Abstract
Vitamin D may be of interest in the prevention of cognitive impairment, though previous findings are inconclusive. Participants were 1766 adults aged 65 years and older from the Health Survey for England 2000, a nationally representative population-based study. Cognitive impairment was assessed using the Abbreviated Mental Test Score. The cross-sectional relation of serum 25-hydroxyvitamin D quartiles to cognitive impairment was modeled using logistic regression. In all, 212 participants (12%) were cognitively impaired. Odds ratios (95% confidence intervals) for cognitive impairment in the first (8–30 nmol/L), second (31–44 nmol/L), and third (45–65 nmol/L) quartiles of serum 25-hydroxyvitamin D compared with the fourth (66–170 nmol/L) were 2.3 (1.4–3.8), 1.4 (0.8–2.4), and 1.1 (0.6–1.9), after adjustment for age, sex, education, ethnicity, season of testing, and additional risk factors for cognitive impairment (P for linear trend = .001). Our data suggest low serum 25-hydroxyvitamin D is associated with increased odds of cognitive impairment.
Keywords: cognitive impairment, dementia, risk factors, vitamin D, serum 25-hydroxyvitamin D
Introduction
Vitamin D deficiency is common in older adults as a result of restricted sunlight exposure, reduced capacity of the skin to produce vitamin D, and reduced dietary intake.1 Vitamin D deficiency in elderly adults is associated with fracture risk, mortality, and various chronic conditions such as type 2 diabetes, although oral supplementation appears to be effective, well tolerated, and economical.2–4 Vitamin D may also be of interest in the prevention of neurodegenerative diseases as ample evidence from in vitro and animal experiments suggests an important role in the expression of neurotrophic factors, the stimulation of adult neurogenesis, calcium homeostasis, and detoxification.5–7 Cognitive impairment is common in elderly adults, although its etiology remains unclear,8,9 and a causal relationship between vitamin D status and brain dysfunction would have major public health implications.5
A number of small clinical studies suggest that serum 25-hydroxyvitamin D [25(OH)D] concentration, an effective indicator of vitamin D status,10 may be associated with dementia and cognitive function. For example, serum 25(OH)D concentrations were lower in 20 female mild dementia cases in comparison with 40 cognitively normal female controls.11 Similarly, a monotonic decrease in serum 25(OH)D was observed when 58 patients with severe Alzheimer’s disease (AD) and 42 mild AD cases were compared with 100 controls.12 However, no significant difference in levels of serum 25(OH)D was observed when comparing 16 dementia cases and 16 controls. 13 Levels of serum 25(OH)D were positively associated with Mini-Mental State Examination (MMSE) scores in 225 outpatients with AD,14 32 patients attending a memory clinic,15 and 2 of 4 measures of cognitive performance in 40 patients with AD and 40 controls.16 However, no association was observed between MMSE scores and serum 25(OH)D concentration in 44 self-neglecting older adults,17 or between serum 25(OH)D and cognitive function across a range of neuropsychological tests in 148 nonsmokers.18
To our knowledge, only 1 previous analysis on this topic has incorporated a large representative population-based sample. McGrath and colleagues observed no linear association between serum 25(OH)D and verbal memory performance in 4809 noninstitutionalized older adults.19 Evidence from well-designed trials is also lacking, though vitamin D supplementation was associated with a significant improvement in choice reaction time after 6 months in 139 ambulatory participants with a history of falls.20 Following vitamin D supplementation, a modest increase in clock drawing test performance, though not verbal fluency, was also observed over 4 weeks in 25 nursing home residents with low 25(OH)D status at baseline.21 Given the paucity of reliable existing evidence, we examined whether low levels of serum 25(OH)D were associated with increased odds of cognitive impairment in a large representative sample of elderly adults.
