Abstract
Evidence has emerged in the last two decades that at the molecular level most chronic diseases, including cancer, are caused by a dysregulated inflammatory response. The identification of transcription factors such as NF-kB, AP-1 and STAT3 and their gene products such as tumor necrosis factor, interleukin-1, interleukin-6, chemokines, cyclooxygenase-2, 5 lipooxygenase, matrix metalloproteases, and vascular endothelial growth factor, adhesion molecules and others have provided the molecular basis for the role of inflammation in cancer. These inflammatory pathways are activated by tobacco, stress, dietary agents, obesity, alcohol, infectious agents, irradiation, and environmental stimuli, which together account for as much as 95% of all cancers. These pathways have been implicated in transformation, survival, proliferation, invasion, angiogenesis, metastasis, chemoresistance, and radioresistance of cancer, so much so that survival and proliferation of most types of cancer stem cells themselves appear to be dependent on the activation of these inflammatory pathways. Most of this evidence, however, is from preclinical studies. Whether these pathways have any role in prevention, progression, diagnosis, prognosis, recurrence or treatment of cancer in patients, is the topic of discussion of this review. We present evidence that inhibitors of inflammatory biomarkers may have a role in both prevention and treatment of cancer.
2. Introduction
Cancer is one disease that fits the paradigm that “more we know, less we understand its intricacies”. That continuous irritation over long periods of time can lead to cancer (called arbuda), has been described in Ayurveda (means the science of long life), written as far back as 5000 years ago. Whether this irritation is the same as that Rudolf Virchow referred to as inflammation in the nineteenth century is uncertain. The observable consequences of irritation were first described by Aulus Cornelius Celsus, a Roman medical writer and possibly a physician in the first century (ca 25BC-50 AD), who characterized inflammation as “redness (rubor) and swelling (tumor) with heat (calor) and pain (dolor)”. Virchow postulated that microinflammation that results from irritation leads to the development of most chronic diseases including cancer. This inflammation is now regarded as a “secret killer” for diseases such as atherosclerosis, rheumatoid arthritis, multiple sclerosis, asthma, Alzheimer's, depression, fatigue, neuropathic pain, lack of appetite, and cancer (1). With the recent advent of molecular biology, cell signaling, recombinant DNA, and genomics, there has been reawakening and tremendous interest in the role of inflammation in cancer and other diseases. This review will focus primarily on the role of inflammation in cancer.
3. Inflammatory network in cancer
In the last two decades numerous molecules have been identified that play a critical role in inflammation. These include tumor necrosis factor (TNF), interleukin-1 (IL-1), interleukin-6 (IL-6), chemokines, cyclooxygenase (COX)-2, 5 lipooxygenase (LOX), matrix metalloproteases (MMP), vascular endothelial growth factor (VEGF), TWIST and cell surface adhesion molecules. What is common to all these molecules is that they are regulated by the transcription factor NF-κB (Fig. 1). Although initially discovered in the kappa chain of immunoglobulin and in nucleus of B cells, NF-κB is now known to be a transcription factor that is ubiquitous to all cell types and present in the cytoplasm in its resting stage. Soon after its discovery, certain NF-κB proteins were shown to exhibit oncogenic activity e.g; v-rel. The activity of NF-κB itself is regulated by other transcription factors such Notch-1 (2), PPAR-g (3), STAT3 (4), beta-catenin (5) and p53 (6). NF-κB has been shown to regulate AP-1 through ELK-1-mediated expression of c-fos (7) (Fig. 2).
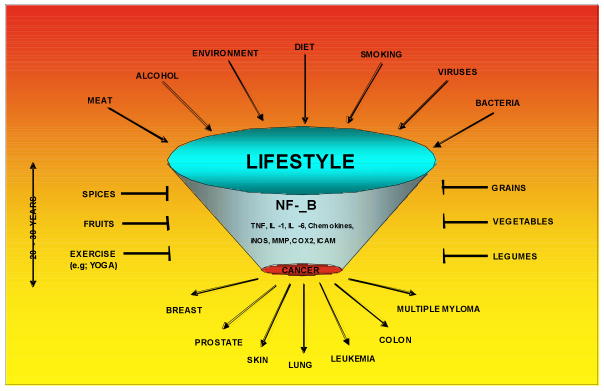
Fig.1.
Activation of inflammatory pathway mediated through NF-κB by life-style related factors such as tobacco, stress, dietary agents, obesity, alcohol, infectious agents, irradiation and environmental stimuli that account for as much as 95% of all cancers. Suppression of inflammatory pathway by life style –related agents such as vegetables, fruits, legumes, grains, spices and exercise (such as Yoga), is indicated.
Fig. 2.
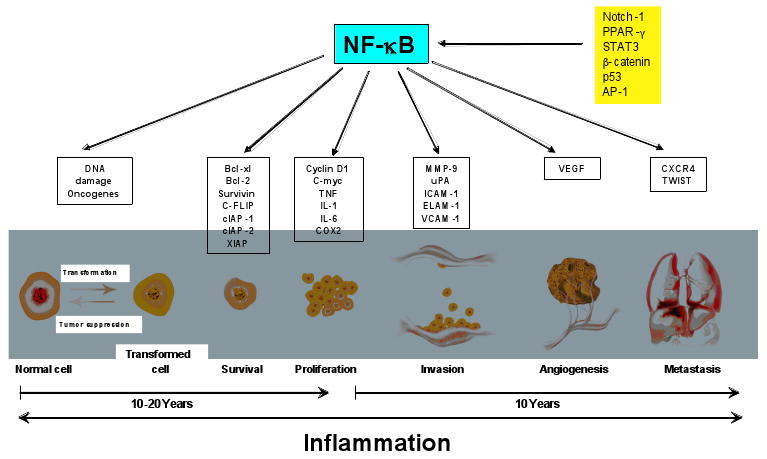
Activation of various inflammatory pathways that lead to expression of gene products linked to cellular transformation, survival, proliferation, invasion, angiogenesis and metastasis of cancer.
For many reasons NF-κB and gene products regulated by it play a critical role in tumorigenesis (8). First, almost all gene products linked with inflammation are regulated by the activation of NF-κB (e.g; TNF, IL-1, IL-6, chemokines, COX2, 5LOX, CRP). Second, NF-κB is activated in response to tobacco, stress, dietary agents, obesity, alcohol, infectious agents, irradiation and environmental stimuli, which together account for as much as 95% of all cancers. Third, NF-κB has been linked with transformation of cells (8). Fourth, NF-κB is constitutively active in most tumor cells. Fifth, NF-κB has also been linked with the survival of cancer stem cells, an early progenitor cells that have acquired self-renewal potential (9-14). Sixth, NF-κB regulates the expression of most antiapoptotic gene products (bcl-2, bcl-xl, c-FLIP, XIAP, IAP-1, IAP-2, and survivin) associated with the survival of the tumor. Seventh, NF-κB also regulates the gene products linked with proliferation of tumors such as c-myc, cyclin D1, and COX2. Additionally most growth factors (e.g; EGF, TNF, IL-6) linked with proliferation of tumors either activate NF-κB or are regulated by this transcription factor. Eighth, NF-κB controls the expression of gene products linked with invasion, angiogenesis and metastasis of cancer (e.g; MMP, adhesion molecules, VEGF, TWIST, CXCR4). Ninth, while most carcinogens activate NF-κB, most chemopreventive agents have been shown to suppress NF-κB activation (Fig. 1). Thus based on cell culture and animal models, the role of NF-κB and the gene products regulated by it are very well established. Because very rarely discoveries in cell culture or animals models translate directly into treatments or preventive for patients (8), we decided to focus in this review on the role of the NF-κB-regulated inflammatory network in progression, diagnosis, prognosis, recurrence, and treatment of cancer in the patients.
4. Evidence that inflammatory genes/products are overexpressed in cancer patients
Both TNF and NF-κB, two major mediators of inflammation (isolated around 1984 and 1986, respectively) are now the subjects of around 73,000 and 27,000 citations. The Pubmed database also shows 2300 citation for NF-κB in patients in general and around 800 in cancer patients alone. Thus it is not possible to cover all this information. Both NF-κB and NF-κB gene products have been, however, linked with prognosis and response to therapy in patients with different cancers (see Tables 1-3 (18-270).
Table 1. Overexpression of constitutive active NF-kB is linked to the progression of cancer in patients.
| Leukemia: |
|
| Gastrointestinal Cancers: |
|
| Genitourinary Cancers: |
|
| Brain Cancers: |
|
| Breast Cancers: |
|
| Gyneoncologic Cancers: |
|
| Head & Neck Cancers: |
|
| Melanoma: |
ALL-Acute Lymphoblastic Leukemia, ATL-Acute T cell Leukemia, AML-Acute Myelogenous Leukemia, HCC-Hepatocellular Carcinoma, MALT-Mucosa-Associated Lymphoid Tissue, NSCLC- Non-Small-Cell Lung Cancer
Tables 3. Expression of cytokines and chemokines and their receptors is linked to the development of cancer in patients.
| CXCR-4 |
| Leukemia: |
| Gastrointestinal Cancers: |
|
| Genitourinary Cancers: |
|
| Breast Cancers: |
|
| Gyneoncologic Cancers: |
| Head & Neck Cancers: |
| VEGF |
| Leukemia: |
| Gastrointestinal Cancers: |
|
| Breast Cancers: |
| Gyneoncologic Cancers: |
|
| Head & Neck Cancers: |
| Melanoma: |
|
| STAT-3 |
|
| IL-1 and 6 |
|
| IL-8 |
|
| MMP-9 |
|
| NOS |
|
| LOX |
|
ALL-Acute Lymphoblastic Leukemia, ATL-Acute T cell Leukemia, AML-Acute Myelogenous Leukemia, HCC-Hepatocellular Carcinoma, MALT-Mucosa-Associated Lymphoid Tissue, NSCLC- Non-Small-Cell Lung Cancer
4A. Role of NF-κB in cancer patients
The presence of constitutively active NF-κB has now been identified in tissue of most cancers including leukemia, lymphoma, and cancers of the prostate, breast, oral cavity, liver, pancreas, colon, and ovary (Table 1;). The role of NF-κB in cancer patients has also been examined. Activation of NF-κB has been linked with metastasis of prostate cancer to the lymph nodes (15) and it predicts patient outcomes in prostate cancer (16). Similarly, it is associated with high recurrence and poor survival in squamous cell carcinoma of the tonsil (17) and with oral tumor progression (18); it is a prognostic indicator in esophageal cancer (19), and is associated with chemoradiation resistance in patients with this tumor (20). Progression of lung cancer (21) reduced survival in ovarian cancer (22), aggressiveness of breast cancer (23, 24)and response to chemotherapy in breast cancer (25) have all been linked to NF-κB activation. Why NF-κB is expressed constitutively in these tissues is not clear. Whole-genome structure analysis revealed that the kinase needed for NF-κB activation (IKKe) is amplified and overexpressed in patient -derived breast cancer tissue (7 of 20) (26). In multiple myeloma, high-density oligonucleotide array CGH and gene expression profiling data revealed ten genes causing the inactivation of TRAF2, TRAF3, CYLD, and IAP1/cIAP2, and activation of NFKB1, NFKB2, CD40, LTBR, TACI, and NIK was linked to constitutive activation of the noncanonical NF-κB pathway (27). Constitutive activation of NF-κB in multiple myeloma patients has also been ascribed to elevated expression of NIK due to genomic alterations or protein stabilization ((28). All these studies suggest that NF-κB plays important in most human cancers and thus that suppression of NF-κB should have therapeutic potential.
