Abstract
Infectious etiologies for certain human cancers have long been suggested by epidemiological studies and studies with animals. Important support for this concept came from the discovery by Harald zur Hausen’s group that human cervical carcinoma almost universally contains certain “high-risk” human papillomavirus (HPV) types. Over the years, much has been learned about the carcinogenic activities of high-risk HPVs. These studies have revealed that two viral proteins, E6 and E7, that are consistently expressed in HPV-associated carcinomas, are necessary for induction and maintenance of the transformed phenotype. Hence, HPV-associated tumors are unique amongst human solid tumors in that they are universally caused by exposure to the same, molecularly defined oncogenic agents, and the molecular signal transduction pathways subverted by these viral transforming agents are frequently disrupted in other, non-virus associated human cancers.
1. Introduction
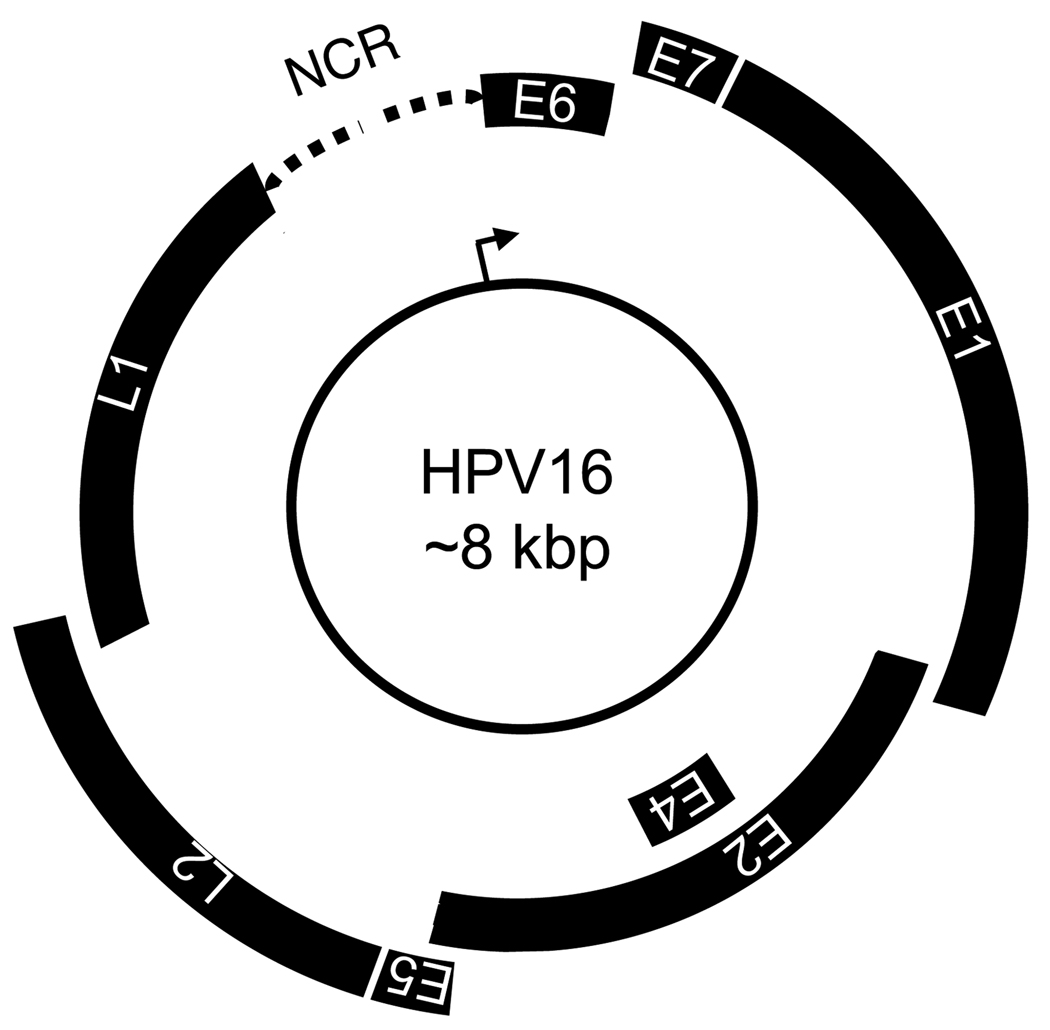
Human papillomaviruses (HPVs) are a group of small, non-enveloped, double-stranded DNA tumor viruses of which approximately 200 types have now been identified. Their genomes are less than 8,000 base pairs. HPVs are not only species specific but also display a tropism for squamous epithelia; a large group of HPVs infects cutaneous epithelia, whereas another sizable group infects mucosal epithelia. Papillomavirus genomes can be separated into two coding regions (early “E” and late “L”) and a non-coding regulatory region “NCR” (also referred to as “Long Control Region”, “LCR” or “Upstream Regulatory Region”, “URR”) (Figure 1). HPVs can be clinically classified as “low-risk” and “high-risk” depending on the relative propensity of the HPV-associated lesions to undergo malignant progression; this classification has been most compellingly established with the mucosal HPVs. Low-risk HPVs, such as HPV6 and 11, are associated with a spectrum of generally benign warts, whereas infections with high-risk HPVs, such as HPV16 and 18, are manifested by intraepithelial neoplasias that can undergo malignant progression (de Villiers et al., 2004). Infections with high-risk mucosal HPVs are associated with a variety of cancers, most notably cervical cancer, which are almost uniformly caused by high-risk HPVs. High-risk HPV associated premalignant lesions represent productive infections whereas tumors often are non-productive; viral proteins are produced but no viral progeny is generated. This switch frequently arises as a consequence of integration of HPV genome sequences into a host cell chromosome; as a result only two viral proteins, E6 and E7, are consistently expressed in HPV-positive cervical cancers. The E6 and E7 oncoproteins contribute to tumor initiation and also play an important role in malignant progression through the induction of genomic instability and other mechanisms. As HPV E6 and E7 expression is necessary for the induction and the maintenance of the transformed phenotype, HPV associated tumors are valuable tools to investigate important aspects of human carcinogenesis. This article provides an overview of HPV-associated lesions and cancers and reviews the main concepts of HPV-associated carcinogenesis.
Figure 1.
Schematic representation of the HPV16 genome. The double stranded circular DNA genome is represented by the central circle. Early (E) and late (L) genes are encoded on a single DNA strand in all three possible open reading frames as indicated. The major early promoter in the non-coding region (NCR) (also referred to as Long Control Region, LCR or Upstream Regulatory Region, URR) is represented by an arrow. Early and late genes are transcribed unidirectionally. See text for details.
2. Human diseases associated with HPVs
Infections with mucosal HPVs represent the most common sexually transmitted infection worldwide; by the age of 50 up to 80% of women will have been exposed to HPV (Myers et al., 2000). Approximately 630 million individuals are currently infected with HPV worldwide, with 30 million genital HPV infections diagnosed each year (Scheurer et al., 2005). Moreover, it is estimated that in the United States alone there are 20 million people currently infected with HPV (Cates, 1999; Koutsky, 1997); almost half of these are in the 15–24 year age group (Weinstock et al., 2004). The estimated total cost for the clinical management of HPV-related diseases in the United States is greater than $3 billion per year (Chesson et al., 2004); the majority of this sum is spent on the treatment of premalignant lesions.
2.1 High-risk mucosal HPV associated lesions and cancers
2.1.1 Carcinomas of the cervix and anogenital region
Mucosal HPV infections are associated with a variety of diseases, ranging from benign genital warts to frank carcinomas of the cervix and anogenital region. Even though high-risk HPV infections can cause intraepithelial lesions that are at risk for malignant progression, most high-risk HPV infections do not result in clinically apparent lesions. Those that do develop spontaneously regress at a very high frequency. Moreover, malignant progression is often a slow process and high-risk HPV associated tumors arise years to decades after the original infection. This provides an extended window of opportunity for detection of HPV-associated lesions before invasive carcinomas have developed. In addition, the viral etiology of these tumors provides unique opportunities for prevention and treatment. Prevention has recently been realized by prophylactic vaccines, which prevent infections with the most abundant mucosal high-risk and low-risk HPV types. It should be emphasized, though, that given the slow progression of HPV associated diseases, the potential clinical benefit of prophylactic vaccines will not be apparent for several decades (reviewed in Frazer, 2004) and, moreover, due to their high cost these vaccines have not been widely used in those countries with the highest incidence of HPV-associated cancers. No HPV specific antiviral treatments or therapeutic vaccines are currently available.
The slow rate of malignant progression has been key for efficient detection of potentially premalignant HPV associated cervical lesions by the Papanicolaou (“Pap”) smear. The Pap smear is a simple and well-accepted procedure that allows for cytological examination of exfoliated cervical cells. In countries where there are no extensive Pap smear programs, cervical cancer is highly prevalent and accounts for ~10–25% of all cancers in females. Worldwide, cervical cancer is the third most frequent cancer in females with an incidence of approximately 500,000 new cases per year and represents the second leading cause of cancer death in females with approximately 288,000 deaths per year (Ferlay et al., 2001). Despite widespread Pap smear programs and a resulting 70% decrease in cervical cancer incidence, cervical cancer remains the second leading cause of cancer death in US women of 20–39 years of age (Jemal et al., 2005). A total of 11,150 cervical cancers have been diagnosed in the US in 2007, and more than 10 women succumb to cervical cancer every day (3,670/year) (Ries et al., 2007). HPVs are associated with greater than 99% of all cervical cancer cases, with high-risk HPV types 16, 18, 31, 33, and 45 detected in up to 97% of cervical cancer cases worldwide (Clifford et al., 2003; Walboomers et al., 1999). In addition to squamous cell carcinomas, HPV DNA has also been detected in most cervical adenocarcinomas, adenosquamous carcinomas, and carcinomas with neuroendocrine differentiation (reviewed in Howley and Lowy, 2007). HPV16 is most commonly associated with squamous cell carcinomas, while HPV18 is the predominant type found in adenocarcinomas and neuroendocrine carcinomas.