Methods
Participants
Participants were from the Health Survey for England 2000 (HSE), a nationally representative population-based study of the adult and child population living in private households plus adults aged 65 years and older resident in institutions.22 For community residents, a stratified sample of 360 postcode sectors (geographic areas) were selected as primary sampling units, and 19 addresses were selected from each of these, giving a total selected sample of 6840 addresses, of which 91% contained private households. All people within eligible households were invited to participate, and 7988 interviews were conducted, of which 1677 were aged 65 years or older (the response rate for this group was 71%). For the institutionalized sample, care homes were selected through multilevel stratified sampling by geographic area. A total of 6 residents aged 65 years and older were randomly selected from each care home. Interviews were conducted in person or using proxies where appropriate. Of the 607 care homes sampled, 544 (90%) took part. Of the 2493 interviews, 1220 (49%) were conducted in person and 1273 (51%) by proxy. A blood sample was obtained from 1818 participants who gave written informed consent, and a valid serum 25(OH)D sample was obtained from 1766 participants (708 men and 1058 women). The HSE was approved by the London Multicentre Research Ethics Committee and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Serum 25(OH)D Concentration
Nonfasting blood samples were collected in a standardized fashion into 6-mL plain tubes (no anticoagulant) by nurses during the HSE.22 Specimens were then dispatched to the Royal Victoria Infirmary in Newcastle, England, for analysis. Analyses were carried out according to standard operating procedures by State Registered Medical Laboratory Scientific Officers under the supervision of the senior officer. Analyses were carried out by the supraregional assay service through a manual 2-step procedure using the DiaSorin Kit (Stillwater, Minn). Step 1 involved rapid extraction of 25(OH)D and related metabolites from serum using acetonitrile. Following extraction, the sample was assayed using an equilibrium radiolabeled antibody procedure whereby an antibody specific for 25(OH)D was incubated with the sample and radiolabeled 125I for 90 minutes at 20°C to 25°C. Separation of bound from free 25(OH)D was achieved by a second 20-minute incubation with an antibody-precipitating complex (donkey antigoat complex/polyethylene glycol). Finally, nonspecific binding (NSB)/addition buffer was added prior to centrifugation and decanting to reduce the interference by NSB. Monthly internal quality control results and external quality assessment results for serum 25(OH)D were acceptable.22
Cognitive Impairment
Cognitive function was measured using the Abbreviated Mental Test (AMT), a widely used neurocognitive screening instrument composed of 10 items primarily covering attention, orientation in time and space, and memory.23 Cognitive impairment was defined on the basis of 3 or more incorrect responses of 10. Such a cut-point has been used in previous studies and is a sensitive (81%) and specific (85%) marker of cognitive impairment.24–26 The AMT is also highly correlated with the Mini-Mental State Examination (r = .86–.87).27
Statistical Analysis
Two-sample t tests (equal variances not assumed) and Fisher exact tests were used as appropriate to compare unadjusted continuous and categorical characteristics of the study population by cognitive status. Multivariable logistic regression models were used to determine the relationship of serum 25(OH)D to cognitive impairment. We divided levels of serum 25(OH)D (nmol/L) into quartiles to aid clinical interpretation. In basic adjusted models, we adjusted for age in years, sex, education, and ethnicity (collapsed into white and ethnic minorities) as these are established potential confounders in cognitive research28 and season during which testing occurred to account for seasonal variation in sunlight exposure. In fully adjusted models, we also adjusted for additional correlates of cognitive impairment: current smoking status, alcohol consumption (g/d), psychiatric morbidity (>3 symptoms on the 12-item General Health Questionnaire),29 hypoalbuminemia (serum albumin <3.5 g/dL), and self-reported medical history (diagnosed diabetes, cardiovascular disease, stroke, and hypertension). We also adjusted for impaired mobility (those reportingmoderate or severe difficulty walking) as reduced outdoor activity may result in lowered serum 25(OH)D concentrations.30
Possible 2-way interactions between serum 25(OH)D and age or sex were tested by including product terms in a fully adjusted logistic regression model. As a secondary analysis, we examined whether the same pattern of associations was observed when men and women were analyzed separately or whether institutionalized participants were excluded. An alternative cut-point of 4 or more incorrect responses is sometimes used to define cognitive impairment using the AMT,23,31 so we examined the effect of changing the cut-point used to define cognitive impairment in a sensitivity analysis. Information on body mass index (BMI [kg/m2]) was available for a subsample of 1279 participants, and we examined whether the same pattern of associations was observed with additional adjustment for BMI as vitamin D status is known to vary on the basis ofBMI,32 and BMI is a potential confounder in cognitive research.28 We also examined the odds ratios for cognitive impairment in participants who were severely 25(OH)D deficient (defined as <25 nmol/L), 25(OH)D deficient (defined as ≥25 nmol/L and <50 nmol/L), 25(OH)D insufficient (defined as ≥50 nmol/L and <75 nmol/L) compared with participants with sufficient 25(OH)D (defined as ≥75 nmol/L).33 Clusters, strata, and survey weights derived using inverse probability weighting were used to adjust for the survey design, sampling strategy, and possible nonresponse bias. We used Stata SE version 9.2 for all analyses (StataCorp, College Station, Tex).