4B. Role of STAT3 in cancer patients
Unlike NF-κB, very little is known about the status of STAT3 in cancer patients. Various growth factors for cancer cells can activate STAT3, including IL-6 and EGF. Constitutively active STAT3 has been reported in multiple myeloma (29), chronic lymphocytic leukemia (30), gastric cancer (31), lung cancer (32), and laryngeal carcinoma (33). Its constitutive activity has been correlated with unfavorable treatment outcome in acute myelogenous leukemia (34): constitutive STAT3 activity was detected in samples from 44% of patients. Disease-free survival (DFS) was significantly shorter in patients with constitutive STAT3 activity (median 8.7 vs 20.6 months), although overall survival did not differ significantly. The subgroup of patients with constitutive STAT3 activity and the STAT3 beta isoform had the shortest DFS and overall survival of all the patients.
4C. Role of COX2 in cancer patients
COX2 has emerged as another major mediator of inflammation, accounting for over 15,000 citations in the medical literature. Overexpression of COX2 has been shown in patients with various cancers (Table 2). Its overexpression has been linked with poor survival in prostate cancer (35), with a high probability of recurrence of this cancer (36), with shorter survival of lung cancer patients (37), with pathogenesis and progression of oral cancer (38) and progression of esophageal cancer (39). Angiogenesis in Hodgkin's disease (40), shorter progression-free survival in multiple myeloma (41), and lymphoangiogenesis and poor prognosis in invasive breast cancer (42) likewise have been linked to COX overexpression. Levels of COX-2 expression were significant prognostic factors for patients with multiple myeloma (43): overall survival of those patients with negative or weak-moderate COX-2 expression was significantly better than that of patients with strong COX-2 immunoreactivity. Overexpression of COX-2 is also associated with a poor prognosis in patients with SCC of the uterine cervix treated with radiation and concurrent chemotherapy (44). COX-2 expression was also found to be related to nuclear grade in ductal carcinoma in situ, and it was increased in its normal adjacent epithelium (45).
Table 2. Effect of Overexpression of Cyclooxygenase-2 in Progression of Cancer in Patients.
| Leukemia: |
|
| Gastrointestinal Cancers: |
|
| Genitourinary Cancers: |
|
| Breast Cancer: |
|
| Gyneoncologic Cancers: |
|
| Head & Neck Cancers: |
|
ALL-Acute Lymphoblastic leukemia, ATL-Acute T cell leukemia, AML-Acute myelogenous leukemia, HCC-Hepatocellular carcinoma, MALT-Mucosa-associated lymphoid tissue, NSCLC- non-small-cell lung cancer
4D. Role of TNF in cancer patients
Although TNF is the most potent activator of NF-kB, elevated levels of TNF in tissue or serum are not very common in cancer patients. One of the first reports of a possible role for TNF in cancer was presented by (46). TNF was detected in 50% of 226 freshly obtained serum samples from cancer patients with active disease. In contrast, only 3% of 32 samples from normal subjects and 18% of 39 samples from cancer patients with no clinically evident disease were positive for this factor, with low activity. Greater proportions of serum samples from patients with ovarian or oat-cell carcinoma were positive (69% and 63%) than those from patients with lymphoma (26%). TNF mRNA was found in 8 of 11 samples of PBMC from cancer patients, but only 1 of 8 normal subjects, and in 2 of 6 colorectal tumors. The similarity in production of TNF both in patients with ovarian tumors (47) and in patients with benign tumors supports the conclusion that the production of these cytokines is more a nonspecific indicator of an inflammatory process than a specific response to a malignant process. In contrast to these studies, the clinical significance of TNF plasma level in patients with chronic lymphocytic leukemia has been reported (48); and acts as an autocrine and paracrine growth factor in this disease. In CLL patients TNF was significantly higher than in the healthy control population (16.4 versus 8.7 pg/mL). The TNF-alpha level was a predictor of survival, and in fact patients with a TNF level above the mean value of14 pg/mL had significantly shorter survival duration.
4E. Role of IL-1 in cancer patients
IL-1β is another cytokine that is regulated by NF-κB. This cytokine has been associated with growth and progression of human gastric carcinoma (49), colorectal cancer (50), esophageal cancer (51) and ovarian cancer (47). In one study, surgical removal of the ovarian tumor and resolution of ascites was followed by declines in serum levels of IL-1beta (52).
4F. Role of IL-6 in cancer patients
IL-6 is another cytokine that is regulated by NF-κB and serves as a growth factor for various tumors through the upregulation of STAT3., An overproduction of IL-6, indicated by increased plasma C-reactive protein levels, is found in 37% of multiple myeloma patients at diagnosis and is associated with disease aggressiveness, myeloma-cell proliferation, and poor prognosis (53). A systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. (54). Inflammatory markers were measured at baseline in 52 patients with stage IV colorectal cancer. Significantly elevated levels of IL-6 and sgp130 were observed in patients with colon cancer, and inflammatory markers paralleled clinical outcome. Use of these markers could improve prognostication and allow for intervention strategies to reduce tumor-associated inflammation. Malignant ascites of epithelial ovarian cancer patients contains high levels of IL-6 (55).
4G. Role of chemokines in cancer patients
Among the chemokines, IL-8 has been linked with progression of colorectal cancer (CRC) (56), metastasis of CRC to liver (57, 58), metastasis of hepatocellular carcinoma (HCC) (59), progression of prostate cancer (60), poor prognosis of nasopharyngeal carcinoma (61), prognotc maker for gastric cancer (62), and tumor progression and time to relapse of lung cancer (63), ovarian cancer (64) and malignant melanoma (65). The expression of the chemokine receptor CXCR4, which binds to stem cell-derived factor (SDF)-1, has also been linked with metastasis, poor prognsis, and short survival of patients with a wide variety of cancers (Table 3). These include breast (66), prostate (67), esophagus (68), CRC (69), lung (70), thyroid (71), astrocytoma (72) and neuroblastoma (73). In addition, the metastatic potential of a cancer stem cell is determined by the expression of CXCR4 (74, 75). The SDF-1 and the G-protein-coupled seven-span transmembrane receptor CXCR4 axis regulates the trafficking of both normal and cancer stem cells. Moreover, functional CXCR4 is also expressed on nonhematopoietic tissue-committed stem/progenitor cells (TCSCs); hence, the SDF-1-CXCR4 axis emerges as a pivotal regulator of trafficking of various types of stem cells in the body. Furthermore, because most if not all malignancies originate in the stem/progenitor cell compartment, cancer stem cells also express CXCR4 on their surface and, as a result, the SDF-1-CXCR4 axis is also involved in directing their trafficking/metastasis to organs that highly express SDF-1 (e.g., lymph nodes, lungs, liver, and bones). Consequently, strategies aimed at modulating the SDF-1-CXCR4 axis could have important clinical applications both in regenerative medicine to deliver normal stem cells to the tissues/organs and in clinical hematology/oncology to inhibit metastasis of cancer stem cells.
4H. Role of 5LOX in cancer patients
The expression of 5-lipooxygenase is also regulated by NF-κB and it has been linked with progression and development of cancer of the kidney ((76), breast (77) and pancreas (78). Besides 5-LOX, 12 –LOX, has also been linked with progression of breast (79) and prostate cancer (80).
4I. Role of VEGF in cancer patients
VEGF is another cytokine that plays an essential role in proliferation of endothelial cells and angiogenesis. The expression of VEGF has been linked with metastasis, poor prognosis and relapse of numerous cancers in pts including those of the lung (81, 82), liver (83), CRC (84), ovary (85), papillary thyroid (86), stomach (87), nasopharyngeal space (88) and pancreas (89) and melanoma (90). Four most common organs of tumor metastasis are lung, bone, lymph node, and brain. Whether VEGF expression in the tumor tissue or the surrounding normal tissue can selectively regulate metastasis to any of these organs is a subjeect of investigation. VEGF has also been found to activate NF-κB in hematopoietic progenitor cells and mediate their survival (91).
4J. Role of iNOS in cancer patients
iNOS, whoseexpression is regulated by NF-κB, mediates the production of NO. Overexpression of iNOS has been linked with gastric cancer progression (92), brain tumor (93), Barrett's associated neoplastic progression (94), poor survival for stage III melanoma patients (95) and progression of transitional cell carcinoma (96). Some of the effects of iNOS are mediated through the suppression of apoptosis.
4K. Role of CRP in cancer patients
C-reactive protein (CRP), an NF-κB-regulated gene product first linked to cardiovascular diseases, has recently been linked with prognosis of cancers of the breast (97), colon (98), kidney (99), ovary (100)lung (101) and stomach (51), and multiple myeloma (102), melanoma (103), and non-Hodgkin's lymphoma (104). Thus CRP is emerging as an important prognostic marker in a wide variety of cancers.
4L. Role of MMP-9 and UPA in cancer patients
The invasion of vital organs by a tumor is regulated by matrix metalloproteases (MMP) and urinary plasminogen activator (UPA). Both of these proteins are regulated by NF-κB. Expression of MMP-9 has been correlated with prognosis, aggressiveness, and survival in cancer of the lung (105, 106), stomach (107), and esophagus (108), and in non-Hodgkin's lmphoma NHL (109), renal cell carcinoma (110), and liposarcoma (111). The role of UPA under these conditions is less well understood.
4M. Role of cyclin D1 in cancer patients
Cyclin D1, whose expression is regulated by NF-κB, is involved in the transition of cells from G1 to S phase. Mantle cell lymphoma (MCL), which accounts for 5-10% of all non-Hodgkin lymphomas and has the worst prognosis among all lymphomas, The hallmark of MCL is a t(11;14) translocation that results in overexpression of cyclin D1 by tumor cells of virtually all patients (5). Cyclin D1 is also a prognostic marker for other type of cancers but the relationship is highly variable. Approximately 30% of myeloma patients express cyclin D1. The low incidence of translocation t(11;14) detected by conventional cytogenetics suggests that the up-regulation of cyclin D1 protein might result from other mechanisms as well as from gene amplification(112).
5. Inhibitors of inflammation in the clinic for treatment of cancer patients
From these studies, it is clear that various inflammatory markers are expressed in various cancers and they mediate progression of the diseases. Thus agents which suppress these inflammatory markers or the pathways activated by them have a potential for prevention and treatment of cancer (Fig. 3). Some of the agents that have potential to suppress these pathways and are being tested include steroids (such as dexamethasone and predensilon), proteasome inhibitors (such as velcade), TNF inhibitors (such as thalidomide, enbrel, humira and remicade), NF-κB inhibitors (such as curcumin), and COX2 inhibitors (such as aspirin and celecoxib). In addition most nutraceuticals derived from fruits, vegetables, legumes, and spices have been shown to suppress both constitutive and inducible NF-κB activation pathways, thus leading to suppression of various inflammatory biomarkers.
Fig. 3.
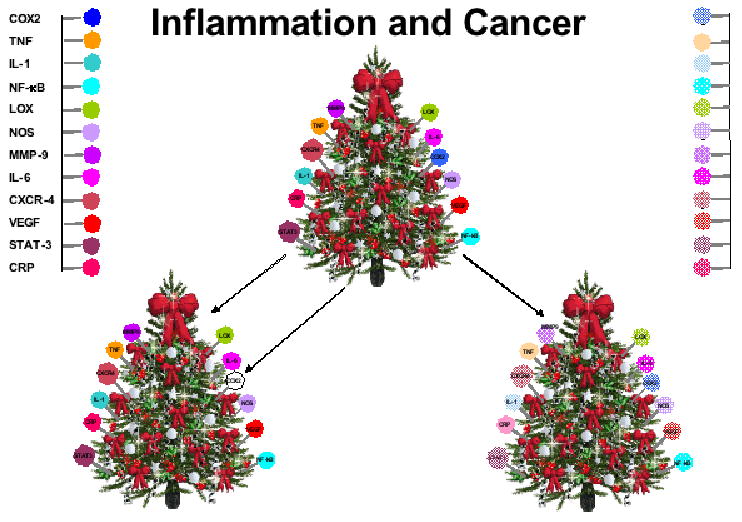
A model for regulation of inflammatory biomarkers. Disease is normally due to dysregulation of numerous inflammatory biomarkers (represented by each bulb). Complete inhibition of a single biomarker (such as COX2) is more likely to be toxic and unlikely to cure the disease. However, downregulation of several biomarkers partially is more likely to inhibit the dysregulated inflammation, be less toxic and more efficient in treating the disease.
