Abstract
Botulinum neurotoxins (BoNTs) elicit flaccid paralysis by cleaving SNARE proteins within peripheral neurons. BoNTs are classified into seven serotypes, termed A-G, based upon antibody cross neutralization. Clostridia produce BoNTs are single chain toxins that are cleaved into a di-chain protein that comprises an N-terminal zinc metalloprotease domain that is linked by a disulfide bond to the C-terminal translocation/receptor binding domain. BoNT/A and BoNT/B utilize synaptic vesicle protein 2 (SV2) and synaptotagmin, respectively, as receptors for entry into neurons. Using affinity chromatography, BoNT/A and BoNT/B were found to bind a synaptic vesicle protein complex in CHAPS extracts of synaptic vesicles. Mass spectroscopy identified synaptic vesicle protein 2, synaptotagmin I, synaptophysin, vesicle-associated membrane protein 2, and the vacuolar ATPase-proton pump as components of the BoNT - synaptic vesicle protein complex. BoNT/A and BoNT/B possessed unique density gradient profiles when bound to synaptic vesicle protein complexes. The identification of BoNT/A and BoNT/B bound to synaptic vesicle protein complexes provides insight into the interactions of BoNT and neuronal receptors.
Keywords: Botulinum neurotoxins, SV2, synaptotagmin, VAMP-2
The botulinum neurotoxins (BoNTs) are the most potent protein toxins for humans and have been designated by the CDC as category A agents (Shapiro et al., 1997; Sobel, 2005). BoNTs elicit flaccid paralysis by blocking acetylcholine release at the neuromuscular junction in peripheral α-motor neurons through the cleavage of SNARE proteins. In contrast, tetanus toxin elicits spastic paralysis by blocking glycine release in the central nervous system through the cleavage of the SNARE protein, vesicle-associated membrane protein 2 (VAMP-2). Recent studies have identified the neuronal receptors for these neurotoxins, our studies have identified a neuronal vesicle protein complex that BoNT/A and BoNT/B bind with high affinity.
Structure-function properties of the Botulinum neurotoxins
BoNTs are classified into seven serotypes (designated A-G) based upon the neutralizing capacity of α-sera, where neutralizing α-sera to BoNT/A does not neutralize BoNT serotypes B-G (Smith et al., 2005). BoNTs serotypes differ up to 70% at the primary amino acid level and up to 32 % within a serotype (Lacy and Stevens, 1999; Smith et al., 2005). Clostridia produce BoNTs as single-chain proteins that are cleaved to di-chain proteins that are linked through a disulfide bond. BoNT are AB toxins that comprise a catalytic light-chain (LC, ~50 kDa) and a heavy chain (HC, ~100 kDa) (DasGupta and Dekleva, 1990). LC comprises a zinc metalloprotease domain that cleaves SNARE proteins; serotypes A, C, and E cleave SNAP-25, serotypes B, D, F, G, and tetanus toxin cleave VAMP-2, and serotype C also cleaves syntaxin 1a (Blasi et al., 1993a; Blasi et al., 1993b; Schiavo et al., 1992; Schiavo et al., 1994; Schiavo et al., 1993). HC comprises two domains: an N-terminal translocation domain (HCT, ~50 kDa) and a C-terminal receptor binding domain (HCR, ~50 kDa) (Lomneth et al., 1993). The HCR domain is further divided into two smaller domains termed HCRN and HCRC (Figure 1a). HCRN does not have a functional activity, but may be involved in aligning the HCT for optimal translocation of the LC into the host cell, while HCRC mediates the binding of BoNTs to host receptors (Lalli et al., 1999; Rummel et al., 2004). Lacy and Stevens solved the crystal structure of BoNT/A (Lacy et al., 1998) (Figure 1b), which provided the first insight into the structure-function organization of this family of toxins and provided a foundation to conduct reverse genetic studies on toxin action. The three domains of BoNT are organized into distinct structures that accounts for the compartmentalization of protein function. One of the unusual aspects of the holo-BoNT structure is that the N-terminal region of the HC wraps around the LC in a manner that is analogous to the mechanism that is utilized by the LC to bind SNARE proteins (Breidenbach and Brunger, 2004; Chen and Barbieri, 2007). The extended substrate binding regions of the BoNTs explain how these proteases efficiently recognize and cleave their coiled SNARE substrates.