Results
The characteristics of the study population are described in Table 1. Unadjusted serum 25(OH)D levels were higher in the cognitively normal than the cognitively impaired, and about half of the people with cognitive impairment had levels of serum 25(OH)D in the first quartile of the distribution (8–30 nmol/L). The cognitively normal participants were also generally younger than the cognitively impaired, more likely to have educational qualifications, consume alcohol, have a higher BMI, and less likely to have impaired mobility, hypoalbuminemia, or a history of stroke.
Table 1.
Characteristics of the Study Population
| Variables | All Participants (n = 1766) | Cognitively Normal (n = 1554) | Cognitively Impaired (n = 212) | P Valuea |
|---|---|---|---|---|
| Quartiles of serum 25(OH)D, n (%) | <.001 | |||
| First (8–30 nmol/L) | 458 (25.9) | 358 (23.0) | 100 (47.2) | |
| Second (31–44 nmol/L) | 453 (25.7) | 398 (25.6) | 55 (25.9) | |
| Third (45–65 nmol/L) | 423 (24.0) | 391 (25.2) | 32 (15.1) | |
| Fourth (66–170 nmol/L) | 432 (24.5) | 407 (26.2) | 25 (11.8) | |
| Additional variables | ||||
| Mean age (SD), years | 78.2 (8.6) | 77.6 (8.5) | 83.3 (7.9) | <.001 |
| Women, n (%) | 1058 (59.9) | 914 (58.8) | 144 (67.9) | .011 |
| No educational qualifications, n (%) | 1191 (67.4) | 1015 (65.3) | 176 (82.0) | <.001 |
| White ethnic origin, n (%) | 1738 (98.5) | 1533 (98.8) | 205 (96.7) | .029 |
| Current smokers, n (%) | 206 (11.7) | 182 (11.7) | 24 (11.3) | 1.000 |
| Alcohol consumption, n (%) | <.001 | |||
| 0 g/d | 436 (24.7) | 347 (22.3) | 89 (42.0) | |
| 0.1–29.9 g/d | 1236 (70.0) | 1121 (72.1) | 115 (54.3) | |
| ≥30 g/d | 94 (5.3) | 86 (5.5) | 8 (3.8) | |
| Psychiatric morbidity,b n (%) | 278 (15.3) | 237 (15.3) | 41 (19.3) | .132 |
| Impaired mobility, n (%) | 739 (41.9) | 616 (39.6) | 123 (58.0) | <.001 |
| Hypoalbuminemia, n (%) | 113 (6.4) | 91 (5.9) | 22 (10.4) | .016 |
| Stroke, n (%) | 168 (9.5) | 128 (8.2) | 40 (18.9) | <.001 |
| Heart disease, n (%) | 249 (14.1) | 219 (14.1) | 30 (14.2) | 1.000 |
| Diabetes, n (%) | 154 (8.7) | 129 (8.3) | 25 (11.8) | .093 |
| Hypertension, n (%) | 358 (20.3) | 306 (19.7) | 52 (24.5) | .102 |
| Mean BMI (SD)c, kg/m2 | 27.0 (4.7) | 27.2 (4.6) | 25.2 (5.0) | <.001 |
| Season tested, n (%) | .002 | |||
| Winter (Dec–Feb) | 378 (21.4) | 347 (22.3) | 31 (14.6) | |
| Spring (Mar–May) | 440 (24.9) | 368 (23.7) | 72 (34.0) | |
| Summer (Jun–Aug) | 406 (23.0) | 365 (23.5) | 41 (19.3) | |
| Autumn (Sep–Nov) | 542 (30.7) | 474 (30.5) | 68 (32.1) | |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index.