Since high-risk HPVs are sexually transmitted, the incidence of HPV-associated lesions and cancers is correlated to early onset of sexual activity and the number of lifetime sex partners (reviewed in Schiffman et al., 2007). Males obviously play an important role as vectors and/or reservoirs of high-risk HPVs and can develop penile intraepithelial lesions (PIN). Interestingly, these lesions are far less commonly detected than CINs in females, but they can undergo malignant progression to penile carcinoma. Although penile cancers are rare, overall, their incidence-rate is correlated to that of cervical carcinoma in many parts of the world (Franceschi et al., 2002).
Integration of HPV genome sequences into a host cell chromosome represents a frequent event during malignant progression. There are no apparent host chromosomal hotspots for HPV genome integration (Ziegert et al., 2003), but common fragile sites are frequently targeted (Smith et al., 1992). Integration often leads to deletions of viral sequences, and the E6 and E7 proteins are the only viral proteins that are consistently expressed in cervical carcinomas (Baker et al., 1987; Schwarz et al., 1985). HPV E6/E7 gene expression is dysregulated upon integration due to frequent loss of the viral transcriptional repressor, E2. Moreover, since HPV E6/E7 mRNAs are spliced to cellular sequences when they are produced from integrated copies, they are more stable thereby contributing to increased E6/E7 protein expression (Jeon et al., 1995; Jeon and Lambert, 1995).
Several lines of experimental evidence support the concept that high-risk HPV E6/E7 expression is rate limiting for cervical cancer development. High-risk HPV E6/E7 expression causes life span extension and leads to cellular immortalization of primary human epithelial cells (Bedell et al., 1989; Hawley-Nelson et al., 1989; Münger et al., 1989a), and high-risk HPV immortalized cells exhibit many of the histopathological abnormalities of high-risk HPV-associated premalignant lesions when they are grown in “organotypic” raft cultures (McCance et al., 1988). Perhaps most compelling, expression of HPV16 E6/E7 in basal epithelial cells in transgenic mice in combination with low-dose estrogen treatment causes cervical cancer development (Arbeit et al., 1996). Moreover, HPV16 E6/E7 expression is necessary for the maintenance of the transformed phenotype of cervical carcinoma cell lines (Francis et al., 2000; Goodwin and DiMaio, 2000; Thierry and Yaniv, 1987). As will be discussed later, this makes HPV-associated tumors an outstanding model to study the molecular mechanisms that are commonly targeted during development of human solid tumors (reviewed in zur Hausen, 2002).
In addition to the vast majority of cervical cancers, a fraction of other anogenital tract cancers has been convincingly linked to high-risk HPV infections (reviewed in Schiffman et al., 2007; zur Hausen, 2002). High-risk HPVs are associated with 40–60% of vaginal carcinomas, vaginal intraepithelial neoplasias, and penile carcinomas, up to 50% of vulvar carcinomas, and approximately 90% of anal carcinomas (reviewed in Parkin and Bray, 2006). Similar to cervical carcinoma, HPV-associated anal carcinoma develops in the transition zone between columnar and squamous epithelia (reviewed in Howley and Lowy, 2007). The risk for developing anal cancer is higher among HIV-infected individuals than in the general population (Frisch et al., 2000).
2.1.2 Oropharyngeal carcinomas
While most oropharyngeal cancers arise in patients with a long-term history of alcohol and tobacco abuse, approximately 25% of oral carcinomas are associated with high-risk HPV infections (Gillison et al., 2000), and these often arise in younger patients who lack the classical risk factors (Fakhry and Gillison, 2006). Oral sex practices have been implicated as a mode of transmission (Kreimer et al., 2004). Infection with HPV may also contribute to some cases of esophageal cancer (Sur and Cooper, 1998), but the role of the virus is less clear in these cancers. Unlike the case with cervical cancers, there are currently no screening programs that would aid in the early detection of oropharyngeal premalignant lesions.
2.2 Low-risk mucosal HPV associated neoplasias
Papillomas, known as ‘warts’ when found on the skin or ‘condylomas’ when found on genitalia, are benign proliferative epithelial lesions that are composed of all the layers of the differentiated epithelium (reviewed in Howley and Lowy, 2007); the majority are associated with low-risk HPVs such as HPV6 and 11 (Greer et al., 1995). Warts and condylomas frequently regress spontaneously but may also persist indefinitely. While malignant progression of such lesions is exceedingly unlikely, in rare cases they can give rise to life-threatening conditions, including recurrent respiratory papillomatosis (RRP) and the giant condyloma of Buschke-Lowenstein. RRP is a rare disease caused by HPV6 or HPV11 infection at birth. RRP most commonly arises in young children, with an average age of 3.8 years at diagnosis, but there are also adult cases. Because such patients are unable to control or clear low-risk HPV infections, they develop rapidly growing exophytic lesions in the larynx and around the vocal cords that can spread to the trachea and lungs. RRP is associated with considerable morbidity and mortality and these patients require very frequent surgeries to keep their airways clear (reviewed in Tasca and Clarke, 2006).
Anogenital HPV6 or 11 infections can give rise to the giant condyloma of Buschke-Lowenstein in patients who have a genetic defect that makes them unable to effectively control or clear these infections. These lesions are slow-growing but are extremely destructive to adjacent uninfected tissues and can eventually give rise to local and distant metastases (reviewed in Frega et al., 2002; Rhea et al., 1998).
2.3 Malignancies associated with cutaneous HPVs
Some cutaneous HPVs, most prominently HPV5 and HPV8, are associated with skin cancers in patients suffering from a rare, genetically determined skin disease, epidermodysplasia verruciformis. (EV) (Ramoz et al., 2002; Ramoz et al., 2000). Indeed, EV-associated skin cancer was the first human tumor type that was compellingly associated with HPV infection. (Jablonska et al., 1966; Orth et al., 1978; Pass et al., 1977; Pfister et al., 1981). Two human susceptibility genes, EVER1 and 2, have been identified (Ramoz et al., 2002) and others likely exist. Similar to RRP, EV patients do not efficiently clear HPV5/HPV8 infections and develop prominent wart like lesions that can eventually progress to skin cancers, particularly in sun-exposed areas of the body (reviewed in Majewski et al., 1997).
HPV5 and HPV8, as well as other related cutaneous HPVs, have also been linked to development of non-melanoma skin cancers (NMSC) in non-EV patients. This has been well established for immune-suppressed patients (Shamanin et al., 1994) but remains controversial in the non-immune-suppressed population (reviewed in Akgul et al., 2006). Unlike with cervical carcinoma, cutaneous HPV genomes are not detected in every NMSC cell. It has thus been suggested that these HPVs may contribute to initiation but may not be necessary for maintenance of NMSCs.
3. Transforming mechanisms of high-risk mucosal HPVs
High-risk mucosal HPVs encode 3 transforming proteins, E5, E6 and E7. The HPV E5 protein may contribute to early steps of cancer initiation (Talbert-Slagle and DiMaio, 2009) and can contribute to cancer formation in a transgenic mouse model (Maufort et al., 2007). Due to frequent integration of HPV genomes during malignant progression, E5 expression is generally lost in cervical carcinoma and, thus, it is not necessary for maintenance of the transformed phenotype. Hence the following discussion will be focused on the high-risk HPV E6 and E7 oncoproteins, which are regularly expressed in HPV associated lesions and cancers.
The major hallmarks of human tumors as catalogued by Hanahan and Weinberg in their classic review article include acquisition of unlimited proliferative potential, growth factor independence, evasion of apoptosis and sensitivity to cytostatic signals, induction of invasive and metastatic properties, and sustained angiogenesis. Each of these characteristics emerges as a result of genomic alterations (Hanahan and Weinberg, 2000). In high-risk HPV associated cancers, E6 and E7 oncogenes target critical regulators of each of these processes (Figure 2 and Figure 3) and hence, high-risk HPV oncoprotein expression substitutes for multiple oncogenic hits that are normally acquired by mutations due to genetic predisposition and/or exposure to environmental carcinogens. In addition, the E6 and E7 oncoproteins act as potent mitotic mutators, thereby increasing the probability of acquiring additional cellular mutations that contribute to carcinogenic progression during each round of cell division (Figure 4). Whereas HPV immortalized human epithelial cells are initially non-tumorigenic, they acquire a fully transformed phenotype upon continuous passaging in vitro (Hurlin et al., 1991). Consistent with this model, an infection with a high-risk HPV type is a far more significant relative risk factor for the development of cervical cancer (RR=50–100) than cigarette smoking is for developing lung cancer (RR=10) (Franco and Harper, 2005). Moreover, classical cancer risk factors such as smoking only play a minor role for cervical cancer development (RR=1.6) (Appleby et al., 2006).
