Abstract
In this review we will describe eight commonly used rat brain tumor models and their application for the development of novel therapeutic and diagnostic modalities. The C6, 9L and T9 gliomas were induced by repeated injections of methylnitrosourea (MNU) to adult rats. The C6 glioma has been used extensively for a variety of studies, but since it arose in an outbred Wistar rat, it is not syngeneic to any inbred strain, and its potential to evoke an alloimmune response is a serious limitation. The 9L gliosarcoma has been used widely and has provided important information relating to brain tumor biology and therapy. The T9 glioma, although not generally recognized, was and probably still is the same as the 9L. Both of these tumors arose in Fischer rats and can be immunogenic in syngeneic hosts, a fact that must be taken into consideration when used in therapy studies, especially if survival is the endpoint. The RG2 and F98 gliomas were both chemically induced by administering ethylnitrosourea (ENU) to pregnant rats, the progeny of which developed brain tumors that subsequently were propagated in vitro and cloned. They are either weakly or non-immunogenic and have an invasive pattern of growth and uniform lethality, which make them particularly attractive models to test new therapeutic modalities. The CNS-1 glioma was induced by administering MNU to a Lewis rat. It has an infiltrative pattern of growth and is weakly immunogenic, which should make it useful in experimental neuro-oncology. Finally, the BT4C glioma was induced by administering ENU to a BD IX rat, following which brain cells were propagated in vitro until a tumorigenic clone was isolated. This tumor has been used for a variety of studies to evaluate new therapeutic modalities. The Avian Sarcoma Virus (ASV) induced tumors, and a continuous cell line derived from one of them designated RT-2, have been useful for studies in which de novo tumor induction is an important requirement. These tumors also are immunogenic and this limits their usefulness for therapy studies. It is essential to recognize the limitations of each of the models that have been described, and depending upon the nature of the study to be conducted, it is important that the appropriate model be selected.
Keywords: C6, 9L, T9, RG2, F98, BT4C, RT-2, CNS-1, Rat brain tumor models
Introduction
There is a continuing need in experimental neuro-oncology for animal models that can be used to assess the efficacy of innovative approaches for the treatment of brain tumors. The rat has been one of the most widely used experimental animals, and rat brain tumor models have been used extensively since the mid 1970s. This review will focus on rat brain tumor models and their utility in evaluating the efficacy of various therapeutic modalities. Until recently, murine models [1] were used less frequently than rat models, but the ability to produce genetically engineered cell lines [2] has increased the use of murine models over the past five years. The relative advantages of rat and murine tumor models are summarized in Table 1. Feline and canine models have been used less frequently [3, 4], but nevertheless, still provide an intermediate between rodent models and humans. It was first reported in the early 1970s that central nervous system (CNS) tumors could be induced reproducibly and selectively in adult rats that had been given repeated, weekly intravenous injections of N-methylnitrosourea (MNU) or a single dose of N-ethyl-N-nitrosourea (ENU). These studies led to the development of a number of rat brain tumor models that were highly reproducible and did not require the topical application of a chemical carcinogen to the brain [5]. In this review, we first will summarize some general principles relating to the use of brain tumor models. Although widely used, xenograft models based on the intracerebral (i.c.) implantation of human brain tumor cell lines into immunologically deficient rodents will not be discussed by us, and interested readers are referred to the recent review of Candolfi et al. [6]. The utility of these models notwithstanding, it is important to recognize that no currently available animal tumor model exactly simulates human high grade brain tumors such as glioblastoma multiforme (GBM) or anaplastic astrocytomas.
Table 1.
Advantages and disadvantages of rat brain tumor models compared to mouse models
| Advantages | Disadvantages |
|---|---|
| 1. Larger size of the rat brain (compared to the mouse brain ~1200 mg vs ~ 400 mg) permits more precise stereotactic implantation than in mice, a longer interval of time until death and a thicker skull essentially eliminates osseous invasion and s.c. growth. | Rat brain tumor models cannot be as easily genetically engineered and manipulated as mouse models in order to elucidate the importance of genetic factors, signaling pathways, cell types and stroma in tumor growth and invasion. |
| 2. Larger tumor size prior to death permits better in vivo localization and imaging by a variety of diagnostic modalities in the rat. | The potential to produce genetically engineered tumor cell lines is less in the rat than in the mouse. |
| 3. Larger tumor size prior to death permits the administration of larger amounts of various therapeutic agents, especially if administered i.c. by CED and more critical evaluation of their effectiveness. | There are a smaller number of mAbs directed against rat surface antigens and chemokines compared to the mouse. |
| 4. More extensive literature on in vitro and in vivo studies of rat brain tumors compared to mouse tumors. | Rats are more expensive to purchase and maintain than mice. |
The cellular signaling pathways important for the genesis of brain tumor are multiple, with feedback mechanisms that can dramatically affect the efficacy of molecularly targeted therapeutic strategies. The heterogeneous composition of human high grade gliomas, which consists of tumor stem cells and differentiated tumor cells with varying characteristics, further complicates their susceptibility to treatment. Brain tumors also can evolve within their microenvironment, adapting to changes that produce epigenetic effects thereby altering their biology, but concomitantly providing additional targets for therapeutic intervention. Finally, genetic variations between individuals can dictate how tumors initiate, progress, and respond to treatment. Rat brain tumor models have provided a wealth of information on the in vitro and in vivo responses to various therapeutic modalities. The larger rat brain (~1200 mg) compared to that of the mouse (~400 mg) allows for more precise tumor cell implantation, and relatively larger volumes (~20 μl) that can be injected versus mice (5 μl; Table 1). Mouse models, on the other hand, have allowed researchers to rigorously test hypotheses developed from examining human tumors by genetically manipulating them and controlling specific variables such as environmental influences, in order to better understand the roles of different pathways, cell types, stromal factors and genetic variation [7]. Mouse tumor models (Table 1) also have allowed researchers to test hypotheses derived from examining human tumors, in a controlled environment with specific genetic alterations and controlled environmental influences [7].
There is a general consensus that valid brain tumor models should fulfill the following criteria: (1) they should be derived from glial cells; (2) it should be possible to grow and clone them in vitro as continuous cell lines and propagate them in vivo by serial transplantation; (3) tumor growth rates should be predictable and reproducible; (4) the tumors should have glioma-like growth characteristics within the brain including neovascularization, alteration of the blood-brain barrier (BBB), an invasive pattern of growth, and lack of encapsulation; (5) host survival time following i.c. tumor implantation should be of sufficient duration to permit therapy and determination of efficacy; (6) for therapy studies, the tumors should be either non or weakly immunogenic in syngeneic hosts; (7) they should not grow into the epidural space or extend beyond the brain and finally (8) their response or lack thereof to conventional treatment should be predictive of the response in human brain tumors.
In studies carried out prior to the 1970s, either cells or tumor fragments were injected i.c. using a free hand approach, which generally lacked reproducibility and precision. A stereotactic implantation procedure using suspensions of tissue-culture-derived brain tumor cells was more successful [8]. This procedure was further improved by the use of concentrated cell suspensions in small volumes, improved injection needles, better stereotactic localization to structures deeper in the white matter such as the caudate nucleus, the use of slower injection rates [9], 0.5–1.0% low gelling agarose to prevent backflow of tumor cells through the injection track [9] and cleansing of the operative field with a solution of Betadine. Finally, rinsing the surface of the brain with sterile water destroys extravasated tumor cells by osmosis prior to closure of the skull with bone wax has also been recommended [10]. This implantation procedure resulted in high success rates of i.c. tumor growth with the elimination of spinal and extracranial dissemination. The implantation of plastic [9] or metallic screws [11] with an entry port, which are permanently implanted in the skull to inject tumor cells, have been very useful [11, 12]. Such devices can be left in place either at the time of or after tumor cell implantation in order to facilitate future administration of therapeutic agents at the same location without further stereotactic surgery. These are well tolerated and non-irritating in rats, but they cannot be as easily used in mice due to the thinness of their skulls [12]. Keeping these general principles of tumor cell implantation in mind, we will now discuss the currently available rat glioma models that have been used in immunocompetent animals.
C6 glioma
The C6 glioma was produced by Benda et al. [13] and Schmidek et al. [14], in Sweet’s laboratory at the Massachusetts General Hospital (MGH) by repetitively administering MNU to outbred Wistar rats over a period of approximately 8 months. When animals developed neurological signs, they were euthanized, and the tumors were excised and explanted into tissue culture. Among these was a tumor designated as “#6”, which was subsequently cloned by Benda et al. [13] and was shown to produce S-100 protein. Following cloning, it was re-designated “C6” [15]. The C6 glioma is composed of a pleomorphic population of cells with variably shaped nuclei. There is focal invasion into contiguous normal brain (Fig. 1a). Initially, the tumor was histopathologically classified as an astrocytoma, and eventually it was designated as a glial tumor following accession by the American Type Culture Collection, Rockville, MD (ATCC# CCL-107). The cells have been reported to have a mutant p16/Cdkn2a/Ink4a locus [16] with no expression of p16 and p19ARF mRNAs, and a wildtype p53 [17]. More recent molecular characterization, which compared changes in gene expression between the C6 glioma and rat stem cell-derived astrocytes, revealed that the changes in gene expression observed in the C6 cell line were the most similar to those reported in human brain tumors [18]. Compared to astrocytes, they also had increased expression of the PDGFβ, IGF-1, EGFR and Erb3/Her3 genes, which are frequently overexpressed in human gliomas [19–21]. In a recent study, the significance of PDGF in gliomagenesis in adult rats was established by infecting white matter with a retrovirus encoding for PDGF and GFP. Within 2 weeks 100% of the animals had tumors derived from both infected and uninfected glial progenitors, thereby implicating PDGF in both autocrine and paracrine stimulation of glial progenitor cells [22]. Although, IGF-1 was overexpressed in C6 glioma cells, there was reduced expression of IGF-2, FGF-9 and FGF-10 relative to astrocytes. Similar to the increased activity of the Ras pathway observed in human gliomas [23], C6 cells also had an increase in both Ras expression and Ras guanine triphosphate activator protein [18]. However, contrary to what has been reported for human GBM, there was an increase in expression of Rb in these cells [18]. A subclone of C6 cells, stably expressing β-galactosidase, subsequently was described [24] and this has permitted in vivo immunohistochemical analysis of these tumors in the brain. This clone is available through the ATCC (# CRL-2303). However, it must be noted that the β-galactosidase marker protein itself can serve as a tumor antigen, and immunization of rats against the reporter gene protected the animals against tumor growth [24].