P value for group difference between cognitively normal and cognitively impaired participants.
Four or more symptoms on the 12-item General Health Questionnaire.29
Available for a subsample of 1279 participants.
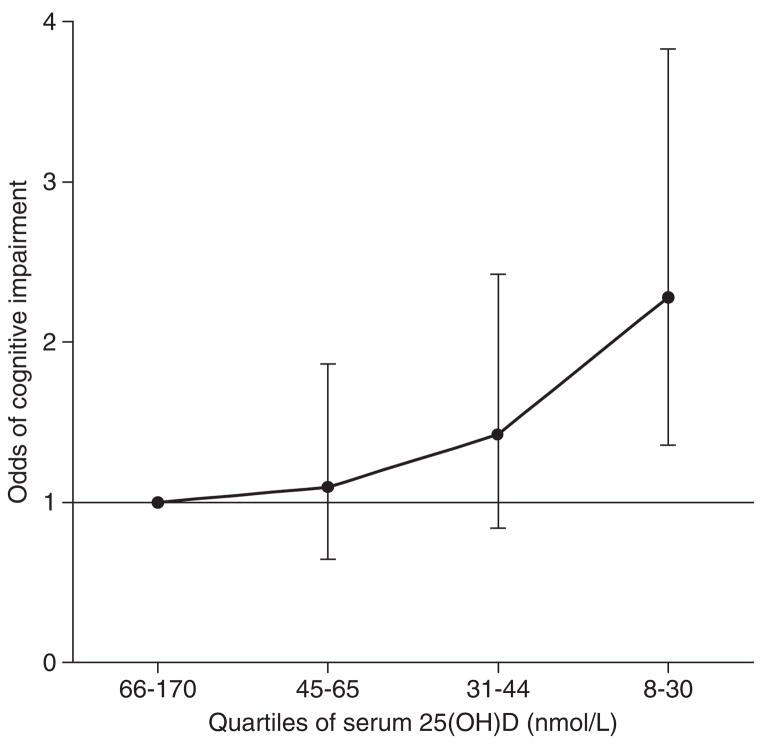
In an unadjusted logistic regression model, those in the lowest quartile of serum 25(OH)D concentration were more than 4 times more likely to be cognitively impaired, and there was clear evidence of a monotonic relationship (Table 2). This association was attenuated following adjustment for age, sex, education, ethnicity, and season tested, though remained significant. Adjustment for additional correlates of cognitive impairment had little effect, and in the fully adjusted model, those with the lowest levels of serum 25(OH)D were more than twice as likely to be cognitively impaired (Figure 1). No significant 2-way interaction between serum 25(OH)D and age was observed (P = .558).
Table 2.
Logistic Regression Models Illustrating the Odds of Cognitive Impairment (95% Confidence Intervals) by Quartiles of Serum 25-hydroxyvitamin D3 [25(OH)D] for 1766 Older Personsa
| Unadjusted Model | Basic Adjusted Modelb | Fully Adjusted Modelc | |
|---|---|---|---|
| Serum 25(OH)D (nmol/L) | |||
| Fourth (66–170) | Ref | Ref | Ref |
| Third (45–65) | 1.29 (0.74–2.25) | 1.09 (0.63–1.87) | 1.09 (0.64–1.86) |
| Second (31–44) | 1.98 (1.12–3.34) | 1.39 (0.81–2.39) | 1.43 (0.84–2.42) |
| First (8–30) | 4.17 (2.56–6.78) | 2.49 (1.49–4.18) | 2.28 (1.36–3.83) |
| P for linear trend | <.001 | <.001 | .001 |
Population weights are used to adjust for the sampling design. Cognitive impairment was defined as a score <8 on the Abbreviated Mental Test.23
Adjusted for age, sex, education, ethnicity, and season tested.