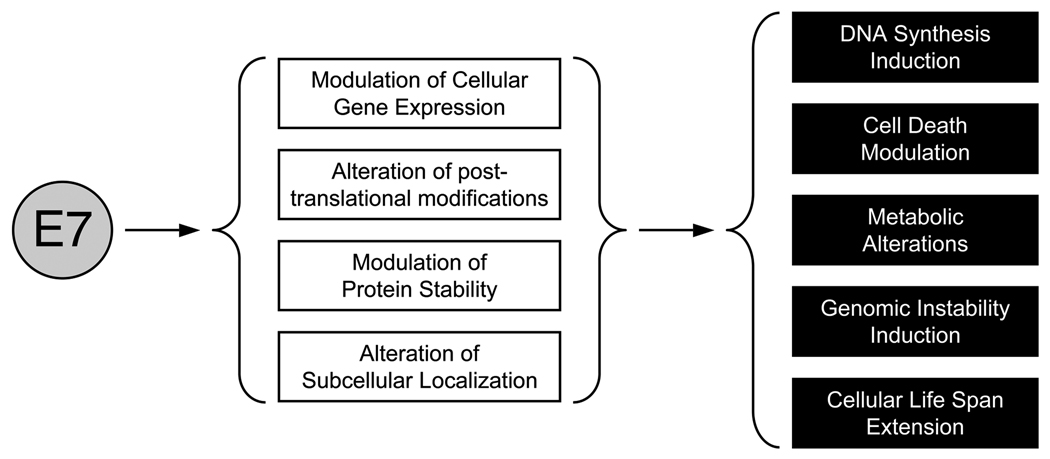
Figure 2.
Major mechanisms (white boxes) utilized by the HPV E7 oncoprotein to induce specific hallmarks of human tumors (black boxes). See text for details.
Figure 3.
Major mechanisms (white boxes) utilized by the HPV E6 oncoprotein to induce specific hallmarks of human tumors (black boxes). See text for details.
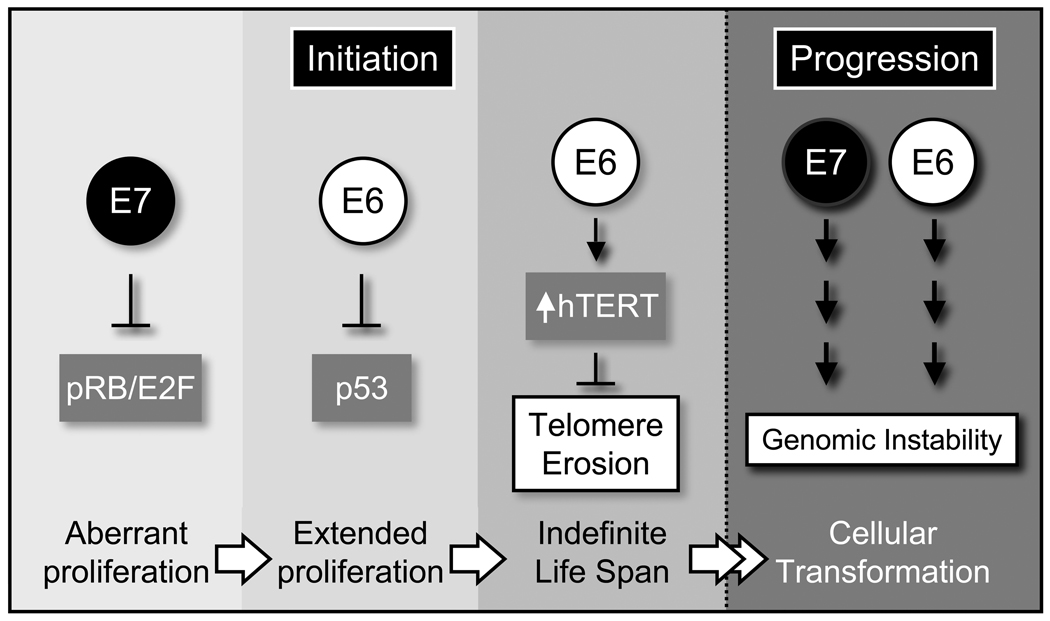
Figure 4.
High-risk HPV E6 and E7 oncoproteins contribute to early and late steps of tumor development. The HPV E7 oncoprotein subverts G1/S restriction through degradation of the retinoblastoma tumor suppressor, pRB. This causes aberrant cell cycle entry, which would normally result in activation of the p53 tumor suppressor, causing cell death. High-risk HPV E6 oncoproteins target p53 for degradation thereby allowing cells to undergo persistent, aberrant proliferation. To avoid telomere erosion as a result of extensive cell division, HPV16 E6 activates hTERT transcription causing cellular immortalization. In addition high-risk HPV oncoproteins also contribute to malignant progression through induction of mitotic abnormalities, which result in genomic instability. See text for detail
3.1 Unlimited proliferative potential
The HPV life cycle is closely linked to the differentiation program of infected epithelial cells. The virus must infect the proliferating basal cells in order to establish a persistent infection (Munger, 2002; Stubenrauch and Laimins, 1999). After the initial infection the viral episomes are maintained in the basal cells. Basal cells divide asymmetrically and perpendicularly to the basement membrane; one daughter cells retains its basal cell characteristics whereas the other daughter cell is committed to differentiation, withdraws from the cell division cycle, and is subsequently pushed upwards within the stratified tissue. HPV genomes are partitioned between the two daughter cells, and one of the major challenges of the papillomavirus life cycle is to establish and/or sustain a replication competent cellular milieu in these differentiated keratinocytes. Studies with organotypic “raft” cultures have revealed that HPV E7 expression can induce differentiated cells to undergo DNA synthesis (Cheng et al., 1995).
The HPV E7 protein associates with the retinoblastoma tumor suppressor protein pRB (Dyson et al., 1989), which controls S-phase entry through association with E2F transcription factor family members (Figure 4). In normal cells, pRB phosphorylation by cdk4 and/or cdk6/cyclin D complexes in G1 results in the disruption and/or functional inactivation of the pRB/E2F transcriptional repressor complexes, and dissociated E2Fs act as transcriptional activators of genes that are rate limiting for S-phase entry and progression (reviewed in Dyson, 1998). HPV E7 proteins associate with pRB through LXCXE amino acid sequence motifs (L, leucine; C, cysteine; E, glutamate; X, any amino acid) (Münger et al., 1989b) (Figure 5). The SV40 large tumor antigen (TAg) and the Adenovirus (Ad) E1A proteins also target pRB family members through LXCXE motifs (DeCaprio et al., 1988; Whyte et al., 1988) to induce S-phase competence in infected cells (Chellappan et al., 1992). High-risk mucosal HPV E7 proteins bind to pRB more efficiently than low-risk HPV E7s. The difference in binding has been attributed to the amino acid residue preceding the LXCXE motif; low-risk HPV E7 proteins contain a glycine residue, while high-risk HPV E7 proteins contain an aspartate residue at this position (Heck et al., 1992; Sang and Barbosa, 1992). However, intriguingly, the presence of an aspartate residue at this position in cutaneous HPV E7 proteins and the accompanying efficient pRB binding does not predict transforming potential in vitro or in vivo (Ciccolini et al., 1994).
Figure 5.
Schematic representation of the HPV16 E6 (top) and E7 (bottom) oncoproteins. Sequences involved in cellular transformation are marked by gray bars, and some of the major cellular targets that associate with the viral oncoproteins through such sequences are indicated. See text for details
The HPV16 E7 protein preferentially associates with hypophosphorylated pRB (Dyson et al., 1992) and, unlike Ad E1A and SV40 TAg, targets it for proteasomal degradation (Boyer et al., 1996; Jones et al., 1997b) through association with a cullin 2 containing ubiquitin ligase complex (Huh et al., 2007) (Figure 5; Figure 6A). The ability of high-risk HPV E7 proteins to target pRB for degradation tightly correlates with its ability to transform cells (Jones and Münger, 1997).
Figure 6.
Reprogramming of cellular ubiquitin ligases by HPV16 oncoproteins. (A) HPV16 E7 retargets the cullin 2 ubiquitin ligase complex to ubiquitinate the retinoblastoma tumor suppressor and potentially other HPV16 E7 associated cellular protein. (B) High-risk HPV E6 oncoproteins associate with the ubiquitin ligase E6AP to ubiquitinate the p53 tumor suppressor protein. The E6/E6AP complex has also been implicated in mediating ubiquitination of high-risk HPV E6 associated cellular PDZ proteins. See text for details.
Non-stoichiometric, enzymatic pRB inactivation by high-risk HPV E7 proteins has two important implications. First, a non-stoichiometric mechanism ensures effective pRB inactivation even when E7 is expressed at low levels; this may be necessary so as to avoid detection and elimination of infected cells by the immune system during persistent infection. Second, high-risk HPV E7-mediated pRB degradation results in subversion of all the biological activities of pRB, not just those related to cell cycle regulation. This is significant as pRB has also been implicated in regulating multiple cellular processes including cell death, mitotic checkpoint control, and cellular differentiation (reviewed in Du and Pogoriler, 2006).
HPV E7 proteins cause aberrant activation of cyclin dependent kinase 2 (cdk2), which is regulated by association with the positive regulatory subunits, cyclins E and A, as well as cdk inhibitors (CKIs), most prominently p21CIP1 and p27KIP1. HPV E7 expression results in dysregulated expression of cyclins E and A through E2F–dependent pathways (Zerfass et al., 1995) and also inactivates the inhibitory activities of the CKIs p21CIP1 and p27KIP1 (Funk et al., 1997; Jones et al., 1997a; Zerfass-Thome et al., 1996), which are induced by anti-proliferative signals such as growth factor withdrawal (Firpo et al., 1994), activation of p53 (El-Deiry et al., 1993), and loss of cellular adhesion (Assoian, 1997; Fang et al., 1996). These CKIs have also been implicated in coupling cell cycle arrest and differentiation in human and mouse keratinocytes (Alani et al., 1998; Di Cunto et al., 1998; Missero et al., 1996). Therefore, inhibition of p21CIP1 and p27KIP1 E7 may play an important role in the HPV-mediated uncoupling of cellular differentiation and proliferation necessary for viral replication.