Figure 1. Structure-function properties of the botulinum neurotoxins.
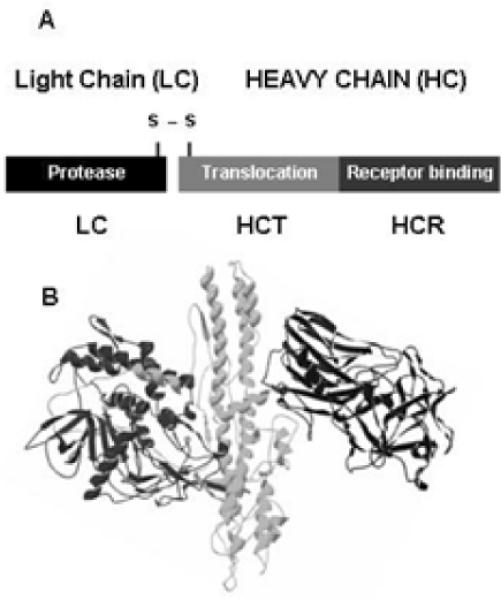
(A) Ribbon diagram of BoNT/A (pdb: 3bta). (B) Linear diagram of the di-chain activated BoNT molecule consisting of a light (LC) and heavy chain (HC) bound by a single disulfide bond. HC is divided into an N-terminal translocation domain (HCT) and a C-terminal receptor binding domain (HCR).
Botulinum Neurotoxin Host Receptor Interactions
BoNTs preferentially intoxicate neurons at the presynaptic membrane of α-motor-neurons (Johnson and Bradshaw, 2001). Recent studies have begun to unravel the steps involved in the binding and entry of the BoNTs into neurons. BoNTs intoxicate neurons through a multi-step mechanism that involves neuronal cell binding, vesicle internalization by receptor-mediated endocytosis and/or synaptic vesicle uptake, and LC translocates across membranes in acidified endosomes to target and cleave the SNARE substrate (Figure 2). In vivo stability and resistance to degradation of internalized LC contributes to the longevity of BoNT morbidity. The current model for BoNT binding to peripheral neurons follows the model proposed by Montecucco and colleagues (Montecucco et al., 2004) where BoNTs bind neurons through dual-receptor interactions that involve an initial binding to ganglioside, which increases the BoNT membrane concentration with subsequent binding to a protein receptor(s). Kozaki, S., et al observed that BoNT/B bound to lipid vesicles containing synaptotagmin and GT1b or GD1a and that a monoclonal antibody against GT1b inhibited BoNT/B binding to brain synaptosomes and inhibited the action of BoNT/B on synaptic transmission of neurons (Kozaki et al., 1998; Ochanda et al., 1986). The nature of the protein component of the BoNT/B receptor was determined by Chapman and coworkers (Dong et al., 2003) who observed that entry of BoNT/B into PC12 cells and motor neurons was activity dependent. Subsequent studies showed that peptide fragments of the luminal loops of synaptotagmin functioned as physiological BoNT/B receptors. Using a similar approach, Chapman and coworkers (Dong et al., 2006) and Binz and coworkers (Mahrhold et al., 2006) reported that the luminal loop of synaptic vesicle protein 2 (SV2) was the receptor for BoNT/A. The BoNT-receptor complex enters neurons by receptor-mediated endocytosis. Upon acidification of the early endosome by the proton pump, the HCT domain of BoNT inserts into the endosome membrane and forms a pore that allows the translocation of the LC into the cytosol. Koriazova and Montal (Koriazova and Montal, 2003) detected currents in BoNT/A and HC/A-treated bi-layers and cleavage of BoNT substrate in the trans-compartment, which implicated a role for the HCT in LC translocation across the endosome membrane.
Figure 2. Model for binding and entry of BoNTs at the Neuromuscular junction.