Fig. 1.
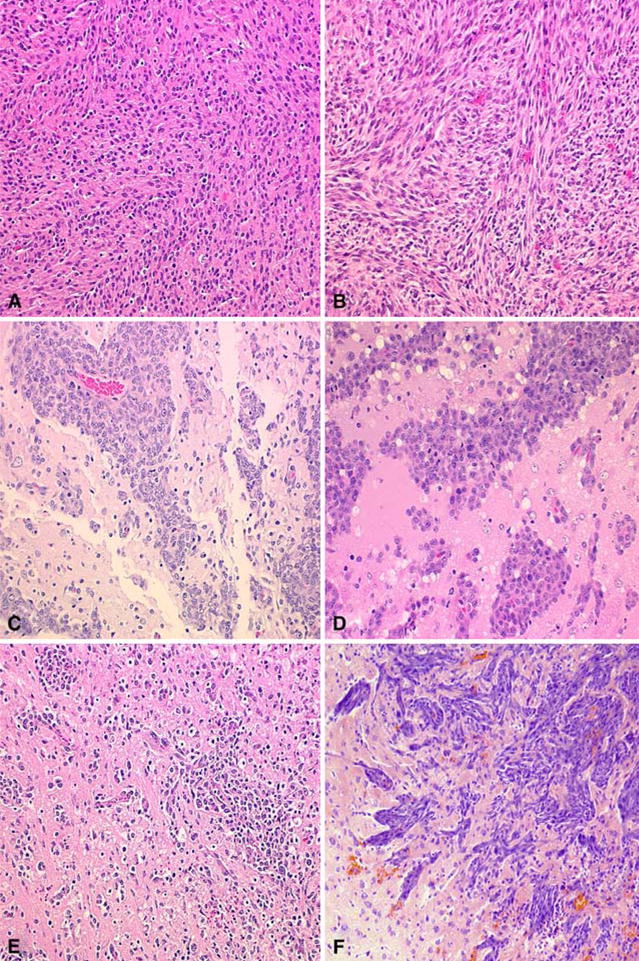
Histopathologic features of the C6, 9L, RG2, F98, CNS-1, and BT4C brain tumors. A The C6 glioma is composed of a pleomorphic population of cells with nuclei ranging from round to oblong. A herring-bone pattern of growth is seen in some areas and there is focal invasion of contiguous normal brain. There are scattered foci of necrosis with pseudo-palisading of tumor cells at the periphery. B The 9L gliosarcoma is composed of spindle-shaped cells with a sarcomatoid appearance. A whorled pattern of growth is seen with sharp delineation of the margins of the tumor with little invasion of contiguous normal brain. C The RG2 glioma is very similar in appearance to the F98 glioma and also has a highly invasive pattern of growth. D The F98 glioma is composed of a mixed population of spindle-shaped cells with fusiform nuclei, frequently forming a whorled pattern of growth, and a smaller subpopulation of polygonal cells with round to oval nuclei. There is extensive invasion of contiguous normal brain with islands of tumor cells at varying distances from the main tumor mass, which form perivascular clusters. Usually, there is a central area of necrosis filled with tumor cell ghosts. E The CNS-1 glioma is composed of a pleomorphic population of cells that show great variation in size and shape. There is extensive invasion of contiguous normal brain with dense infiltrates in some areas and in others, more circumscribed clusters of tumor cells. Small foci of hemorrhage are scattered through the tumor. F The BT4C glioma is composed of a pleomorphic population of tumor cells with a sarcomatous pattern of growth. Scattered tumor giant cells are seen and mitotic figures are frequent. The tumor grows expansively and invades the surrounding normal brain along perivascular tracts and occasional tumor cell nests are seen in the surrounding normal brain. There is neo-vascularization, especially in the tumor periphery, where microhemorrhages are frequent. Central necrosis is usually not present but occasionally scattered areas of necrosis may be seen in larger tumors. (Photomicrograph of the BT4C was kindly provided by M. Sandström and description by M. Johansson. Representative microscope slides of the CNS-1 glioma were kindly provided by Dr. Carol Kruse). All photomicrographs are at a magnification of 200×, except for F
The C6 rat glioma model has been widely used in experimental neuro-oncology to evaluate the therapeutic efficacy of a variety of modalities, including chemotherapy [25], anti-angiogenic therapy [26], proteosome inhibitors [27], treatment with toxins [28], radiation therapy [29], photodynamic therapy [30], oncolytic viral therapy [31] and gene therapy [32]. Since this tumor arose in an outbred Wistar rat, however, there is no syngeneic host in which it can be propagated. This is a very serious limitation that diminishes its usefulness for survival studies since the tumor is immunogenic, even in Wistar rats. The C6 glioma has been demonstrated to be immunogenic in Wistar and BDX rats [33], and it therefore is not useful for evaluating the efficacy of immunotherapy. This problem is exemplified by prior studies in which C6 glioma cells were transfected with an antisense cDNA expression vector that downregulated the constitutive production of IGF-1 [34, 35]. Not recognizing that the tumor was of Wistar origin, the authors unfortunately used BD IX rats, which they thought was the strain of origin, due to some ambiguity in the literature. Subsequently, it was reported that BD IX rats, which had been immunized with the C6 anti-sense IGF-1 transfected cells, were resistant to both s.c. and i.c. challenge of the C6 glioma. Similarly, Wistar rats, bearing C6 gliomas (s.c. or i.c.), developed potent humoral and cellular immune responses to the tumor, and rats challenged simultaneously with s.c. and i.c. tumors, had a survival rate of 100% [33]. Since C6 glioma cells are allogeneic in all inbred strains, this should provide a strong cautionary note for studies employing this tumor model and they should not be used for immunotherapy studies. Despite this limitation, the C6 glioma model continues to be used for a variety of studies related to brain tumor biology [36]. These have included studies on tumor growth, invasion, migration, BBB disruption, neovascularization, growth factor regulation and production, and biochemical studies [37–39]. Finally, single-cell clonal analysis has revealed that C6 cells also have cancer stem cell-like characteristics, including self-renewal, the potential for multi-lineage differentiation in vitro and tumor formation in vivo [40].
9L gliosarcoma
The 9L gliosarcoma has been the most widely used experimental rat brain tumor model. It was produced in Fisher 344 rats by the intravenous injection of 5 mg/kg of MNU for 26 weeks [14, 41]. The original tumor was designated as tumor #9, which subsequently was cloned at the Brain Tumor Research Center, University of California, San Francisco, and then was designated “9L” [8, 13, 14]. These tumor cells could be propagated in vitro, which made them very useful for in vivo studies to investigate the effects of various therapeutic modalities on brain tumors. 9L cells can be implanted i.c. into syngeneic Fischer rats, following which they give rise to rapidly growing tumors. These are composed of spindle-shaped cells with a sarcomatoid appearance. The tumor margins are sharply delineated with little obvious invasion into the contiguous normal brain (Fig. 1b). The 9L gliosarcoma has a mutant p53 gene [17], but there is normal expression of p16 and p19ARF mRNAs, indicating that there is a wild type p16/Cdkn2a/INK4α locus [16]. Molecular characterization of the 9L relative to rat stem cell-derived astrocytes revealed an increased expression of the genes encoding TGFα and its receptor, EGFR [18]. Interestingly, decreased expression of FGF-2, FGF-9, and FGFR-1 and PDGFRβ also was noted [18]. Recently, cancer stem-like cells (CSLCs) have been demonstrated in the 9L cell line. These CSLCs grow as neurospheres in chemically defined medium and express the neural stem cell markers Nestin and Sox2. They are self-renewable and differentiate in vitro into neuron- and glial-like cells [42]. The neurospheres have a lower proliferation rate and express several anti-apoptotic and drug related genes. Furthermore, these cells form tumors that are more aggressive than the parental 9L tumor [42], which could be an important property in future studies.
The 9L gliosarcoma model has been used extensively to investigate mechanisms and development of drug resistance [43, 44], transport of drugs across the blood-brain and blood-tumor barrier [45–48], imaging of brain tumors including radiological techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) and imaging to evaluate tumor hypoxia and metabolism [49, 50], pharmacokinetic studies of nitrosourea [48], mechanisms and effects of anti-angiogenic drugs [51, 52], effects of radiation [53], chemotherapy [54, 55], gene therapy [56–59], cancer stem cells [42], immunotoxin treatment [60], immunotherapy and cytokine therapy [57, 61] and oncolytic viral therapy [62, 63].