E7 expression also causes increased expression of cdc25, a cellular phosphatase that removes an inhibitory phosphorylation on cdk2 (Katich et al., 2001; Nguyen et al., 2002). Moreover, HPV16 E7 has been reported to directly associate with cdk2 and enhance its enzymatic activity (He et al., 2003); this association is independent of pRB, p107, and p130 (Nguyen and Munger, 2008).
3.2 Evasion of apoptosis
Apoptosis can be triggered by a number of cell autonomous and non-autonomous mechanisms. Cell autonomous initiation of apoptosis is an important tumor suppressive mechanism, as it ensures that aberrantly proliferating, and therefore potentially premalignant cells are permanently removed from the proliferative pool (reviewed in Lowe et al., 2004). Non-cell autonomous mechanisms can be triggered by cytokines that are produced in response to viral infection and/or viral gene expression. In addition to apoptosis, several other abortive mechanisms exist, including cell cycle arrest, senescence, autophagy and terminal differentiation.
3.2.1 Trophic sentinel signaling – abrogation of p53 tumor suppressor protein activity
Trophic sentinel signaling represents a cellular defense mechanism whereby aberrantly proliferating cells are recognized and eliminated. Conceptually, this pathway functions as a rheostat and assures that the proliferative state of the cell is consistent with extracellular growth factor availability and presumably the intracellular metabolic state. Cells that undergo aberrant proliferation as a consequence of oncogenic injury are eliminated from the proliferative pool through this tumor suppressive pathway (Evan and Vousden, 2001). The p53 tumor suppressor is thought to represent an important nexus in this process and can signal cell cycle arrest and/or cell death. The trophic sentinel response was initially discovered through studies where the Ad E1A and c-myc oncogene were ectopically expressed in normal diploid cells (Evan et al., 1992; Rao et al., 1992). Similarly, HPV16 E7 expressing normal human fibroblasts undergo cell death when they reach confluence or are maintained in culture medium that lacks serum. Trophic sentinel signaling in this system is correlated with the ability of HPV16 E7 to induce pRB degradation (Jones et al., 1997b). Although the mechanism of cell death depends on the integrity of the p53 tumor suppressor pathway, it does not involve transcriptional activation of canonical p53 transcriptional targets. Intriguingly, HPV16 E7 expression generally interferes with transcriptional activity of p53 (Eichten et al., 2002). Furthermore, the mechanism of cell death triggered by HPV16 E7 expression in primary human fibroblasts appears to be distinct from classic apoptosis; although caspases are activated and DNA is degraded to nucleosomal fragments, cell death is mostly caspase independent (Eichten et al., 2004). While low-risk HPV E7 proteins associate with pRB, they do not induce trophic sentinel signaling under similar assay conditions. Although the signaling pathways have not yet been molecularly analyzed, HPV16 E7 expressing normal human epithelial cells also undergo cell death in response to growth factor deprivation. Interestingly, there is evidence for autophagy in HPV16 E7 expressing keratinocytes even when the cells are maintained under normal culture conditions, suggesting the HPV16 E7 expressing cells may have increased metabolic requirements. This may be a consequence of E7-induced metabolic alterations (see below) and/or increased proliferative activity (Zhou and Munger, 2009). It will be interesting to determine whether and how autophagy induction may be mechanistically linked to trophic sentinel signaling in epithelial cells.
Presumably at least in part to avoid elimination through trophic sentinel signaling, high-risk HPV E6 proteins target p53 for proteasome-mediated degradation (Scheffner et al., 1990). E6 associates with the cellular ubiquitin ligase E6AP, and the E6/E6AP complex binds and ubquitinates p53 (Huibregtse et al., 1991; Scheffner et al., 1993) (Figure 5; Figure 6B). Whereas low-risk HPV E6 proteins can also associate with E6AP, the ability to target p53 for ubiquitination is unique to E6 proteins encoded by high-risk HPVs (Scheffner et al., 1990).
3.2.2 Anoikis signaling
Anoikis is a form of apoptosis that is triggered when normal cells attempt to divide in the absence of a matrix (reviewed in Frisch and Screaton, 2001). Resistance to anoikis is a hallmark of cellular transformation and is manifested by the ability of transformed cells to overcome density mediated growth arrest and form foci and to proliferate in an anchorage independent manner. As pointed out above, one of the major requirements of the HPV life cycle is that differentiated keratinocytes remain DNA synthesis competent, and cell division in suprabasal layers is regularly observed in HPV associated lesions. Hence, abrogation of anoikis may be key to the viral replication strategy. The p600 protein has been implicated as a regulator of this process. High- and low-risk HPV E7 proteins, as well as the E7 protein encoded by bovine papillomavirus 1 (BPV1), associate with p600 (DeMasi et al., 2005; Huh et al., 2005). Expression of papillomavirus E7 proteins results in anoikis resistance in a number of different cell types: in the case of BPV1 E7 this is at least in part due to p600 association (DeMasi et al., 2007). Depletion of p600 causes loss of anchorage independent growth in human cancer cells (Huh et al., 2005). Thus, the interaction between E7 and p600 may inhibit anoikis and protect detached cells from apoptosis, thereby contributing to viral transformation (DeMasi et al., 2007; Huh et al., 2005). Consistent with this hypothesis, HPV16 E7 associates with p600 through an amino terminal domain, which is necessary for the transformation capability of HPV16 E7 (Gulliver et al., 1997; Phelps et al., 1992) (Figure 5). Although the biological functions of p600 remain enigmatic, the protein has been shown to function as a ubiquitin ligase in the N-end rule pathway (Tasaki et al., 2005). It will be interesting to determine whether and how ubiquitin ligase activity may contribute to anoikis signaling.
3.2.3 Subversion of cytotoxic/cytostatic cytokine signaling
Acquisition of transforming growth factor β (TGF-β) resistance is a hallmark of epithelial tumors, and cervical carcinoma cell lines are TGF-β resistant (De Geest et al., 1994). Ectopic HPV16 E7 expression abrogates TGF-β mediated growth inhibition (Pietenpol et al., 1990); acquisition of TGF-β resistance is a multi-step process, as TGF-β can repress HPV16 E6/E7 expression (Creek et al., 1995; Woodworth et al., 1990). The emergence of TGF-β resistance in such cells has been correlated to Ski overexpression, but the exact mechanism of this is not yet known (Baldwin et al., 2004).
Additionally, HPV positive cervical carcinoma cell lines are resistant to the cytostatic effects of tumor necrosis factor α (TNF) (Villa et al., 1992), a major inflammatory cytokine in the skin and mucosa that causes G1 growth arrest and cellular differentiation in normal keratinocytes (Basile et al., 2001). This effect is keratinocyte-specific and involves NF-kB-mediated induction of p21CIP1 (Basile et al., 2003). The TNF-related cytokines Fas ligand and TNF-related apoptosis inducing ligand (TRAIL) do not inhibit keratinocyte proliferation (Basile et al., 2001). When co-administered with cycloheximide, TNF treatment can also trigger apoptosis. HPV E7 expressing keratinocytes exhibit increased apoptosis upon TNF/cycloheximide treatment (Basile et al., 2001; Stoppler et al., 1998), whereas in contrast, apoptosis is inhibited in HPV16 E7 expressing diploid human fibroblasts (Thompson et al., 2001).
Interferons (IFNs) are cytostatic cytokines produced as part of the innate immune response to viral infections. The expression of IFN is activated by Interferon regulatory factors (IRFs). HPV16 E7 has been reported to associate with IRF-1 and impair its transcriptional activity (Park et al., 2000; Perea et al., 2000), and HPV16 E6 can associate with and inhibit the transcriptional activity of IRF-3 (Ronco et al., 1998). Moreover, HPV16 E7 has been reported to form a complex with p48 (the DNA binding component of the transcription factor ISGF-3, a transcriptional mediator of IFNs) and perturb its nuclear translocation upon stimulation (Barnard and McMillan, 1999; Barnard et al., 2000). Therefore, HPV16 E6 and E7 may cooperate to subvert innate immune signaling.
HPV16 E7 may also interfere with Insulin-like growth factor (IGF) signaling, which regulates cell survival. HPV16 E7 can associate with IGFBP3 (Mannhardt et al., 2000) and accelerate its proteasome-mediated degradation (Santer et al., 2007). In particular under conditions of growth factor depletion, HPV16 E7 expressing cells express increased IGFBP-2 and IGFBP-5 levels through an NF-kB dependent mechanism (Eichten et al., 2004).
3.3 Transcriptional activities of HPV oncoproteins
Despite the fact that HPV E6 and E7 oncoproteins lack specific DNA binding activities, they can associate with transcription factor complexes and alter their transcriptional activities. The best-documented examples are the p53 and E2F transcription factor complexes. As described above, high-risk HPV E6 proteins target p53 for proteasomal degradation, whereas E7 expression results in p53 stabilization but inhibits its transcriptional activity. Most of the known E2F family members contain a pRB binding site, and cell-cycle dependent association with pRB family members regulates their transcriptional activities. As discussed earlier, HPV E7 proteins dysregulate E2F activity by association with and/or disruption of pRB family member containing E2F complexes. Some HPV E7 proteins have also been reported to directly associate with E2F family members, resulting in enhanced transcriptional activity (Hwang et al., 2002). HPV E7 proteins also associate with the non-canonical E2F family member E2F6; this association is mediated by C-terminal HPV E7 sequences. E2F6 is a transcriptional repressor that is induced late during S-phase through “activating” E2Fs such as E2F1, and its role is to repress E2F target genes that are necessary during S-phase phase so as to allow S-phase exit and G2 entry (Lyons et al., 2006). E2F6 lacks a pRB-binding site, acts as transcriptional repressor, and pRB family members do not modulate its transcriptional activity. The association with HPV16 E7 has been shown to abrogate the repressive activity of E2F6; SV40 TAg and Ad E1A also interact with E2F6 (McLaughlin-Drubin et al., 2008).