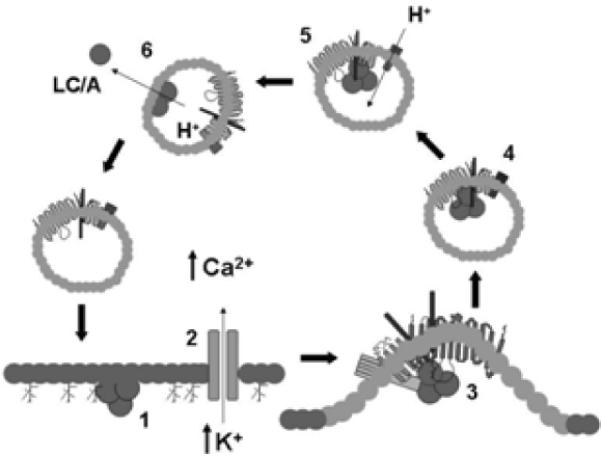
BoNT/A associates with the presynaptic membrane of α-motor neurons through interactions with oligosaccharides such as ganglioside GT1b (1). Calcium influx stimulates synaptic vesicle membrane fusion (2). BoNT/A interacts with the exposed receptor complex, stimulating synaptic vesicle recycling (3). Recycled synaptic vesicles are acidified through the activity of the v-ATPase (4). Vesicle acidification drives the translocation of the BoNT/A light chain (LC/A) into the cytosol (5) completing the cycle (6).
Synaptic Vesicle Protein Complexes
Neuronal synaptic transmission is initiated when an action potential induces the elevation of intracellular Ca2+ which stimulates synaptic vesicle exocytosis and neurotransmitter release. After plasma membrane fusion, synaptic vesicles undergo endocytosis, recycling to refill with neurotransmitters, and can initiate another round of exocytosis. Synaptic vesicles contain transporter proteins that are composed of the v-type ATPase to generate an electrochemical gradient, neurotransmitter transporter proteins that load neurotransmitters into the vesicle, and trafficking proteins. Scheller and colleagues demonstrated physical interactions among five synaptic vesicle proteins (Bennett et al., 1992). In these experiments, CHAPS extraction of synaptic vesicle yielded a protein complex that included SV2, Synaptotagmin I (Syt), synaptophysin (Syp), VAMP2, and the v-type ATPase, while octylglucoside extraction preserved an SV2 / Syt complex and Triton X-100 extraction yielded SV2 / Syt and Syp / VAMP2 complexes. The authors proposed that these protein complexes contributed to neurotransmitter secretion, vesicle trafficking and spatial organization within the neuron. Other laboratories confirmed the presence of the Syt I / SV2 and Syp / VAMP2 complexes, but the physiological significance of these synaptic vesicle protein complexes remains unclear. Recent studies in our laboratory (Baldwin and Barbieri, 2007) identified a synaptic vesicle protein complex as a high affinity receptor for BoNT/A and BoNT/B. The complex included SV2, Syt, Syp, VAMP2, and the v-type ATPase. This implicated a physiological role for these protein complexes as BoNT receptors.
BoNT HCR/A and HCR/B Co-purify with Synaptic Vesicle Protein Complexes
Physical interactions between synaptic vesicle proteins and BoNT HCRs were analyzed LC-MS/MS. A synaptic vesicle fraction from rat brain cortex was extracted with CHAPS or Triton X-100, since CHAPS extraction had been shown to retain synaptic vesicle protein complexes, while Triton X-100 extraction dissociated these protein complexes (Hjelmeland and Chrambach, 1984). Co-IPs were facilitated by using a 3xFLAG epitope as an efficient target for immunoprecipitation, with recovery rates typically >90% and by incubating the immunoprecipitate with the 3xFLAG peptide to release the 3-FLAG-HCR, along with associated vesicle proteins, from beads to reduce the non-specific binding of proteins to HCRs (Figure 3).
Figure 3. Protocol for the identification of BoNT-HCR-binding proteins.