A number of these studies have yielded impressive therapeutic results, including apparent cures of tumor bearing animals. However, it must be emphasized that this tumor has been shown to be highly immunogenic. Animals immunized with X-irradiated 9L cells were resistant to both subcutaneous (s.c.) as well as i.c. tumor challenge, compared to 100% tumor takes in immunologically naïve animals [64]. This report was first published in the proceedings of a meeting, which did not receive wide circulation, but subsequent studies have confirmed the immunogenicity of this model [65, 66]. Expression of the s-Myc gene under the control of a CMV promotor resulted in complete suppression of 9L tumor growth, as well as rejection of subsequent challenges of tumor cells. Histological examination of the tumors after s-Myc therapy revealed massive mononuclear cell infiltration with CD8 + T lymphocytes, which accounted for >70% of these infiltrating cells. These observations suggested that tumor rejection was due to a potent T-cell mediated, anti-tumor immune response. This, and several more recent studies, have underscored the significance of the anti-tumor immune response following gene therapy induced tumor eradication observed with 9L model. It is now recognized that in vivo bystander cell killing [67], which has been observed with the 9L gliosarcoma following delivery of the Herpes simplex virus thymidine kinase gene (hsv-tk), [62, 68] followed by treatment with ganciclovir, was due in part to an anti-tumor immune response. The immunogenicity of the 9L glioma must be kept in mind when utilizing this model to evaluate the efficacy of novel therapeutic agents. Early studies employing radiation or chemotherapy alone were largely unsuccessful in curing the 9L tumor. However, the success obtained by boron neutron capture therapy and gene therapy highlights the importance of utilizing anti-tumor treatments that can destroy individual cancer cells and simultaneously spare host immune effector cells that can eradicate residual tumor cells [69–72].
Despite the fact that the 9L arose in a Fischer rat, 9L gliosarcoma cells also can form i.c. tumors in allogeneic Wistar rats [73]. Histopathological evaluation revealed that these tumors formed circumscribed masses that were not infiltrative and did not spread into the subarachnoid space or ventricles [73]. Immunostaining of the tumors revealed the presence of glial fibrillary acidic protein (GFAP-positive, infiltrating astrocytic cells, and activated ED1 positive macrophages/microglia [73]. Higher numbers of K(ATP) and K(Ca) channels have been observed in 9L tumors grown in allogeneic Wistar rats compared to those grown in syngeneic Fischer rats. Furthermore, the allogeneic tumors showed a greater increase in brain tumor permeability upon treatment with potassium channel agonists, compared to those grown in syngeneic hosts [45]. The 9L tumor model also has been used following treatment to study the effect of BBB disruption [45], implantation of devices for repeated intratumoral delivery [12] and imaging [74].
The 9L gliomaosarcoma model also has been used to develop a model for brainstem tumors [75]. Progression to hemiparesis with the onset of symptoms occurred 17 days post-implantation into the brainstem. This model has been used to evaluate the efficacy of convection enhanced delivery (CED) of carboplatin to the brainstem, [75] and to study the response of recurrent, chemo-resistant gliomas. Two bis-chloroethyl nitrosourea (BCNU) resistant cell lines were derived from 9L cells by treating them with BCNU in vitro or in vivo. Both of these cell lines formed tumors in a 100% of the animals following i.c. implantation, and were much more invasive than the parental 9L cells [76]. The 9L gliosarcoma also has been used as a model to evaluate drug-resistant and invasive recurrent gliomas [77], but as previously indicated, caution must be used in evaluating results obtained with such a highly immunogenic tumor.
T9 glioma
Although not fully appreciated, the T9 glioma was at one time, and still may be the same as the 9L gliosarcoma [5]. The original stock of T9 cells was obtained from Sweet’s laboratory at the MGH by Denlinger, and Koestner, and it was renamed T9 by them [65]. Similar to the immunogenicity of the 9L gliomosarcoma, [64], the T9 glioma also was found to be highly immunogenic [65]. Kida et al. found that rats immunized with irradiated T9 cells or T9 cells mixed with C. parvum rejected subsequent s.c. implants of T9 glioma cells [78]. However, in order to immunize against intracranial tumors, rats initially had to reject intradermal T9 cells [78]. As might have been predicted, these results indicated that, similar to the 9L gliosarcoma, the T9 glioma also was immunogenic. The T9 cell line subsequently has been shared among numerous investigators and has been used for many studies, including the evaluation of anti-angiogenic [79], and chemotherapeutic agents [80], immunotherapy [81], and gene therapy with interferon-β [82]. Although tumor specific or tumor associated antigens have yet to be identified, for the 9L gliosarcoma and T9 glioma, it is only a matter of time before they are identified.
RG2 glioma
The RG2 glioma (ATCC #CRL-2433) was produced in Koestner’s laboratory at The Ohio State University by the i.v. administration of ENU (50 mg/kg body weight) to a pregnant Fischer 344 rat on the 20th day of gestation. Subsequently, the in vitro growth and morphology of the F98 glioma was described in detail [83], and based on its histopathology it was classified as an anaplastic or undifferentiated glioma [9]. The progeny of ENU-injected rats subsequently developed tumors, and following cloning by Wechsler in Germany, one of these clones was designated as “RG2” (rat glioma 2). The same clone was called the “D74-RG2” or “D74” in Koestner’s laboratory at The Ohio State University. The RG2 glioma (Fig. 1c) is similar in microscopic appearance to the F98 glioma (Fig. 1d), and also has a highly invasive pattern of growth, which has made it a good representative model for GBM [84]. Gene expression profiling of these cells established that they had increased gene expression of PDGFβ, IGF-1, Ras, Erb3/HER3 precursor mRNA and cyclin D2 [18]. They express a wildtype p53 and a concurrent loss in the expression of the p16/Cdkn2a/Ink4 gene locus, [16]. It has been used for a variety of preclinical studies to evaluate changes in vascular permeability [85], disruption of the BBB [86, 87], anti-angiogenic therapy [88], gene therapy [89], chemotherapy [90, 91] and radionuclide therapy [92].
The RG2 glioma is non-immunogenic in syngeneic Fischer rats [84] and has low levels of MHC-1 expression compared to the C6 and 9L gliomas [93]. However, in vitro treatment with IFN-γ upregulated MHC class I antigen expression and also resulted in a significant in vivo anti-tumor immune response with increased survival of treated animals [93]. More recently, the RG2 glioma has been stably transfected with human Herpes virus Entry Mediator C (HveC) to facilitate HSV infection and has been used to study the therapeutic effects of oncolytic Herpes simplex virus-1 treatment [94]. The transfected cells retained their tumorigenicity following i.c. implantation in Fischer rats, and transfection of the HveC gene did not affect i.c. tumor growth [95]. However, it has not been determined if HveC can cause these cells to become immunogenic, and therefore, this must be taken into account when using the RG2 for immunotherapy studies.
F98 glioma
The F98 glioma (ATCC # CRL-2397) was produced by Wechsler in Koestner’s laboratory at the same time as the RG2 glioma. It is composed of a mixed population of spindle-shaped cells, the majority of which have fusiform nuclei, and a smaller number of polygonal cells with round to oval nuclei. There is extensive invasion of contiguous normal brain with islands of tumor cells at varying distances from the tumor mass, many of which form perivascular clusters (Fig. 1d). Similar to human GBM, these cells overexpress PDGFβ, and Ras along with an increase in EGFR, cyclin D1 and cyclin D2 expression relative to rat astrocytes [18]. Like the C6 glioma, they also have increased expression of Rb relative to rat astrocytes [18]. Immunofluorescence studies of F98 cells also revealed low expression of BRCA1, and a lack of radiation and cisplatin induced BRCA1 foci in these cells [54]. Usually, there is a necrotic core, scattered mitotic cells and non-glomeruloid neovascular proliferation [96]. The tumor is GFAP and vimentin positive with negligible staining for CD3 + T cells [96].
Since it simulates the behavior of human GBMs in a number of important ways, such as its highly invasive pattern of growth and low immunogenicity, it has been used to evaluate the efficacy of a variety of experimental therapeutic agents. It is refractory to a number of therapeutic modalities, including systemic chemotherapy with paclitaxel, and carboplatin [97], and it is poorly responsive to photon-irradiation alone [5], which in part may be related to its functionally impaired BRCA1 status that can favor genomic instability and impaired DNA repair [54]. Recently, it has been shown to be responsive to a combination of synchrotron radiation with cisplatin [98], and to convection enhanced delivery (CED) of carboplatin in combination with 6 MV photon-irradiation in rats bearing i.c. tumors [99, 100]. This model has been used extensively by Barth et al. to evaluate the efficacy of boron neutron capture therapy (BNCT) [101, 102]. Elleaume and her coworkers have evaluated cisplatin, carboplatin and iodine enhanced synchrotron stereotactic radiotherapy [103] in F98 glioma bearing rats [104]. It has also been used to evaluate non-invasive MRI to visualize tumor growth [105], diffusion tensor imaging [106], tumor angiogenesis [107] and the tumor tropism of mesenchymal stem cells [108].
The F98 glioma is very weakly immunogenic [109] and transfection with the gene encoding B7.1 co-stimulatory molecule [110], or syngeneic cellular vaccination combined with GM-CSF, did not enhance its immunogenicity [110, 111]. This makes it a very attractive model to investigate the mechanisms underlying glioma resistance to immunotherapy. It has also been used to study the molecular genetic alterations in GBMs [112], effects of infusion rates on drug distribution in i.c. tumors [47], and for suicide gene therapy with Herpes simplex virus-1 thymidine kinase (HSV-TK) [113]. Like the 9L gliosarcoma, F98 cells also have been injected into the pontine tegmentum of the brainstem of Fischer rats to produce a model for brainstem tumors [75]. The histopathological and radiobiological characteristics of these tumors were comparable to aggressive, primary human brainstem tumors, which could facilitate preclinical testing of therapeutics to treat these lethal tumors.
