did not find any differences between the sham and tSOS conditions (each P > 0.1) (Table 2; Table S2). Recognition performance on the number list learning task was not sensitive to tSOS (F1,11 < 0.74, P > 0.41) (Table 2), supporting other studies on differential mechanisms underlying free-recall and recognition (22–24). Control measures of working memory, vigilance, and alertness [digit span, Positive and Negative Affect Schedule (PANAS), Stanford Sleepiness Scale] were not changed by tSOS (P > 0.2) (Table 2; Fig. S1).
Fig. 2.
Learning performance on the VLMT. Number of words recalled on the five learning trials on the standard list (R1-R5), the IL, and at immediate recall (R6) of the standard list. Encoding performance on the VLMT was significantly increased during tSOS (F1,11 = 5.49, P < 0.05) especially during the later presentations of the standard list. *, P < 0.05 for post hoc comparisons between the stimulation and sham condition (n = 12).
Table 2.
Performance measures in experiment 2 (n = 12; mean ± SEM)
| Test | Sham | Stimulation |
|---|---|---|
| VLMT, errors | ||
| R1 (standard list) | 0.33 ± 0.19 | 0.17 ± 0.11 |
| R2 | 0.83 ± 0.24 | 0.33 ± 0.14 |
| R3 | 0.92 ± 0.31 | 0.42 ± 0.19 |
| R4 | 0.58 ± 0.26 | 0.92 ± 0.34 |
| R5 | 0.58 ± 0.26 | 0.33 ± 0.19 |
| IL | 0.17 ± 0.11 | 0.67 ± 0.36 |
| R6 | 0.33 ± 0.26 | 0.17 ± 0.11 |
| Delayed recall | 0.75 ± 0.28 | 0.33 ± 0.14 |
| No. list learning, recognized nos. | ||
| List 1, nos.; % | 83.6 ± 3.1 | 82.6 ± 3.1 |
| List 2, nos.; % | 83.3 ± 2.7 | 79.9 ± 4.7 |
| Psychometric tests | ||
| Digit span forward (digits) | 8.16 ± 0.60 | 8.92 ± 0.69 |
| Digit span backward (digits) | 7.58 ± 0.62 | 8.00 ± 0.79 |
| Control tests, before learning | ||
| Stanford Sleepiness Scale | 2.25 ± 0.21 | 2.08 ± 0.34 |
| PANAS, positive score | 4.61 ± 0.68 | 4.82 ± 0.82 |
| PANAS, negative score | 2.88 ± 0.56 | 3.02 ± 0.62 |
| Control tests, after learning | ||
| Stanford Sleepiness Scale | 2.25 ± 0.30 | 2.08 ± 0.33 |
| PANAS, positive score | 4.21 ± 0.51 | 4.57 ± 0.65 |
| PANAS, negative score | 2.46 ± 0.40 | 3.06 ± 0.64 |
For all measures, sham vs. stimulation comparisons were nonsignificant.
Common EEG Effects Are Produced by tSOS in Attentive and Quiet Wakefulness.
The Supplementary experiment was performed to assure that EEG changes induced by tSOS during attentive wakefulness, i.e., during encoding of declarative memories in Exp. 2, were comparable with those revealed during quiet wakefulness in Exp. 1. Indeed, like during quiet wakefulness, tSOS during learning of word lists induced a significant increase in slow oscillation power (0.4–1.2 Hz) during the 1-min intervals after each of the five stimulation intervals, with this increase again restricted to sites closest to the stimulating electrodes, i.e., at frontal locations F7, Fz, and F8 (stimulation × lead: F10,120 = 2.49, P < 0.05; P < 0.05 for post hoc comparisons at these leads) (Fig. 3B). Notably, tSOS was also accompanied by a prominent and widespread increase in theta (4–8 Hz) activity distributed almost equally across electrode sites (stimulation main effect: F1,12 = 12.71, P < 0.005; Fig. 3B) (stimulation × lead: F10,120 = 2.10, P > 0.05). During attentive wakefulness, tSOS did not modify beta activity (15–25 Hz; stimulation: F1,12 = 0.32, P > 0.5). Both slow oscillation power and theta band activity were increased only during the five stimulation-free intervals, but not at 30 or 60 min after the stimulation period (stimulation × time, frontal slow oscillation, F7,84 = 1.84, P < 0.1, theta, F7,84 = 7.82, P < 0.001) (data not shown). All other frequency bands were not significantly influenced.