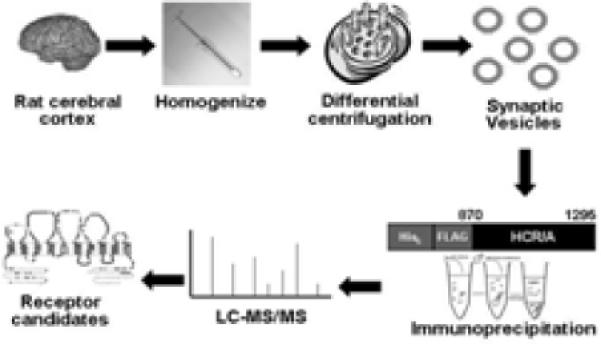
Rat brain cortex are homogenized and subjected to differential centrifugation. Synaptic vesicles are lysed and extracted with either CHAPS or Triton-X100. A 3-FLAG epitope IP is performed to isolate HCR binding proteins. 3FLAG peptide is added to the IP to release #-FLAG-HCR form the bead along with HCR biding proteins, which are identified by MALDI-MS or Western blotting.
Initial experiments showed that HCR/A co-purified with a CHAPS-extracted synaptic vesicle protein complex that included SV2, Syt I, Syp, Synaptogyrin 3, VAMP-2, and the v-ATPase. In contrast, HCR/A co-purified with primarily with SV2 in a Triton X-100-extracted synaptic vesicle extract. This suggested that the BoNT receptor, SV2, may be a component of a larger protein complex. Like, HCR/A, HCR/B co-purified with a CHAPS extracted synaptic vesicle protein complex. HCR/B co-purified Triton X-100 extracted synaptotagmin I only. Again, suggesting the Synaptotagmin 1 may be a component of the larger protein complex. While several of the synaptic vesicle proteins that co-purified with HCR/A and HCR/B were conserved, there was some heterogeneity of the synaptic vesicles that co-purified with HCR/A and HCR/B, which may contribute to the serotype specificity of the BoNTs. The absence of Syt II, as determined by LC-MS/MS and Western blotting, in the protein complex that co-purified with HCR/B was unexpected, since Syt II was reported have high affinity binding to BoNT/B (Dong et al., 2003). This may have been due to differential expression of Syt I and Syt II in the cerebral cortex (Ullrich and Sudhof, 1995). In contrast, HCR/C did not co-purify with either isolated SV2 / synaptotagmin I from the Triton X-100 extract or co-purify with the CHAPS stabilized protein complex. Thus, HCR/A and HCR/B, but not HCR/C, associate with synaptic vesicle protein complexes (Figure 4).
Figure 4. Model for binding of BoNTs to the synaptic vesicle protein complex.
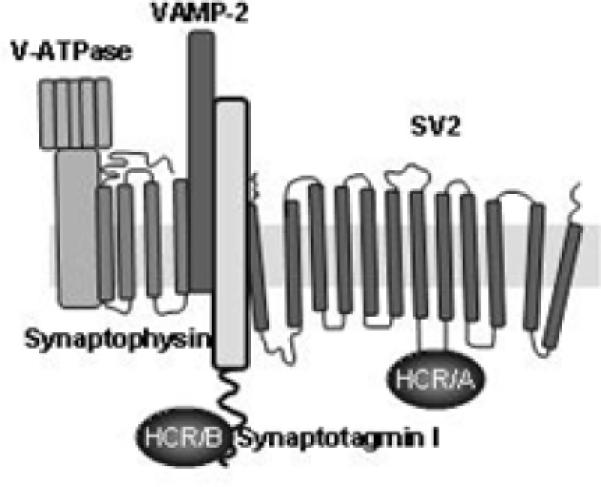
Synaptic vesicle proteins forms a large protein complex that includes synaptic vesicle protein 2 (SV2), synaptotagmin I, synaptophysin (Syp), vesicle-associated membrane protein 2 (VAMP 2) and the vacuolar proton pump (v-ATPase). The synaptic receptor complex is stable following solubilization with CHAPS and HCR/A binds to SV2 and HCR/B binds synaptotagmin I within the complex.
