Abstract
Background
The International Neuroblastoma Pathology Classification (INPC) was the first to clearly define prognostic subgroups in ganglioneuroma (GN) and ganglioneuroblastoma (GNB).
Procedure
Histopathology and tumor resectability of 552 GN/GNB cases from the CCG (Children’s Cancer Group) and COG (Children’s Oncology Group) neuroblastoma studies were reviewed. The results were analyzed along with clinical information and biological data of the cases.
Results
According to the INPC, 300 tumors were classified into the Favorable Histology (FH) group and 252 were into the Unfavorable Histology (UH) group. Tumors in the FH group included 43 ganglioneuroma-maturing (GN-M), 198 ganglioneuroblastoma-intermixed (GNB-I), and 59 ganglioneuroblastoma-nodular, favorable subset (GNB-N-FS), and were often (91%) resected completely by single or multiple surgical procedures. Patients with the FH tumors had an excellent prognosis with no tumor-related deaths. The UH group included ganglioneuroblastoma-nodular, unfavorable subset (GNB-N-US) tumors. Patients with the UH tumors had a high incidence (53%) of distant metastasis at the time of diagnosis, and their prognosis significantly depended on clinical stage (5-year EFS: 80.1% for non-stage 4 patients; 16.7% for stage 4 patients): Complete primary tumor resection was not beneficial to those GNB-N-US patients, regardless of whether metastasis was present or not. MYCN amplification was detected in 4 tumors in the FH group and 6 tumors in the UH group. The majority (160/191, 84%) of GN-M and GNB-I tumors had a diploid pattern determined by flow cytometry.
Conclusions
Stringent application of the INPC along with clinical staging was critical for prognostic evaluation of the patients with this group of tumors.
Keywords: Ganglioneuroma, Ganglioneuroblastoma, International Neuroblastoma Pathology Classification, Clinical Staging, Tumor Resectability, Prognosis
INTRODUCTION
Historically there are three categories in peripheral neuroblastic tumors (pNTs): Neuroblastoma (NB), Ganglioneuroblastoma (GNB), and Ganglioneuroma (GN). These tumor categories represent a spectrum of maturation from the most primitive form, NB to the most mature form, GN. A series of reports on pathology, biology, and clinical aspects of NB, the most common form in pNTs, have been published (1)-(10). GN is recognized as a benign tumor usually diagnosed in older children and in adults (11) (12). GNB was defined as “a transitional tumor of sympathetic cell origin that contained malignant neuroblastomatous and benign ganglioneuromatous element” by Robertson in 1915 (13). For decades, GNB caused some consternation for both pathologists and oncologists for several reasons: Firstly, there were no clear and objective delineations between NB and GNB, and between GNB and GN, and secondly, there were no well-defined guidelines to predict tumor biology and clinical outcome of the patients with GNB. In an attempt to provide clarity, Stout subclassified the tumor into composite and diffuse GNB (14). Others such as Bove et al. reported their experience with composite GNB, but failed to provide a conclusive statement in predicting clinical outcome of their patients (15).
The International Neuroblastoma Pathology Classification (INPC), established in 1999 and revised in 2003, re-defined the histologic features of pNTs, and proposed 4 tumor categories in two distinct prognostic groups; i.e., Favorable Histology (FH) and Unfavorable Histology (UH)(7) (8) (16). The INPC was the first to define the tumor categories using histologic indicators of both grade of neuroblastic differentiation and Schwannian stromal development by accommodating the system developed by Shimada et al. in 1984(3). NB (Schwannian stroma-poor tumor) is classified into FH or UH depending on age-linked evaluation of grade of neuroblastic differentiation and mitosis-karyorrhexis index (MKI). GNB-Intermixed (Schwannian stroma-rich) and GN (Schwannian stroma-dominant) tumors are always classified into the FH group. GNB-Nodular (composite, Schwannian stroma-rich/stroma-dominant and stroma-poor) tumors are classified into the FH or UH group based on the characteristics of the neuroblastic component. The revised INPC has been used by national and international cooperative studies for pathology evaluation of pNTs.
Here we report the clinicopathological characteristics of GNB and GN with analysis of the world’s largest series of 552 cases from the CCG (Children’s Cancer Group) and COG (Children’s Oncology Group) studies by using the revised INPC (16). According to the INPC, tumors in the GN category are further classified into GN-Maturing subtype (the same tumor group described as “Ganglioneuroblastoma, well differentiated” in the Shimada classification system3) and GN-Mature subtype (completely mature form of neuroblastic tumor). Cases with the diagnosis of GN-Mature subtype were excluded from the analysis, since they were not eligible for the CCG/COG studies.