A model for regulation of inflammatory biomarkers is proposed (Fig. 3). Disease is normally due to dysregulation of numerous inflammatory biomarkers (represented by each bulb). Complete inhibition of a single biomarker (such as COX2) is more likely to be toxic and unlikely to cure the disease. However, downregulation of several biomarkers “partially” is more likely to inhibit the dysregulated inflammation and be less toxic and more efficient in treating the disease.
6. Conclusion
All the studies described above provide conclusive proof that inflammation is a critical mediator of cancer. Thus antinflammatory agents should be explored for both prevention and treatment of cancer. Although numerous cell culture and animal studies have identified several natural anti-inflammatory agents, their true potential will be recognized only through well-controlled clinical trials. Such studies are urgently needed. Curcumin, a component of turmeric, is one such agent that has been shown to suppress all the pathways indicated above. But its full clinical potential remains to be recognized.
Acknowledgments
We thank Walter Pagel for carefully proofreading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and grant from Center for Targeted Therapy of M.D. Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heidland A, Klassen A, Rutkowski P, Bahner U. The contribution of Rudolf Virchow to the concept of inflammation: what is still of importance? J Nephrol. 2006;19 10:S102–9. [PubMed] [Google Scholar]
- 2.Vilimas T, Mascarenhas J, Palomero T, et al. Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nat Med. 2007;13:70–7. doi: 10.1038/nm1524. [DOI] [PubMed] [Google Scholar]; **An important evidence for the role of NF-kB in leukemia.
- 3.Lee KJ, Kim HA, Kim PH, et al. Ox-LDL suppresses PMA-induced MMP-9 expression and activity through CD36-mediated activation of PPAR-g. Exp Mol Med. 2004;36:534–44. doi: 10.1038/emm.2004.68. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Sun L, Qian J, et al. Cyclin D1 as a universally expressed mantle cell lymphoma-associated tumor antigen for immunotherapy. Leukemia. 2009 doi: 10.1038/leu.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteghirfo S, Tosetti F, Ambrosini C, et al. Antileukemia effects of xanthohumol in Bcr/Abl-transformed cells involve nuclear factor-kappaB and p53 modulation. Mol Cancer Ther. 2008;7:2692–702. doi: 10.1158/1535-7163.MCT-08-0132. [DOI] [PubMed] [Google Scholar]
- 7.Fujioka S, Niu J, Schmidt C, et al. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004;24:7806–19. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 9.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–7. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 10.Guzman ML, Rossi RM, Neelakantan S, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–35. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun T, Carvalho G, Coquelle A, et al. NF-kappaB constitutes a potential therapeutic target in high-risk myelodysplastic syndrome. Blood. 2006;107:1156–65. doi: 10.1182/blood-2005-05-1989. [DOI] [PubMed] [Google Scholar]
- 12.Birnie R, Bryce SD, Roome C, et al. Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 2008;9:R83. doi: 10.1186/gb-2008-9-5-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widera D, Kaus A, Kaltschmidt C, Kaltschmidt B. Neural stem cells, inflammation and NF-kappaB: basic principle of maintenance and repair or origin of brain tumours? J Cell Mol Med. 2008;12:459–70. doi: 10.1111/j.1582-4934.2007.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Zhang Y. Cancer stem cells: Models, mechanisms and implications for improved treatment. Cell Cycle. 2008;7:1360–70. doi: 10.4161/cc.7.10.5953. [DOI] [PubMed] [Google Scholar]
- 15.Ismail HA, Lessard L, Mes-Masson AM, Saad F. Expression of NF-kappaB in prostate cancer lymph node metastases. Prostate. 2004;58:308–13. doi: 10.1002/pros.10335. [DOI] [PubMed] [Google Scholar]; **Provides first evidence for the role of NF-kB in prostate cancer.
- 16.Lessard L, Begin LR, Gleave ME, Mes-Masson AM, Saad F. Nuclear localisation of nuclear factor-kappaB transcription factors in prostate cancer: an immunohistochemical study. Br J Cancer. 2005;93:1019–23. doi: 10.1038/sj.bjc.6602796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang PL, Pellitteri PK, Law A, et al. Overexpression of phosphorylated nuclear factor-kappa B in tonsillar squamous cell carcinoma and high-grade dysplasia is associated with poor prognosis. Mod Pathol. 2005;18:924–32. doi: 10.1038/modpathol.3800372. [DOI] [PubMed] [Google Scholar]
- 18.Mishra A, Bharti AC, Varghese P, Saluja D, Das BC. Differential expression and activation of NF-kappaB family proteins during oral carcinogenesis: Role of high risk human papillomavirus infection. Int J Cancer. 2006;119:2840–50. doi: 10.1002/ijc.22262. [DOI] [PubMed] [Google Scholar]
- 19.Izzo JG, Malhotra U, Wu TT, et al. Clinical biology of esophageal adenocarcinoma after surgery is influenced by nuclear factor-kappaB expression. Cancer Epidemiol Biomarkers Prev. 2007;16:1200–5. doi: 10.1158/1055-9965.EPI-06-1083. [DOI] [PubMed] [Google Scholar]
- 20.Izzo JG, Malhotra U, Wu TT, et al. Association of activated transcription factor nuclear factor kappab with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24:748–54. doi: 10.1200/JCO.2005.03.8810. [DOI] [PubMed] [Google Scholar]
- 21.Tang X, Liu D, Shishodia S, et al. Nuclear factor-kappaB (NF-kappaB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer. 2006;107:2637–46. doi: 10.1002/cncr.22315. [DOI] [PubMed] [Google Scholar]
- 22.Niesporek S, Weichert W, Sinn B, et al. NF-kappaB subunit p65/RelA expression in ovarian carcinoma: prognostic impact and link to COX-2 overexpression. Verh Dtsch Ges Pathol. 2007;91:243–9. [PubMed] [Google Scholar]
- 23.Lerebours F, Vacher S, Andrieu C, et al. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer. 2008;8:41. doi: 10.1186/1471-2407-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Eppenberger-Castori S, Marx C, et al. Activation of nuclear factor-kappaB (NFkappaB) identifies a high-risk subset of hormone-dependent breast cancers. Int J Biochem Cell Biol. 2005;37:1130–44. doi: 10.1016/j.biocel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Garg AK, Hortobagyi GN, Aggarwal BB, Sahin AA, Buchholz TA. Nuclear factor-kappa B as a predictor of treatment response in breast cancer. Curr Opin Oncol. 2003;15:405–11. doi: 10.1097/00001622-200311000-00001. [DOI] [PubMed] [Google Scholar]; **Shows NF-kB as a predictor of response in breast cancer.
- 26.Boehm JS, Zhao JJ, Yao J, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–79. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Keats JJ, Fonseca R, Chesi M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–44. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–30. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 30.Frank DA, Mahajan S, Ritz J. B lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J Clin Invest. 1997;100:3140–8. doi: 10.1172/JCI119869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanda N, Seno H, Konda Y, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–9. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- 32.Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–94. doi: 10.1158/1078-0432.CCR-05-0827. [DOI] [PubMed] [Google Scholar]
- 33.Liu B, Ren Z, Shi Y, Guan C, Pan Z, Zong Z. Activation of signal transducers and activators of transcription 3 and overexpression of its target gene CyclinD1 in laryngeal carcinomas. Laryngoscope. 2008;118:1976–80. doi: 10.1097/MLG.0b013e31817fd3fa. [DOI] [PubMed] [Google Scholar]
- 34.Benekli M, Xia Z, Donohue KA, et al. Constitutive activity of signal transducer and activator of transcription 3 protein in acute myeloid leukemia blasts is associated with short disease-free survival. Blood. 2002;99:252–7. doi: 10.1182/blood.v99.1.252. [DOI] [PubMed] [Google Scholar]
- 35.Khor LY, Bae K, Pollack A, et al. COX-2 expression predicts prostate-cancer outcome: analysis of data from the RTOG 92-02 trial. Lancet Oncol. 2007;8:912–20. doi: 10.1016/S1470-2045(07)70280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen BL, Gomez P, Omori Y, et al. Cyclooxygenase-2 (COX-2) expression is an independent predictor of prostate cancer recurrence. Int J Cancer. 2006;119:1082–7. doi: 10.1002/ijc.21749. [DOI] [PubMed] [Google Scholar]
- 37.Khuri FR, Wu H, Lee JJ, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861–7. [PubMed] [Google Scholar]
- 38.Pannone G, Bufo P, Caiaffa MF, et al. Cyclooxygenase-2 expression in oral squamous cell carcinoma. Int J Immunopathol Pharmacol. 2004;17:273–82. doi: 10.1177/039463200401700307. [DOI] [PubMed] [Google Scholar]
- 39.Yang GZ, Li L, Ding HY, Zhou JS. Cyclooxygenase-2 is over-expressed in Chinese esophageal squamous cell carcinoma, and correlated with NF-kappaB: an immunohistochemical study. Exp Mol Pathol. 2005;79:214–8. doi: 10.1016/j.yexmp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Ohsawa M, Fukushima H, Ikura Y, et al. Expression of cyclooxygenase-2 in Hodgkin's lymphoma: its role in cell proliferation and angiogenesis. Leuk Lymphoma. 2006;47:1863–71. doi: 10.1080/10428190600685442. [DOI] [PubMed] [Google Scholar]
- 41.Trojan A, Tinguely M, Vallet S, et al. Clinical significance of cyclooxygenase-2 (COX-2) in multiple myeloma. Swiss Med Wkly. 2006;136:400–3. doi: 10.4414/smw.2006.11467. [DOI] [PubMed] [Google Scholar]
- 42.Zhang XH, Huang DP, Guo GL, et al. Coexpression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC Cancer. 2008;8:4. doi: 10.1186/1471-2407-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cetin M, Buyukberber S, Demir M, et al. Overexpression of cyclooxygenase-2 in multiple myeloma: association with reduced survival. Am J Hematol. 2005;80:169–73. doi: 10.1002/ajh.20460. [DOI] [PubMed] [Google Scholar]
- 44.Kim YB, Kim GE, Cho NH, et al. Overexpression of cyclooxygenase-2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer. 2002;95:531–9. doi: 10.1002/cncr.10684. [DOI] [PubMed] [Google Scholar]
- 45.Shim SJ, Yang WI, Shin E, et al. Clinical significance of cyclooxygenase-2 expression in extranodal natural killer (NK)/T-cell lymphoma, nasal type. Int J Radiat Oncol Biol Phys. 2007;67:31–8. doi: 10.1016/j.ijrobp.2006.07.1387. [DOI] [PubMed] [Google Scholar]
- 46.Balkwill F, Osborne R, Burke F, et al. Evidence for tumour necrosis factor/cachectin production in cancer. Lancet. 1987;2:1229–32. doi: 10.1016/s0140-6736(87)91850-2. [DOI] [PubMed] [Google Scholar]; *An earliest reported indication about the role of inflammation in cancer.