Scheller and colleagues identified a similar protein complex, comprising SV2, Syt I, Syp, VAMP-2 and the v-ATPase, in CHAPS-extracts of synaptic vesicles (Bennett et al., 1992). In addition to the core proteins described by Scheller, the BoNT receptor complexes contained several additional proteins, including SNAP25, synaptogyrin 3, GAPDH, and vGLUT1/2, In addition to the v-ATPase V0 subunit d isoform 2, V0 subunit a and V1 subunits A1, B1, B2, D1, E1, and H were identified. Rat neurons express three different V0 a subunits - a1-I, a1-IV and a2, which are located on different neuronal membrane, with the a1-I isoform the only a subunit specifically located in nerve terminals (Poea-Guyon et al., 2006). This is consistent with the BoNT receptor protein complex being derived from synaptic vesicles. Earlier studies determined that v-ATPase V0 d1 associated with the synaptophysin / VAMP2 complex in the presence of Triton X-100 (Galli et al., 1996). The receptor protein complex associated with HCR/A and HCR/B also contained synaptogyrin 3. Synaptogyrins are a family of tyrosine-phosphorylated proteins with two neuronal isoforms (synaptogyrins 1 and 3) and one general isoform (Stenius et al., 1995). In neurons synaptogyrin1 is more widely expressed than synaptogyrin 3 (Belizaire et al., 2004), which may indicate that BoNT receptor protein complexes represent a distinct subpopulation of synaptic vesicles. The BoNT receptor complexes also contained Vesicular Glutamate Transporter 2 (vGLUT-2) in addition to the main transporter, vGLUT-1. In mice, vGLUT1 is expressed in the cortex, while vGLUT2 is expressed in the brainstem (Hioki et al., 2003; Kaneko et al., 2002; Nakamura et al., 2005). The presence of vGLUT-1 and vGLUT-2 in the BoNT receptor protein complex suggests that BoNTs may target specific subsets of glutamatergic neurons.
HCR/A and HCR/B form unique stable complexes with CHAPS-extracted synaptic vesicles
The results of the BoNT-synaptic vesicle extracts immunoprecipitations indicated the specific association of HCR/A and HCR/B with a synaptic vesicle protein complex. Buoyant density centrifugation was used to further characterize the interaction between synaptic vesicle protein complexes and the HCRs. HCR/A associated with dense protein complex fractions of CHAPS-extracted synaptic vesicles. The HCR/A-dense protein complex was not observed in Triton-X100 extracted synaptic vesicles. In both CHAPS- and Triton X-100- synaptic vesicle gradients, a fraction of the vesicle proteins were detected in the light density fractions of the gradient, which could result from complex dissociation. The fractionation of HCR/A with synaptic vesicle protein complexes, but not light density fractions, indicated that HCR/A had a preferred association with dense synaptic vesicle protein complexes. Controls showed that HCR/A did not migrate into the gradient in the absence of synaptic vesicle proteins and that Rab3a was excluded from the dense synaptic vesicle protein complex, which indicated that indicated that intact synaptic vesicles were not present in the gradient fractions. This also indicated that the HCR/A fractionation was due the association of HCR/A with the synaptic vesicle protein complex. Relative to HCR/A, HCR/B had a unique fractionation pattern and was associated with both dense- and light- synaptic vesicle protein complexes in either CHAPS- or Triton X-100- extracted synaptic vesicle extracts. This was interpreted as HCR/B associating with synaptotagmin I in both dense vesicle protein complexes and with synaptotagmin 1 monomers. This interpretation is supported by Kozaki and colleagues who previously reported that BoNT/B bound both rat synaptosomes and purified rat synaptotagmin II with similar affinity (Kd ≈ 0.4 nM) (Kozaki et al., 1998; Nishiki et al., 1994; Nishiki et al., 1996a; Nishiki et al., 1996b).