METHODS
Patient Cohort
Among a total of 3,953 patients registered in CCG and COG neuroblastoma studies from August 1, 1991 to December 31, 2006, there were 552 cases whose diagnosis was either GN or GNB. About 60% of patients (329/552) enrolled on at least one of 24 different therapeutic studies in which various different treatments were offered. Patients in this study included 70 GNB-N cases reported by Peuchmaur et al. in 2003(16). Patient data including age at diagnosis, clinical stage according to the International Neuroblastoma Staging System (INSS)(17), and follow-up information were centrally collected at the COG Statistics and Data Center, University of Florida (Gainesville, FL). Results of MYCN status of the individual tumors, either amplified or non-amplified, determined with Southern blot, PCR, and/or FISH by the Reference Laboratories at St. Jude Research Hospital (Memphis, TN), Dana Farber Cancer Institute (Boston, MA), Childrens Hospital Los Angeles (Los Angeles, CA), and Nationwide Children’s Hospital (Columbus, OH) were also collected from the patients (N=500) of both CCG and COG studies. DNA indices of either diploid or hyperdiploid determined with flow cytometry by the Reference Laboratory at Dana Farber Cancer Institute or Nationwide Children’s Hospital were available only for the tumors of the COG patients (N=416).
Case Review
Careful review of pathology slides along with determination of primary tumor resectability of the individual cases was performed at the COG Neuroblastoma Pathology Reference Laboratory (CO and HS) at the Department of Pathology and Laboratory Medicine, Childrens Hospital Los Angeles, Los Angeles, CA. H&E stained slides (varying from 1 to 50 slides per case, average 8 slides) from the 552 tumor tissues, obtained by either biopsy or surgery prior to starting chemotherapy/irradiation and filed in the repository of the Pathology Reference Laboratory were available. Those tumors were classified into Ganglioneuroma-Maturing subtype (GN-M), Ganglioneuroblastoma-Intermixed (GNB-I), Ganglioneuroblastoma-Nodular, Favorable Subset (GNB-N-FS), or Ganglioneuroblastoma-Nodular, Unfavorable Subset (GNB-N-US) according to the revised INPC (16). The definition of the tumor diagnoses used in this study is summarized in Table I (7), (16). Tumor resectability for individual cases was determined by reviewing operative/pathology reports and confirmed by the pathology slide review. Each case was classified into one of three categories; i.e., Complete Resection (Co-R), Incomplete Resection (Inco-R), or Resectability Unknown (R-UK), based on the primary tumor resectability (Table II for the definition of Resectability in this study).
Table I. Definition: Ganglioneuroma-Maturing, Ganglioneuroblastoma Intermixed, and Ganglioneuroblastoma-Nodular.
According to the International Neuroblastoma Pathology Classification (7)
| Ganglioneuroma-Maturing (Schwannian stroma-dominant): Composed predominantly of ganglioneuromatous stroma with a minor component of individually scattered, evenly or unevenly distributed differentiating neuroblasts and/or maturing ganglion cells, in addition to fully mature ganglion cells. Fully mature ganglion cells are always covered with satellite cells. Tumors in this category are classified into a Favorable Histology Group. |
| Ganglioneuroblastoma-Intermixed (Schwannian stroma-rich): A transitional form between Neuroblastoma and Ganglioneuroma. Tumor is on its way toward full differentiation/maturation; however, the process is not complete, as evidenced by scattered “residual” microscopic neuroblastomatous foci presenting as pockets of neuropil containing varying numbers of neuroblastic cells with various stages of maturation including neuroblasts, differentiating neuroblasts, and/or ganglion cells. The proportion of the ganglioneuromatous component to “residual” neuroblastomatous foci should exceed 50% of the total volume in microscopic field(s) from representative section(s) of the tumor. Tumors in this category are classified into a Favorable Histology Group. |
| Ganglioneuroblastoma-Nodular (composite, Schwannian stroma- rich/stroma- dominant and stroma-poor): Composed of biologically different clones characterized by the presence of macroscopic, usually hemorrhagic neuroblastomatous nodule(s) of Schwannian stroma-poor component coexisting Ganglioneuroblastoma-intermixed of stroma- rich component or Ganglioneuroma of stroma- dominant component. The proportion of neuroblastomatous nodule(s) to Ganglioneuroblastoma- intermixed/Ganglioneuroma area varies from case to case (7) (16). Tumors in this category, once all were Unfavorable Histology, are now classified into favorable subset (Favorable Histology) and unfavorable subset (Unfavorable Histology) based on the age-linked evaluation of grade of neuroblastic differentiation and mitosis-karyorrhexis index of the neuroblastomatous nodule(s). |
Table II.
Definition: Resectability on Primary Tumor
| Complete resection: Complete or gross (≥95% of volume) tumor resection was achieved by single or multiple surgeries from the primary site before starting chemotherapy/irradiation therapy. When completely resected, tumor surfaces were smooth and well encapsulated. When surgical margin was positive, it had to be composed of ganglioneuromatous tissue absence of neuroblastoma tissue in surgical margin was confirmed by careful microscopic examination. Paraspinal tumors in this group often had minimal residual tumor tissue (<5%) in the intervertebral canal. |
| Incomplete resection: Only biopsy or partial resection (<95% of volume) of the primary tumor was performed. |
| Resectability Unknown: No information was available on resectability of the primary site in this group. |
Clinical protocol assignment
Tumors in the GN and GNB-I categories were classified into the FH group in both the CCG and COG studies (18). Since the revised INPC was not available before the year of 2003 (16), all CCG tumors in the GNB-N category had once been classified into the UH group (3). While the revised INPC distinguished FS tumor in the FH group from US tumor in the UH group in the GNB-N category for the COG study at the time of patient enrollment.