Discussion
The application of tSOS during a wake retention interval (i) enhanced frontal EEG slow oscillation activity, but was otherwise associated with distinctly different effects than those observed previously after tSOS during sleep (18); (ii) tSOS during quiet and attentive wakefulness enhanced global activity in the theta (4–8 Hz) frequency band [beta activity (15–25 Hz) was additionally enhanced during quiet wakefulness]; (iii) retention of declarative memories during the wake retention interval was not improved; (iv) instead, tSOS during the process of encoding improved learning performance as assessed by immediate recall of words learned in the presence of stimulation. Our findings indicate that the effects of tSOS on memory function critically depend on the functional brain state (19, 25). The presence of slowly oscillating potential fields restricted to prefrontal cortical tissue does not support memory consolidation beyond sleep. Instead, tSOS during wakefulness promotes memory encoding presumably by globally increasing theta EEG oscillations.
Increased EEG Slow Oscillation Activity.
The ability of tSOS to enhance slow oscillatory activity even during wakefulness in the prefrontal cortex, which is the preferential source of the endogenous sleep slow oscillation (26, 27), may indicate increased frontocortical susceptibility to electric field fluctuations in this frequency. It has been suggested that the initiation of the slow oscillation results from a focus of increased excitability arising from the coincidence of spontaneously occurring miniature depolarizing events (28). This activity may then spread to neighboring neurons; thus, incorporating previously silent cells into an emergent oscillating network. Our tSOS appears sufficient to induce an avalanche response measurable during a short time period in the immediate aftermath of stimulation.
The amplitude of the slow oscillations is thought to reflect global synaptic strength in underlying circuitry with stronger synaptic connectivity resulting in enhanced oscillations with steeper wave slopes (29–31). Global synaptic strength in underlying cortical networks may be low in the beginning of the wake phase, i.e., the time the experiments were conducted, in comparison with conditions in the evening when synaptic potentiation has accumulated over the day (32). On this background, the stimulation-induced increase in slow oscillation activity would be expected to be more pronounced after prolonged waking. This time effect, together with a generally increased inhibitory level of neocortical networks during the wake state, could also explain that the tSOS induced increase in endogenous slow oscillation activity was not only less pronounced, but also topographically more restricted to prefrontal cortical areas here than observed after tSOS applied during sleep (18).
Increased EEG Theta Activity and Improved Encoding.
Transcranial SOS during waking enhanced encoding rather than consolidation of declarative memories, and this effect was associated with a widespread increase in EEG theta activity, but only a restricted increase in slow oscillation activity. Why was specifically theta oscillation enhanced by tSOS and not, for example, alpha activity dominant during quiet wakefulness (33)? One simple reason may be that during quiet wakefulness, alpha activity has a posterior focus that was less affected by tSOS location over the dorsolateral prefrontal cortex than EEG theta activity for which a prefrontal current source has been estimated (34). A preferred coupling between slowly oscillating potential fields ≈0.75 Hz and oscillations at theta frequency may, however, reflect a basic functional relationship between the cortical networks underlying these oscillatory frequencies. Spontaneous slow oscillatory EEG activity emerges on a global scale at the transition into SWS, whereby theta activity prevails in the active waking state. Based on the correlation between EEG theta activity in waking and slow wave activity in sleep, it has been proposed that both oscillations are markers of a common homeostatic sleep process (35, 36). Neocortical slow oscillatory and hippocampal theta activity are also proposed to have complementary roles in the coupling of neocortical and hippocampal systems during hippocampus-dependent memory formation: Whereas theta is functionally related to working memory processes that enable the explicit encoding of episodic memories during active wakefulness, slow oscillations during SWS reveal a distinct grouping influence on hippocampal sharp-wave ripples and an associated neuronal replay of memory representations, which is thought to promote the redistribution of these representations to neocortical networks for long-term storage (9, 37–40). The generation of neocortical EEG slow oscillations depends at least partially on prior use of the same networks during encoding (11, 12, 41). Thus, in prefrontal-hippocampal networks involved in the formation of hippocampus-dependent memories, theta activity critical to explicit encoding in the waking brain may translate into slow oscillation activity during SWS, supporting the consolidation of these memories. However, the cellular mechanisms involved in the induction by tSOS of enhanced theta and, to a lesser extent, beta frequency activity and the wide-spread nature of these responses are presently unclear. The dependence of the theta enhancement by tSOS on the waking as opposed to the sleeping state points toward the relevance of a general increase in network excitability conveyed, among others, by increased cholinergic tone (42, 43). Increased network excitability might have been additionally promoted by the anodal component of tSOS.