In addition, high-risk HPV E6 proteins can bind c-myc (Veldman et al., 2003) and a repressor of hTERT expression, NFX1 (Gewin et al., 2004; Katzenellenbogen et al., 2007), resulting in increased hTERT transcription (McMurray and McCance, 2003) (Figure 4). HPV E7 proteins have been reported to bind the forkhead transcription factor MPP2 (Luscher-Firzlaff et al., 1999), AP1 (Antinore et al., 1996), and interfere with NF-kB activation (Huang and McCance, 2002; Spitkovsky et al., 2002). HPV E6 and E7 proteins may also associate with components of the basal transcription factor machinery, but the potential functional relevance of these associations is not clear (Massimi et al., 1996; Mazzarelli et al., 1995).
3.4 Association of HPV oncoproteins with histone modifying enzymes
HPV E6 and E7 proteins have also been reported to associate with histone modifying enzymes and associated transcriptional co-factors (Bernat et al., 2003; Brehm et al., 1999; Huang and McCance, 2002; Patel et al., 1999; Zimmermann et al., 1999). HPV E7 interacts with class I histone deacetylases (HDACs) (Brehm et al., 1999; Longworth and Laimins, 2004), which has been linked to increased levels of E2F2-mediated transcription in differentiating cells (Longworth and Laimins, 2004). In addition, E7 can also associate histone acetyl transferases (HATs), including p300, pCAF, and SRC1 (Avvakumov et al., 2003; Baldwin et al., 2006; Bernat et al., 2003; Huang and McCance, 2002) and has been shown to abrogate SRC1 associated HAT activity (Baldwin et al., 2006). More recently, the histone methyl transferase, enhancer of zeste homologue 2 (EZH2), has been identified as a transcriptional target of HPV E7 proteins (Holland et al., 2008).
3.5 Cellular metabolism
The “Warburg effect” refers to the metabolic shift from mitochondrial respiration to glycolytic fermentation that occurs in many human tumor cells (reviewed in Aisenberg, 1961; Warburg, 1936). A similar metabolic shift occurs in HPV transformed cells and HPV16 E7 can associate with and alter the activity of the M2 embryonic splice variant of the metabolic enzyme pyruvate kinase (M2-PK) (Zwerschke et al., 1999). Accordingly, HPV16 E7 transformed cells derive their metabolic energy mostly from glycolytic processes rather than from oxidative phosphorylation (Mazurek et al., 2001). Therefore, HPV16 E7 expressing cells may provide a useful model to determine the biochemical basis of this metabolic shift, which is critical for tumor maintenance (Fantin et al., 2006). HPV16 E7 has also been found to associate with and allosterically activate α-glucosidase (Zwerschke et al., 2000), an enzyme that causes glycogen catabolism. Similar to many other tumor types, HPV-associated cervical cancers contain low glycogen levels (Bannasch et al., 1997; Pedersen, 1975).
Transformed cells often have a higher intracellular pH, and the expression of HPV E7 has been shown to cause cytoplasmic alkalinization due to increased activity of the Na+/H+ exchanger protein. Inhibition of this process decreased cell proliferation and inhibited anchorage independent growth of HPV16 E7 transformed NIH3T3 cells (Reshkin et al., 2000).
As mentioned previously, there is evidence for autophagy in HPV16 E7 expressing keratinocytes (Zhou and Munger, 2009). While the biochemical basis of this has not been determined, it is tempting to speculate that the autophagy pathway is engaged at least in part as a consequence of some of these metabolic alterations.
3.6 Cytoskeleton and cell polarity
E6 proteins encoded by high-risk, but not low-risk, HPVs contain a (S/T)-X-V-I-L sequence motif at their extreme carboxyl termini that acts as a ligand for cellular PDZ domain containing proteins (Kiyono et al., 1997; Lee et al., 1997) (Figure 5). PDZ proteins are concentrated in areas of cell-to-cell contact and may act as scaffolds to spatially organize important cellular signal transduction processes, which may be involved in establishing cell polarity (Craven and Bredt, 1998). A number of putative PDZ targets of high-risk HPV E6 proteins have been reported, including MAGI-1, −2, and −3, hDLG, MUPP-1, hSCRIB, PATJ, and PTPN3, and some of them may be targeted for E6/E6AP mediated ubiquitination (Gardiol et al., 1999; Glaunsinger et al., 2000; Jing et al., 2007; Lee et al., 2000; Nakagawa and Huibregtse, 2000; Storrs and Silverstein, 2007; Thomas et al., 2002). The integrity of the PDZ domain binding sequence is important for E6 activities during the viral life cycle (Lee and Laimins, 2004) and for the transforming activities of high-risk HPV E6 proteins (Kiyono et al., 1997; Nguyen et al., 2003). Other viral oncoproteins, such as Adenovirus type 9 E4 ORF1 and HTLV-1 tax, also associate with PDZ proteins (Gardiol et al., 1999). In each case, association with PDZ proteins has been linked to transforming activities of these viral oncoproteins (reviewed in Hall and Fujii, 2005; Liu and Baleja, 2008; Tauber and Dobner, 2001).
3.7 Induction of Genomic Instability
The expression of HPV16 E6 and E7 in primary human keratinocytes causes cellular immortalization; these cells exhibit many hallmarks of premalignant lesions (Figure 4). However, HPV-immortalized cells are not fully transformed, as they do not form tumors when injected into nude mice. A fully transformed phenotype is only acquired upon prolonged passaging in cell culture (Hurlin et al., 1991) or when additional oncogenes are expressed (Dürst et al., 1989; Pei et al., 1993). Likewise, in transgenic mice where HPV16 E6 and E7 are expressed in basal epithelial cells, the development of cervical cancer is dependent upon long-term exposure to low doses of estrogen that mimic persistent estrus (Arbeit, 1996). This is reminiscent of the often decades long latency period between initial high-risk HPV infection and the development of invasive cervical carcinoma (reviewed in Schiffman et al., 2007).
Thus, although HPV oncogene expression is necessary and sufficient for initiation of cervical carcinogenesis, additional mutations in the host genome must be acquired for malignant progression to occur. Consistent with this notion, cervical cancer cells have accumulated a plethora of numerical and structural chromosomal aberrations (Mitelman et al.). The development of genomic instability is associated with lesions caused by high-risk, but not low-risk, HPV infections (Rihet et al., 1996) and can be detected in premalignant lesions (Bibbo et al., 1989; Steinbeck, 1997) prior to the integration of HPV genomes into host chromosomes (Bulten et al., 1998; Southern et al., 1997). This suggests a causal role for high-risk HPV in subversion of genomic stability (Figure 4).
In agreement with the concept that high-risk HPV oncogene expression may facilitate genomic destabilization, a variety of cytogenetic abnormalities have been detected in HPV immortalized keratinocytes (Cottage et al., 2001; Smith et al., 1989); indeed high-risk HPV E6 and/or E7 expression in primary human cells increases genomic instability (Hashida and Yasumoto, 1991; White et al., 1994). Aneuploidy, gains and/or losses of entire chromosomes, in HPV E6 and/or E7 expressing cells arises as a consequence of multiple types of mitotic abnormalities, including multipolar mitoses, anaphase bridges and disorganized mitoses with lagging chromosomal material (Duensing et al., 2000; Duensing and Münger, 2002).
3.7.1 Multipolar Mitoses
Abnormal, multipolar mitoses are histopathological hallmarks of high-risk HPV-associated premalignant lesions and cancers (Winkler et al., 1984) and are caused by supernumerary centrosomes (Duensing et al., 2000). Centrosomes function as microtubule organizing centers (MTOCs) and form the mitotic spindle poles. Centrosomes are duplicated during S-phase, in synchrony with DNA replication to ensure bipolar, symmetric chromosome segregation during mitosis (reviewed in Stearns, 2001).
High-risk, but not low-risk, HPV E6 and E7 proteins cooperate to generate mitotic defects and aneuploidy through the induction of supernumerary centrosomes in primary human epithelial cells (Duensing et al., 2000); in fact, the characteristic, multipolar mitoses in high-risk HPV associated cervical lesions (Crum et al., 1984) represent a direct manifestation of HPV E6/E7 expression (Duensing et al., 2000). Centrosome abnormalities and associated mitotic defects similar to those found in high-risk associated premalignant lesions are apparent in cells that express episomal HPV16 at low copy number and E7 plays a major role in generating these defects (Duensing et al., 2001b). The incidence of centrosome abnormalities and mitotic defects increases in cells with integrated HPV genomes (Pett et al., 2004; Skyldberg et al., 2001). In addition, centrosome abnormalities have been documented in HPV16 E6 and/or E7 expressing transgenic mice that develop cervical (Balsitis et al., 2003; Riley et al., 2003) or skin lesions (Schaeffer et al., 2004).