Conclusions
The association of HCR/A and HCR/B with a synaptic vesicle protein complex that included SV2, synaptotagmin I, synaptophysin, synaptogyrin 3, VAMP2 and multiple subunits of the vacuolar type ATPase (v-ATPase) was demonstrated. The CHAPS extracted vesicle protein complex resembled a previously identified protein complex from synaptic vesicles identified by Scheller and colleagues who reported that synaptic vesicle proteins complexes was CHAPS-dependent and that Triton X-100 disrupted the complex (Bennett et al., 1992). Immuno-gold electron microscopy suggested these complexes existed in native synaptic vesicles. Subsequent studies have confirmed interactions between specific components of the protein complex and demonstrated how these interactions regulate synaptic vesicle activities (Galli et al., 1996; Khvotchev and Sudhof, 2004; Schivell et al., 1996; Schivell et al., 2005; Yelamanchili et al., 2005). The observation that BoNTs bind these complexes supports a physiological function for the complexes in synaptic vesicle biology.
The data presented in this study are consistent with the double receptor model proposed by Montecucco and colleagues (Montecucco et al., 2004). In this model (Figure 2), BoNT initially interacts with lipid- and/or protein-linked oligosaccharides such as ganglioside GT1b, concentrating the toxin on the presynaptic membrane. This initial interaction appears important, since ganglioside synthesis knockout mice show reduced toxin sensitivity (Bullens et al., 2002; Bullens et al., 2003; Kitamura et al., 1999) and pre-incubation with ganglioside reduces BoNT binding to cultured neurons. Subqequent to ganglioside binding, an action potential across the synaptic vesicle membrane stimulates Ca2+ influx and synaptic vesicle membrane fusion, exposing the protein receptor complexes. BoNT /A and BoNT/B associate with the synaptic vesicle protein complexes on the exposed cell surface and the BoNT-synaptic vesicle protein complexes are taken up through clathrin-mediated endocytosis. Future studies will elucidate the role of individual proteins in the vesicle protein complex in the binding and entry process.
Acknowledgements
We thank William Tepp, Cristina Pier, Marita Brashaw, and Eric A. Johnson, ScD for providing BoNT heavy chain DNA and native BoNTs. LC-MS/MS analysis was performed by John Leszyk, PhD, Core Laboratory for Proteomic Mass Spectrometry, University of Massachusetts Medical School. This work was supported by grants from the Great Lakes Regional Center of Excellence (GLRCE) from NIH-NIAID U54 AI057153 (JTB) and NIH-NINDS K99 NS061763 (MRB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin MR, Barbieri JT. Association of botulinum neurotoxin serotypes a and B with synaptic vesicle protein complexes. Biochemistry. 2007;46:3200–3210. doi: 10.1021/bi602396x. [DOI] [PubMed] [Google Scholar]
- Belizaire R, Komanduri C, Wooten K, Chen M, Thaller C, Janz R. Characterization of synaptogyrin 3 as a new synaptic vesicle protein. J Comp Neurol. 2004;470:266–281. doi: 10.1002/cne.20008. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Kreiner T, Scheller RH. Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol. 1992;116:761–775. doi: 10.1083/jcb.116.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993a;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. Embo J. 1993b;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach MA, Brunger AT. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature. 2004;432:925–929. doi: 10.1038/nature03123. [DOI] [PubMed] [Google Scholar]
- Bullens RW, O'Hanlon GM, Wagner E, Molenaar PC, Furukawa K, Plomp JJ, Willison HJ. Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J Neurosci. 2002;22:6876–6884. doi: 10.1523/JNEUROSCI.22-16-06876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullens RW, O'Hanlon GM, Wagner E, Molenaar PC, Furukawa K, Plomp JJ, Willison HJ. Roles of complex gangliosides at the neuromuscular junction. Ann N Y Acad Sci. 2003;998:401–403. doi: 10.1196/annals.1254.051. [DOI] [PubMed] [Google Scholar]