A large body of data on performance-related EEG (and MEG) theta activity in rodents and humans has indicated its involvement in various aspects of memory processing, as well as in working memory, attention, and motivation-related functions (44, 45). In humans, in relation to episodic memory, enhanced EEG theta power and/or coherence have mostly been reported during encoding, but also during retrieval of verbal and spatial memories (3, 6, 7, 15, 46–48). During spatial navigation, theta activity increased with maze length, whereas at difficult maze junction, gamma rather than theta band activity was increased (48, 49), suggesting a permissive attention-related role for theta activity, whereas gamma activity (which for technical reasons was not measured here) more directly relates to the encoding and retrieval of specific information. On this background, the improved encoding performance observed here during tSOS is well in line with reported EEG theta enhancement in association with increased sustained attentional processes and working memory demands, although a more immediate contribution to the encoding process cannot be ruled out. A closer relation of theta activity to attentional aspects of stimulus processing might also explain why the overall moderate size of the tSOS effect on encoding became more robust for the last repetitions of the word list. The effect size might furthermore be related to the fact that our subjects were healthy university students showing optimum encoding capabilities under sham conditions.
Of note, the encoding improvement by stimulation was observed primarily for free recall measures (of the VLMT), but not for recognition measures (number recognition task). Unlike recognition, free recall requires the subject to generate his own retrieval cues and, hence, profits particularly from a high level of integration of an acquired memory representations into preexisting knowledge networks (24). Thus, tSOS enhancing later free recall, but not recognition may improve encoding efficiency primarily by increasing associative connectivity of acquired representations rather than by increasing the strength of associations per se. This view is also consistent with the concept of EEG theta oscillations during waking reflecting long-range cortical network activity (49). Increased associative connectivity as measured by long-range theta coherence during working memory and encoding tasks has been reported between prefrontal and temporoparietal neocortical regions (1, 4, 34, 48), and is interpreted as a functional binding of widely distributed cortical assemblies (49–51). Enhanced associative connectivity has likewise been observed between hippocampus and occipital-temporal neocortex using the same encoding paradigm as used in the present study (52). However, it has to be emphasized that these previous studies were all correlative in nature, not excluding that theta activity represents a mere epiphenomenon of task performance. By contrast, here, EEG theta activity was increased by electrical stimulation independent of the subject's engagement in any task performance, providing evidence in humans for a functional significance of the EEG theta rhythm in facilitating processes of encoding (53). On this background, the finding of enhanced verbal encoding after tSOS provides strong evidence for a functional role of theta oscillations in the encoding of hippocampus-dependent memories.
In conclusion, the present study contributes essentially to the debate on the functional relevance of EEG oscillatory activity (54). Our findings support the concept that neocortical EEG theta activity is not merely an epiphenomenon of activity subserving sustained attention and encoding, but that cortical networks oscillating in theta frequency range are functionally relevant. Also, a physiological link between potential fields and networks oscillating at slow oscillation and theta frequencies is implicated.
Methods
Subjects and General Procedure.
Twenty-eight subjects (19 to 35 years), all native German speakers, nonsmokers, medication-free, and of comparable education level (students or highly educated professionals) participated in two main experiments after having given informed written consent. Presence or history of epilepsy, paroxysms, cognitive impairments, mental, hormonal, metabolic, circulatory disorders, or sleep disturbances formed the exclusion criteria. The experimental protocol was approved by the ethics committee of the University of Lübeck.