Patients were treated by risk group: low-risk patients had surgery alone; intermediate-risk patients had a moderate chemotherapy treatment in addition to surgery; and high-risk patients had intensive chemotherapy, surgery, radiotherapy, and in many cases, myeloablative therapy. Please refer to our previous publications for detailed description of risk stratification and protocol assignment for the patients of CCG and COG studies (18) (21). Informed consent approved by the institutional review board was obtained for all patients at the time of enrollment on either COG or CCG biology or therapeutic study.
Statistical Analysis
The results of pathology review were analyzed with age at diagnosis, clinical stage(17), MYCN status, DNA ploidy pattern, primary tumor resectability, and clinical outcome of the patients. Event-Free Survival (EFS) and Overall Survival (OS) analysis for patients in each histologic category were performed using the methods of Kaplan and Meier (22), with standard errors per the methods of Peto (23). Survival curves were compared using a log-rank test. P-values less than 0.05 were considered statistically significant. Time to event was defined as the time from enrollment on any study, biologic classification or therapeutic, whichever occurred first, until the time of first occurrence of relapse, progressive disease, secondary malignancy, or death, or until the time of last contact of no event occurred. For overall survival, death was the only event considered. In a total of 552 cases, there were 519 cases with survival data available for this study. Among those 519 cases, 483 cases also had tumor resectability information.
RESULTS
There were 43 GN-M tumors, 198 GNB-I tumors, 59 GNB-N-FS tumors, and 252 GNB-N-US tumors in this series. Clinical characteristics of the patients and biological risk factors of their tumors are summarized in Table III. All patients with GN-M (Age between 1 year 10 months and 17 years, mean: 6 years 5 months, median: 5 years 6 months), and the majority of patients with GNB-I (Age between 10 months and 16 years 3 months, mean: 4 years 10 months, median: 4 years 1 months) and with GNB-N-US (Age between 6 months and 18 years 9 months, mean: 4 years 10 months, median: 3 years 9 months) were diagnosed after 18 months of age. More than half of the patients with GNB-N-FS tumor were diagnosed before 18 months of age (Age between 4 months and 4 years 11 months, mean: 1 year 11 months, median: 1 year 5 months). By definition of the INPC, all GNB-N tumors diagnosed over 60 months of age were classified into US. The majority of GN-M, GNB-I, and GNB-N-FS patients had localized disease (stage 1, 2, 3) and low or intermediate-risk tumors. In contrast, more than half of the GNB-N-US patients had distant metastasis (stage 4) and high risk tumors at the time of diagnosis. No patients were presented with stage 4S disease. Tumors in this series, regardless of their histologic categories, had a low incidence of MYCN amplification. The majority of tumors in the GN-M and GNB-I categories had a diploid pattern, while DNA indices of the tumors in GNB-N categories were almost evenly distributed to both the diploid and hyperdiploid groups, regardless of prognostic subsets (FS or US).
Table III.
Clinical and Biological Risk Factors for Ganglioneuroma and Ganglioneuroblastoma
| GN-maturing N (%) | GNB-intermixed N (%) | GNB-nodular FS N (%) | GNB-nodular US N (%) | |
|---|---|---|---|---|
| Age (months) | ||||
| < 18 | 0 (0%) | 9 (5%) | 31 (53%) | 4 (2%) |
| ≥ 18 | 43 (100%) | 189 (95%) | 28 (47%) | 248 (98%) |
|
| ||||
| INSS stage | ||||
| 1, 2, 3 | 36 (100%) | 187 (99%) | 56 (95%) | 116 (47%) |
| 4 | 0 (0%) | 2 (1%) | 3 (5%) | 132 (53%) |
| Unknown | 7 | 9 | 0 | 4 |
|
| ||||
| Risk group | ||||
| Low/Intermed* | 32 (97%) | 181 (99%) | 48 (84%) | 92 (37%) |
| High | 1 (3%) | 2 (1%) | 9 (16%) | 154 (63%) |
| Unknown | 10 | 15 | 2 | 6 |
|
| ||||
| MYCN status | ||||
| Not Amp** | 32 (94%) | 174 (99%) | 53 (100%) | 231 (97%) |
| Amp** | 2 (6%) | 2 (1%) | 0 (0%) | 6 (3%) |
| Unknown | 9 | 22 | 6 | 15 |
|
| ||||
| Ploidy*** | ||||
| Hyperdiploid | 4 (13%) | 27 (17%) | 20 (56%) | 86 (46%) |
| Diploid | 28 (87%) | 132 (83%) | 16 (44%) | 103 (54%) |
| Unknown | 11 | 39 | 23 | 63 |
|
| ||||
| Total # Patients | 43 | 198 | 59 | 252 |
Intermed = Intermediate;
Amp = Amplification;
Ploidy, data available only for the COG cases; GN-maturing = Ganglioneuroma, maturing subtype; GNB-intermixed = Ganglioneuroblastoma, intermixed; GNB-nodular FS = Ganglioneuroblastoma, nodular Favorable Subset; GNB-nodular US = Ganglioneuroblastoma, nodular Unfavorable Subset
As shown in Table IV, the majority (258/285, 90.5%) of GN-M, GNB-I and GNB-N-FS cases were found in the Co-R group, and their FH diagnosis was made after reviewing the completely resected primary tumor. In order to assure the FH classification for those cases in the Inco-R group, close discussion took place between the central review pathologist and the oncology team from the contributing institutions to ensure adequate sampling (see discussion). In contrast, significantly higher proportion of the GNB-N-US patients had incompletely resected primary tumor (67/226, 29.6%; p<0.0001 by Chi-test), and their UH diagnosis was made by identifying histologically unfavorable NB nodule(s) suggesting aggressive clone(s) (24). It was also noted that 44.0% (70/159) of GNB-N-US cases with completely resected primary tumor had already established distant metastasis from unfavorable NB nodule at the time of diagnosis. The following is a summary of the cases in each histologic category.