- 47.Punnonen J, Heinonen PK, Kuoppala T, Jansen CT, Punnonen R. Production of interleukin-1 beta and tumour necrosis factor-alpha in patients with benign or malignant ovarian tumours. J Cancer Res Clin Oncol. 1991;117:587–92. doi: 10.1007/BF01613293. [DOI] [PubMed] [Google Scholar]
- 48.Ferrajoli A, Keating MJ, Manshouri T, et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100:1215–9. [PubMed] [Google Scholar]
- 49.Kai H, Kitadai Y, Kodama M, et al. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005;25:709–13. [PubMed] [Google Scholar]
- 50.Miki C, Konishi N, Ojima E, Hatada T, Inoue Y, Kusunoki M. C-reactive protein as a prognostic variable that reflects uncontrolled up-regulation of the IL-1-IL-6 network system in colorectal carcinoma. Dig Dis Sci. 2004;49:970–6. doi: 10.1023/b:ddas.0000034556.48527.6e. [DOI] [PubMed] [Google Scholar]
- 51.Deans DA, Wigmore SJ, Gilmour H, Paterson-Brown S, Ross JA, Fearon KC. Elevated tumour interleukin-1beta is associated with systemic inflammation: A marker of reduced survival in gastro-oesophageal cancer. Br J Cancer. 2006;95:1568–75. doi: 10.1038/sj.bjc.6603446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. The role of inflammatory cytokines in Meigs' syndrome. Obstet Gynecol. 2002;99:917–9. doi: 10.1016/s0029-7844(01)01602-7. [DOI] [PubMed] [Google Scholar]
- 53.Klein B, Bataille R. Cytokine network in human multiple myeloma. Hematol Oncol Clin North Am. 1992;6:273–84. [PubMed] [Google Scholar]
- 54.Sharma R, Zucknick M, London R, Kacevska M, Liddle C, Clarke SJ. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clin Colorectal Cancer. 2008;7:331–7. doi: 10.3816/CCC.2008.n.044. [DOI] [PubMed] [Google Scholar]
- 55.Offner FA, Obrist P, Stadlmann S, et al. IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine. 1995;7:542–7. doi: 10.1006/cyto.1995.0073. [DOI] [PubMed] [Google Scholar]
- 56.Rubie C, Frick VO, Pfeil S, et al. Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer. World J Gastroenterol. 2007;13:4996–5002. doi: 10.3748/wjg.v13.i37.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terada H, Urano T, Konno H. Association of interleukin-8 and plasminogen activator system in the progression of colorectal cancer. Eur Surg Res. 2005;37:166–72. doi: 10.1159/000085964. [DOI] [PubMed] [Google Scholar]
- 58.Haraguchi M, Komuta K, Akashi A, Matsuzaki S, Furui J, Kanematsu T. Elevated IL-8 levels in the drainage vein of resectable Dukes' C colorectal cancer indicate high risk for developing hepatic metastasis. Oncol Rep. 2002;9:159–65. [PubMed] [Google Scholar]
- 59.Kubo F, Ueno S, Hiwatashi K, et al. Interleukin 8 in human hepatocellular carcinoma correlates with cancer cell invasion of vessels but not with tumor angiogenesis. Ann Surg Oncol. 2005;12:800–7. doi: 10.1245/ASO.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 60.Murphy C, McGurk M, Pettigrew J, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin Cancer Res. 2005;11:4117–27. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- 61.Horikawa T, Kaizaki Y, Kato H, Furukawa M, Yoshizaki T. Expression of interleukin-8 receptor A predicts poor outcome in patients with nasopharyngeal carcinoma. Laryngoscope. 2005;115:62–7. doi: 10.1097/01.mlg.0000150675.37860.f7. [DOI] [PubMed] [Google Scholar]
- 62.Kido S, Kitadai Y, Hattori N, et al. Interleukin 8 and vascular endothelial growth factor -- prognostic factors in human gastric carcinomas? Eur J Cancer. 2001;37:1482–7. doi: 10.1016/s0959-8049(01)00147-2. [DOI] [PubMed] [Google Scholar]
- 63.Yuan A, Yang PC, Yu CJ, et al. Interleukin-8 messenger ribonucleic acid expression correlates with tumor progression, tumor angiogenesis, patient survival, and timing of relapse in non-small-cell lung cancer. Am J Respir Crit Care Med. 2000;162:1957–63. doi: 10.1164/ajrccm.162.5.2002108. [DOI] [PubMed] [Google Scholar]
- 64.Kassim SK, El-Salahy EM, Fayed ST, et al. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin Biochem. 2004;37:363–9. doi: 10.1016/j.clinbiochem.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Nurnberg W, Tobias D, Otto F, Henz BM, Schadendorf D. Expression of interleukin-8 detected by in situ hybridization correlates with worse prognosis in primary cutaneous melanoma. J Pathol. 1999;189:546–51. doi: 10.1002/(SICI)1096-9896(199912)189:4<546::AID-PATH487>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 66.Woo SU, Bae JW, Kim CH, Lee JB, Koo BW. A significant correlation between nuclear CXCR4 expression and axillary lymph node metastasis in hormonal receptor negative breast cancer. Ann Surg Oncol. 2008;15:281–5. doi: 10.1245/s10434-007-9595-1. [DOI] [PubMed] [Google Scholar]
- 67.Darash-Yahana M, Pikarsky E, Abramovitch R, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. Faseb J. 2004;18:1240–2. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 68.Kaifi JT, Yekebas EF, Schurr P, et al. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J Natl Cancer Inst. 2005;97:1840–7. doi: 10.1093/jnci/dji431. [DOI] [PubMed] [Google Scholar]
- 69.Yoshitake N, Fukui H, Yamagishi H, et al. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer. 2008;98:1682–9. doi: 10.1038/sj.bjc.6604363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su L, Zhang J, Xu H, et al. Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin Cancer Res. 2005;11:8273–80. doi: 10.1158/1078-0432.CCR-05-0537. [DOI] [PubMed] [Google Scholar]
- 71.Wagner PL, Moo TA, Arora N, et al. The chemokine receptors CXCR4 and CCR7 are associated with tumor size and pathologic indicators of tumor aggressiveness in papillary thyroid carcinoma. Ann Surg Oncol. 2008;15:2833–41. doi: 10.1245/s10434-008-0064-2. [DOI] [PubMed] [Google Scholar]
- 72.Woerner BM, Warrington NM, Kung AL, Perry A, Rubin JB. Widespread CXCR4 activation in astrocytomas revealed by phospho-CXCR4-specific antibodies. Cancer Res. 2005;65:11392–9. doi: 10.1158/0008-5472.CAN-05-0847. [DOI] [PubMed] [Google Scholar]
- 73.Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg. 2004;39:1506–11. doi: 10.1016/j.jpedsurg.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 74.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 75.Gelmini S, Mangoni M, Castiglione F, et al. The CXCR4/CXCL12 axis in endometrial cancer. Clin Exp Metastasis. 2009;26:261–8. doi: 10.1007/s10585-009-9240-4. [DOI] [PubMed] [Google Scholar]
- 76.Faronato M, Muzzonigro G, Milanese G, et al. Increased expression of 5-lipoxygenase is common in clear cell renal cell carcinoma. Histol Histopathol. 2007;22:1109–18. doi: 10.14670/HH-22.1109. [DOI] [PubMed] [Google Scholar]
- 77.Jiang WG, Douglas-Jones AG, Mansel RE. Aberrant expression of 5-lipoxygenase-activating protein (5-LOXAP) has prognostic and survival significance in patients with breast cancer. Prostaglandins Leukot Essent Fatty Acids. 2006;74:125–34. doi: 10.1016/j.plefa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Hennig R, Grippo P, Ding XZ, et al. 5-Lipoxygenase, a marker for early pancreatic intraepithelial neoplastic lesions. Cancer Res. 2005;65:6011–6. doi: 10.1158/0008-5472.CAN-04-4090. [DOI] [PubMed] [Google Scholar]
- 79.Jiang WG, Douglas-Jones A, Mansel RE. Levels of expression of lipoxygenases and cyclooxygenase-2 in human breast cancer. Prostaglandins Leukot Essent Fatty Acids. 2003;69:275–81. doi: 10.1016/s0952-3278(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 80.Gao X, Grignon DJ, Chbihi T, et al. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–37. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 81.Maekawa S, Iwasaki A, Shirakusa T, et al. Correlation between lymph node metastasis and the expression of VEGF-C, VEGF-D and VEGFR-3 in T1 lung adenocarcinoma. Anticancer Res. 2007;27:3735–41. [PubMed] [Google Scholar]
- 82.Li J, Li BL, Zhang HQ, et al. Relationship between vascular endothelial growth factor C expression level and lymph node metastasis in non small cell lung cancer. Zhonghua Yi Xue Za Zhi. 2008;88:2982–5. [PubMed] [Google Scholar]
- 83.Jia JB, Zhuang PY, Sun HC, et al. Protein expression profiling of vascular endothelial growth factor and its receptors identifies subclasses of hepatocellular carcinoma and predicts survival. J Cancer Res Clin Oncol. 2009;135:847–54. doi: 10.1007/s00432-008-0521-0. [DOI] [PubMed] [Google Scholar]
- 84.Kim JG, Chae YS, Sohn SK, et al. Vascular endothelial growth factor gene polymorphisms associated with prognosis for patients with colorectal cancer. Clin Cancer Res. 2008;14:62–6. doi: 10.1158/1078-0432.CCR-07-1537. [DOI] [PubMed] [Google Scholar]
- 85.Duncan TJ, Al-Attar A, Rolland P, et al. Vascular endothelial growth factor expression in ovarian cancer: a model for targeted use of novel therapies? Clin Cancer Res. 2008;14:3030–5. doi: 10.1158/1078-0432.CCR-07-1888. [DOI] [PubMed] [Google Scholar]
- 86.Tian X, Cong M, Zhou W, Zhu J, Liu Q. Relationship between protein expression of VEGF-C, MMP-2 and lymph node metastasis in papillary thyroid cancer. J Int Med Res. 2008;36:699–703. doi: 10.1177/147323000803600411. [DOI] [PubMed] [Google Scholar]
- 87.Tang H, Wang J, Bai F, et al. Positive correlation of osteopontin, cyclooxygenase-2 and vascular endothelial growth factor in gastric cancer. Cancer Invest. 2008;26:60–7. doi: 10.1080/07357900701519279. [DOI] [PubMed] [Google Scholar]
- 88.Li YH, Hu CF, Shao Q, et al. Elevated expressions of survivin and VEGF protein are strong independent predictors of survival in advanced nasopharyngeal carcinoma. J Transl Med. 2008;6:1. doi: 10.1186/1479-5876-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang B, Zhao WH, Zhou WY, Yu WS, Yu JM, Li S. Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. Cancer Detect Prev. 2007;31:436–42. doi: 10.1016/j.cdp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 90.Boone B, Blokx W, De Bacquer D, Lambert J, Ruiter D, Brochez L. The role of VEGF-C staining in predicting regional metastasis in melanoma. Virchows Arch. 2008;453:257–65. doi: 10.1007/s00428-008-0641-6. [DOI] [PubMed] [Google Scholar]
- 91.Dikov MM, Oyama T, Cheng P, et al. Vascular endothelial growth factor effects on nuclear factor-kappaB activation in hematopoietic progenitor cells. Cancer Res. 2001;61:2015–21. [PubMed] [Google Scholar]
- 92.Rajnakova A, Moochhala S, Goh PM, Ngoi S. Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer Lett. 2001;172:177–85. doi: 10.1016/s0304-3835(01)00645-0. [DOI] [PubMed] [Google Scholar]
- 93.Broholm H, Rubin I, Kruse A, et al. Nitric oxide synthase expression and enzymatic activity in human brain tumors. Clin Neuropathol. 2003;22:273–81. [PubMed] [Google Scholar]
- 94.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–34. [PubMed] [Google Scholar]
- 95.Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer. 2006;119:861–6. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- 96.Hayashi H, Kuwahara M, Fujisaki N, Furihata M, Ohtsuki Y, Kagawa S. Immunohistochemical findings of nitric oxide synthase expression in urothelial transitional cell carcinoma including dysplasia. Oncol Rep. 2001;8:1275–9. doi: 10.3892/or.8.6.1275. [DOI] [PubMed] [Google Scholar]
- 97.O'Hanlon DM, Lynch J, Cormican M, Given HF. The acute phase response in breast carcinoma. Anticancer Res. 2002;22:1289–93. [PubMed] [Google Scholar]
- 98.Cahlin C, Lonnroth C, Arvidsson A, Nordgren S, Lundholm K. Growth associated proteins in tumor cells and stroma related to disease progression of colon cancer accounting for tumor tissue PGE2 content. Int J Oncol. 2008;32:909–18. [PubMed] [Google Scholar]
- 99.Jabs WJ, Busse M, Kruger S, Jocham D, Steinhoff J, Doehn C. Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney Int. 2005;68:2103–10. doi: 10.1111/j.1523-1755.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 100.Zakrzewska I, Poznanski J. Changes of serum il-6 and CRP after chemotherapy in patients with ovarian carcinoma. Pol Merkur Lekarski. 2001;11:210–3. [PubMed] [Google Scholar]
- 101.Yudoh K, Matsui H, Kamanori M, et al. Prognostic value of the doubling time of serum C-reactive protein and alkaline phosphatase levels in primary bone and soft tissue tumors. Jpn J Cancer Res. 1996;87:1288–95. doi: 10.1111/j.1349-7006.1996.tb03145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamble R, Wilson CS, Fassas A, et al. Malignant pleural effusion of multiple myeloma: prognostic factors and outcome. Leuk Lymphoma. 2005;46:1137–42. doi: 10.1080/10428190500102845. [DOI] [PubMed] [Google Scholar]