For all experiments, subjects were first introduced to the experimental setup. They were asked to sit relaxed in a chair in a sound-attenuated room with dimmed light and a PC monitor. The experiments proper consisted each of a stimulation condition and a sham stimulation condition, separated by an interval of at least 10 days and balanced in order across subjects. Sixteen subjects (nine female, seven male), mean age 23.8 ± 4.3 years (range: 19.4–34.7 years) participated in Exp. 1, and 12 subjects (nine female, three male), mean age of 23.8 ± 2.2 years (range 20.6–27.5 years) in Exp. 2. Thirteen subjects (six female, seven male; mean age of 24.08 ± 2.69 years; range 19.6–27.2 years) participated in a supplementary experiment.
tSOS.
Transcranial SOS was identical to that described previously by Marshall et al. (17, 18). Stimulation current fluctuated between 0 and 260 μA, with 0.33 s-on/0.33 s-off periods, and rising and falling slopes of 0.33 s; thus, resulting in a 0.75-Hz oscillating stimulation (tSOS). Anodal electrodes (8-mm diameter) were positioned bilaterally at fronto-lateral locations (F3 and F4) according to the international 10–20 system, with reference electrodes placed at both mastoids. Electrode resistance was <2 kOhm. The estimated maximum current density at the stimulating electrodes corresponded to 0.517 mA/cm2. Pilot experiments and postexperimental debriefing of subjects assured that stimulation was not felt by the subjects so that they were completely unaware of being stimulated or not. Stimulation consisted of five 5-min epochs of stimulation separated by 1-min stimulation-free intervals. In the sham sessions, electrodes were positioned on the scalp as in the stimulation sessions, but the stimulator remained off.
Procedure of Experiment 1.
Subjects arrived at the laboratory at 8:00 h. After preparation for tSOS and EEG recordings, subjects performed on tasks of declarative memory (verbal and nonverbal paired-associate learning) and procedural memory (finger sequence tapping and mirror tracing) between 9:00 and 10:00 h (learning period). For details, see SI Methods. The order of tasks was balanced across subjects, with two different versions of each task also balanced across stimulation and sham conditions. Subjects were then seated in the recording room for continuous EEG recordings and tSOS. Stimulation started ≈20 min after the end of the learning period, with this postlearning delay approximately equivalent to that of a previous study with tSOS during postlearning sleep (18). EEG was recorded continuously between 20 min before stimulation/sham stimulation until 1 h after stimulation had ended while subjects watched dynamically shifting abstract color images on a PC monitor and listened to relaxing instrumental music. EEG recordings lasted until shortly before 12:00 h. Retrieval performance on the memory tasks was tested 7 h after the learning period, i.e., at 17:00 h. During the last 5 h of the 7-h retention period, participants could choose to engage in different standardized activities involving low cognitive demand and/or slightly exerting physical activity (e.g., taking a walk, bicycle riding). They were not allowed to take in coffee, tea, chocolate, or any kind of drugs, to read, write, or watch television or to nap. Also, they were asked not to rehearse any of the learned material. Adherence to these instructions was confirmed in a post experimental interview (Fig. S1A).
To control for possible confounding influences of changes in arousal, mood, and motivation, before the learning and retrieval periods, the PANAS (55), and an adjective check list describing the subjects' current mood and motivation on various dimensions (56) were applied (psychometric control tests; Fig. S1A). At retrieval, testing subjects performed on a word fluency task to assess the general capability to retrieve information from long-term memory (57), and on the digit span test of the Wechsler Adult Intelligence Scale to control for global changes in working memory function. Activation level was assessed by the Stanford Sleepiness Scale.
EEG Recordings and Analyses in Experiment 1.
The EEG was recorded using Ag/AgCl electrodes placed according to the 10–20 system (at F7, Fz, F8, T3, C3, Cz, C4, T4, P3, Pz, and P4), all referenced to an electrode attached to the nose. The ground was placed on the forehead. Recordings were amplified (1,000 μV/V), filtered between 0.05 and 30 Hz (5083 Syn-Amps; Neuroscan), and sampled with 200 Hz (5 ms) and 16-bit precision. Also, horizontal and vertical eye movements and the electromyogram (from the chin) were recorded for artifact detection. All recordings were stored on a PC for later off-line analyses.