Table IV.
Summary of Tumor Resectability
| Complete | Incomplete | Unknown/No Report | Total | |
|---|---|---|---|---|
| GN-maturing | 40 | 3 | 43 | |
| GNB-intermixed | 165 | 24 | 9 | 198 |
| GNB-nodular FS | 53* | 0 | 6 | 59 |
| GNB-nodular US | 159** | 67*** | 26 | 252 |
including 3 cases having complete primary tumor resection with distant metastasis of favorable histology neuroblastoma to BM (2 cases) or liver (one case) at the time of diagnosis;
including 74 cases having complete primary tumor resection but metastatic spread of unfavorable histology neuroblastoma already established at the time of diagnosis;
including one case with no surgery to the primary tumor; ; GN-maturing = Ganglioneuroma, maturing subtype; GNB-intermixed = Ganglioneuroblastoma, intermixed; GNB-nodular FS = Ganglioneuroblastoma, nodular Favorable Subset; GNB-nodular US = Ganglioneuroblastoma, nodular Unfavorable Subset
GN-M
Among 43 cases in this category, 40 were Co-R and 3 were Inco-R. As for the Co-R group, 34 patients had a single surgery, one had multiple surgeries for double primaries (2 procedures to the mediastinal primary and one procedure to the adrenal primary), and 5 had an initial biopsy (including one laminectomy due to neurological emergency) with a subsequent total tumor resection before starting chemotherapy/irradiation therapy.
GNB-I
Among 198 cases in this category, 165 were Co-R, 24 were Inco-R, and 9 were R-UK. As for the Co-R group, 154 patients had a single surgery alone to the primary site and one patient had an additional surgery for complete removal of metastatic GNB-I in the adenoid region. Ten patients had multiple procedures (2 surgeries in 9 cases and 4 surgeries in one case) for complete removal of their tumor: The initial procedure was a biopsy from the primary tumor in 9 cases (3 tumors showed an appearance of GN-M and 6 tumors showed an appearance of GNB-I) and from the metastatic lymph node in one case (showed an appearance of transitional form between NB and GNB-I). Among the 24 cases in the Inco-R group, 23 patients had a single procedure, and one patient had a biopsy with a subsequent surgery.
GNB-N-FS
Among 59 cases in this category, 50 were Co-R: 48 patients had a single surgery, and 2 patients had a biopsy (showed an appearance of GN-M and GNB-I histology, respectively) from the primary site before complete excision revealing FH neuroblastoma clone. Additional 3 patients, whose primary tumor was also completely resected (Co-R), had distant metastasis to the bone marrow (2 cases) or the liver (one case) from the NB component of FH. Six cases were classified into the R-UK group.
GNB-N-US
Among 252 cases in this category, 159 were Co-R, 67 were Inco-R, and 26 were R-UK. As for the Co-R group, 74 had distant metastasis at the time of diagnosis: Unfavorable NB nodule(s) were detected in both primary and metastatic (if present) site in 153 cases and only in the metastatic site in 6 cases. As for the Inco-R group, 47 had distant metastasis at the time of diagnosis: Unfavorable NB nodule(s) were detected in both primary and metastatic (if present) site in 62 cases and only in the metastatic site in 4 cases. The last one case in this Inco-R group was unique, since the patient had no surgery to the retroperitoneal primary tumor and the diagnosis was made by identifying 2 different clones in the skin nodule (GN-M) and bone marrow metastasis (NB of Unfavorable Histology). As for the R-UK group, 4 had distant metastasis at the time of diagnosis and metastatic status was unknown in 4 cases: All cases in this group had unfavorable NB nodule(s) in both primary and metastatic site (if present). It was noted that 2 cases in the R-UK group had had a FH diagnosis (One with GNB-I and the other with GNB-N-FS) before reaching the final diagnosis of GNB-N-US by detecting aggressive NB nodule in the primary tumor site after multiple surgical procedures.