- 103.Reichle A, Bross K, Vogt T, et al. Pioglitazone and rofecoxib combined with angiostatically scheduled trofosfamide in the treatment of far-advanced melanoma and soft tissue sarcoma. Cancer. 2004;101:2247–56. doi: 10.1002/cncr.20574. [DOI] [PubMed] [Google Scholar]
- 104.Bien E, Balcerska A. Clinical significance of erythrocyte sedimentation rate, C-reactive protein and serum lactate dehydrogenase levels in the diagnosis, prognosis and treatment monitoring of children suffering from cancer. Med Wieku Rozwoj. 2004;8:1081–9. [PubMed] [Google Scholar]
- 105.Chen XL, Wang LC, Zhang WG, Chen XY, Sun ZM. Correlations of S100A4 and MMP9 expressions to infiltration, metastasis and prognosis of non-small cell lung cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1254–8. [PubMed] [Google Scholar]
- 106.Guo CB, Wang S, Deng C, Zhang DL, Wang FL, Jin XQ. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol Diagn Ther. 2007;11:183–92. doi: 10.1007/BF03256240. [DOI] [PubMed] [Google Scholar]
- 107.Hu ZL, Wen JF, Shen M, Liu Y. Expressions of TGIF, MMP9 and VEGF proteins and their clinicopathological relationship in gastric cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:70–4. [PubMed] [Google Scholar]
- 108.Gu ZD, Li JY, Li M, et al. Matrix metalloproteinases expression correlates with survival in patients with esophageal squamous cell carcinoma. Am J Gastroenterol. 2005;100:1835–43. doi: 10.1111/j.1572-0241.2005.50018.x. [DOI] [PubMed] [Google Scholar]
- 109.Sakata K, Satoh M, Someya M, et al. Expression of matrix metalloproteinase 9 is a prognostic factor in patients with non-Hodgkin lymphoma. Cancer. 2004;100:356–65. doi: 10.1002/cncr.11905. [DOI] [PubMed] [Google Scholar]
- 110.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113–9. [PubMed] [Google Scholar]
- 111.Benassi MS, Gamberi G, Magagnoli G, et al. Metalloproteinase expression and prognosis in soft tissue sarcomas. Ann Oncol. 2001;12:75–80. doi: 10.1023/a:1008318614461. [DOI] [PubMed] [Google Scholar]
- 112.Hoechtlen-Vollmar W, Menzel G, Bartl R, Lamerz R, Wick M, Seidel D. Amplification of cyclin D1 gene in multiple myeloma: clinical and prognostic relevance. Br J Haematol. 2000;109:30–8. doi: 10.1046/j.1365-2141.2000.02007.x. [DOI] [PubMed] [Google Scholar]
- 113.Munzert G, Kirchner D, Ottmann O, Bergmann L, Schmid RM. Constitutive NF-kappab/Rel activation in philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) Leuk Lymphoma. 2004;45:1181–4. doi: 10.1080/10428190310001657326. [DOI] [PubMed] [Google Scholar]
- 114.Zaninoni A, Imperiali FG, Pasquini C, Zanella A, Barcellini W. Cytokine modulation of nuclear factor-kappaB activity in B-chronic lymphocytic leukemia. Exp Hematol. 2003;31:185–90. doi: 10.1016/s0301-472x(02)01046-9. [DOI] [PubMed] [Google Scholar]
- 115.Hewamana S, Lin TT, Jenkins C, et al. The novel nuclear factor-kappaB inhibitor LC-1 is equipotent in poor prognostic subsets of chronic lymphocytic leukemia and shows strong synergy with fludarabine. Clin Cancer Res. 2008;14:8102–11. doi: 10.1158/1078-0432.CCR-08-1673. [DOI] [PubMed] [Google Scholar]
- 116.Hewamana S, Lin TT, Rowntree C, et al. Rel a is an independent biomarker of clinical outcome in chronic lymphocytic leukemia. J Clin Oncol. 2009;27:763–9. doi: 10.1200/JCO.2008.19.1114. [DOI] [PubMed] [Google Scholar]
- 117.Martinez-Delgado B, Cuadros M, Honrado E, et al. Differential expression of NF-kappaB pathway genes among peripheral T-cell lymphomas. Leukemia. 2005;19:2254–63. doi: 10.1038/sj.leu.2403960. [DOI] [PubMed] [Google Scholar]
- 118.Kuo SH, Chen LT, Yeh KH, et al. Nuclear expression of BCL10 or nuclear factor kappa B predicts Helicobacter pylori-independent status of early-stage, high-grade gastric mucosa-associated lymphoid tissue lymphomas. J Clin Oncol. 2004;22:3491–7. doi: 10.1200/JCO.2004.10.087. [DOI] [PubMed] [Google Scholar]
- 119.Yeh KH, Kuo SH, Chen LT, et al. Nuclear expression of BCL10 or nuclear factor kappa B helps predict Helicobacter pylori-independent status of low-grade gastric mucosa-associated lymphoid tissue lymphomas with or without t(11;18)(q21;q21) Blood. 2005;106:1037–41. doi: 10.1182/blood-2005-01-0004. [DOI] [PubMed] [Google Scholar]
- 120.Valnet-Rabier MB, Challier B, Thiebault S, et al. c-Flip protein expression in Burkitt's lymphomas is associated with a poor clinical outcome. Br J Haematol. 2005;128:767–73. doi: 10.1111/j.1365-2141.2005.05378.x. [DOI] [PubMed] [Google Scholar]
- 121.Ruiz-Ballesteros E, Mollejo M, Rodriguez A, et al. Splenic marginal zone lymphoma: proposal of new diagnostic and prognostic markers identified after tissue and cDNA microarray analysis. Blood. 2005;106:1831–8. doi: 10.1182/blood-2004-10-3898. [DOI] [PubMed] [Google Scholar]
- 122.Bargou RC, Leng C, Krappmann D, et al. High-level nuclear NF-kappa B and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood. 1996;87:4340–7. [PubMed] [Google Scholar]
- 123.Emmerich F, Meiser M, Hummel M, et al. Overexpression of I kappa B alpha without inhibition of NF-kappaB activity and mutations in the I kappa B alpha gene in Reed-Sternberg cells. Blood. 1999;94:3129–34. [PubMed] [Google Scholar]
- 124.Houldsworth J, Petlakh M, Olshen AB, Chaganti RS. Pathway activation in large B-cell non-Hodgkin lymphoma cell lines by doxorubicin reveals prognostic markers of in vivo response. Leuk Lymphoma. 2008;49:2170–80. doi: 10.1080/10428190802428369. [DOI] [PubMed] [Google Scholar]
- 125.Santhi WS, Sebastian P, Varghese BT, Prakash O, Pillai MR. NF-kappaB and COX-2 during oral tumorigenesis and in assessment of minimal residual disease in surgical margins. Exp Mol Pathol. 2006;81:123–30. doi: 10.1016/j.yexmp.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 126.Logan RM, Gibson RJ, Sonis ST, Keefe DM. Nuclear factor-kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007;43:395–401. doi: 10.1016/j.oraloncology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 127.Rhodus NL, Cheng B, Myers S, Bowles W, Ho V, Ondrey F. A comparison of the pro-inflammatory, NF-kappaB-dependent cytokines: TNF-alpha, IL-1-alpha, IL-6, and IL-8 in different oral fluids from oral lichen planus patients. Clin Immunol. 2005;114:278–83. doi: 10.1016/j.clim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 128.Rhodus NL, Ho V, Miller CS, Myers S, Ondrey F. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29:42–5. doi: 10.1016/j.cdp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 129.Izzo JG, Correa AM, Wu TT, et al. Pretherapy nuclear factor-kappaB status, chemoradiation resistance, and metastatic progression in esophageal carcinoma. Mol Cancer Ther. 2006;5:2844–50. doi: 10.1158/1535-7163.MCT-06-0351. [DOI] [PubMed] [Google Scholar]
- 130.Gan H, Ouyang Q, Chen Y, Xia Q. Activation of nuclear factor-kappaB and effects of anti-inflammatory treatment thereon in intestinal mucosa of patients with ulcerative colitis. Zhonghua Yi Xue Za Zhi. 2002;82:384–8. [PubMed] [Google Scholar]
- 131.Tai DI, Tsai SL, Chang YH, et al. Constitutive activation of nuclear factor kappaB in hepatocellular carcinoma. Cancer. 2000;89:2274–81. [PubMed] [Google Scholar]
- 132.Weichert W, Boehm M, Gekeler V, et al. High expression of RelA/p65 is associated with activation of nuclear factor-kappaB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer. 2007;97:523–30. doi: 10.1038/sj.bjc.6603878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O'Neil BH, Buzkova P, Farrah H, et al. Expression of nuclear factor-kappaB family proteins in hepatocellular carcinomas. Oncology. 2007;72:97–104. doi: 10.1159/000111116. [DOI] [PubMed] [Google Scholar]
- 134.Asakawa M, Kono H, Amemiya H, et al. Role of interleukin-18 and its receptor in hepatocellular carcinoma associated with hepatitis C virus infection. Int J Cancer. 2006;118:564–70. doi: 10.1002/ijc.21367. [DOI] [PubMed] [Google Scholar]
- 135.Cascinu S, Scartozzi M, Carbonari G, et al. COX-2 and NF-KB overexpression is common in pancreatic cancer but does not predict for COX-2 inhibitors activity in combination with gemcitabine and oxaliplatin. Am J Clin Oncol. 2007;30:526–30. doi: 10.1097/COC.0b013e318054675c. [DOI] [PubMed] [Google Scholar]
- 136.Yamanaka N, Sasaki N, Tasaki A, et al. Nuclear factor-kappaB p65 is a prognostic indicator in gastric carcinoma. Anticancer Res. 2004;24:1071–5. [PubMed] [Google Scholar]
- 137.Levidou G, Korkolopoulou P, Nikiteas N, et al. Expression of nuclear factor kappaB in human gastric carcinoma: relationship with I kappaB a and prognostic significance. Virchows Arch. 2007;450:519–27. doi: 10.1007/s00428-007-0396-5. [DOI] [PubMed] [Google Scholar]
- 138.Wu L, Pu Z, Feng J, Li G, Zheng Z, Shen W. The ubiquitin-proteasome pathway and enhanced activity of NF-kappaB in gastric carcinoma. J Surg Oncol. 2008;97:439–44. doi: 10.1002/jso.20952. [DOI] [PubMed] [Google Scholar]
- 139.Voboril R, Voborilova J, Rychterova V, Jirasek T, Dvorak J. Dissociated invasively growing cancer cells with NF-kappaB/p65 positivity after radiotherapy: a new marker for worse clinical outcome in rectal cancer? Preliminary data. Clin Exp Metastasis. 2008;25:491–6. doi: 10.1007/s10585-008-9155-5. [DOI] [PubMed] [Google Scholar]
- 140.Levidou G, Saetta AA, Korkolopoulou P, et al. Clinical significance of nuclear factor (NF)-kappaB levels in urothelial carcinoma of the urinary bladder. Virchows Arch. 2008;452:295–304. doi: 10.1007/s00428-007-0560-y. [DOI] [PubMed] [Google Scholar]
- 141.Riemann K, Becker L, Struwe H, Rubben H, Eisenhardt A, Siffert W. Insertion/deletion polymorphism in the promoter of NFKB1 as a potential molecular marker for the risk of recurrence in superficial bladder cancer. Int J Clin Pharmacol Ther. 2007;45:423–30. doi: 10.5414/cpp45423. [DOI] [PubMed] [Google Scholar]
- 142.Fradet V, Lessard L, Begin LR, Karakiewicz P, Masson AM, Saad F. Nuclear factor-kappaB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res. 2004;10:8460–4. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- 143.Domingo-Domenech J, Mellado B, Ferrer B, et al. Activation of nuclear factor-kappaB in human prostate carcinogenesis and association to biochemical relapse. Br J Cancer. 2005;93:1285–94. doi: 10.1038/sj.bjc.6602851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Korkolopoulou P, Levidou G, Saetta AA, et al. Expression of nuclear factor-kappaB in human astrocytomas: relation to pIkappaBa, vascular endothelial growth factor, Cox-2, microvascular characteristics, and survival. Hum Pathol. 2008;39:1143–52. doi: 10.1016/j.humpath.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 145.Yamamoto M, Fukushima T, Hayashi S, et al. Correlation of the expression of nuclear factor-kappa B, tumor necrosis factor receptor type 1 (TNFR 1) and c-Myc with the clinical course in the treatment of malignant astrocytomas with recombinant mutant human tumor necrosis factor-alpha (TNF-SAM2) Anticancer Res. 2000;20:611–8. [PubMed] [Google Scholar]
- 146.Conti A, Ageunnouz M, La Torre D, et al. Expression of the tumor necrosis factor receptor-associated factors 1 and 2 and regulation of the nuclear factor-kappaB antiapoptotic activity in human gliomas. J Neurosurg. 2005;103:873–81. doi: 10.3171/jns.2005.103.5.0873. [DOI] [PubMed] [Google Scholar]
- 147.Hou MF, Lin SB, Yuan SS, et al. The clinical significance between activation of nuclear factor kappa B transcription factor and overexpression of HER-2/neu oncoprotein in Taiwanese patients with breast cancer. Clin Chim Acta. 2003;334:137–44. doi: 10.1016/s0009-8981(03)00196-7. [DOI] [PubMed] [Google Scholar]
- 148.Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005;12 1:S37–46. doi: 10.1677/erc.1.00977. [DOI] [PubMed] [Google Scholar]
- 149.Biswas DK, Shi Q, Baily S, et al. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci U S A. 2004;101:10137–42. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]; **An important evidence for the role of NF-kB in breast cancer.