There are two distinct mechanisms by which centrosome abnormalities can arise: either as a consequence of cytokinesis failure or cell fusion or directly through aberrant centriole synthesis (reviewed in Duensing, 2005). Centrosome abnormalities that arise indirectly as a consequence of defects in cytokinesis and/or cell division or as a result of cell fusion are accompanied by nuclear abnormalities and/or tetraploidy (reviewed in Storchova and Pellman, 2004). However, it has been shown that HPV16 E7 expression uncouples centriole synthesis from the cell division cycle and induces supernumerary centrosomes through primary centrosome and centriole duplication errors in normal diploid cells (Duensing et al., 2001a; Guarguaglini et al., 2005). HPV16 E7 induces supernumerary centrosomes through concurrent synthesis of multiple daughter centrioles from a single maternal template (Duensing et al., 2007b). Evidence for aberrant centriole synthesis has also been detected in high-risk HPV positive tumor tissues; however, in most cases cells will undergo pseudo-bipolar mitosis (Duensing et al., 2007a; Duensing et al., 2008). This is due to a mechanism referred to as centrosome clustering or coalescence where multiple centrosomes form a single MTOC. Due to uneven forces at individual spindle poles, such mitotic events, even though they appear phenotypically normal, may give rise to chromosome missegregation and generate aneuploid progeny (Grill et al., 2001).
Although it has been postulated that aneuploidy generally arises from a tetraploid intermediate (Fujiwara et al., 2005), it has in fact been documented that aneuploid cell populations can arise directly from diploid cells. This discovery was made in mammary epithelial cells where loss of p16INK4A gives rise to aberrant centriole splitting. This caused formation of multipolar mitoses and aneuploid progeny (McDermott et al., 2006). Hence, even though HPV16 E7 induces centrosome duplication errors in diploid cells through a different mechanism, the presence of supernumerary centrosomes in diploid cells suggests development of aneuploidy in the absence of a tetraploid intermediate.
Unlike normal centrosome duplication, the ability of HPV16 E7 to induce aberrant centriole synthesis is acutely dependent on cdk2 activity. Cdk2 inhibition interferes specifically with E7 mediated induction of supernumerary centrosomes and decreases the incidence of centrosome abnormalities and aneuploidy in E7 expressing cells but does not affect cell cycle progression and normal centrosome duplication (Duensing et al., 2006; Duensing et al., 2004). HPV16 E7 expression causes aberrant cdk2 activation through multiple pathways, including increased expression of the regulatory cdk2 subunits cyclins E and A through aberrant E2F activation as a consequence of pRB family member degradation. Since HPV16 E7 expression augments the incidence of supernumerary centrosomes in mouse embryo fibroblasts that lack pRB, p107, and p130, the ability of HPV16 E7 to induce supernumerary centrosomes is at least in part independent of the ability to target pRB family members (Duensing and Munger, 2003). A possible pRB/p107/p130 independent mechanism for the emergence of supernumerary centrosomes in HPV16 E7 expressing cells involves pRB/p107/p130-independent association of E7 with the centrosomal regulator γ-tubulin through sequences that overlap the pRB core-binding site. The association of HPV16 E7 with γ-tubulin correlated with the ability of HPV16 E7 to induce supernumerary centrosome abnormalities in pRB/p107/p130 deficient cells. Moreover, HPV16 E7 expression significantly inhibited γ-tubulin recruitment to the centrosome (Nguyen et al., 2007).
Supernumerary centrosomes do not necessarily result in multipolar mitoses. A cellular defense mechanism, centrosome coalescence, causes formation of a single mitotic spindle pole by multiple centrosomes. As pointed out above, such cells undergo pseudo-bipolar mitoses, although such mitotic events may lead to chromosome segregation errors. Nonetheless, tripolar mitoses are hallmarks of high-risk HPV-associated lesions and cancers (Crum et al., 1984; Skyldberg et al., 2001), and a significant fraction of HPV16 E6/E7 expressing keratinocytes undergo multipolar mitoses in vitro (Duensing et al., 2000).
3.7.2 Anaphase Bridges
Anaphase bridges in HPV oncoprotein expressing cells most likely represent chromosomal fusions caused by double strand DNA breaks (Duensing and Münger, 2002). The presence of DNA repair foci in HPV16 E7 expressing cells indicates that E7 expression may either induce double strand DNA breaks or interfere with break repair, thus facilitating viral genome integration. Consequently, E7 may be a driving force for integration of high-risk HPV genomes into host cellular chromosomes (Kessis et al., 1996), thereby accelerating malignant progression. Induction of genomic instability and HPV genome integration may also arise as a direct consequence of HPV genome replication through “onion skin” DNA replication, where multiple replication initiation events occur on a single viral genome template so that replication is occurring at different stages simultaneously (Kadaja et al., 2007; Mannik et al., 2002). This may result in double stranded HPV DNA fragments breaking off of the circular genome and being integrated into the host genome via the endogenous DNA double strand break (DSB) repair machinery (Kadaja et al., 2007). If the upstream regulatory region is integrated into the host DNA, it may be the site of continued “onion skin” replication as long as the viral E1 and E2 replication proteins are expressed (Kadaja et al., 2007). This abnormal generation of multiple double stranded DNA fragments can also initiate DNA DSB repair, which may result in several possible outcomes: excision of HPV DNA, excision of host DNA, rearrangement of host or HPV DNA, repeated integration of HPV DNA, or repeated fragments of host DNA.
Fanconi anemia (FA) patients have an increased incidence of squamous cell carcinomas at sites that are infected by HPVs (reviewed in zur Hausen, 2002), and one study has suggested that oral cancers arising in FA patients are HPV positive at a significantly higher rate than in the general population (Kutler et al., 2003). The FA pathway is activated by DNA cross-linking agents and stalled replication forks (reviewed in Kennedy and D’Andrea, 2005), and it was recently demonstrated that the FA pathway is activated by HPV16 E7 expression and that the capacity of HPV16 E7 to induce DNA repair foci is enhanced in FA patient derived cell lines (Spardy et al., 2007). Hence it is conceivable that FA patients may be particularly vulnerable to high-risk HPV E7 induced genomic instability, which may accelerate malignant progression.
3.7.3 Lagging chromosomes
The detection of mitotic figures with lagging chromosomes in high-risk HPV oncoprotein expressing cells may be linked to the ability of HPV E7 proteins to associate with the Nuclear and Mitotic Apparatus Protein-1 (NuMA) (Nguyen and Munger, 2009). NuMA is nuclear during interphase but in metaphase it relocalizes to centrosomes and mitotic spindles where it associates with the microtubule motor protein dynein and not only functions in microtubule binding but also in recognition of specific cargoes that are transported. Recent studies showed that there is an increased incidence of NuMA (Nguyen and Munger, 2009) and dynein (Nguyen et al., 2008) delocalized from mitotic spindles during mitosis in HPV E7 expressing cells.
Disorganized chromosome alignment during metaphase in HPV16 E7-expressing cells was correlated with the ability of E7 to associate with NuMA and delocalize dynein from mitotic spindles. The nature of these mitotic abnormalities was assessed by live cell fluorescence videomicroscopy and revealed that the disorganized metaphases that were observed with fixed cells (Duensing and Münger, 2002) corresponded to cells that were in prometaphase, and metaphase was significantly extended in E7 expressing cells (Nguyen and Munger, 2009).
3.8 Epithelial to mesenchymal transition
Expression of high-risk HPV oncoproteins has also been implicated in dysregulation of other cellular pathways that are associated with malignant progression, including epithelial to mesenchymal transition (EMT) and angiogenesis.
Cancer associated EMT is a reversible process through which epithelial cells acquire certain characteristics of fibroblasts and is thought to play a key role in carcinogenesis and metastasis. It may reflect the final step of tumor de-differentiation, which is generally associated with high invasion potential and chemoresistance. The molecular hallmarks of EMT are down-regulation of the epithelial adhesion molecule E-cadherin, de novo expression of N-cadherin, nuclear translocation of β-catenin, and expression of mesenchymal proteins including vimentin, smooth muscle actin, and fibronectin (reviewed in Moustakas and Heldin, 2007). HPV16 expressing cells have been shown to undergo EMT-related processes (Gilles et al., 1994), and this has been reported to be associated with E6 expression (Watson et al., 2003), although a recent manuscript has also implicated E7 expression with EMT development (Hellner et al., In Press).
3.9 Angiogenesis
Angiogenesis is a critical process in tumor progression that ensures that rapid tumor growth can be sustained by an adequate supply of oxygen and nutrients. HPV16 E6 has been shown to induce expression of vascular endothelial growth factor (VEGF) through a p53-independent transcriptional pathway (Lopez-Ocejo et al., 2000). Moreover, HPV16 E6 expression also resulted in increased expression of fibroblast growth factor binding protein (FGF-BP), which has been implicated as a modulator of the angiogenic switch (Stoppler et al., 2001). Consistent with these results, transcriptional analyses of HPV16 E6/E7 expressing human keratinocytes demonstrated increased expression of VEGF and interleukin-8 and decreased expression of the two angiogenesis inhibitors thrombospondin-1 and maspin (Chen et al., 2007; Toussaint-Smith et al., 2004).