F98 cells have been stably transfected with expression vectors encoding for wildtype EGFR and EGFRvIII, and the resulting cell lines have been designated F98EGFR (ATCC# CRL-2948) and F98npEGFRvIII (ATCC# CRL-2949). They each express ~105 non-functional (i.e. non-phosphorylatable) receptor sites per cell. This is below the threshold number of 106 sites per cell that can evoke a xeno-immune response against human EGFR in rats [114]. These cell lines have been used in Fischer rats for studies on molecular targeting [115] to evaluate the therapeutic efficacy of boronated mAbs and EGF for neutron capture therapy (NCT) [102, 116]. The boronated mAbs, L8A4, which is specific for EGFRvIII, and cetuximab, which recognizes wild type EGFR, specifically targeted their respective receptor positive i.c. tumors after CED and they were therapeutically effective following NCT [102, 115–117].
A bioluminescent F98 cell line recently was constructed by stably transfecting F98 cells with the luciferase gene. When implanted i.c. into the brains of Fischer rats, tumor size could be monitored by measuring luminescence. This model should permit rapid, non-invasive imaging of i.c. tumor growth to evaluate novel therapeutic modalities [118]. Finally, F98 cells also are capable of growing as i.c. xenografts in cats [119], but since these cells can evoke a xenoimmune response, this model is of limited usefulness. It is important to note that, what may be therapeutically effective in the rat, may not be in the human. However, it probably is safe to say that if a particular therapeutic approach is ineffective in a rat model, it is even more unlikely to be so in humans.
CNS-1 glioma
The CNS-1 glioma was derived from an inbred Lewis rat that had received weekly i.v. injections of MNU for 6 months [120]. Following i.c. implantation into Lewis rats, it demonstrated an infiltrative pattern of growth with leptomeningeal, perivascular, and periventricular spread and extension of the tumor into the choroid plexus [120]. Histologically, these tumors exhibited hypercellularity, nuclear atypia and pleomorphism, and had necrotic foci. These were surrounded by glioma cells arranged in a pseudopalisading pattern (Fig. 1e), although to a lesser extent than that seen in human GBM [6]. Like human GBMs, these tumors also were infiltrated with macrophages and T-cells, but did not have extensive glomeruloid endothelial/microvascular proliferation [6]. Kielian et al. identified the constitutive expression of monocyte chemotactic factor 1 (MCP-1) by CNS-1 cells [121]. In vivo, CNS-1 tumors also showed extensive infiltration by macrophages, which might confer a growth advantage [122]. This model has been useful to study glioma invasion [123], changes in the biology of glioma cells and their extracellular matrix [124–126], and gene therapy [127]. It also has been used to study the efficacy of immunotherapy as a potential treatment for human GBM [128] although its immunogenicity has not been studied in great detail.
BT4C glioma
The BT4C glioma was derived by giving a single transplacental administration of N-ethyl-N-nitrosourea (ENU) to pregnant BD IX rats. Dissociated brain tumor cells from one of these animals were propagated in vitro and after 200 days in culture they became tumorigenic [129]. The cells subsequently were implanted s.c. into BD IX rats and the resulting tumors contained a mixture of multipolar glia-like cells and flattened cells with fewer and shorter cytoplasmic processes and occasional giant cells [130]. BT4C glioma-derived tumors show high cellularity and have pleomorphic nuclei and numerous mitotic figures and the tumor blood vessels are irregular, dilated and show areas of proliferation (Fig. 1f) [131]. At the molecular level, BT4C cells express VEGF, tPA, uPA and MVD in the periphery of the growing tumor and are S100 positive by immunohistochemistry (M. Johansson, Personal communication). This model has been useful to test novel chemotherapeutic targeting strategies [132], antitumor effects of gene therapy [133], anti-angiogenic agents alone [134] and in combination with radiation and temozolomide [135]. BT4C gliomas also have been used to investigate the impact of hyperoxia on tumor bearing rats. This resulted in slower growth accompanied by increased apoptosis of tumor cells and reduced microvessel density (MVD). Apart from studies to evaluate therapeutic efficacy, the BT4C glioma model also has been used to study the molecular and biological changes induced by chemotherapy [136, 137], radiation therapy [138] and suicide gene therapy [139]. BT4C cells, stably transfected with cDNA encoding β-galactosidase, have been used to evaluate the migration of single migrating tumor cell glioma spheroids and fetal brain aggregate coculture systems in vitro and in rat brains in vivo [140, 141].
Avian sarcoma virus induced and RT-2 glioma
The induction of experimental brain tumors by the injection of Rous sarcoma virus has been described in canines, rats, and monkeys [5]. Tumors were induced by inoculating neonatal Fischer rats i.c. with purified avian sarcoma virus (ASV) suspensions [142]. All of the animals developed tumors within 2 weeks following ASV injection, 94% of which were anaplastic astrocytomas, and the remainder were low grade gliomas or sarcomas [143]. This model has been used to study the effects of chemo- and radiotherapy, BBB disruption, and tumor permeability [5]. The response to immunotherapy indicated that these tumors were immunogenic, and expressed a variety of virally encoded tumor specific antigens. A continuous cell line, designated “RT-2”, was derived from an ASV-induced Fischer rat tumor, and this has been used to study tumor growth [143], photochemotherapy [144], cytotoxic gene therapy [145] and radio-sensitization [146]. The RT2 tumor appears to be immunogenic, as evidenced by its ability to evoke a CD8 + T cell-mediated anti-tumor immune response [147], and this must be taken into account if it is used for immunotherapy studies. RT-2 cells expressing GFP have been used for quantitative assessment of glioma invasion in the rat brain [148]. The RT-2 glioma model also has been used to evaluate the therapeutic efficacy of oncolytic adenoviruses. Although they can be efficiently infected they do not permit efficient replication of E1-attenuated adenoviruses [63]. These cells also have been transfected with cDNA encoding heat shock protein 72 (HSP72), which was thought to be necessary for replication of E1 deleted adenoviruses [63]. These transfectants have been found to be permissive for replication of E1-deleted, conditionally replication-competent adenoviruses [63]. The inherent immunogenicity of the RT-2 glioma may limit its usefulness for survival studies, but nevertheless it still may be a useful model for other types of studies.
Concluding comments
Rat brain tumor models have provided a wealth of information on the biology, biochemistry, imaging and experimental therapeutics of brain tumors in experimental neuro-oncology, and there is every reason to believe that they will continue to do so. However, it is essential to recognize the limitations of each of the models that have been described, and depending on the nature of the study to be conducted, it is important that the appropriate model be selected. It now has become clear that immunogenic tumors such as the C6, 9L and T9 are not good choices for studies in immunocompetent rats, if the endpoint is prolongation of survival time or cure of the tumor. Destruction of tumor cells in these models, which have tumor infiltrating host immune effector cells within the tumor, can lead to significant amplification of an antitumor response. This may be the single most important in vivo contributor to the bystander effect that has been observed with gene therapy of the C6 and 9L gliomas following transfection with the HSV-tK gene and the lack of such immune amplification with the weakly immunogenic RG2 glioma. Anti-tumor immune response following transfection with suicide genes such as HSV-tK initially was unanticipated, but it is an important effect associated with both gene therapy and boron neutron capture therapy, but not with conventional chemo- and radiotherapy of the 9L gliosarcoma. Since human high grade brain tumors generally are regarded as being either non- or weakly immunogenic, therapeutic exploitation of this using modalities that spare tumor infiltrating host immune effector cells could have important therapeutic implications. Undoubtedly other rat brain tumor models will be developed, especially cell lines derived from genetically engineered rats that will expand the types of studies that can be carried out in this very important laboratory animal.
Table 2.