- 150.Guo RX, Qiao YH, Zhou Y, Li LX, Shi HR, Chen KS. Increased staining for phosphorylated AKT and nuclear factor-kappaB p65 and their relationship with prognosis in epithelial ovarian cancer. Pathol Int. 2008;58:749–56. doi: 10.1111/j.1440-1827.2008.02306.x. [DOI] [PubMed] [Google Scholar]
- 151.O'Riordan JM, Abdel-latif MM, Ravi N, et al. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol. 2005;100:1257–64. doi: 10.1111/j.1572-0241.2005.41338.x. [DOI] [PubMed] [Google Scholar]
- 152.Meteoglu I, Erdogdu IH, Meydan N, Erkus M, Barutca S. NF-KappaB expression correlates with apoptosis and angiogenesis in clear cell renal cell carcinoma tissues. J Exp Clin Cancer Res. 2008;27:53. doi: 10.1186/1756-9966-27-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kourelis K, Sotiropoulou-Bonikou G, Vandoros G, Repanti M, Varakis I, Goumas P. Coordinated upregulation of COX-2 and NF-kappaB is a steady feature of laryngeal carcinogenesis. ORL J Otorhinolaryngol Relat Spec. 2007;69:181–9. doi: 10.1159/000099229. [DOI] [PubMed] [Google Scholar]
- 154.Zhang D, Jin X, Wang F, et al. Combined prognostic value of both RelA and IkappaB-alpha expression in human non-small cell lung cancer. Ann Surg Oncol. 2007;14:3581–92. doi: 10.1245/s10434-007-9560-z. [DOI] [PubMed] [Google Scholar]
- 155.Bhojani MS, Chen G, Ross BD, Beer DG, Rehemtulla A. Nuclear localized phosphorylated FADD induces cell proliferation and is associated with aggressive lung cancer. Cell Cycle. 2005;4:1478–81. doi: 10.4161/cc.4.11.2188. [DOI] [PubMed] [Google Scholar]
- 156.Jin X, Wang Z, Qiu L, et al. Potential biomarkers involving IKK/RelA signal in early stage non-small cell lung cancer. Cancer Sci. 2008;99:582–9. doi: 10.1111/j.1349-7006.2007.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang HP, Xu YJ, Zhang ZX, Ni W, Chen SX. Expression of protein kinase C and nuclear factor kappa B in lung tissue of patients with chronic obstructive pulmonary disease. Zhonghua Nei Ke Za Zhi. 2004;43:756–9. [PubMed] [Google Scholar]
- 158.Gasparian AV, Fedorova MD, Kisselev FL. Regulation of matrix metalloproteinase-9 transcription in squamous cell carcinoma of uterine cervix: the role of human papillomavirus gene E2 expression and activation of transcription factor NF-kappaB. Biochemistry (Mosc) 2007;72:848–53. doi: 10.1134/s0006297907080068. [DOI] [PubMed] [Google Scholar]
- 159.Kashani-Sabet M, Shaikh L, Miller JR, 3rd, et al. NF-kappa B in the vascular progression of melanoma. J Clin Oncol. 2004;22:617–23. doi: 10.1200/JCO.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 160.Gao K, Dai DL, Martinka M, Li G. Prognostic significance of nuclear factor-kappaB p105/p50 in human melanoma and its role in cell migration. Cancer Res. 2006;66:8382–8. doi: 10.1158/0008-5472.CAN-05-4402. [DOI] [PubMed] [Google Scholar]
- 161.Bao ZH, Li GL, Yu JH. Expression of cyclooxygenase-2 in bone marrow cells of chronic leukemia and its significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:923–6. [PubMed] [Google Scholar]
- 162.Li HL, Sun BZ, Ma FC. Expression of COX-2, iNOS, p53 and Ki-67 in gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol. 2004;10:1862–6. doi: 10.3748/wjg.v10.i13.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chang BW, Kim DH, Kowalski DP, et al. Prognostic significance of cyclooxygenase-2 in oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2004;10:1678–84. doi: 10.1158/1078-0432.ccr-03-0354. [DOI] [PubMed] [Google Scholar]
- 164.Ling FC, Baldus SE, Khochfar J, et al. Association of COX-2 expression with corresponding active and chronic inflammatory reactions in Barrett's metaplasia and progression to cancer. Histopathology. 2007;50:203–9. doi: 10.1111/j.1365-2559.2007.02576.x. [DOI] [PubMed] [Google Scholar]
- 165.Miyashita M, Makino H, Katsuta M, et al. Cyclo-oxygenase-2 over-expression is associated with human esophageal squamous cell carcinoma. J Nippon Med Sch. 2006;73:308–13. doi: 10.1272/jnms.73.308. [DOI] [PubMed] [Google Scholar]
- 166.Alici S, Ugras S, Bayram I, Izmirli M. Prognostic factors and COX-2 expression in advanced stage esophageal squamous cell carcinoma. Adv Ther. 2006;23:672–9. doi: 10.1007/BF02850306. [DOI] [PubMed] [Google Scholar]
- 167.France M, Drew PA, Dodd T, Watson DI. Cyclo-oxygenase-2 expression in esophageal adenocarcinoma as a determinant of clinical outcome following esophagectomy. Dis Esophagus. 2004;17:136–40. doi: 10.1111/j.1442-2050.2004.00390.x. [DOI] [PubMed] [Google Scholar]
- 168.Takatori H, Natsugoe S, Okumura H, et al. Cyclooxygenase-2 expression is related to prognosis in patients with esophageal squamous cell carcinoma. Eur J Surg Oncol. 2008;34:397–402. doi: 10.1016/j.ejso.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 169.Yoshikawa R, Fujiwara Y, Koishi K, et al. Cyclooxygenase-2 expression after preoperative chemoradiotherapy correlates with more frequent esophageal cancer recurrence. World J Gastroenterol. 2007;13:2283–8. doi: 10.3748/wjg.v13.i16.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cadden I, Johnston BT, Turner G, McCance D, Ardill J, McGinty A. An evaluation of cyclooxygenase-2 as a prognostic biomarker in mid-gut carcinoid tumours. Neuroendocrinology. 2007;86:104–11. doi: 10.1159/000107555. [DOI] [PubMed] [Google Scholar]
- 171.Karamitopoulou E, Tornillo L, Zlobec I, et al. Clinical significance of cell cycle- and apoptosis-related markers in biliary tract cancer: a tissue microarray-based approach revealing a distinctive immunophenotype for intrahepatic and extrahepatic cholangiocarcinomas. Am J Clin Pathol. 2008;130:780–6. doi: 10.1309/AJCP35FDCAVANWMM. [DOI] [PubMed] [Google Scholar]
- 172.Gao YW, Chen YX, Wang ZM, et al. Correlation between expression of cyclooxygenase-2 and the presence of CD4+ infiltrating T-lymphocyte in human primary hepatocellular carcinoma. Hepatogastroenterology. 2008;55:345–50. [PubMed] [Google Scholar]
- 173.El-Bassiouny AE, Zoheiry MM, Nosseir MM, El-Ahwany EG, Ibrahim RA, El-Bassiouni NE. Expression of cyclooxygenase-2 and transforming growth factor-beta1 in HCV-induced chronic liver disease and hepatocellular carcinoma. MedGenMed. 2007;9:45. [PMC free article] [PubMed] [Google Scholar]
- 174.Iwamoto A, Ikeguchi M, Matsumoto S, et al. Tumor cyclooxygenase-2 gene suppresses local immune responses in patients with hepatocellular carcinoma. Tumori. 2006;92:130–3. doi: 10.1177/030089160609200208. [DOI] [PubMed] [Google Scholar]
- 175.Tang TC, Poon RT, Lau CP, Xie D, Fan ST. Tumor cyclooxygenase-2 levels correlate with tumor invasiveness in human hepatocellular carcinoma. World J Gastroenterol. 2005;11:1896–902. doi: 10.3748/wjg.v11.i13.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fumino S, Tokiwa K, Ono S, Iwai N. Cyclooxygenase-2 expression in the gallbladder of patients with anomalous arrangement of the pancreaticobiliary duct. J Pediatr Surg. 2003;38:585–9. doi: 10.1053/jpsu.2003.50127. [DOI] [PubMed] [Google Scholar]
- 177.Schlosser W, Schlosser S, Ramadani M, Gansauge F, Gansauge S, Beger HG. Cyclooxygenase-2 is overexpressed in chronic pancreatitis. Pancreas. 2002;25:26–30. doi: 10.1097/00006676-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 178.Niijima M, Yamaguchi T, Ishihara T, et al. Immunohistochemical analysis and in situ hybridization of cyclooxygenase-2 expression in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2002;94:1565–73. doi: 10.1002/cncr.10358. [DOI] [PubMed] [Google Scholar]
- 179.Santini D, Vincenzi B, Tonini G, et al. Cyclooxygenase-2 overexpression is associated with a poor outcome in resected ampullary cancer patients. Clin Cancer Res. 2005;11:3784–9. doi: 10.1158/1078-0432.CCR-04-2136. [DOI] [PubMed] [Google Scholar]
- 180.Nakamoto RH, Uetake H, Iida S, et al. Correlations between cyclooxygenase-2 expression and angiogenic factors in primary tumors and liver metastases in colorectal cancer. Jpn J Clin Oncol. 2007;37:679–85. doi: 10.1093/jjco/hym080. [DOI] [PubMed] [Google Scholar]
- 181.Ogino S, Kirkner GJ, Nosho K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res. 2008;14:8221–7. doi: 10.1158/1078-0432.CCR-08-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]; *An important evidence for the role of COX2 in colon cancer.