4. Concluding Remarks
Cancer is a complex disease that arises as a consequence of multiple genetic aberrations. Emerging high-throughput/high-content analytical “omics” technologies have made it possible to rapidly and effectively catalogue aberrations at very high resolution at the genome, epigenome and transcriptome levels. Such approaches have already yielded clinically useful insights; the promise of “personalized medicine”, the ability to design efficacious therapeutic strategies based on defining molecular aberrations, however, has not yet been fully realized for most human solid tumors. Given the large number of genetic alterations that human solid tumors accumulate, one of the major challenges has been to identify genetic aberrations that mechanistically contribute to some stage of tumor development (“drivers”) as opposed to those that are generated due to increased genomic instability and do not directly contribute to the cancer process (“passengers”). Moreover, rapid, affordable, and comprehensive analyses of many functionally important parameters of the tumor proteome caused by alterations in protein translation, stability, subcellular localization, or posttranslational modifications remain elusive.
Virus-associated cancers present unique experimental systems to address some of these issues. Very much like hematological tumors that are often driven by a single initiating mutation, such as a specific chromosomal translocation, virus-associated cancers are initiated by uniform oncogenic hits: viral oncogene expression. This has been most impressively demonstrated for HPV-associated cervical cancers where E6 and E7 oncogene expression drive cancer initiation and progression (Figure 4). As pointed out in this review, much has already been learned from in depth molecular analyses of specific pathways that the viral oncoproteins are known or predicted to target. The major cellular targets of the HPV16 E6 and E7 oncoproteins, the p53 and pRB tumor suppressor pathways, are dysfunctional at some level in almost every human solid tumor and these aberrations are well-accepted to represent almost universal “driver mutations”.
This leaves us with several major challenges and opportunities for the future. The first is to design viable therapeutic strategies that target tumor suppressor loss. Thus far, the concept of restoring tumor suppressor activity by gene therapy-based strategies have been disappointing; whereas proof of principle gene transfer experiments clearly showed that restoring p53 or pRB tumor suppressor function inhibits tumorigenesis in vitro, attempts to translate this to practicable therapeutic approaches have been largely futile. Alternative approaches will need to be explored. One exciting possibility may be to perform genetic screens to identify druggable modifier genes of tumor suppressor pathways. One of the challenges is to define the appropriate genetic background for such screens. This is complicated by the fact that normal and cancer genomes are vastly different.
A second challenge will to identify additional “universal” drivers of human solid tumors. Researchers who study viral oncogenesis should be able to play a vital role in this endeavor by harnessing some of the same “omics” technologies that have been so productively used by several research groups who study non-virus associated human cancers. For these approaches, too, much thought needs to be given to choosing an appropriate genetic background.
Systems and network biology based comparisons of molecular aberrations induced by specific viral oncoproteins with those detected in non virus-associated human solid tumors should prove a highly fruitful area of investigation to identify consistent aberrations in signaling networks that drive cancer development.
Acknowledgments
Supported by NIH grants R01CA066980 and R01CA081135 (KM) and American Cancer Society Postdoctoral Fellowship PF-07-072-01-MBC (MEM-D). We thank Ari Czykler for editorial help and the two anonymous reviewers for their excellent comments on the initial version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisenberg AC. The Glycolysis and Respiration of Tumors. New York and London: Academic Press; 1961. [Google Scholar]
- Akgul B, Cooke JC, Storey A. HPV-associated skin disease. J Pathol. 2006;208(2):165–175. doi: 10.1002/path.1893. [DOI] [PubMed] [Google Scholar]
- Alani RM, Hasskarl J, Munger K. Alterations in cyclin-dependent kinase 2 function during differentiation of primary human keratinocytes. Mol Carcinog. 1998;23:226–233. doi: 10.1002/(sici)1098-2744(199812)23:4<226::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Antinore MJ, Birrer MJ, Patel D, Nader L, McCance DJ. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO Journal. 1996;15:1950–1960. [PMC free article] [PubMed] [Google Scholar]
- Appleby P, Beral V, Berrington de Gonzalez A, Colin D, Franceschi S, Goodill A, Green J, Peto J, Plummer M, Sweetland S. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118(6):1481–1495. doi: 10.1002/ijc.21493. [DOI] [PubMed] [Google Scholar]
- Arbeit JM. Transgenic models of epidermal neoplasia and multistage carcinogenesis. Cancer Surv. 1996;26:7–34. [PubMed] [Google Scholar]
- Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci U S A. 1996;93(7):2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK. Anchorage-dependent cell cycle progression. J Cell Biol. 1997;136(1):1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvakumov N, Torchia J, Mymryk JS. Interaction of the HPV E7 proteins with the pCAF acetyltransferase. Oncogene. 2003;22(25):3833–3841. doi: 10.1038/sj.onc.1206562. [DOI] [PubMed] [Google Scholar]
- Baker CC, Phelps WC, Lindgren V, Braun MJ, Gonda MA, Howley PM. Structural and translational analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. Journal of Virology. 1987;61:962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A, Huh KW, Munger K. Human papillomavirus E7 oncoprotein dysregulates steroid receptor coactivator 1 localization and function. J Virol. 2006;80(13):6669–6677. doi: 10.1128/JVI.02497-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A, Pirisi L, Creek KE. NFI-Ski interactions mediate transforming growth factor beta modulation of human papillomavirus type 16 early gene expression. J Virol. 2004;78(8):3953–3964. doi: 10.1128/JVI.78.8.3953-3964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsitis SJ, Sage J, Duensing S, Munger K, Jacks T, Lambert PF. Recapitulation of the effects of the human papillomavirus type 16 E7 oncogene on mouse epithelium by somatic Rb deletion and detection of pRb-independent effects of E7 in vivo. Mol Cell Biol. 2003;23(24):9094–9103. doi: 10.1128/MCB.23.24.9094-9103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannasch P, Klimek F, Mayer D. Early bioenergetic changes in hepatocarcinogenesis: preneoplastic phenotypes mimic responses to insulin and thyroid hormone. J Bioenerg Biomembr. 1997;29:303–313. doi: 10.1023/a:1022438528634. [DOI] [PubMed] [Google Scholar]
- Barnard P, McMillan NA. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-alpha. Virology. 1999;259:305–313. doi: 10.1006/viro.1999.9771. [DOI] [PubMed] [Google Scholar]
- Barnard P, Payne E, McMillan NA. The human papillomavirus E7 protein is able to inhibit the antiviral and anti-growth functions of interferon-alpha. Virology. 2000;277(2):411–419. doi: 10.1006/viro.2000.0584. [DOI] [PubMed] [Google Scholar]
- Basile JR, Eichten A, Zacny V, Munger K. NF-kB-mediated induction of p21cip1/Waf1 by tumor necrosis factor a induces growth arrest and cytoprotection in normal human keratinocytes. Mol Cancer Res. 2003;1:262–270. [PubMed] [Google Scholar]
- Basile JR, Zacny V, Munger K. The cytokines tumor necrosis factor-alpha (TNF-alpha ) and TNF-related apoptosis-inducing ligand differentially modulate proliferation and apoptotic pathways in human keratinocytes expressing the human papillomavirus-16 E7 oncoprotein. J Biol Chem. 2001;276:22522–22528. doi: 10.1074/jbc.M010505200. [DOI] [PubMed] [Google Scholar]
- Bedell MA, Jones KH, Grossman SR, Laimins LA. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. Journal of Virology. 1989;63:1247–1255. doi: 10.1128/jvi.63.3.1247-1255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat A, Avvakumov N, Mymryk JS, Banks L. Interaction between the HPV E7 oncoprotein and the transcriptional coactivator p300. Oncogene. 2003;22(39):7871–7881. doi: 10.1038/sj.onc.1206896. [DOI] [PubMed] [Google Scholar]
- Bibbo M, Dytch HE, Alenghat E, Bartels PH, Wied GL. DNA ploidy profiles as prognostic indicators in CIN lesions. Am J Clin Pathol. 1989;92:261–265. doi: 10.1093/ajcp/92.3.261. [DOI] [PubMed] [Google Scholar]
- Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- Brehm A, Nielsen SJ, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. Embo J. 1999;18:2449–2458. doi: 10.1093/emboj/18.9.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulten J, Poddighe PJ, Robben JCM, Gemmink JH, de Wilde PCM, Hanselaar AGJM. Interphase cytogenetic analysis of cervical intraepithelial neoplasia. Am J Pathol. 1998;152:495–503. [PMC free article] [PubMed] [Google Scholar]
- Cates W., Jr Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. American Social Health Association Panel. Sex Transm Dis. 1999;26(4 Suppl):S2–S7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- Chellappan S, Kraus VB, Kroger B, Münger K, Howley PM, Phelps WC, Nevins JR. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between the transcription factor E2F and the retinoblastoma gene product. Proceedings of the National Academy of Sciences USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Li F, Mead L, White H, Walker J, Ingram DA, Roman A. Human papillomavirus causes an angiogenic switch in keratinocytes which is sufficient to alter endothelial cell behavior. Virology. 2007;367(1):168–174. doi: 10.1016/j.virol.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes & Development. 1995;9:2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- Chesson HW, Blandford JM, Gift TL, Tao G, Irwin KL. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health. 2004;36(1):11–19. doi: 10.1363/psrh.36.11.04. [DOI] [PubMed] [Google Scholar]
- Ciccolini F, Dipasquale G, Carlotti F, Crawford L, Tommasino M. Functional studies of E7 proteins from different HPV types. Oncogene. 1994;9:2633–2638. [PubMed] [Google Scholar]
- Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89(1):101–105. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottage A, Dowen S, Roberts I, Pett M, Coleman N, Stanley M. Early genetic events in HPV immortalised keratinocytes. Genes Chromosomes Cancer. 2001;30(1):72–79. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1060>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93(4):495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- Creek KE, Geslani G, Batova A, Pirisi L. Progressive loss of sensitivity to growth control by retinoic acid and transforming growth factor-beta at late stages of human papillomavirus type 16-initiated transformation of human keratinocytes. Adv Exp Med Biol. 1995;375:117–135. doi: 10.1007/978-1-4899-0949-7_11. [DOI] [PubMed] [Google Scholar]