Survival Analysis
Table V summarizes the 5-year EFS and OS rates by clinical stage (non-stage 4 vs. stage 4) for patients within the individual tumor categories (INSS Staging data and survival data available for 532 and 519 patients respectively). Patients with tumors in the FH group (GN-M, GNB-I, or GN-N-FS), while vast majority of them were presented with non-stage 4 disease, had an excellent prognosis and no one died of tumor in our series: (1) GN-M (survival data available in 31 Co-R and 3 Inco-R cases): All patients were alive without event; (2) GNB-I (survival data available in 149 Co-R and 24 Inco-R cases): Among 5 patients (3 Co-R and 2 Inco-R) who had an event within 5 years after diagnosis, only one Co-R patient died after developing secondary acute myeloid leukemia. Rest of the patients was alive at the time of last follow-up; and (3) GNB-N-FS (survival data available in 52 Co-R): Four Co-R patients had an event, and one died of non-tumor related cause 10 years after the diagnosis. All others including 3 patients with distant metastasis of FH neuroblastoma nodule had no events within 5 years after diagnosis.
Table V.
Event-free and Overall Survival for Tumor Category by Clinical Stage
| Histological Category | Overall Number of Patients | Number with Survival data | 5-year EFS ± SE (%) | EFS p-value | 5-year OS ± SE (%) | OS p-value |
|---|---|---|---|---|---|---|
| GN-maturing | ||||||
| Stage 1, 2, 3 | 36 | 34 | 100 | 100 | ||
| Stage 4 | 0 | 0 | --- | NA | --- | NA |
|
| ||||||
| GNB-intermixed | ||||||
| Stage 1, 2, 3 | 187 | 179 | 94.1 ± 4.3 | 97.0 ± 3.2 | ||
| Stage 4 | 2 | 2 | --- | NA | --- | NA |
|
| ||||||
| GNB-nodular FS | ||||||
| Stage 1, 2, 3 | 56 | 55 | 92.6 ± 6.3 | 100 | ||
| Stage 4 | 3 | 3 | 100 | NA | 100 | NA |
|
| ||||||
| GNB-nodular US | ||||||
| Stage 1, 2, 3 | 116 | 114 | 80.1 ± 8.0 | 86.2 ± 7.0 | ||
| Stage 4 | 132 | 132 | 16.7 ± 5.8 | <.0001 | 35.9 ± 7.0 | <.0001 |
EFS = Event-free survival; OS = Overall survival; SE = standard error; NA = not applicable due to no event; --- = No patients at risk at 5 years; GN-maturing = Ganglioneuroma, maturing subtype; GNB-intermixed = Ganglioneuroblastoma, intermixed; GNB-nodular FS = Ganglioneuroblastoma, nodular Favorable Subset; GNB-nodular US = Ganglioneuroblastoma, nodular Unfavorable Subset
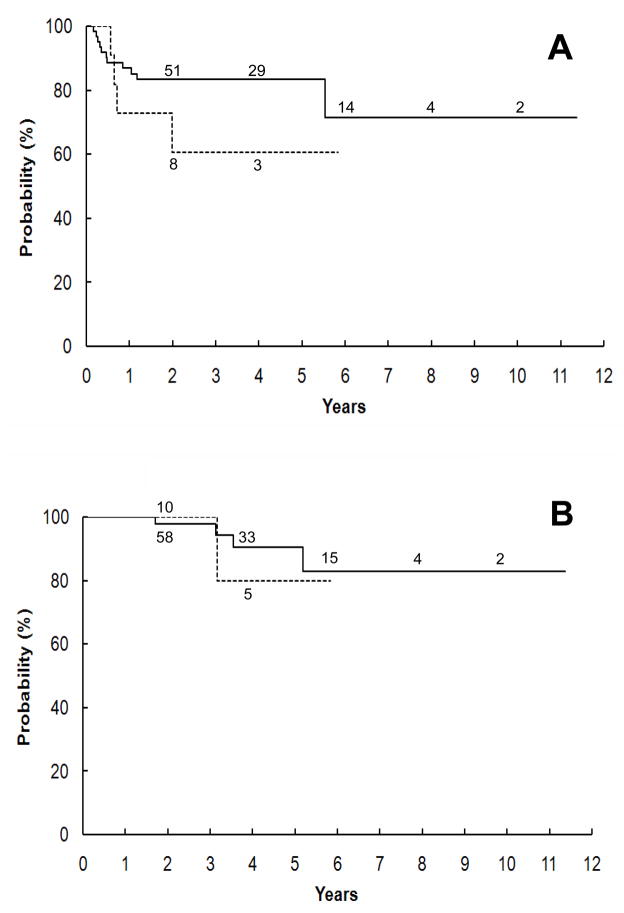
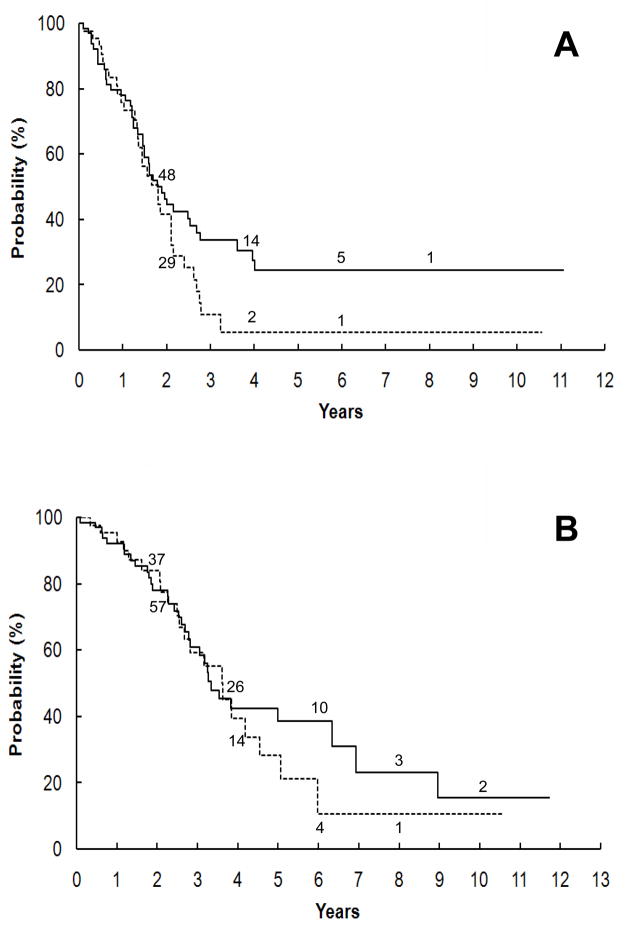
In contrast, the prognosis of patients with GNB-N-US was significantly dependent on clinical stage. Further analysis on those cases demonstrated that the degree of resectability on primary tumor did not have an impact on survival probabilities. For non-stage 4 patients, there was no significant difference in survival between patients with complete primary resection (85 cases) and with incomplete primary resection (16 cases), with 5-year EFS of 83.4% ± 9.1% vs. 60.6% ± 26.9% (p = 0.2227) and OS of 90.5% ± 7.2% vs. 80.0% ± 25.3% (p = 0.7176), respectively (Figure 1). Survival was equally poor for stage 4 patients irrespective of primary tumor resectability (Figure 2): The 5-year EFS for stage 4 patients with completely (74 cases) and incompletely resected (51 cases) primary tumors, respectively, was 24.3% ± 9.5% vs. 5.4% ± 5.2% (p = 0.1068) and OS was 38.5% ± 9.6% vs. 28.2% ± 11.9% (p = 0.5797).