Adjusted for age, sex, education, ethnicity, season tested, current smoking status, alcohol consumption, psychiatric morbidity, impaired mobility, hypoalbuminemia, and medical history (stroke, heart disease, diabetes, and hypertension).
Figure 1.
Odds of cognitive impairment by quartiles of serum 25-hydroxyvitamin D [25(OH)D] for 1766 older persons. Bars indicate 95% confidence intervals. Cognitive impairment was defined as a score <8 on the Abbreviated Mental Test.23 Population weights are used to allow for the sampling design. Results are also adjusted for age, sex, education, ethnicity, season tested, current smoking status, alcohol consumption, psychiatric morbidity, impaired mobility, hypoalbuminemia, and medical history (stroke, heart disease, diabetes, and hypertension).
A different pattern of associations was observed for women and men when examined separately using logistic regression models (Table 3), and there was a 2-way interaction between serum 25(OH)D and sex of borderline statistical significance (P = .048). There was evidence for a linear increase in the odds of cognitive impairment by serum 25(OH)D quartiles for men (P for linear trend < .001), but a smaller association that became nonsignificant after full adjustment was observed for women (P for linear trend = .139). When the sample was restricted to noninstitutionalized participants (n = 1050), a monotonic increase in the odds of cognitive impairment by serum 25(OH)D quartiles was observed (P for linear trend < .001; Table 4). Using an alternative cut-point of 4 or more incorrect responses on the AMT to define cognitive impairment had little effect on the pattern of results observed (results not shown). Odds ratios (95% confidence intervals) for cognitive impairment in participants who were severely 25(OH)D deficient (<25 nmol/L), 25(OH)D deficient (≥25 nmol/L and <50 nmol/L), and 25(OH)D insufficient (≥50 nmol/L and <75 nmol/L) compared with participants with sufficient 25(OH)D (≥75 nmol/L) were 2.72 (1.48–5.00), 1.37 (0.81–2.31), and 0.89 (0.50–1.56) in fully adjusted models (P for linear trend <.001). Additional adjustment for BMI did not change the pattern of associations observed for men, though the association became stronger for women (P for linear trend = .082), and there was no longer a suggestion of a 2-way interaction between serum 25(OH)D and sex (P = .512).
Table 3.
Logistic Regression Models Illustrating the Odds of Cognitive Impairment (95% Confidence Intervals) by Quartiles of Serum 25-hydroxyvitamin D [25(OH)D] for Men and Women Separatelya
| Men (n = 708) |
Women (n = 1058) |
|||||
|---|---|---|---|---|---|---|
| Unadjusted Model | Basic Adjusted Modelb | Fully Adjusted Modelc | Unadjusted Model | Basic Adjusted Modelb | Fully Adjusted Modelc | |
| Serum 25(OH)D (nmol/L) | ||||||
| Fourth (66–170) | Ref | Ref | Ref | Ref | Ref | Ref |
| Third (45–65) | 2.86 (0.92–8.92) | 2.54 (0.83–7.80) | 2.60 (0.84–8.07) | 0.97 (0.52–1.81) | 0.85 (0.45–1.59) | 0.86 (0.47–1.58) |
| Second (31–44) | 6.91 (2.45–19.55) | 6.01 (2.00–18.11) | 6.83 (2.24–20.80) | 1.16 (0.62–2.19) | 0.82 (0.42–1.60) | 0.88 (0.46–1.66) |
| First (8–30) | 11.37 (4.16–31.09) | 9.39 (3.30–26.67) | 9.57 (3.18–28.79) | 2.73 (1.57–4.76) | 1.61 (0.88–2.95) | 1.43 (0.78–2.61) |
| P for linear trend | <.001 | <.001 | <.001 | <.001 | .056 | .139 |
Population weights are used to adjust for the sampling design. Cognitive impairment was defined as a score <8 on the Abbreviated Mental Test.23
Adjusted for age, education, ethnicity, and season tested.