Comparison of various rat brain tumor models currently in use
| Tumor | Strain of origin/Haplotype* | Mode of tumor induction† | Minimum i.c. inoculum | Immunogenicity | Pattern of growth | Molecular markers | Original reference |
|---|---|---|---|---|---|---|---|
| C6** | Outbred Wistar | MNU | 104 | Strong | Circumscribed | Deletion p16/CDkn2a/Ink4a; no expression of p16 and p19ARF mRNAs or of wildtype p53; increased expression of PDGFβ, IGF-1, EGFR and ErbB3/HER3 precursor proteins, decreased expression of EGF-9 and 10 and IGF-II genes | [41] |
| 9L | Fischer/RT11v1 | MNU | 104 | Strong | Circumscribed | Mutant p53, increased expression of TGFα and EGFR; decreased expression of FGF-2, FGF-9, FGFR-1 and PDGFRβ | [41] |
| T9 | Fischer/RT11v1 | MNU | 104 | Strong | Circumscribed | Presumably similar to 9L | [41] |
| RG2 (D74) | Fischer/RT11v1 | ENU | 101–102 | Weak | Infiltrative | Deletion of p16/Cdkn2a/Ink4a gene; increased expression of PDGFβ, IGF-1, Ras, and Erb3/HER3 precursor mRNA and cyclin D2 | [83] |
| F98 | Fischer/RT11v1 | ENU | 101–102 | Weak | Infiltrative | Deletion of p16/Cdkn2a/Ink4a gene; increased expression of PDGFβ, RbRas, EGFR, cyclin D1 and cyclin D2 | [83] |
| CNS-1 | Lewis/RT11 | MNU | 5 × 103 | Weak | Infiltrative | Expression of vimentin | [120] |
| BT4C | BD IX/RT1dv1 | ENU | 104 | Weak | Infiltrative | Expression of VEGF, tPA, uPA and MVD | [130] |
The haplotype information was kindly provided by Dr. Carol Kruse, Sidney Kimmel Cancer Center, San Diego, CA
C6 cells express RT1u, a haplotype of inbred Wistar-Furth rats [149]
Abbreviations: MNU: methylnitrosourea; ENU: N-ethyl-N-nitrosourea
Contributor Information
R. F. Barth, Department of Pathology, The Ohio State University, 165 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210, USA, e-mail: rolf.barth@osumc.edu
B. Kaur, Department of Neurological Surgery, Dardinger Laboratory for Neuro-Oncology and Neurosciences, The Ohio State University, 385-D OSUCCC, 410 West 12th Avenue, OSUCCC, Columbus, OH 43210, USA, e-mail: balveen.kaur@osumc.edu
References
- 1.Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–5297. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- 2.Lampson LA. New animal models to probe brain tumor biology, therapy, and immunotherapy: advantages and remaining concerns. J Neurooncol. 2001;53:275–287. doi: 10.1023/A:1012230113527. [DOI] [PubMed] [Google Scholar]
- 3.Kimmelman J, Nalbantoglu J. Faithful companions: a proposal for neurooncology trials in pet dogs. Cancer Res. 2007;67:4541–4544. doi: 10.1158/0008-5472.CAN-06-3792. [DOI] [PubMed] [Google Scholar]
- 4.Krushelnycky BW, Farr-Jones MA, Mielke B, et al. Development of a large-animal human brain tumor xenograft model in immunosuppressed cats. Cancer Res. 1991;51:2430–2437. [PubMed] [Google Scholar]
- 5.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/A:1005805203044. [DOI] [PubMed] [Google Scholar]
- 6.Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuro-pathological characterization and tumor progression. J Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reilly KM, Rubin JB, Gilbertson RJ, et al. Rethinking brain tumors: the fourth mouse models of human cancers consortium nervous system tumors workshop. Cancer Res. 2008;68:5508–5511. doi: 10.1158/0008-5472.CAN-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker M, Hoshino T, Gurcay O, et al. Development of an animal brain tumor model and its response to therapy with 1, 3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1973;33:976–986. [PubMed] [Google Scholar]
- 9.Kobayashi N, Allen N, Clendenon NR, et al. An improved rat brain-tumor model. J Neurosurg. 1980;53:808–815. doi: 10.3171/jns.1980.53.6.0808. [DOI] [PubMed] [Google Scholar]
- 10.Landen JW, Hau V, Wang M, et al. Noscapine crosses the blood-brain barrier and inhibits glioblastoma growth. Clin Cancer Res. 2004;10:5187–5201. doi: 10.1158/1078-0432.CCR-04-0360. [DOI] [PubMed] [Google Scholar]
- 11.Lal S, Lacroix M, Tofilon P, et al. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92:326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- 12.Saini M, Roser F, Samii M, et al. A model for intra-tumoural chemotherapy in the rat brain. Acta Neurochir (Wien) 2004;146:731–734. doi: 10.1007/s00701-004-0261-0. [DOI] [PubMed] [Google Scholar]
- 13.Benda P, Lightbody J, Sato G, et al. Differentiated rat glial cell strain in tissue culture. Science. 1968;161:370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- 14.Schmidek HH, Nielsen SL, Schiller AL, et al. Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg. 1971;34:335–340. doi: 10.3171/jns.1971.34.3.0335. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer SE, Herschman HR, Lightbody J, et al. Synthesis by a clonal line of rat glial cells of a protein unique to the nervous system. J Cell Physiol. 1970;75:329–339. doi: 10.1002/jcp. 1040750309. [DOI] [PubMed] [Google Scholar]
- 16.Schlegel J, Piontek G, Kersting M, et al. The p16/Cdkn2a/Ink4a gene is frequently deleted in nitrosourea-induced rat glial tumors. Pathobiology. 1999;67:202–206. doi: 10.1159/000028073. [DOI] [PubMed] [Google Scholar]
- 17.Asai A, Miyagi Y, Sugiyama A, et al. Negative effects of wild-type p53 and s-myc on cellular growth and tumorigenicity of glioma cells. Implication of the tumor suppressor genes for gene therapy. J Neurooncol. 1994;19:259–268. doi: 10.1007/BF01053280. [DOI] [PubMed] [Google Scholar]
- 18.Sibenaller ZA, Etame AB, Ali MM, et al. Genetic characterization of commonly used glioma cell lines in the rat animal model system. Neurosurg Focus. 2005;19:E1. doi: 10.3171/foc. 2005.19.4.2. [DOI] [PubMed] [Google Scholar]
- 19.Guo P, Hu B, Gu W, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimberger AB, Suki D, Yang D, et al. The natural history of EGFR and EGFRvIII in glioblastoma patients. J Transl Med. 2005;3:38. doi: 10.1186/1479-5876-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morford LA, Boghaert ER, Brooks WH, et al. Insulin-like growth factors (IGF) enhance three-dimensional (3D) growth of human glioblastomas. Cancer Lett. 1997;115:81–90. doi: 10.1016/S0304-3835(97)04717-4. [DOI] [PubMed] [Google Scholar]
- 22.Assanah M, Lochhead R, Ogden A, et al. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakada M, Niska JA, Tran NL, et al. EphB2/R-ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 2005;167:565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lampson LA, Lampson MA, Dunne AD. Exploiting the lacZ reporter gene for quantitative analysis of disseminated tumor growth within the brain: use of the lacZ gene product as a tumor antigen, for evaluation of antigenic modulation, and to facilitate image analysis of tumor growth in situ. Cancer Res. 1993;53:176–182. [PubMed] [Google Scholar]
- 25.Doblas S, Saunders D, Kshirsagar P, et al. Phenyl-tert-butylnitrone induces tumor regression and decreases angiogenesis in a C6 rat glioma model. Free Radic Biol Med. 2008;44:63–72. doi: 10.1016/j.freeradbiomed.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Solly F, Fish R, Simard B, et al. Tissue-type plasminogen activator has antiangiogenic properties without effect on tumor growth in a rat C6 glioma model. Cancer Gene Ther. 2008;15:685–692. doi: 10.1038/cgt.2008.36. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed AE, Jacob S, Nagy AA, et al. Dibromoacetonitrile-induced protein oxidation and inhibition of proteasomal activity in rat glioma cells. Toxicol Lett. 2008;179:29–33. doi: 10.1016/j.toxlet.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Zhao S, Zhang X, Zhang J, et al. Intravenous administration of arsenic trioxide encapsulated in liposomes inhibits the growth of C6 gliomas in rat brains. J Chemother. 2008;20:253–262. doi: 10.1179/joc.2008.20.2.253. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan J, Ionescu A, Pouratian N, et al. Use of trans sodium crocetinate for sensitizing glioblastoma multiforme to radiation: laboratory investigation. J Neurosurg. 2008;108:972–978. doi: 10.3171/JNS/2008/108/5/0972. [DOI] [PubMed] [Google Scholar]
- 30.Mannino S, Molinari A, Sabatino G, et al. Intratumoral vs systemic administration of meta-tetrahydroxyphenylchlorin for photodynamic therapy of malignant gliomas: assessment of uptake and spatial distribution in C6 rat glioma model. Int J Immunopathol Pharmacol. 2008;21:227–231. doi: 10.1177/039463200802100126. [DOI] [PubMed] [Google Scholar]
- 31.Yang WQ, Lun X, Palmer CA, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004;10:8561–8576. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 32.Tanriover N, Ulu MO, Sanus GZ, et al. The effects of systemic and intratumoral interleukin-12 treatment in C6 rat glioma model. Neurol Res. 2008;30:511–517. doi: 10.1179/174313208X289516. [DOI] [PubMed] [Google Scholar]
- 33.Parsa AT, Chakrabarti I, Hurley PT, et al. Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery. 2000;47:993–999. doi: 10.1097/00006123-200010000-00050. discussion 999–1000. [DOI] [PubMed] [Google Scholar]