Figure 1.
Kaplan-Meier survival curves for GNB-N-US (Ganglioneuroblastoma- Nodular, Unfavorable Subset) patients with non-stage 4 (stage 1, 2, or 3) disease. No significant difference in both expected event-free (A) and overall (B) survival rates was seen between patients with complete primary tumor resection (solid line) and with incomplete primary resection (interrupted line), p=0.2227 and 0.7176, respectively.
Figure 2.
Kaplan-Meier survival curves for GNB-N-US (Ganglioneuroblastoma, Nodular Unfavorable Subset) patients with stage 4 disease. No significant difference in both event-free (A) and overall (B) survival rates was seen between patients with complete primary tumor resection (solid line) and incomplete primary resection (interrupted line), p=0.1068 and 0.5797, respectively.
There were 10 patients whose tumor had amplified MYCN in this series: all 4 with FH tumors (2 cases with GN-M; 2 cases with GNB-I) were alive and well at the time of last follow-up: 3 of them had more than one year of follow-up period, while 2 of 6 patients whose tumor had UH (GNB-N-US) and amplified MYCN died of tumor.
DISCUSSION
This study summarizes the clinicopathological characteristics of ganglioneuroma and ganglioneuroblastoma cases by using the stringent criteria of the INPC. FH tumors, which include GN-M, GNB-I, and GNB-N-FS, usually presented as localized disease (stage 1, 2, or 3), and were often amenable to complete resection by either single or multiple surgical procedures. None of the patients with FH tumors died of tumor. In contrast, the prognosis of patients with UH tumor; i.e., GNB-N-US, was generally poor, and more than half of them had distant metastasis (stage 4) at the time of diagnosis.
In order to assure the diagnosis of FH tumors with excellent prognosis, there was a close communication and discussion between the central review pathologist and oncology team (institutional pathologist, oncologist, surgeons, and radiologist) at the participating institutions during the course of those CCG and COG Neuroblastoma studies. The discussion included reassessment/re-evaluation of primary tumor status and metastatic disease, if any, and planning of additional surgery, if necessary. As you see in Table II, we clearly defined tumor resectability, and surgical margins were always examined carefully to rule out any possibility of aggressive NB growth. It was always ideal for pathologist to have a completely resected sample for the final diagnosis, so as not to miss any unfavorable nodules or different clones. However, in real life, we sometimes encountered the situation/clinical decision where we had to evaluate a biopsy or incompletely resected material. For those Inco-R cases, FH diagnosis of GN-M, GNB-I, or GNB-N-FS was accompanied by the remark of “the diagnosis is made based on review of a limited material”, and followed by a team effort to search for any hidden aggressive NB growth and to rule-in or -out a possibility of GNB-N-US.