Analyses were conducted using Brain Vision Analyzer (Version 1.05; Brain Products). After applying an ocular correction to the entire EEG raw data signal (58), eight EEG intervals were selected for further EEG analyses: a 1-min interval before tSOS, five 1-min stimulation-free intervals immediately after each 5-min period of stimulation (including the interval after the last stimulation), and 1-min EEG epochs 30 and 60 min after the end of the fifth stimulation. EEG signals during corresponding time intervals after recording begin were taken in the sham condition. For each 1-min interval, fast Fourier Transformations (frequency resolution = 0.195 Hz) were applied to 8–12 artifact-free (based on visual inspection) EEG segments of 5.12 s duration (1,024 points × 5 ms), using a Hanning window of 20% before power spectra calculation. After individual mean power spectra across all segments of each time interval were calculated and subjected to a three-point moving average, mean EEG power in the following six frequency bands were calculated for all eight time intervals: slow oscillations (0.4–1.2 Hz), delta (1–4 Hz), theta (4–8 Hz), slow alpha (8–12 Hz), fast alpha (12–15 Hz), and beta (15–25 Hz).
Procedure of Experiment 2.
Here, tSOS was applied during learning, i.e., while subjects encoded declarative memories. Subjects arrived at the laboratory at 9:00 h. After preparation for tSOS, they performed a modified version of the VLMT. Before and after the VLMT, subjects conducted a number list learning task. Immediately after the second number list learning task, the digit span test was applied to control for working memory function. Before and after the experiments, the subject's arousal level, mood, and motivation were assessed by PANAS, an adjective check list, and the Stanford Sleepiness Scale as described in the procedures of Exp. 1. The VLMT and number list learning were performed during the 5-min intervals of stimulation. Parallel versions of the tasks were balanced across the stimulation and sham conditions. In these experiments EEG was not recorded (Fig. S1B).
Learning Tasks in Experiment 2.
In the VLMT (i.e., the German version of the Rey Auditory Verbal Learning Test), a standard word list consisting of 15 semantically unrelated words (nouns) was orally presented five times, with each run followed immediately by a free recall, in which the subject had to orally recall as many of the presented words as possible (R1-R5). Words from all lists were presented each for 1 s by a prerecorded neutral male voice. Subjects had unlimited time to recall the words. Immediately after the fifth run, a second list of 15 semantically unrelated words different from those in the standard word list (i.e., the IL) was presented in the same way as the standard list, and again, the subject had to recall as many of the words as possible. After the IL, subjects were requested to recall again the 15 words from the standard list, now without prior presentation of the list words (R6 in Fig. 2). Performance measures were the number of recalled words and the number of errors (defined as words named by the subjects but not contained in the corresponding list).
In the number list learning task, adapted from ref. 59, each of 16 two-digit numbers ranging from 12 to 99 were presented on a monitor sequentially for 2 s in randomized order, with interstimulus intervals of 500 ms. Each test consisted of two runs, in which 1 min after presentation, recognition was tested by presenting all 16 numbers of the old list randomly interspersed among 16 new numbers. The subjects had to report orally whether or not a number belonged to the old list.
EEG Recording and Analyses During Learning in the Supplementary Experiment.
Because in Exp. 2 EEG was not recorded while subjects were attending to task performance, the objective of this supplementary experiment was to measure whether tSOS during attentive wakefulness induces the same EEG responses as during quiet wakefulness in Exp. 1. EEG recording was conducted as in Exp. 1, while subjects performed the modified version of the VLMT as in Exp. 2. During EEG recording, subjects were to fixate a large red dot on a black monitor screen ≈100 cm in front of them, and requested to suppress eye movements and blinking. Transcranial SOS and EEG recordings were conducted as described above with the exception that the length of stimulation-free intervals was not strictly 1-min, to obtain EEG epochs free of speech. VLMT performance confirmed results of Exp. 2, and are not reported here.
Statistical Analyses.
Data were analyzed using SPSS version 11.5 for Windows. Normal distribution of data were assured by Kolmogorov–Smirnov test. EEG power was separately analyzed in the six frequency bands of interest using three-way analyses of variance (ANOVA) with the factors stimulation (stimulation vs. sham), time (baseline, 1-min stimulation-free intervals 1–5, 30 and 60 min after stimulation), and lead (F7, Fz, F8, T3, C3, Cz, C4, T4, P3, Pz, and P4). Three-way ANOVAs were also conducted using the difference in EEG power at the 7 time points referenced to the 1-min prestimulation baseline interval. Only robust results showing significance in analyses of both absolute and difference power values (and surviving Bonferroni correction) are reported here (referring to the analyses of absolute values). Post hoc t tests were used to specify significant interactions.