- 34.Trojan J, Johnson TR, Rudin SD, et al. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993;259:94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- 35.Johnson TR, Trojan J, Rudin SD, Ilan J, Tykocinski ML. Evoking an immune response to glioblastoma cells transfected with episome-based plasmid expressing insulin-like growth factor I. In: Levine AJ, Schmidek HH, editors. Molecular genetics of nervous system tumors. Wiley-Liss Inc.; New York: 1993. pp. 387–400. [Google Scholar]
- 36.Karmakar S, Olive MF, Banik NL, et al. Intracranial stereotaxic cannulation for development of orthotopic glioblastoma allograft in Sprague-Dawley rats and histoimmunopathological characterization of the brain tumor. Neurochem Res. 2007;32:2235–2242. doi: 10.1007/s11064-007-9450-6. [DOI] [PubMed] [Google Scholar]
- 37.Assadian S, Aliaga A, Del Maestro RF, et al. FDG-PET imaging for the evaluation of antiglioma agents in a rat model. Neuro-oncol. 2008;10:292–299. doi: 10.1215/15228517-2008-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valable S, Lemasson B, Farion R, et al. Assessment of blood volume, vessel size, and the expression of angiogenic factors in two rat glioma models: a longitudinal in vivo and ex vivo study. NMR Biomed. 2008;21:1043–1056. doi: 10.1002/nbm. 1278. [DOI] [PubMed] [Google Scholar]
- 39.Valable S, Barbier EL, Bernaudin M, et al. In vivo MRI tracking of exogenous monocytes/macrophages targeting brain tumors in a rat model of glioma. Neuroimage. 2008;40:973–983. doi: 10.1016/j.neuroimage.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Shen G, Shen F, Shi Z, et al. Identification of cancer stem-like cells in the C6 glioma cell line and the limitation of current identification methods. In Vitro Cell Dev Biol Anim. 2008;44:280–289. doi: 10.1007/s11626-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 41.Benda P, Someda K, Messer J, et al. Morphological and immunochemical studies of rat glial tumors and clonal strains propagated in culture. J Neurosurg. 1971;34:310–323. doi: 10.3171/jns.1971.34.3.0310. [DOI] [PubMed] [Google Scholar]
- 42.Ghods AJ, Irvin D, Liu G, et al. Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells. 2007;25:1645–1653. doi: 10.1634/stemcells.2006-0624. [DOI] [PubMed] [Google Scholar]
- 43.Barcellos-Hoff MH, Linfoot PA, Marton LJ, et al. Production of stable phenotypes from 9L rat brain tumor multicellular spheroids treated with 1, 3-bis(2-chloroethyl)-1-nitrosourea. Int J Cancer. 1992;52:409–413. doi: 10.1002/ijc.2910520314. [DOI] [PubMed] [Google Scholar]
- 44.Schepkin VD, Lee KC, Kuszpit K, et al. Proton and sodium MRI assessment of emerging tumor chemotherapeutic resistance. NMR Biomed. 2006;19:1035–1042. doi: 10.1002/nbm.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Black KL, Yin D, Konda BM, et al. Different effects of KCa and KATP agonists on brain tumor permeability between syngeneic and allogeneic rat models. Brain Res. 2008;1227:198–206. doi: 10.1016/j.brainres.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fross RD, Warnke PC, Groothuis DR. Blood flow and blood-to-tissue transport in 9L gliosarcomas: the role of the brain tumor model in drug delivery research. J Neurooncol. 1991;11:185–197. doi: 10.1007/BF00165526. [DOI] [PubMed] [Google Scholar]
- 47.Khan A, Jallo GI, Liu YJ, et al. Infusion rates and drug distribution in brain tumor models in rats. J Neurosurg. 2005;102:53–58. doi: 10.3171/ped.2005.102.1.0053. [DOI] [PubMed] [Google Scholar]
- 48.Warnke PC, Blasberg RG, Groothuis DR. The effect of hyperosmotic blood-brain barrier disruption on blood-to-tissue transport in ENU-induced gliomas. Ann Neurol. 1987;22:300–305. doi: 10.1002/ana.410220304. [DOI] [PubMed] [Google Scholar]
- 49.Bansal A, Shuyan W, Hara T, et al. Biodisposition and metabolism of [(18)F]fluorocholine in 9L glioma cells and 9L glioma-bearing Fisher rats. Eur J Nucl Med Mol Imaging. 2008;35:1192–1203. doi: 10.1007/s00259-008-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan H, Schroeder T, Bowsher JE, et al. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) J Nucl Med. 2006;47:989–998. [PubMed] [Google Scholar]
- 51.Wolff JE, Molenkamp G, Hotfilder M, et al. Dexamethasone inhibits glioma-induced formation of capillary like structures in vitro and angiogenesis in vivo. Klin Padiatr. 1997;209:275–277. doi: 10.1055/s-2008-1043962. [DOI] [PubMed] [Google Scholar]
- 52.Yang H, Chopp M, Zhang X, et al. Using behavioral measurement to assess tumor progression and functional outcome after antiangiogenic treatment in mouse glioma models. Behav Brain Res. 2007;182:42–50. doi: 10.1016/j.bbr.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 53.Regnard P, Le Duc G, Brauer-Krisch E, et al. Irradiation of intracerebral 9L gliosarcoma by a single array of microplanar x-ray beams from a synchrotron: balance between curing and sparing. Phys Med Biol. 2008;53:861–878. doi: 10.1088/0031-9155/53/4/003. [DOI] [PubMed] [Google Scholar]
- 54.Bencokova Z, Pauron L, Devic C, et al. Molecular and cellular response of the most extensively used rodent glioma models to radiation and/or cisplatin. J Neurooncol. 2008;86:13–21. doi: 10.1007/s11060-007-9433-0. [DOI] [PubMed] [Google Scholar]
- 55.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 56.Barba D, Hardin J, Ray J, et al. Thymidine kinase-mediated killing of rat brain tumors. J Neurosurg. 1993;79:729–735. doi: 10.3171/jns.1993.79.5.0729. [DOI] [PubMed] [Google Scholar]
- 57.Iwadate Y, Inoue M, Saegusa T, et al. Recombinant Sendai virus vector induces complete remission of established brain tumors through efficient interleukin-2 gene transfer in vaccinated rats. Clin Cancer Res. 2005;11:3821–3827. doi: 10.1158/1078-0432.CCR-04-1485. [DOI] [PubMed] [Google Scholar]
- 58.Kumar S, Brown SL, Kolozsvary A, et al. Efficacy of suicide gene therapy in hypoxic rat 9L glioma cells. J Neurooncol. 2008;90:19–24. doi: 10.1007/s11060-008-9635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miletic H, Fischer Y, Litwak S, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- 60.Chignola R, Foroni R, Franceschi A, et al. Heterogeneous response of individual multicellular tumour spheroids to immunotoxins and ricin toxin. Br J Cancer. 1995;72:607–614. doi: 10.1038/bjc.1995.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Wang Q, Kleinschmidt-DeMasters BK, et al. TGF-beta2 inhibition augments the effect of tumor vaccine and improves the survival of animals with pre-established brain tumors. J Neurooncol. 2007;81:149–162. doi: 10.1007/s11060-006-9222-1. [DOI] [PubMed] [Google Scholar]
- 62.Aghi M, Chou TC, Suling K, et al. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 1999;59:3861–3865. [PubMed] [Google Scholar]
- 63.Madara J, Krewet JA, Shah M. Heat shock protein 72 expression allows permissive replication of oncolytic adenovirus dl1520 (ONYX-015) in rat glioblastoma cells. Mol Cancer. 2005;4:12. doi: 10.1186/1476-4598-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blume MR, Wilson CP, Vasquez DA. Immune response to a transplantable intracerebral glioma in rats. In: Sane K, Ishi S, Le Vey D, editors. Recent progress in neurological surgery. Excerpta Medica; Amsterdam: 1974. pp. 129–134. [Google Scholar]
- 65.Denlinger RH, Axler DA, Koestner A, et al. Tumor-specific transplantation immunity to intracerebral challenge with cells from a methylnitrosourea-induced brain tumor. J Med. 1975;6:249–259. [PubMed] [Google Scholar]
- 66.Morantz RA, Wood GW, Foster M, et al. Macrophages in experimental and human brain tumors. Part 1: Studies of the macrophage content of experimental rat brain tumors of varying immunogenicity. J Neurosurg. 1979;50:298–304. doi: 10.3171/jns.1979.50.3.0298. [DOI] [PubMed] [Google Scholar]
- 67.Chen CY, Chang YN, Ryan P, et al. Effect of herpes simplex virus thymidine kinase expression levels on ganciclovir-mediated cytotoxicity and the “bystander effect”. Hum Gene Ther. 1995;6:1467–1476. doi: 10.1089/hum.1995.6.11-1467. [DOI] [PubMed] [Google Scholar]
- 68.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 69.Coderre J, Rubin P, Freedman A, et al. Selective ablation of rat brain tumors by boron neutron capture therapy. Int J Radiat Oncol Biol Phys. 1994;28:1067–1077. doi: 10.1016/0360-3016(94)90480-4. [DOI] [PubMed] [Google Scholar]
- 70.Moriuchi S, Wolfe D, Tamura M, et al. Double suicide gene therapy using a replication defective herpes simplex virus vector reveals reciprocal interference in a malignant glioma model. Gene Ther. 2002;9:584–591. doi: 10.1038/sj.gt.3301693. [DOI] [PubMed] [Google Scholar]
- 71.Namba H, Tagawa M, Miyagawa T, et al. Treatment of rat experimental brain tumors by herpes simplex virus thymidine kinase gene-transduced allogeneic tumor cells and ganciclovir. Cancer Gene Ther. 2000;7:947–953. doi: 10.1038/sj.cgt.7700172. [DOI] [PubMed] [Google Scholar]
- 72.Smilowitz HM, Micca PL, Nawrocky MM, et al. The combination of boron neutron-capture therapy and immunoprophylaxis for advanced intracerebral gliosarcomas in rats. J Neurooncol. 2000;46:231–240. doi: 10.1023/A:1006409721365. [DOI] [PubMed] [Google Scholar]
- 73.Stojiljkovic M, Piperski V, Dacevic M, et al. Characterization of 9L glioma model of the Wistar rat. J Neurooncol. 2003;63:1–7. doi: 10.1023/A:1023732619651. [DOI] [PubMed] [Google Scholar]