Adjusted for age, education, ethnicity, season tested, current smoking status, alcohol consumption, psychiatric morbidity, impaired mobility, hypoalbuminemia, and medical history (stroke, heart disease, diabetes, and hypertension).
Table 4.
Logistic Regression Models Illustrating the Odds of Cognitive Impairment (95% Confidence Intervals) by Quartiles of Serum 25-hydroxyvitamin D3 [25(OH)D] for 1050 Older Noninstitutionalized Personsa
| Unadjusted Model | Basic Adjusted Modelb | Fully Adjusted Modelc | |
|---|---|---|---|
| Serum 25(OH)D (nmol/L) | |||
| Fourth (66–170) | Ref | Ref | Ref |
| Third (45–65) | 0.99 (0.29–3.40) | 0.95 (0.28–3.24) | 0.92 (0.27–3.17) |
| Second (31–44) | 2.03 (0.66–6.23) | 2.10 (0.68–6.53) | 2.04 (0.61–6.80) |
| First (8–30) | 7.47 (2.90–19.19) | 5.63 (2.22–14.26) | 4.83 (1.86–12.52) |
| P for linear trend | <.001 | <.001 | .001 |
Population weights are used to adjust for the sampling design. Cognitive impairment was defined as a score <8 on the Abbreviated Mental Test.23
Adjusted for age, sex, education, ethnicity, and season tested.
Adjusted for age, sex, education, ethnicity, season tested, current smoking status, alcohol consumption, psychiatric morbidity, impaired mobility, hypoalbuminemia, and medical history (stroke, heart disease, diabetes, and hypertension).
Discussion
The aim of this study was to examine the association between serum 25(OH)D and cognitive impairment in a large population-based sample of elderly adults. Our results suggest that levels of serum 25(OH)D were generally lower in the cognitively impaired general population, and there was evidence for a monotonic relationship. The association between serum 25(OH)D and cognitive impairment was stronger in men, which appeared to be partly attributable to differences in BMI. Thus, our results suggest that high levels of serum 25(OH)D are associated with lower odds of cognitive impairment.
To date, evidence for the beneficial effect of vitamin D on cognitive function comes mainly from animal and in vitro experiments.5–7 A number of studies suggest that low levels of serum 25(OH)D are associated with poor cognitive function14–16 and dementia,11,12 whereas others have reported no such association.13,17,18 However, these studies incorporated relatively small highly selected clinical samples, were not population based, and did not adjust for important potential confounders. McGrath and colleagues recently reported no linear association between serum 25(OH)D and verbal memory performance in 4809 noninstitutionalized older adults.19 However, given that cognitive impairment and dementia are highly prevalent in those residing in institutions,34,35 it is not clear whether the lack of association in their study reflects cognitive homogeneity within their sample. Alternatively, the briefmeasure of verbal memory incorporated in their analysis may not provide a sensitive measure of cognitive function, or vitamin D may be primarily associated with cognitive domains other than memory. Two trials also suggest that vitamin D supplementation may be associated with improved choice reaction time over 6 months,20 and clock drawing test performance over 4 weeks.21 Taken together with the results from this study, it appears that low serum 25(OH)D may be a risk factor for cognitive impairment.
The bioactive form of 25(OH)D, calcitriol (1,25-dihydroxycholecalciferol), has long been known for its important role in regulating levels of calcium, phosphate homeostasis, and bone mineralization. More recently, it has become clear that receptors for vitamin D are present in a wide variety of cells, including neurons and glial cells, and genes encoding the enzymes involved in the metabolism of calcitriol are also expressed in brain cells.5,36 Calcitriol reduces cellular calcium, inhibits the synthesis of inducible nitric oxide synthase, and increases levels of the antioxidant glutathione, suggesting a role in brain detoxification pathways.6,37 Calcitriol stimulates neurogenesis and regulates the synthesis of neurotrophic factors such as nerve growth factor (NGF), neurotrophin (NT) 3, NT 4, and glial cell line–derived neurotrophic factor (GDNF), which are important for cell differentiation and survival.5,38,39 Calcitriol is also an effective immunosuppressor and may be neuroprotective by inhibiting autoimmune damage to the nervous system.6,38 Low circulating levels of 25(OH)D may be associated with a higher risk of multiple sclerosis,40 and 25(OH)D has also recently been hypothesized to play a role in the pathogenesis of Parkinson disease.41 Accumulating data therefore provide evidence for previously unsuspected roles for vitamin D in brain development and neuroprotection.