As for the patients with GNB-N-US, this study revealed that their prognosis significantly depended on clinical stage, but not on the resectability of their primary tumor. It was of interest to note that almost half of the patients (74/159, 47%) included in this group had complete resection of their primary tumor with already established distant metastasis at the time of diagnosis. In rare cases (6 cases in our series), unfavorable NB nodule was detected only in the metastatic site even after complete pathology evaluation of their primary tumors. Aggressive primary tumor resection did not seem to be beneficial to GNB-N-US patients, regardless of whether metastasis was present or not.
The next consideration is the relationship between molecular/genetic markers (MYCN status, DNA index) and histologic features of ganglioneuroma and ganglioneuroblastoma. MYCN amplification, detected in about 15 to 20% of all pNTs, is a well-established indicator for a poor prognosis of the patients and almost exclusively found in the UH group (25) (26). As expected, there were only 4 FH tumors (4/210, 1.9%) that had amplified MYCN in our series of GN-M (2 cases) and GNB-I (2 cases), and all 4 patients were alive and well at the time of last follow-up. It is suggested that amplified MYCN may not have an adverse prognostic effect in these rare FH cases. No tumor in GNB-N-FH and only 6 tumors (3%) in GNB-N-UH had amplified MYCN: Since these tumors are composed of different tumor histologies suggesting multiple distinct clones (24), analysis of actual incidence and prognostic impact of MYCN status cannot yield a conclusive statement in this study.
DNA index is another known prognostic factor useful for prognostic prediction of peripheral neuroblastic tumors especially in infants with pNTs. DNA index can distinguish biologically favorable tumors composed of hyperdiploid neuroblasts for a good prognosis from biologically unfavorable tumor composed of diploid neuroblasts for a poor prognosis(25) (27). In our series, the majority of GN-M and GNB-I tumors, even though they were biologically favorable with an excellent prognosis, had a diploid pattern determined by flow cytometry. This could be attributable to the fact that those tumors were mainly or predominantly composed of Schwannian stromal cells, and neuroblastic cells were not the main source of cells to be tested. Though there have been controversial reports on cellular origin of Schwannian stromal cells (28) (29), Ambros et al. report that Schwannian cells in GN-M and GNB-I are not neoplastic and do not share the same cellular origin of neural crest with neuroblastic cells, but they are normal diploid cells recruited by biologically favorable hyperdiploid neuroblastic cells. Recent study by Du et al. also suggested a possibility of bone marrow stroma cell origin of Schwannian stroma in neuroblastoma (30)
CONCLUSION
Since there is a significant prognostic difference among the patients with ganglioneuroma and ganglioneuroblastoma, it is very important to classify those tumors into the FH (GN-M, GNB-I, GNB-N-FS) and UH (GNB-N-US) group according to the International Neuroblastoma Pathology Classification along with clinical staging. Close communication among members of the oncology team is important for better management of patients with this disease, especially when their tumors are not completely resected at the initial surgery.
Acknowledgments
This work was supported in part by grant U10 CA98413 from the Division of Cancer Treatment, National Cancer Institute, National Institute of Health, Department of Health and Human Services. The authors express their cordial thanks to Drs. Lisa Shane and Ignacio Gonzalez at Childrens Hospital Los Angeles, Los Angeles, California, for their support and useful discussion.