Memory retention (defined by the difference in performance at retrieval testing and during learning) was analyzed by one-way ANOVA with the factor stimulation (stimulation vs. sham). One-way ANOVAs were performed separately for performance at learning and retrieval testing. Numbers of recalled words on the VLMT were first analyzed by two-way ANOVA with the factors stimulation and list (R1-R6). Because the number of errors on the VLMT was not normally distributed, these data were analyzed using Wilcoxon's tests. Analyses of all control tests (digit span, word fluency, etc.) were based on ANOVA.
Supplementary Material
Acknowledgments.
We thank Dr. Matthias Mölle for discussion of the manuscript, Prof. Vasil Kolev for help with data analyses, and Sophie Peron, Max Rohwer, and Teodora Lambrinova for technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 654.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904438106/DCSupplemental.
References
- 1.Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von SA. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95:7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mölle M, Marshall L, Fehm HL, Born J. EEG theta synchronization conjoined with alpha desynchronization indicate intentional encoding. Eur J Neurosci. 2002;15:923–928. doi: 10.1046/j.1460-9568.2002.01921.x. [DOI] [PubMed] [Google Scholar]
- 3.Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauseng P, et al. Theta coupling in the human electroencephalogram during a working memory task. Neurosci Lett. 2004;354:123–126. doi: 10.1016/j.neulet.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Kahana MJ. The cognitive correlates of human brain oscillations. J Neurosci. 2006;26:1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osipova D, et al. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci. 2006;26:7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caplan JB, Glaholt MG. The roles of EEG oscillations in learning relational information. NeuroImage. 2007;38:604–616. doi: 10.1016/j.neuroimage.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 8.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 9.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Rasch B, Born J. Maintaining memories by reactivation. Curr Opin Neurobiol. 2007;17:698–703. doi: 10.1016/j.conb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 12.Mölle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci USA. 2004;101:13963–13968. doi: 10.1073/pnas.0402820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman KL, et al. The upshot of up states in the neocortex: From slow oscillations to memory formation. J Neurosci. 2007;27:11838–11841. doi: 10.1523/JNEUROSCI.3501-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achermann P, Borbely AA. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 15.Mölle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci. 2002;22:10941–10947. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steriade M, Nunez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall L, Mölle M, Born J. Oscillating current stimulation - slow oscillation stimulation during sleep. Nat Protoc. 2006 doi: 10.1038/nprot.2006.299. [DOI] [Google Scholar]
- 18.Marshall L, Helgadottir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 19.Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Boggio PS, et al. Temporal lobe cortical electrical stimulation during the encoding and retrieval phase reduces false memories. PLoS ONE. 2009;4:e4959. doi: 10.1371/journal.pone.0004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallate J, Chi R, Ellwood S, Snyder A. Reducing false memories by magnetic pulse stimulation. Neurosci Lett. 2009;449:151–154. doi: 10.1016/j.neulet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Hasselmo ME, Wyble BP. Free recall and recognition in a network model of the hippocampus: Simulating effects of scopolamine on human memory function. Behav Brain Res. 1997;89:1–34. doi: 10.1016/s0166-4328(97)00048-x. [DOI] [PubMed] [Google Scholar]
- 23.Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tulving E, Madigan SA. Memory and verbal learning. Annu Rev Psychol. 1970;21:437–484. [Google Scholar]
- 25.Kanai R, Chaieb L, Antal A, Walsh V, Paulus W. Frequency-dependent electrical stimulation of the visual cortex. Curr Biol. 2008;18:1839–1843. doi: 10.1016/j.cub.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy M, et al. Source modeling sleep slow waves. Proc Natl Acad Sci USA. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- 29.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol. 2005;93:1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 30.Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30:1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer J, Schuster HG, Claussen JC, Mölle M. Corticothalamic projections control synchronization in locally coupled bistable thalamic oscillators. Phys Rev Lett. 2007;99 doi: 10.1103/PhysRevLett.99.068102. 068102. [DOI] [PubMed] [Google Scholar]