A number of methodological issues should be considered when interpreting our findings. Although information on cognitive impairment from a widely used neurocognitive screening test was available, a clinical examination was not incorporated in the HSE. The cross-sectional design of this study means that we cannot determine whether low levels of serum 25(OH)D cause cognitive impairment. However, a causal relation between these factors has been suggested in animal and in vitro experiments5–7 and clinical trials.20,21 Reverse causality also appeared unlikely as additional adjustment for factors that may lead to reduced outdoor activity (notably psychiatric morbidity19 and impaired mobility30) had little effect, though a direct measure of sunlight exposure was not available. There is a potential for nonresponse bias, though the HSE response rate was acceptable and survey weights were used to adjust for possible bias. Given that the elderly English population is predominantly of white ethnic origin, further research is necessary to examine the degree to which our findings generalize to more diverse populations. Although unlikely, a genetic predisposition to both cognitive impairment and low serum25(OH)D might confound the association observed in our study.
In conclusion, we provide new evidence to suggest that serum 25(OH)D is related to cognitive impairment in the elderly population and a potential diagnostic aid for screening or differential diagnosis.42 This is important because serum 25(OH)D may play an important role in the expression of neurotrophic factors, the stimulation of adult neurogenesis, calcium homeostasis, and detoxification.5–7 Furthermore, the association between serum 25(OH)D levels and cognitive impairment underlines the importance of micronutrients in the elderly population. Further research is warranted to investigate if vitamin D supplementation is a cost-effective and safe way of reducing the incidence of cognitive impairment in the growing elderly population around the world.
Acknowledgments
The HSE is funded by the UK Department of Health. Dr KML was supported by grants from the NIA (K08 AG019180 and R01 AG027010) and a Paul Beeson Physician Faculty Scholars in Aging Research award. Dr IAL is an Academic Speciality Registrar in Public Health supported by the NHS South-West Region Public Health Training Scheme. The sponsors played no part in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the article for publication.
References
- 1.van der Wielen RP, Lowik MR, van den Berg H, et al. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 2.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 4.Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185–197. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 5.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 6.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 7.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. 2006;10:377–385. [PubMed] [Google Scholar]
- 8.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(suppl 6):1706S–1709S. doi: 10.1093/ajcn/80.6.1706S. [DOI] [PubMed] [Google Scholar]
- 11.Kipen E, Helme RD, Wark JD, Flicker L. Bone density, vitamin D nutrition, and parathyroid hormone levels in women with dementia. J Am Geriatr Soc. 1995;43:1088–1091. doi: 10.1111/j.1532-5415.1995.tb07005.x. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Honda Y, Hayashida N, Iwamoto J, Kanoko T, Satoh K. Vitamin K deficiency and osteopenia in elderly women with Alzheimer’s disease. Arch Phys Med Rehabil. 2005;86:576–581. doi: 10.1016/j.apmr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Nes M, Sem SW, Rousseau B, et al. Dietary intakes and nutritional status of old people with dementia living at home in Oslo. Eur J Clin Nutr. 1988;42:581–593. [PubMed] [Google Scholar]
- 14.Oudshoorn C, Mattace-Raso FU, van der Velde N, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25:539–543. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- 15.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460:202–205. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 17.Aung K, Burnett J, Smith SM, Dyer CB. Vitamin D deficiency associated with self-neglect in the elderly. J Elder Abuse Negl. 2006;18:63–78. doi: 10.1300/j084v18n04_07. [DOI] [PubMed] [Google Scholar]