References
- 1.Beckwith JB, Martin RF. Observations on the histopathology of neuroblastomas. J Pediatr Surg. 1968;3:106–10. doi: 10.1016/0022-3468(68)90989-5. [DOI] [PubMed] [Google Scholar]
- 2.Hughes M, Marsden HB, Palmer MK. Histologic patterns of neuroblastoma related to prognosis and clinical staging. Cancer. 1974;34:1706–1711. doi: 10.1002/1097-0142(197411)34:5<1706::aid-cncr2820340519>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Shimada H, Chatten J, Newton WA, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 4.Dehner LP. Classic neuroblastoma: histopathologic grading as a prognostic indicator, The Shimada System and its progenitors. Am J Pediatr Hematol Oncol. 1988;10:143–154. [Google Scholar]
- 5.Joshi VV, Rao PV, Cantor AB, Altshuler G, Shuster JJ, Castleberry RP. Modified histologic grading of neuroblastomas by replacement of mitotic rate with mitosis karyorrhexis index. Cancer. 1996;77:1582–1588. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1582::AID-CNCR24>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Joshi VV, Tsongalis GJ. Correlation between morphologic and nonmorphologic prognostic markers of neuroblastoma. Ann NY Acad Sci. 1997;824:71–83. doi: 10.1111/j.1749-6632.1997.tb46210.x. [DOI] [PubMed] [Google Scholar]
- 7.Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349–63. [PubMed] [Google Scholar]
- 8.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–72. [PubMed] [Google Scholar]
- 9.Ambros IM, Hata J, Joshi VV, et al. Morphologic features of neuroblastoma (Schwannian stroma-poor tumors) in clinically favorable and unfavorable groups. Cancer. 2002;94:1574–1583. doi: 10.1002/cncr.10359. [DOI] [PubMed] [Google Scholar]
- 10.Brodeur GM. Neuroblastoma: Biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 11.Hayes FA, Green AA, Rao BN. Clinical manifestations of ganglioneuroma. Cancer. 1989;63:1211–1214. doi: 10.1002/1097-0142(19890315)63:6<1211::aid-cncr2820630628>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.De Bernardi B, Gambini C, Haupt R, et al. Retrospective study of Childhood Ganglioneuroma. J Clin Oncol. 2008;26:1710–1716. doi: 10.1200/JCO.2006.08.8799. [DOI] [PubMed] [Google Scholar]
- 13.Robertson HE. Das Ganglioneuroblastom ein besonederer Typus im System der Neurome. Virchows Arch [Pathol Anat] 1915;63:147–168. [Google Scholar]
- 14.Stout AP. Ganglioneuroma of the sympathetic nervous system. Surg Gynecol Obstet. 1947;83:101–110. [PubMed] [Google Scholar]
- 15.Bove KE, McAdams AJ. Composite ganglioneuroblastoma, an assessment of the significance of histological maturation in neuroblastoma diagnosed beyond infancy. Arch Pthol Lab Med. 1981;105:325–330. [PubMed] [Google Scholar]
- 16.Peuchmar M, d’Amore ESG, Joshi VV, et al. Revision of the International Neuroblastoma Pathology Classification. Confirmation of Favorable and Unfavorable Prognostic Subsets in Ganglioneuroblastoma. Nodular Cancer. 2003;98:2274–81. doi: 10.1002/cncr.11773. [DOI] [PubMed] [Google Scholar]
- 17.Brodeur GM, Prichard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein JL, Katzenstein HM, Cohn SL. Advances in the Diagnosis and Treatment of Neuroblastoma. Oncologist. 2003;8:278–292. doi: 10.1634/theoncologist.8-3-278. [DOI] [PubMed] [Google Scholar]
- 19.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 20.Matthay KK, Perez K, Seeger RC, et al. Successful treatment of stage III neuroblastoma baced on prospective biologic staging: A Children’s Cancer Group study. J Clin Oncol. 1998;16:1256–1264. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 21.Perez C, Matthay KK, Atkinson JB, et al. Biologic variables in the outcome of stage I and II neuroblastoma treated with surgery as primary therapy: A Children’s Cancer Group study. J Clin Oncol. 2000;18:18–26. doi: 10.1200/JCO.2000.18.1.18. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Non- parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part II: Analysis and Examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt ML, Salwen HR, Chagnovich D, Bauer KD, Crawford SE, Cohn SL. Evidence of molecular heterogeneity in human ganglioneuroblastoma. Pediatr Pathol. 1993;13:787–796. doi: 10.3109/15513819309048265. [DOI] [PubMed] [Google Scholar]
- 25.Look AT, Hayes AF, Shuster JJ, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: A Pediatric Oncology Group Study. J Clin Oncol. 1991;9:581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- 26.Goto S, Umehara S, Gerbing RB, et al. Histopathology (International Neuroblastoma Pathology Classification) and MYCN Status in Patients with Peripheral Neuroblastic Tumors. A Report from the Children’s Cancer Group. Cancer. 2001;92:2699–2708. doi: 10.1002/1097-0142(20011115)92:10<2699::aid-cncr1624>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Oppedal BR, Storm-Mathisen I, Lie SO, Brandtzaeg P. Prognostic factors in Neuroblastoma: Clinical, histopathologic, and immunohistochemical features and DNA ploidy in relation to prognosis. Cancer. 1988;62:772–780. doi: 10.1002/1097-0142(19880815)62:4<772::aid-cncr2820620422>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 28.Mora J, Cheung NK, Juan G, et al. Neuroblastic and Schwannian Stromal Cells of Neuroblastoma are derived from a tumoral progenitor cell. Cancer Res. 2001;61:6892–6898. [PubMed] [Google Scholar]
- 29.Ambros IM, Zellner A, Roald B, et al. Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N Engl J Med. 1996;334:1501–1511. doi: 10.1056/NEJM199606063342304. [DOI] [PubMed] [Google Scholar]
- 30.Du W, Hozumi N, Sakamoto M, Hata J, Yamada T. Reconstitution of Schwannian Stroma in Neuroblastomas Using Human Bone Marrow Stromal Cells. Am J Pathol. 2008;173:1153–1164. doi: 10.2353/ajpath.2008.070309. [DOI] [PMC free article] [PubMed] [Google Scholar]