- 182.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465–71. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 183.Abe A, Fukui H, Fujii S, et al. Involvement of cyclooxygenase-2 and vascular endothelial growth factor in vascularization and lymph node metastasis of colorectal cancers with submucosal invasion. J Gastroenterol Hepatol. 2007;22:1071–7. doi: 10.1111/j.1440-1746.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- 184.Cressey R, Pimpa S, Tontrong W, Watananupong O, Leartprasertsuke N. Expression of cyclooxygenase-2 in colorectal adenocarcinoma is associated with p53 accumulation and hdm2 overexpression. Cancer Lett. 2006;233:232–9. doi: 10.1016/j.canlet.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 185.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–8. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 186.Konno H, Baba M, Shoji T, Ohta M, Suzuki S, Nakamura S. Cyclooxygenase-2 expression correlates with uPAR levels and is responsible for poor prognosis of colorectal cancer. Clin Exp Metastasis. 2002;19:527–34. doi: 10.1023/a:1020392309715. [DOI] [PubMed] [Google Scholar]
- 187.Masunaga R, Kohno H, Dhar DK, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064–8. [PubMed] [Google Scholar]
- 188.Yamagata R, Shimoyama T, Fukuda S, Yoshimura T, Tanaka M, Munakata A. Cyclooxygenase-2 expression is increased in early intestinal-type gastric cancer and gastric mucosa with intestinal metaplasia. Eur J Gastroenterol Hepatol. 2002;14:359–63. doi: 10.1097/00042737-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 189.Joo YE, Oh WT, Rew JS, Park CS, Choi SK, Kim SJ. Cyclooxygenase-2 expression is associated with well-differentiated and intestinal-type pathways in gastric carcinogenesis. Digestion. 2002;66:222–9. doi: 10.1159/000068366. [DOI] [PubMed] [Google Scholar]
- 190.Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003;9:1421–6. doi: 10.3748/wjg.v9.i7.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tatsuguchi A, Matsui K, Shinji Y, et al. Cyclooxygenase-2 expression correlates with angiogenesis and apoptosis in gastric cancer tissue. Hum Pathol. 2004;35:488–95. doi: 10.1016/j.humpath.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 192.Petersen S, Haroske G, Hellmich G, Ludwig K, Petersen C, Eicheler W. COX-2 expression in rectal carcinoma: immunohistochemical pattern and clinical outcome. Anticancer Res. 2002;22:1225–30. [PubMed] [Google Scholar]
- 193.Hammam OA, Aziz AA, Roshdy MS, Abdel Hadi AM. Possible role of cyclooxygenase-2 in schistosomal and non-schistosomal-associated bladder cancer. Medscape J Med. 2008;10:60. [PMC free article] [PubMed] [Google Scholar]
- 194.Shirahama T, Arima J, Akiba S, Sakakura C. Relation between cyclooxygenase-2 expression and tumor invasiveness and patient survival in transitional cell carcinoma of the urinary bladder. Cancer. 2001;92:188–93. doi: 10.1002/1097-0142(20010701)92:1<188::aid-cncr1308>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 195.Komhoff M, Guan Y, Shappell HW, et al. Enhanced expression of cyclooxygenase-2 in high grade human transitional cell bladder carcinomas. Am J Pathol. 2000;157:29–35. doi: 10.1016/S0002-9440(10)64513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shariat SF, Matsumoto K, Kim J, et al. Correlation of cyclooxygenase-2 expression with molecular markers, pathological features and clinical outcome of transitional cell carcinoma of the bladder. J Urol. 2003;170:985–9. doi: 10.1097/01.ju.0000080401.85145.ee. [DOI] [PubMed] [Google Scholar]
- 197.Tuna B, Yorukoglu K, Gurel D, Mungan U, Kirkali Z. Significance of COX-2 expression in human renal cell carcinoma. Urology. 2004;64:1116–20. doi: 10.1016/j.urology.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 198.Yoshimura R, Sano H, Masuda C, et al. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589–96. [PubMed] [Google Scholar]
- 199.Guo GL, Yang GL, Li ZY, et al. The effect of cyclooxygenase-2 on lymphangiogenesis in breast cancer. Zhonghua Wai Ke Za Zhi. 2008;46:132–5. [PubMed] [Google Scholar]
- 200.Costa C, Soares R, Reis-Filho JS, Leitao D, Amendoeira I, Schmitt FC. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429–34. doi: 10.1136/jcp.55.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Haffty BG, Yang Q, Moran MS, Tan AR, Reiss M. Estrogen-dependent prognostic significance of cyclooxygenase-2 expression in early-stage invasive breast cancers treated with breast-conserving surgery and radiation. Int J Radiat Oncol Biol Phys. 2008;71:1006–13. doi: 10.1016/j.ijrobp.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 202.Nassar A, Radhakrishnan A, Cabrero IA, Cotsonis G, Cohen C. COX-2 expression in invasive breast cancer: correlation with prognostic parameters and outcome. Appl Immunohistochem Mol Morphol. 2007;15:255–9. doi: 10.1097/01.pai.0000213130.63417.b3. [DOI] [PubMed] [Google Scholar]
- 203.Lu S, Yu G, Zhu Y, Archer MC. Cyclooxygenase-2 overexpression in MCF-10F human breast epithelial cells inhibits proliferation, apoptosis and differentiation, and causes partial transformation. Int J Cancer. 2005;116:847–52. doi: 10.1002/ijc.21142. [DOI] [PubMed] [Google Scholar]
- 204.Leo C, Faber S, Hentschel B, Hockel M, Horn LC. The status of cyclooxygenase-2 expression in ductal carcinoma in situ lesions and invasive breast cancer correlates to cyclooxygenase-2 expression in normal breast tissue. Ann Diagn Pathol. 2006;10:327–32. doi: 10.1016/j.anndiagpath.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 205.Gaffney DK, Haslam D, Tsodikov A, et al. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:922–8. doi: 10.1016/s0360-3016(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 206.Ferrandina G, Lauriola L, Zannoni GF, et al. Expression of cyclooxygenase-2 (COX-2) in tumour and stroma compartments in cervical cancer: clinical implications. Br J Cancer. 2002;87:1145–52. doi: 10.1038/sj.bjc.6600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Miyata Y, Koga S, Kanda S, Nishikido M, Hayashi T, Kanetake H. Expression of cyclooxygenase-2 in renal cell carcinoma: correlation with tumor cell proliferation, apoptosis, angiogenesis, expression of matrix metalloproteinase-2, and survival. Clin Cancer Res. 2003;9:1741–9. [PubMed] [Google Scholar]
- 208.Fagotti A, Ferrandina G, Fanfani F, et al. Analysis of cyclooxygenase-2 (COX-2) expression in different sites of endometriosis and correlation with clinico-pathological parameters. Hum Reprod. 2004;19:393–7. doi: 10.1093/humrep/deh054. [DOI] [PubMed] [Google Scholar]
- 209.Fujimoto J, Toyoki H, Sakaguchi H, Jahan I, Alam SM, Tamaya T. Clinical implications of expression of cyclooxygenase-2 related to angiogenesis in ovarian cancer. Oncol Rep. 2006;15:21–5. [PubMed] [Google Scholar]
- 210.Ali-Fehmi R, Che M, Khalifeh I, et al. The effect of cyclooxygenase-2 expression on tumor vascularity in advanced stage ovarian serous carcinoma. Cancer. 2003;98:1423–9. doi: 10.1002/cncr.11650. [DOI] [PubMed] [Google Scholar]
- 211.Denkert C, Kobel M, Pest S, et al. Expression of cyclooxygenase 2 is an independent prognostic factor in human ovarian carcinoma. Am J Pathol. 2002;160:893–903. doi: 10.1016/S0002-9440(10)64912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Seo SS, Song YS, Kang DH, et al. Expression of cyclooxygenase-2 in association with clinicopathological prognostic factors and molecular markers in epithelial ovarian cancer. Gynecol Oncol. 2004;92:927–35. doi: 10.1016/j.ygyno.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 213.Lou WZ, Shen K, Zhang Y, Lang JH. Relationship of cyclooxygenase 2 expression and chemotherapy response and prognosis in human ovarian carcinoma. Zhonghua Fu Chan Ke Za Zhi. 2004;39:529–32. [PubMed] [Google Scholar]
- 214.Guo X, Chen Y, Xu Z, Xu Z, Qian Y, Yu X. Prognostic significance of VEGF-C expression in correlation with COX-2, lymphatic microvessel density, and clinicopathologic characteristics in human non-small cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2009;41:217–22. doi: 10.1093/abbs/gmp004. [DOI] [PubMed] [Google Scholar]
- 215.Dong P, Li X, Yu Z, Lu G. Expression of cyclooxygenase-2, vascular endothelial growth factor and matrix metalloproteinase-2 in patients with primary laryngeal carcinoma: a tissue microarray study. J Laryngol Otol. 2007;121:1177–83. doi: 10.1017/S002221510700031X. [DOI] [PubMed] [Google Scholar]
- 216.Sackett MK, Bairati I, Meyer F, et al. Prognostic significance of cyclooxygenase-2 overexpression in glottic cancer. Clin Cancer Res. 2008;14:67–73. doi: 10.1158/1078-0432.CCR-07-2028. [DOI] [PubMed] [Google Scholar]
- 217.Tjiu JW, Liao YH, Lin SJ, et al. Cyclooxygenase-2 overexpression in human basal cell carcinoma cell line increases antiapoptosis, angiogenesis, and tumorigenesis. J Invest Dermatol. 2006;126:1143–51. doi: 10.1038/sj.jid.5700191. [DOI] [PubMed] [Google Scholar]
- 218.Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res. 2001;61:4375–81. [PubMed] [Google Scholar]
- 219.Crazzolara R, Kreczy A, Mann G, et al. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br J Haematol. 2001;115:545–53. doi: 10.1046/j.1365-2141.2001.03164.x. [DOI] [PubMed] [Google Scholar]
- 220.Arai J, Yasukawa M, Yakushijin Y, Miyazaki T, Fujita S. Stromal cells in lymph nodes attract B-lymphoma cells via production of stromal cell-derived factor-1. Eur J Haematol. 2000;64:323–32. doi: 10.1034/j.1600-0609.2000.90147.x. [DOI] [PubMed] [Google Scholar]
- 221.Ottaiano A, Franco R, Aiello Talamanca A, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–803. doi: 10.1158/1078-0432.CCR-05-2142. [DOI] [PubMed] [Google Scholar]
- 222.Mochizuki H, Matsubara A, Teishima J, et al. Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun. 2004;320:656–63. doi: 10.1016/j.bbrc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 223.Holm NT, Byrnes K, Li BD, et al. Elevated levels of chemokine receptor CXCR4 in HER-2 negative breast cancer specimens predict recurrence. J Surg Res. 2007;141:53–9. doi: 10.1016/j.jss.2007.03.015. [DOI] [PubMed] [Google Scholar]; *An importance of CXCR4 for breast cancer recurrence.