- 74.Bhattacharya P, Chekmenev EY, Perman WH, et al. Towards hyperpolarized (13)C-succinate imaging of brain cancer. J Magn Reson. 2007;186:150–155. doi: 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jallo GI, Volkov A, Wong C, et al. A novel brainstem tumor model: functional and histopathological characterization. Childs Nerv Syst. 2006;22:1519–1525. doi: 10.1007/s00381-006-0174-8. [DOI] [PubMed] [Google Scholar]
- 76.Saito R, Bringas J, Mirek H, et al. Invasive phenotype observed in 1, 3-bis(2-chloroethyl)-1-nitrosourea-resistant sub-lines of 9L rat glioma cells: a tumor model mimicking a recurrent malignant glioma. J Neurosurg. 2004;101:826–831. doi: 10.3171/jns.2004.101.5.0826. [DOI] [PubMed] [Google Scholar]
- 77.Schepkin VD, Lee KC, Kuszpit K, et al. Proton and sodium MRI assessment of emerging tumor chemotherapeutic resistance. NMR Biomed. 2006;19:1035–1042. doi: 10.1002/nbm.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kida Y, Cravioto H, Hochwald GM, et al. Immunity to transplantable nitrosourea-induced neurogenic tumors. II. Immunoprophylaxis of tumors of the brain. J Neuropathol Exp Neurol. 1983;42:122–135. doi: 10.1097/00005072-198303000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Jeffes EW, Zhang JG, Hoa N, et al. Antiangiogenic drugs synergize with a membrane macrophage colony-stimulating factor-based tumor vaccine to therapeutically treat rats with an established malignant intracranial glioma. J Immunol. 2005;174:2533–2543. doi: 10.4049/jimmunol.174.5.2533. [DOI] [PubMed] [Google Scholar]
- 80.Pietronigro D, Drnovsky F, Cravioto H, et al. DTI-015 produces cures in T9 gliosarcoma. Neoplasia. 2003;5:17–22. doi: 10.1016/s1476-5586(03)80013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shibuya N, Hochgeschwender U, Kida Y, et al. Immunity to transplantable nitrosourea-induced neurogenic tumors. III. Systemic adoptive transfer of immunity. J Neuropathol Exp Neurol. 1984;43:426–438. doi: 10.1097/00005072-198407000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Harada K, Yoshida J, Mizuno M, et al. Growth inhibition of intracerebral rat glioma by transfection-induced human interferon-beta. J Surg Oncol. 1995;59:105–109. doi: 10.1002/jso.2930590207. [DOI] [PubMed] [Google Scholar]
- 83.Ko L, Koestner A, Wechsler W. Morphological characterization of nitrosourea-induced glioma cell lines and clones. Acta Neuropathol. 1980;51:23–31. doi: 10.1007/BF00688846. [DOI] [PubMed] [Google Scholar]
- 84.Weizsacker M, Nagamune A, Winkelstroter R, et al. Radiation and drug response of the rat glioma RG2. Eur J Cancer Clin Oncol. 1982;18:891–895. doi: 10.1016/0277-5379(82)90200-0. [DOI] [PubMed] [Google Scholar]
- 85.Ferrier MC, Sarin H, Fung SH, et al. Validation of dynamic contrast-enhanced magnetic resonance imaging-derived vascular permeability measurements using quantitative autoradiography in the RG2 rat brain tumor model. Neoplasia. 2007;9:546–555. doi: 10.1593/neo.07289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ningaraj NS, Rao M, Hashizume K, et al. Regulation of blood-brain tumor barrier permeability by calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;301:838–851. doi: 10.1124/jpet.301.3.838. [DOI] [PubMed] [Google Scholar]
- 87.Hashizume K, Black KL. Increased endothelial vesicular transport correlates with increased blood-tumor barrier permeability induced by bradykinin and leukotriene C4. J Neuropathol Exp Neurol. 2002;61:725–735. doi: 10.1093/jnen/61.8.725. [DOI] [PubMed] [Google Scholar]
- 88.Zagorac D, Jakovcevic D, Gebremedhin D, et al. Anti-angiogenic effect of inhibitors of cytochrome P450 on rats with glioblastoma multiforme. J Cereb Blood Flow Metab. 2008;28:1431–1439. doi: 10.1038/jcbfm.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang W, Tai CK, Kershaw AD, et al. Use of replication-competent retroviral vectors in an immunocompetent intracranial glioma model. Neurosurg Focus. 2006;20:E25. doi: 10.3171/foc. 2006.20.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miknyoczki S, Chang H, Grobelny J, et al. The selective poly(ADP-ribose) polymerase-1(2) inhibitor, CEP-8983, increases the sensitivity of chemoresistant tumor cells to temozolomide and irinotecan but does not potentiate myelotoxicity. Mol Cancer Ther. 2007;6:2290–2302. doi: 10.1158/1535-7163. MCT-07-0062. [DOI] [PubMed] [Google Scholar]
- 91.Tsai NM, Lin SZ, Lee CC, et al. The antitumor effects of angelica sinensis on malignant brain tumors in vitro and in vivo. Clin Cancer Res. 2005;11:3475–3484. doi: 10.1158/1078-0432. CCR-04-1827. [DOI] [PubMed] [Google Scholar]
- 92.Shen DH, Marsee DK, Schaap J, et al. Effects of dose, intervention time, and radionuclide on sodium iodide symporter (NIS)-targeted radionuclide therapy. Gene Ther. 2004;11:161–169. doi: 10.1038/sj.gt.3302147. [DOI] [PubMed] [Google Scholar]
- 93.Oshiro S, Liu Y, Fukushima T, et al. Modified immunoregulation associated with interferon-gamma treatment of rat glioma. Neurol Res. 2001;23:359–366. doi: 10.1179/016164101101198569. [DOI] [PubMed] [Google Scholar]
- 94.Kurozumi K, Hardcastle J, Thakur R, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99:1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 95.Wakimoto H, Fulci G, Tyminski E, et al. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide’s enhancement of viral oncolysis. Gene Ther. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathieu D, Lecomte R, Tsanaclis AM, et al. Standardization and detailed characterization of the syngeneic Fischer/F98 glioma model. Can J Neurol Sci. 2007;34:296–306. doi: 10.1017/s0317167100006715. [DOI] [PubMed] [Google Scholar]
- 97.von Eckardstein KL, Patt S, Kratzel C, et al. Local chemotherapy of F98 rat glioblastoma with paclitaxel and carboplatin embedded in liquid crystalline cubic phases. J Neurooncol. 2005;72:209–215. doi: 10.1007/s11060-004-3010-6. [DOI] [PubMed] [Google Scholar]
- 98.Biston MC, Joubert A, Adam JF, et al. Cure of Fischer rats bearing radioresistant F98 glioma treated with cis-platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res. 2004;64:2317–2323. doi: 10.1158/0008-5472.CAN-03-3600. [DOI] [PubMed] [Google Scholar]
- 99.Rousseau J, Boudou C, Barth RF, et al. Enhanced survival and cure of F98 glioma-bearing rats following intracerebral delivery of carboplatin in combination with photon irradiation. Clin Cancer Res. 2007;13:5195–5201. doi: 10.1158/1078-0432.CCR-07-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rousseau R, Barth RF, Moeschberger ML, et al. Efficacy of intracerebral delivery of carboplatin in combination with photon irradiation for treatment of F98 glioma bearing rats. Int J Radiat Oncol Biol Phys. 2008;73:530–536. doi: 10.1016/j.ijrobp.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 101.Barth RF, Yang W, Coderre JA. Rat brain tumor models to assess the efficacy of boron neutron capture therapy: a critical evaluation. J Neurooncol. 2003;62:61–74. doi: 10.1007/BF02699934. [DOI] [PubMed] [Google Scholar]
- 102.Yang W, Wu G, Barth RF, et al. Molecular targeting and treatment of composite EGFR and EGFRvIII-positive gliomas using boronated monoclonal antibodies. Clin Cancer Res. 2008;14:883–891. doi: 10.1158/1078-0432.CCR-07-1968. [DOI] [PubMed] [Google Scholar]
- 103.Cho JY, Shen DH, Yang W, et al. In vivo imaging and radioiodine therapy following sodium iodide symporter gene transfer in animal model of intracerebral gliomas. Gene Ther. 2002;9:1139–1145. doi: 10.1038/sj.gt.3301787. [DOI] [PubMed] [Google Scholar]
- 104.Adam JF, Joubert A, Biston MC, et al. Prolonged survival of Fischer rats bearing F98 glioma after iodine-enhanced synchrotron stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:603–611. doi: 10.1016/j.ijrobp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Blanchard J, Mathieu D, Patenaude Y, et al. MR-pathological comparison in F98-Fischer glioma model using a human gantry. Can J Neurol Sci. 2006;33:86–91. doi: 10.1017/s0317167100004753. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, van Zijl PC, Laterra J, et al. Unique patterns of diffusion directionality in rat brain tumors revealed by high-resolution diffusion tensor MRI. Magn Reson Med. 2007;58:454–462. doi: 10.1002/mrm.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang D, Feng XY, Henning TD, et al. MR imaging of tumor angiogenesis using sterically stabilized gd-DTPA liposomes targeted to CD105. Eur J Radiol. 2008 doi: 10.1016/j.ejrad. 2008.04.022. [DOI] [PubMed] [Google Scholar]
- 108.Wu X, Hu J, Zhou L, et al. In vivo tracking of super-paramagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108:320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 109.Tzeng JJ, Barth RF, Orosz CG, et al. Phenotype and functional activity of tumor-infiltrating lymphocytes isolated from immunogenic and nonimmunogenic rat brain tumors. Cancer Res. 1991;51:2373–2378. [PubMed] [Google Scholar]
- 110.Paul DB, Barth RF, Yang W, et al. B7.1 expression by the weakly immunogenic F98 rat glioma does not enhance immunogenicity. Gene Ther. 2000;7:993–999. doi: 10.1038/sj.gt.3301209. [DOI] [PubMed] [Google Scholar]
- 111.Clavreul A, Delhaye M, Jadaud E, et al. Effects of syngeneic cellular vaccinations alone or in combination with GM- CSF on the weakly immunogenic F98 glioma model. J Neurooncol. 2006;79:9–17. doi: 10.1007/s11060-005-9115-8. [DOI] [PubMed] [Google Scholar]