- Chen S, Barbieri JT. Multiple pocket recognition of SNAP25 by botulinum neurotoxin serotype E. J Biol Chem. 2007;282:25540–25547. doi: 10.1074/jbc.M701922200. [DOI] [PubMed] [Google Scholar]
- DasGupta BR, Dekleva ML. Botulinum neurotoxin type A: sequence of amino acids at the N-terminus and around the nicking site. Biochimie. 1990;72:661–664. doi: 10.1016/0300-9084(90)90048-l. [DOI] [PubMed] [Google Scholar]
- Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA, Chapman ER. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J Cell Biol. 2003;162:1293–1303. doi: 10.1083/jcb.200305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 Is the Protein Receptor for Botulinum Neurotoxin A. Science. 2006 doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- Galli T, McPherson PS, De Camilli P. The V0 sector of the V-ATPase, synaptobrevin, and synaptophysin are associated on synaptic vesicles in a Triton X-100-resistant, freeze-thawing sensitive, complex. J Biol Chem. 1996;271:2193–2198. doi: 10.1074/jbc.271.4.2193. [DOI] [PubMed] [Google Scholar]
- Hioki H, Fujiyama F, Taki K, Tomioka R, Furuta T, Tamamaki N, Kaneko T. Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience. 2003;117:1–6. doi: 10.1016/s0306-4522(02)00943-0. [DOI] [PubMed] [Google Scholar]
- Hjelmeland LM, Chrambach A. Solubilization of functional membrane proteins. Methods Enzymol. 1984;104:305–318. doi: 10.1016/s0076-6879(84)04097-0. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Bradshaw M. Clostridium botulinum and its neurotoxins: a metabolic and cellular perspective. Toxicon. 2001;39:1703–1722. doi: 10.1016/s0041-0101(01)00157-x. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Khvotchev MV, Sudhof TC. Stimulus-dependent dynamic homo- and heteromultimerization of synaptobrevin/VAMP and synaptophysin. Biochemistry. 2004;43:15037–15043. doi: 10.1021/bi048290+. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Takamiya K, Aizawa S, Furukawa K. Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim Biophys Acta. 1999;1441:1–3. doi: 10.1016/s1388-1981(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat Struct Biol. 2003;10:13–18. doi: 10.1038/nsb879. [DOI] [PubMed] [Google Scholar]
- Kozaki S, Kamata Y, Watarai S, Nishiki T, Mochida S. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb Pathog. 1998;25:91–99. doi: 10.1006/mpat.1998.0214. [DOI] [PubMed] [Google Scholar]
- Lacy DB, Stevens RC. Sequence homology and structural analysis of the clostridial neurotoxins. J Mol Biol. 1999;291:1091–1104. doi: 10.1006/jmbi.1999.2945. [DOI] [PubMed] [Google Scholar]
- Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat Struct Biol. 1998;5:898–902. doi: 10.1038/2338. [DOI] [PubMed] [Google Scholar]
- Lalli G, Herreros J, Osborne SL, Montecucco C, Rossetto O, Schiavo G. Functional characterisation of tetanus and botulinum neurotoxins binding domains. J Cell Sci. 1999;112(Pt 16):2715–2724. doi: 10.1242/jcs.112.16.2715. [DOI] [PubMed] [Google Scholar]
- Lomneth R, Gimenez J, Martin TF, DasGupta BR. Calcium-dependent release of norepinephrine from permeabilized PC12 cells is inhibited by approximately 48 and approximately 112 kDa fragments of botulinum neurotoxin type E. Neuropharmacology. 1993;32:285–289. doi: 10.1016/0028-3908(93)90113-h. [DOI] [PubMed] [Google Scholar]
- Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580:2011–2014. doi: 10.1016/j.febslet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Rossetto O, Schiavo G. Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol. 2004;12:442–446. doi: 10.1016/j.tim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGluT)1 and VGluT2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol. 2005;492:263–288. doi: 10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- Nishiki T, Kamata Y, Nemoto Y, Omori A, Ito T, Takahashi M, Kozaki S. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J Biol Chem. 1994;269:10498–10503. [PubMed] [Google Scholar]
- Nishiki T, Tokuyama Y, Kamata Y, Nemoto Y, Yoshida A, Sato K, Sekiguchi M, Takahashi M, Kozaki S. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 1996a;378:253–257. doi: 10.1016/0014-5793(95)01471-3. [DOI] [PubMed] [Google Scholar]
- Nishiki T, Tokuyama Y, Kamata Y, Nemoto Y, Yoshida A, Sekiguchi M, Takahashi M, Kozaki S. Binding of botulinum type B neurotoxin to Chinese hamster ovary cells transfected with rat synaptotagmin II cDNA. Neurosci Lett. 1996b;208:105–108. doi: 10.1016/0304-3940(96)12557-x. [DOI] [PubMed] [Google Scholar]
- Ochanda JO, Syuto B, Ohishi I, Naiki M, Kubo S. Binding of Clostridium botulinum neurotoxin to gangliosides. J Biochem (Tokyo) 1986;100:27–33. doi: 10.1093/oxfordjournals.jbchem.a121702. [DOI] [PubMed] [Google Scholar]
- Poea-Guyon S, Amar M, Fossier P, Morel N. Alternative splicing controls neuronal expression of v-ATPase subunit a1 and sorting to nerve terminals. J Biol Chem. 2006;281:17164–17172. doi: 10.1074/jbc.M600927200. [DOI] [PubMed] [Google Scholar]
- Rummel A, Karnath T, Henke T, Bigalke H, Binz T. Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J Biol Chem. 2004;279:30865–30870. doi: 10.1074/jbc.M403945200. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Malizio C, Trimble WS, Polverino de Laureto P, Milan G, Sugiyama H, Johnson EA, Montecucco C. Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala-Ala peptide bond. J Biol Chem. 1994;269:20213–20216. [PubMed] [Google Scholar]
- Schiavo G, Shone CC, Rossetto O, Alexander FC, Montecucco C. Botulinum neurotoxin serotype F is a zinc endopeptidase specific for VAMP/synaptobrevin. J Biol Chem. 1993;268:11516–11519. [PubMed] [Google Scholar]
- Schivell AE, Batchelor RH, Bajjalieh SM. Isoform-specific, calcium-regulated interaction of the synaptic vesicle proteins SV2 and synaptotagmin. J Biol Chem. 1996;271:27770–27775. doi: 10.1074/jbc.271.44.27770. [DOI] [PubMed] [Google Scholar]
- Schivell AE, Mochida S, Kensel-Hammes P, Custer KL, Bajjalieh SM. SV2A and SV2C contain a unique synaptotagmin-binding site. Mol Cell Neurosci. 2005;29:56–64. doi: 10.1016/j.mcn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Shapiro RL, Hatheway C, Becher J, Swerdlow DL. Botulism surveillance and emergency response. A public health strategy for a global challenge. Jama. 1997;278:433–435. [PubMed] [Google Scholar]
- Smith TJ, Lou J, Geren IN, Forsyth CM, Tsai R, Laporte SL, Tepp WH, Bradshaw M, Johnson EA, Smith LA, Marks JD. Sequence variation within botulinum neurotoxin serotypes impacts antibody binding and neutralization. Infect Immun. 2005;73:5450–5457. doi: 10.1128/IAI.73.9.5450-5457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. Botulism. Clin Infect Dis. 2005;41:1167–1173. doi: 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- Stenius K, Janz R, Sudhof TC, Jahn R. Structure of synaptogyrin (p29) defines novel synaptic vesicle protein. J Cell Biol. 1995;131:1801–1809. doi: 10.1083/jcb.131.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich B, Sudhof TC. Differential distributions of novel synaptotagmins: comparison to synapsins. Neuropharmacology. 1995;34:1371–1377. doi: 10.1016/0028-3908(95)00132-p. [DOI] [PubMed] [Google Scholar]
- Yelamanchili SV, Reisinger C, Becher A, Sikorra S, Bigalke H, Binz T, Ahnert-Hilger G. The C-terminal transmembrane region of synaptobrevin binds synaptophysin from adult synaptic vesicles. Eur J Cell Biol. 2005;84:467–475. doi: 10.1016/j.ejcb.2004.11.007. [DOI] [PubMed] [Google Scholar]