- 18.Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. The Tromso study. J Neurol. 2006;253:464–470. doi: 10.1007/s00415-005-0027-5. [DOI] [PubMed] [Google Scholar]
- 19.McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29:49–54. doi: 10.1159/000108918. [DOI] [PubMed] [Google Scholar]
- 20.Dhesi JK, Jackson SH, Bearne LM, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–595. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 21.Przybelski R, Agrawal S, Krueger D, Engelke JA, Walbrun F, Binkley N. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int. 2008;19:1621–1628. doi: 10.1007/s00198-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 22.Prior G, Teers R, Brookes M, Primatesta P. Health Survey for England 2000: Methodology and Documentation. London: The Stationery Office; 2002. [Google Scholar]
- 23.Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing. 1972;1:233–238. doi: 10.1093/ageing/1.4.233. [DOI] [PubMed] [Google Scholar]
- 24.Jitapunkul S, Pillay I, Ebrahim S. The abbreviated mental test: its use and validity. Age Ageing. 1991;20:332–336. doi: 10.1093/ageing/20.5.332. [DOI] [PubMed] [Google Scholar]
- 25.Stewart R, Hirani V. Dental health and cognitive impairment in an English national survey population. J Am Geriatr Soc. 2007;55:1410–1414. doi: 10.1111/j.1532-5415.2007.01298.x. [DOI] [PubMed] [Google Scholar]
- 26.Iseli RK, Brand C, Telford M, LoGiudice D. Delirium in elderly general medical inpatients: a prospective study. Intern Med J. 2007;37:806–811. doi: 10.1111/j.1445-5994.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 27.Flicker L, Logiudice D, Carlin JB, Ames D. The predictive value of dementia screening instruments in clinical populations. Int J Geriatr Psychiatry. 1997;12:203–209. doi: 10.1002/(sici)1099-1166(199702)12:2<203::aid-gps603>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project: Report of the Critical Evaluation Study Committee. Alzheimer’s & Dementia. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg D, Williams P. A User’s Guide to the General Health Questionnaire. Windsor, Berkshire: NFERNELSON; 1988. [Google Scholar]
- 30.Scragg R, Holdaway I, Jackson R, Lim T. Plasma 25-hydroxyvitamin D3 and its relation to physical activity and other heart disease risk factors in the general population. Ann Epidemiol. 1992;2:697–703. doi: 10.1016/1047-2797(92)90014-h. [DOI] [PubMed] [Google Scholar]
- 31.Zuccala G, Marzetti E, Cesari M, et al. Correlates of cognitive impairment among patients with heart failure: results of a multicenter survey. Am J Med. 2005;118:496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 34.Feldman H, Clarfield AM, Brodsky J, King Y, Dwolatzky T. An estimate of the prevalence of dementia among residents of long-term care geriatric institutions in the Jerusalem area. Int Psychogeriatr. 2006;18:643–652. doi: 10.1017/S1041610205003091. [DOI] [PubMed] [Google Scholar]
- 35.Matthews FE, Dening T. Prevalence of dementia in institutional care. Lancet. 2002;360:225–226. doi: 10.1016/S0140-6736(02)09461-8. [DOI] [PubMed] [Google Scholar]
- 36.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Ibi M, Sawada H, Nakanishi M, et al. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40:761–771. doi: 10.1016/s0028-3908(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 38.Kalueff AV, Eremin KO, Tuohimaa P. Mechanisms of neuroprotective action of vitamin D(3) Biochemistry (Mosc) 2004;69:738–741. doi: 10.1023/b:biry.0000040196.65686.2f. [DOI] [PubMed] [Google Scholar]
- 39.Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 40.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 41.Newmark HL, Newmark J. Vitamin D and Parkinson’s disease—a hypothesis. Mov Disord. 2007;22:461–468. doi: 10.1002/mds.21317. [DOI] [PubMed] [Google Scholar]
- 42.Kasa M, Bierma TJ, Waterstraat F, Jr, Corsaut M, Singh SP. Routine blood chemistry screen: a diagnostic aid for Alzheimer’s disease. Neuroepidemiology. 1989;8:254–261. doi: 10.1159/000110191. [DOI] [PubMed] [Google Scholar]