- 112.Hanissian SH, Teng B, Akbar U, et al. Regulation of myeloid leukemia factor-1 interacting protein (MLF1IP) expression in glioblastoma. Brain Res. 2005;1047:56–64. doi: 10.1016/j.brainres.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 113.von Eckardstein KL, Patt S, Zhu J, et al. Short-term neuropathological aspects of in vivo suicide gene transfer to the F98 rat glioblastoma using liposomal and viral vectors. Histol Histopathol. 2001;16:735–744. doi: 10.14670/HH-16.735. [DOI] [PubMed] [Google Scholar]
- 114.Fenstermaker RA, Capala J, Barth RF, et al. The effect of epidermal growth factor receptor (EGFR) expression on in vivo growth of rat C6 glioma cells. Leukemia. 1995;9(Suppl 1):S106–S112. [PubMed] [Google Scholar]
- 115.Yang W, Barth RF, Wu G, et al. Development of a syngeneic rat brain tumor model expressing EGFRvIII and its use for molecular targeting studies with monoclonal antibody L8A4. Clin Cancer Res. 2005;11:341–350. [PubMed] [Google Scholar]
- 116.Wu G, Yang W, Barth RF, et al. Molecular targeting and treatment of an epidermal growth factor receptor-positive glioma using boronated cetuximab. Clin Cancer Res. 2007;13:1260–1268. doi: 10.1158/1078-0432.CCR-06-2399. [DOI] [PubMed] [Google Scholar]
- 117.Wu G, Barth RF, Yang W, et al. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol Cancer Ther. 2006;5:52–59. doi: 10.1158/1535-7163. MCT-05-0325. [DOI] [PubMed] [Google Scholar]
- 118.Bryant MJ, Chuah TL, Luff J, et al. A novel rat model for glioblastoma multiforme using a bioluminescent F98 cell line. J Clin Neurosci. 2008;15:545–551. doi: 10.1016/j.jocn.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 119.Ernestus RI, Wilmes LJ, Hoehn-Berlage M. Identification of intracranial liqor metastases of experimental stereotactically implanted brain tumors by the tumor-selective MRI contrast agent MnTPPS. Clin Exp Metastasis. 1992;10:345–350. doi: 10.1007/BF00058174. [DOI] [PubMed] [Google Scholar]
- 120.Kruse CA, Molleston MC, Parks EP, et al. A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J Neurooncol. 1994;22:191–200. doi: 10.1007/BF01052919. [DOI] [PubMed] [Google Scholar]
- 121.Kielian T, van Rooijen N, Hickey WF. MCP-1 expression in CNS-1 astrocytoma cells: implications for macrophage infiltration into tumors in vivo. J Neurooncol. 2002;56:1–12. doi: 10.1023/A:1014495613455. [DOI] [PubMed] [Google Scholar]
- 122.Platten M, Kretz A, Naumann U, et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54:388–392. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 123.Owens GC, Orr EA, DeMasters BK, et al. Overexpression of a transmembrane isoform of neural cell adhesion molecule alters the invasiveness of rat CNS-1 glioma. Cancer Res. 1998;58:2020–2028. [PubMed] [Google Scholar]
- 124.Lapointe M, Lanthier J, Moumdjian R, et al. Expression and activity of l-isoaspartyl methyltransferase decrease in stage progression of human astrocytic tumors. Brain Res Mol Brain Res. 2005;135:93–103. doi: 10.1016/j.molbrainres.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Matthews RT, Gary SC, Zerillo C, et al. Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem. 2000;275:22695–22703. doi: 10.1074/jbc.M909764199. [DOI] [PubMed] [Google Scholar]
- 126.Nutt CL, Zerillo CA, Kelly GM, et al. Brain enriched hyaluronan binding (BEHAB)/brevican increases aggressiveness of CNS-1 gliomas in lewis rats. Cancer Res. 2001;61:7056–7059. [PubMed] [Google Scholar]
- 127.Biglari A, Bataille D, Naumann U, et al. Effects of ectopic decorin in modulating intracranial glioma progression in vivo, in a rat syngeneic model. Cancer Gene Ther. 2004;11:721–732. doi: 10.1038/sj.cgt.7700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laerum OD, Rajewsky MF. Neoplastic transformation of fetal rat brain cells in culture after exposure to ethylnitrosourea in vivo. J Natl Cancer Inst. 1975;55:1177–1187. doi: 10.1093/jnci/55.5.1177. [DOI] [PubMed] [Google Scholar]
- 130.Laerum OD, Rajewsky MF, Schachner M, et al. Phenotypic properties of neoplastic cell lines developed from fetal rat brain cells in culture after exposure to ethylnitrosourea in vivo. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1977;89:273–295. doi: 10.1007/BF00283783. [DOI] [PubMed] [Google Scholar]
- 131.Stuhr LE, Raa A, Oyan AM, et al. Hyperoxia retards growth and induces apoptosis, changes in vascular density and gene expression in transplanted gliomas in nude rats. J Neurooncol. 2007;85:191–202. doi: 10.1007/s11060-007-9407-2. [DOI] [PubMed] [Google Scholar]
- 132.Pulkkinen M, Pikkarainen J, Wirth T, et al. Three-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin-biotin technology: formulation development and in vitro anticancer activity. Eur J Pharm Biopharm. 2008;70:66–74. doi: 10.1016/j.ejpb.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 133.Raty JK, Airenne KJ, Marttila AT, et al. Enhanced gene delivery by avidin-displaying baculovirus. Mol Ther. 2004;9:282–291. doi: 10.1016/j.ymthe.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 134.Huszthy PC, Brekken C, Pedersen TB, et al. Antitumor efficacy improved by local delivery of species-specific endostatin. J Neurosurg. 2006;104:118–128. doi: 10.3171/jns.2006.104. 1.118. [DOI] [PubMed] [Google Scholar]
- 135.Sandstrom M, Johansson M, Bergstrom P, et al. Effects of the VEGFR inhibitor ZD6474 in combination with radiotherapy and temozolomide in an orthotopic glioma model. J Neurooncol. 2008;88:1–9. doi: 10.1007/s11060-008-9527-3. [DOI] [PubMed] [Google Scholar]
- 136.Vallbo C, Bergenheim T, Hedman H, et al. The antimicrotubule drug estramustine but not irradiation induces apoptosis in malignant glioma involving AKT and caspase pathways. J Neurooncol. 2002;56:143–148. doi: 10.1023/A:1014562503097. [DOI] [PubMed] [Google Scholar]
- 137.Yoshida D, Noha M, Watanabe K, et al. The bleb formation of the extracellular pseudopodia; early evidence of microtubule depolymerization by estramustine phosphate in glioma cell; in vitro study. J Neurooncol. 2001;52:37–47. doi: 10.1023/A:1010653613588. [DOI] [PubMed] [Google Scholar]
- 138.Andersson U, Grankvist K, Bergenheim AT, et al. Rapid induction of long-lasting drug efflux activity in brain vascular endothelial cells but not malignant glioma following irradiation. Med Oncol. 2002;19:1–9. doi: 10.1385/MO:19:1:1. [DOI] [PubMed] [Google Scholar]
- 139.Griffin JL, Lehtimaki KK, Valonen PK, et al. Assignment of 1H nuclear magnetic resonance visible polyunsaturated fatty acids in BT4C gliomas undergoing ganciclovir-thymidine kinase gene therapy-induced programmed cell death. Cancer Res. 2003;63:3195–3201. [PubMed] [Google Scholar]
- 140.Garcia-Cabrera I, Edvardsen K, Tysnes BB, et al. The lac-z reporter gene: a tool for in vitro studies of malignant glioma cell invasion. Invasion Metastasis. 1996;16:107–115. [PubMed] [Google Scholar]
- 141.Pedersen PH, Edvardsen K, Garcia-Cabrera I, et al. Migratory patterns of lac-z transfected human glioma cells in the rat brain. Int J Cancer. 1995;62:767–771. doi: 10.1002/ijc.2910620620. [DOI] [PubMed] [Google Scholar]
- 142.Copeland DD, Talley FA, Bigner DD. The fine structure of intracranial neoplasms induced by the inoculation of avian sarcoma virus in neonatal and adult rats. Am J Pathol. 1976;83:149–176. [PMC free article] [PubMed] [Google Scholar]
- 143.Prabhu SS, Broaddus WC, Oveissi C, et al. Determination of intracranial tumor volumes in a rodent brain using magnetic resonance imaging, Evans blue, and histology: a comparative study. IEEE Trans Biomed Eng. 2000;47:259–265. doi: 10.1109/10.821776. [DOI] [PubMed] [Google Scholar]
- 144.Beckman WC, Jr, Powers SK, Brown JT, et al. Differential retention of rhodamine 123 by avian sarcoma virus-induced glioma and normal brain tissue of the rat in vivo. Cancer. 1987;59:266–270. doi: 10.1002/1097-0142(19870115). 59:2<266:: AID-CNCR2820590215>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 145.Valerie K, Hawkins W, Farnsworth J, et al. Substantially improved in vivo radiosensitization of rat glioma with mutant HSV-TK and acyclovir. Cancer Gene Ther. 2001;8:3–8. doi: 10.1038/sj.cgt.7700265. [DOI] [PubMed] [Google Scholar]
- 146.Valerie K, Brust D, Farnsworth J, et al. Improved radio-sensitization of rat glioma cells with adenovirus-expressed mutant herpes simplex virus-thymidine kinase in combination with acyclovir. Cancer Gene Ther. 2000;7:879–884. doi: 10.1038/sj.cgt.7700185. [DOI] [PubMed] [Google Scholar]
- 147.Shah MR, Ramsey WJ. CD8 + T-cell mediated anti-tumor responses cross-reacting against 9L and RT2 rat glioma cell lines. Cell Immunol. 2003;225:113–121. doi: 10.1016/j.cellimm. 2003.10.004. [DOI] [PubMed] [Google Scholar]
- 148.Mourad PD, Farrell L, Stamps LD, et al. Quantitative assessment of glioblastoma invasion in vivo. Cancer Lett. 2003;192:97–107. doi: 10.1016/S0304-3835(02)00637-7. [DOI] [PubMed] [Google Scholar]
- 149.Beutler AS, Banck MS, Wedekind D, et al. Tumor gene therapy made easy: allogeneic major histocompatibility complex in the C6 rat glioma model. Hum Gene Ther. 1999;10:95–101. doi: 10.1089/10430349950019228. [DOI] [PubMed] [Google Scholar]