- 32.Tononi G, Cirelli C. Staying awake puts pressure on brain arousal systems. J Clin Invest. 2007;117:3648–3650. doi: 10.1172/JCI34250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laufs H, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuhara H, Yamaguchi Y. Human cortical circuits for central executive function emerge by theta phase synchronization. NeuroImage. 2007;36:232–244. doi: 10.1016/j.neuroimage.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: Correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 36.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Buzsáki G. Memory consolidation during sleep: A neurophysiological perspective. J Sleep Res. 1998;7:17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 38.Mölle M, Born J. Hippocampus whispering in deep sleep to prefrontal cortex–for good memories? Neuron. 2009;61:496–498. doi: 10.1016/j.neuron.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battaglia FP, Benchenane K, Khamassi M, Peyrache A, Wiener SI. In: Advances in Cognitive Neurodynamics ICCN 2007. Wang R, Gu F, Shen E, editors. Netherlands: Springer; 2008. pp. 285–288. [Google Scholar]
- 41.Huber R, et al. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao W, Wu JY. Propagating wave and irregular dynamics: Spatiotemporal patterns of cholinergic theta oscillations in neocortex in vitro. J Neurophysiol. 2003;90:333–341. doi: 10.1152/jn.00715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukatch HS, MacIver MB. Physiology, pharmacology, and topography of cholinergic neocortical oscillations in vitro. J Neurophysiol. 1997;77:2427–2445. doi: 10.1152/jn.1997.77.5.2427. [DOI] [PubMed] [Google Scholar]
- 44.Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal-midline theta from the perspective of hippocampal “theta.”. Prog Neurobiol. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Klimesch W, et al. Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neurosci Lett. 2001;302:49–52. doi: 10.1016/s0304-3940(01)01656-1. [DOI] [PubMed] [Google Scholar]
- 47.Weiss S, Rappelsberger P. Long-range EEG synchronization during word encoding correlates with successful memory performance. Brain Res Cogn Brain Res. 2000;9:299–312. doi: 10.1016/s0926-6410(00)00011-2. [DOI] [PubMed] [Google Scholar]
- 48.Weiss S, Muller HM, Rappelsberger P. Theta synchronization predicts efficient memory encoding of concrete and abstract nouns. Neuroreport. 2000;11:2357–2361. doi: 10.1097/00001756-200008030-00005. [DOI] [PubMed] [Google Scholar]
- 49.Mizuhara H, Wang LQ, Kobayashi K, Yamaguchi Y. A long-range cortical network emerging with theta oscillation in a mental task. Neuroreport. 2004;15:1233–1238. doi: 10.1097/01.wnr.0000126755.09715.b3. [DOI] [PubMed] [Google Scholar]
- 50.Sammer G, et al. Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Hum Brain Mapp. 2007;28:793–803. doi: 10.1002/hbm.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: Local activity and interregional connectivity in the theta range. Eur J Neurosci. 2007;25:587–593. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- 52.Babiloni C, et al. Hippocampal, amygdala, and neocortical synchronization of theta rhythms is related to an immediate recall during Rey auditory verbal learning test. Hum Brain Mapp. 2008;30:2077–2089. doi: 10.1002/hbm.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNaughton N, Ruan M, Woodnorth MA. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16:1102–1110. doi: 10.1002/hipo.20235. [DOI] [PubMed] [Google Scholar]
- 54.Sejnowski TJ, Paulsen O. Network oscillations: Emerging computational principles. J Neurosci. 2006;26:1673–1676. doi: 10.1523/JNEUROSCI.3737-05d.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1998;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 56.Janke W, Debus G. Die Eigenschaftswörterliste EWL. Eine mehrdimensionale Methode zur Beschreibung von Aspekten des Befindens. Göttingen, Germany: Hogrefe; 1978. [Google Scholar]
- 57.Aschenbrenner S, Tucha KW, Lange W. Regensburg Word Fluency Test. Göttingen, Germany: Hogrefe; 2000. in German. [Google Scholar]
- 58.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 59.Rasch BH, Born J, Gais S. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J Cognit Neurosci. 2006;18:793–802. doi: 10.1162/jocn.2006.18.5.793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.