- 224.Salvucci O, Bouchard A, Baccarelli A, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97:275–83. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 225.Cabioglu N, Sahin AA, Morandi P, et al. Chemokine receptors in advanced breast cancer: differential expression in metastatic disease sites with diagnostic and therapeutic implications. Ann Oncol. 2009 doi: 10.1093/annonc/mdn740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kang H, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The elevated level of CXCR4 is correlated with nodal metastasis of human breast cancer. Breast. 2005;14:360–7. doi: 10.1016/j.breast.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 227.Li YM, Pan Y, Wei Y, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 228.Guo Z, Cai S, Fang R, et al. The synergistic effects of CXCR4 and EGFR on promoting EGF-mediated metastasis in ovarian cancer cells. Colloids Surf B Biointerfaces. 2007;60:1–6. doi: 10.1016/j.colsurfb.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 229.Kodama J, Hasengaowa, Kusumoto T, et al. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann Oncol. 2007;18:70–6. doi: 10.1093/annonc/mdl342. [DOI] [PubMed] [Google Scholar]
- 230.de Bont ES, Rosati S, Jacobs S, Kamps WA, Vellenga E. Increased bone marrow vascularization in patients with acute myeloid leukaemia: a possible role for vascular endothelial growth factor. Br J Haematol. 2001;113:296–304. doi: 10.1046/j.1365-2141.2001.02722.x. [DOI] [PubMed] [Google Scholar]
- 231.Kuramoto K, Sakai A, Shigemasa K, et al. High expression of MCL1 gene related to vascular endothelial growth factor is associated with poor outcome in non-Hodgkin's lymphoma. Br J Haematol. 2002;116:158–61. doi: 10.1046/j.1365-2141.2002.03253.x. [DOI] [PubMed] [Google Scholar]
- 232.Han U, Can OI, Han S, Kayhan B, Onal BU. Expressions of p53, VEGF C, p21: could they be used in preoperative evaluation of lymph node metastasis of esophageal squamous cell carcinoma? Dis Esophagus. 2007;20:379–85. doi: 10.1111/j.1442-2050.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 233.Kimura S, Kitadai Y, Tanaka S, et al. Expression of hypoxia-inducible factor (HIF)-1alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40:1904–12. doi: 10.1016/j.ejca.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 234.Shih CH, Ozawa S, Ando N, Ueda M, Kitajima M. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2000;6:1161–8. [PubMed] [Google Scholar]
- 235.Dassoulas K, Gazouli M, Rizos S, et al. Common polymorphisms in the vascular endothelial growth factor gene and colorectal cancer development, prognosis, and survival. Mol Carcinog. 2008 doi: 10.1002/mc.20495. [DOI] [PubMed] [Google Scholar]
- 236.Romano M, Cuomo A, Tuccillo C, et al. Vascular endothelial growth factor and cyclooxygenase-2 are overexpressed in ileal pouch-anal anastomosis. Dis Colon Rectum. 2007;50:650–9. doi: 10.1007/s10350-006-0807-8. [DOI] [PubMed] [Google Scholar]
- 237.Al-Harris ES, Al-Janabi AA, Al-Toriahi KM, Yasseen AA. Over expression of vascular endothelial growth factor in correlation to Ki-67, grade, and stage of breast cancer. Saudi Med J. 2008;29:1099–104. [PubMed] [Google Scholar]
- 238.Yilmaz A, Ernam D, Unsal E, Demirag F, Atikcan S, Tastepe I. Vascular endothelial growth factor immunostaining correlates with postoperative relapse and survival in non-small cell lung cancer. Arch Med Res. 2007;38:764–8. doi: 10.1016/j.arcmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 239.Vanasse GJ, Winn RK, Rodov S, et al. Bcl-2 overexpression leads to increases in suppressor of cytokine signaling-3 expression in B cells and de novo follicular lymphoma. Mol Cancer Res. 2004;2:620–31. [PubMed] [Google Scholar]
- 240.Ma XT, Wang S, Ye YJ, Du RY, Cui ZR, Somsouk M. Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma. World J Gastroenterol. 2004;10:1569–73. doi: 10.3748/wjg.v10.i11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang H, Wang S, Zhang YC, Ye YJ, Cui ZR, Fang WG. Correlation between Stat3 signal transduction pathway and expression of cyclooxygenase-2 in colorectal cancer cells. Zhonghua Yi Xue Za Zhi. 2005;85:2899–904. [PubMed] [Google Scholar]
- 242.Hbibi AT, Lagorce C, Wind P, et al. Identification of a functional EGF-R/p60c-src/STAT3 pathway in colorectal carcinoma: analysis of its long-term prognostic value. Cancer Biomark. 2008;4:83–91. doi: 10.3233/cbm-2008-4204. [DOI] [PubMed] [Google Scholar]
- 243.Dhir R, Ni Z, Lou W, DeMiguel F, Grandis JR, Gao AC. Stat3 activation in prostatic carcinomas. Prostate. 2002;51:241–6. doi: 10.1002/pros.10079. [DOI] [PubMed] [Google Scholar]
- 244.Mora LB, Buettner R, Seigne J, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–66. [PubMed] [Google Scholar]
- 245.Torres-Roca JF, DeSilvio M, Mora LB, et al. Activated STAT3 as a correlate of distant metastasis in prostate cancer: a secondary analysis of Radiation Therapy Oncology Group 86-10. Urology. 2007;69:505–9. doi: 10.1016/j.urology.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 246.Emilie D, Devergne O, Raphael M, Coumbaras LJ, Galanaud P. Production of interleukin-6 in high grade B lymphomas. Curr Top Microbiol Immunol. 1992;182:349–55. doi: 10.1007/978-3-642-77633-5_45. [DOI] [PubMed] [Google Scholar]
- 247.Seymour JF, Talpaz M, Cabanillas F, Wetzler M, Kurzrock R. Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol. 1995;13:575–82. doi: 10.1200/JCO.1995.13.3.575. [DOI] [PubMed] [Google Scholar]
- 248.Preti HA, Cabanillas F, Talpaz M, Tucker SL, Seymour JF, Kurzrock R. Prognostic value of serum interleukin-6 in diffuse large-cell lymphoma. Ann Intern Med. 1997;127:186–94. doi: 10.7326/0003-4819-127-3-199708010-00002. [DOI] [PubMed] [Google Scholar]
- 249.Shimamoto T, Hayashi S, Ando K, et al. Anaplastic large-cell lymphoma which showed severe inflammatory status and myelodysplasia with increased VEGF and IL-6 serum levels after long-term immunosuppressive therapy. Am J Hematol. 2001;66:49–52. doi: 10.1002/1096-8652(200101)66:1<49::AID-AJH1008>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 250.Kato H, Kinoshita T, Suzuki S, et al. Elevated serum interleukin-6 (IL-6) is derived from neoplastic lymphoid cells in patients with B-cell non-Hodgkin's lymphoma: correlation with extent of IL-6 expression and serum concentration. Br J Haematol. 1996;92:1014–21. doi: 10.1046/j.1365-2141.1996.444983.x. [DOI] [PubMed] [Google Scholar]
- 251.Voorzanger N, Touitou R, Garcia E, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin's lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–505. [PubMed] [Google Scholar]
- 252.Kossakowska AE, Edwards DR, Prusinkiewicz C, et al. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin's lymphomas. Blood. 1999;94:2080–9. [PubMed] [Google Scholar]
- 253.Seymour JF, Talpaz M, Hagemeister FB, Cabanillas F, Kurzrock R. Clinical correlates of elevated serum levels of interleukin 6 in patients with untreated Hodgkin's disease. Am J Med. 1997;102:21–8. doi: 10.1016/s0002-9343(96)00352-x. [DOI] [PubMed] [Google Scholar]
- 254.Ludwig H, Nachbaur DM, Fritz E, Krainer M, Huber H. Interleukin-6 is a prognostic factor in multiple myeloma. Blood. 1991;77:2794–5. [PubMed] [Google Scholar]
- 255.Reibnegger G, Krainer M, Herold M, Ludwig H, Wachter H, Huber H. Predictive value of interleukin-6 and neopterin in patients with multiple myeloma. Cancer Res. 1991;51:6250–3. [PubMed] [Google Scholar]
- 256.Dvorakova K, Payne CM, Ramsey L, et al. Increased expression and secretion of interleukin-6 in patients with Barrett's esophagus. Clin Cancer Res. 2004;10:2020–8. doi: 10.1158/1078-0432.ccr-0437-03. [DOI] [PubMed] [Google Scholar]
- 257.Komoda H, Tanaka Y, Honda M, Matsuo Y, Hazama K, Takao T. Interleukin-6 levels in colorectal cancer tissues. World J Surg. 1998;22:895–8. doi: 10.1007/s002689900489. [DOI] [PubMed] [Google Scholar]
- 258.Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85:2526–31. doi: 10.1002/(sici)1097-0142(19990615)85:12<2526::aid-cncr6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 259.Ito H, Miki C. Profile of circulating levels of interleukin-1 receptor antagonist and interleukin-6 in colorectal cancer patients. Scand J Gastroenterol. 1999;34:1139–43. doi: 10.1080/003655299750024959. [DOI] [PubMed] [Google Scholar]
- 260.Garza-Gonzalez E, Bosques-Padilla FJ, El-Omar E, et al. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer. 2005;114:237–41. doi: 10.1002/ijc.20718. [DOI] [PubMed] [Google Scholar]
- 261.Ashizawa T, Okada R, Suzuki Y, et al. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124–31. doi: 10.1007/s10120-005-0315-x. [DOI] [PubMed] [Google Scholar]
- 262.Chung YC, Chaen YL, Hsu CP. Clinical significance of tissue expression of interleukin-6 in colorectal carcinoma. Anticancer Res. 2006;26:3905–11. [PubMed] [Google Scholar]
- 263.El-Salahy EM. Evaluation of cytokeratin-19 & cytokeratin-20 and interleukin-6 in Egyptian bladder cancer patients. Clin Biochem. 2002;35:607–13. doi: 10.1016/s0009-9120(02)00382-x. [DOI] [PubMed] [Google Scholar]
- 264.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–32. [PubMed] [Google Scholar]
- 265.Wei LH, Kuo ML, Chen CA, et al. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway. Oncogene. 2001;20:5799–809. doi: 10.1038/sj.onc.1204733. [DOI] [PubMed] [Google Scholar]
- 266.Wang L, Tang Z, Sun H. Nitric oxide synthase and vascular endothelial growth factor expression in hepatocellular carcinoma and their relation to angiogenesis. Zhonghua Zhong Liu Za Zhi. 2000;22:301–3. [PubMed] [Google Scholar]
- 267.Klotz T, Bloch W, Jacobs G, Niggemann S, Engelmann U, Addicks K. Immunolocalization of inducible and constitutive nitric oxide synthases in human bladder cancer. Urology. 1999;54:416–9. doi: 10.1016/s0090-4295(99)00212-5. [DOI] [PubMed] [Google Scholar]
- 268.Fujimoto H, Ando Y, Yamashita T, et al. Nitric oxide synthase activity in human lung cancer. Jpn J Cancer Res. 1997;88:1190–8. doi: 10.1111/j.1349-7006.1997.tb00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Natarajan R, Esworthy R, Bai W, Gu JL, Wilczynski S, Nadler J. Increased 12-lipoxygenase expression in breast cancer tissues and cells. Regulation by epidermal growth factor. J Clin Endocrinol Metab. 1997;82:1790–8. doi: 10.1210/jcem.82.6.3990. [DOI] [PubMed] [Google Scholar]
- 270.Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97:1827–36. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]