- Crum CP, Ikenberg H, Richart RM, Gissman L. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984;310(14):880–883. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- De Geest K, Bergman CA, Turyk ME, Frank BS, Wilbanks GD. Differential response of cervical intraepithelial and cervical carcinoma cell lines to transforming growth factor-beta 1. Gynecol Oncol. 1994;55(3 Pt 1):376–385. doi: 10.1006/gyno.1994.1310. [DOI] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- DeCaprio JA, Ludlow JW, Figge J, Shew JY, Huang CM, Lee WH, Marsilio E, Paucha E, Livingston DM. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeMasi J, Chao MC, Kumar AS, Howley PM. Bovine papillomavirus E7 oncoprotein inhibits anoikis. J Virol. 2007;81(17):9419–9425. doi: 10.1128/JVI.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMasi J, Huh KW, Nakatani Y, Munger K, Howley PM. Bovine papillomavirus E7 transformation function correlates with cellular p600 protein binding. Proc Natl Acad Sci U S A. 2005;102(32):11486–11491. doi: 10.1073/pnas.0505322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth PK, Dotto GP. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- Du W, Pogoriler J. Retinoblastoma family genes. Oncogene. 2006;25(38):5190–5200. doi: 10.1038/sj.onc.1209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing A, Chin A, Wang L, Kuan SF, Duensing S. Analysis of centrosome overduplication in correlation to cell division errors in high-risk human papillomavirus (HPV)-associated anal neoplasms. Virology. 2007a doi: 10.1016/j.virol.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing A, Chin A, Wang L, Kuan SF, Duensing S. Analysis of centrosome overduplication in correlation to cell division errors in high-risk human papillomavirus (HPV)-associated anal neoplasms. Virology. 2008;372(1):157–164. doi: 10.1016/j.virol.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing A, Liu Y, Perdreau SA, Kleylein-Sohn J, Nigg EA, Duensing S. Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene. 2007b;26(43):6280–6288. doi: 10.1038/sj.onc.1210456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing A, Liu Y, Tseng M, Malumbres M, Barbacid M, Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25(20):2943–2949. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing A. A tentative classification of centrosome abnormalities in cancer. Cell Biol Int. 2005 doi: 10.1016/j.cellbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Duensing S, Duensing A, Crum CP, Munger K. Human papillomavirus type 16 E7 oncoprotein-induced abnormal centrosome synthesis is an early event in the evolving malignant phenotype. Cancer Res. 2001a;61:2356–2360. [PubMed] [Google Scholar]
- Duensing S, Duensing A, Flores ER, Do A, Lambert PF, Munger K. Centrosome abnormalities and genomic instability by episomal expression of human papillomavirus type 16 in raft cultures of human keratinocytes. J Virol. 2001b;75:7712–7716. doi: 10.1128/JVI.75.16.7712-7716.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S, Duensing A, Lee DC, Edwards KM, Piboonniyom SO, Manuel E, Skaltsounis L, Meijer L, Munger K. Cyclin-dependent kinase inhibitor indirubin-3’-oxime selectively inhibits human papillomavirus type 16 E7-induced numerical centrosome anomalies. Oncogene. 2004;23(50):8206–8215. doi: 10.1038/sj.onc.1208012. [DOI] [PubMed] [Google Scholar]
- Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, Crum CP, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A. 2000;97:10002–10007. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S, Munger K. Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J Virol. 2003;77(22):12331–12335. doi: 10.1128/JVI.77.22.12331-12335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S, Münger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Canc. Res. 2002;62:7075–7082. [PubMed] [Google Scholar]
- Dürst M, Gallahan D, Jay G, Rhim JS. Glucocorticoid enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology. 1989;173:767–771. doi: 10.1016/0042-6822(89)90595-3. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Dyson N, Guida P, Münger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. Journal of Virology. 1992;66:6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N, Howley PM, Münger K, Harlow E. The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Eichten A, Rud DS, Grace M, Piboonniyom SO, Zacny V, Munger K. Molecular pathways executing the “trophic sentinel” response in HPV-16 E7-expressing normal human diploid fibroblasts upon growth factor deprivation. Virology. 2004;319(1):81–93. doi: 10.1016/j.virol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Eichten A, Westfall M, Pietenpol JA, Münger K. Stabilization and functional impairment of the tumor suppressor p53 by the human papillomavirus type 16 E7 oncoprotein. Virology. 2002;295:74–95. doi: 10.1006/viro.2002.1375. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24(17):2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271(5248):499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9(6):425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin DM. IARC CancerBase, 5. Lyon: IARCPress; 2001. GLOBOCAN 2000: Cancer Incidence, Mortality and Prevalence Worldwide, Version 1.0. [Google Scholar]
- Firpo EJ, Koff A, Solomon MJ, Roberts JM. Inactivation of a cdk2 inhibitor during interleukin 2-induced proliferation of human T lymphocytes. Molecular and Cellular Biology. 1994;14:4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, Castellsague X, Dal Maso L, Smith JS, Plummer M, Ngelangel C, Chichareon S, Eluf-Neto J, Shah KV, Snijders PJ, Meijer CJ, Bosch FX, Munoz N. Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer. 2002;86(5):705–711. doi: 10.1038/sj.bjc.6600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DA, Schmid SI, Howley PM. Repression of the integrated papillomavirus E6/E7 promoter is required for growth suppression of cervical cancer cells. J Virol. 2000;74(6):2679–2686. doi: 10.1128/jvi.74.6.2679-2686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005;23(17–18):2388–2394. doi: 10.1016/j.vaccine.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol. 2004;4(1):46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- Frega A, Stentella P, Tinari A, Vecchione A, Marchionni M. Giant condyloma acuminatum or buschke-Lowenstein tumor: review of the literature and report of three cases treated by CO2 laser surgery. A long-term follow-up. Anticancer Res. 2002;22(2B):1201–1204. [PubMed] [Google Scholar]
- Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiencysyndrome. J Natl Cancer Inst. 2000;92(18):1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437(7061):1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes & Development. 1997;11:2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol D, Kuhne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene. 1999;18(40):5487–5496. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- Gewin L, Myers H, Kiyono T, Galloway DA. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 2004;18(18):2269–2282. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles C, Polette M, Piette J, Birembaut P, Foidart JM. Epithelial-to-mesenchymal transition in HPV-33-transfected cervical keratinocytes is associated with increased invasiveness and expression of gelatinase A. Int J Cancer. 1994;59(5):661–666. doi: 10.1002/ijc.2910590514. [DOI] [PubMed] [Google Scholar]
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high- risk papillomavirus E6 oncoproteins. Oncogene. 2000;19(46):5270–5280. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci U S A. 2000;97(23):12513–12518. doi: 10.1073/pnas.97.23.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, Langenberg A, Yen TS, Ralston R. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995;33(8):2058–2063. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill SW, Gonczy P, Stelzer EH, Hyman AA. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature. 2001;409(6820):630–633. doi: 10.1038/35054572. [DOI] [PubMed] [Google Scholar]
- Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA. The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell. 2005;16(3):1095–1107. doi: 10.1091/mbc.E04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver GA, Herber RL, Liem A, Lambert PF. Both conserved region 1 (CR1) and CR2 of the human papillomavirus type 16 E7 oncogene are required for induction of epidermal hyperplasia and tumor formation in transgenic mice. J Virol. 1997;71(8):5905–5914. doi: 10.1128/jvi.71.8.5905-5914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WW, Fujii M. Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene. 2005;24(39):5965–5975. doi: 10.1038/sj.onc.1208975. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hashida T, Yasumoto S. Induction of chromosomal abnormalities in mouse and human epidermal keratinocytes by the human papillomavirus type 16 E7 oncogene. Journal of General Virology. 1991;72:1569–1577. doi: 10.1099/0022-1317-72-7-1569. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. The EMBO Journal. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Staples D, Smith C, Fisher C. Direct activation of cyclin-dependent kinase 2 by human papillomavirus E7. J Virol. 2003;77(19):10566–10574. doi: 10.1128/JVI.77.19.10566-10574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DV, Yee CL, Howley PM, Münger K. Efficiency of binding to the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proceedings of the National Academy of Sciences USA. 1992;89:4442–4446. doi: 10.1073/pnas.89.10.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellner K, Mar JC, Fang F, Quackenbush J, Munger K. HPV16 E7 oncogene expression in normal human epithelial cells causes molecular changes indicative of an epithelial to mesenchymal transition. Virology. In Press doi: 10.1016/j.virol.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Hoppe-Seyler K, Schuller B, Lohrey C, Maroldt J, Durst M, Hoppe-Seyler F. Activation of the enhancer of zeste homologue 2 gene by the human papillomavirus E7 oncoprotein. Cancer Res. 2008;68(23):9964–9972. doi: 10.1158/0008-5472.CAN-08-1134. [DOI] [PubMed] [Google Scholar]
- Howley PM, Lowy DR. Papillomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. pp. 2299–2354. [Google Scholar]
- Huang SM, McCance DJ. Down Regulation of the Interleukin-8 Promoter by Human Papillomavirus Type 16 E6 and E7 through Effects on CREB Binding Protein/p300 and P/CAF. J Virol. 2002;76(17):8710–8721. doi: 10.1128/JVI.76.17.8710-8721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh K, Zhou X, Hayakawa H, Cho JY, Libermann TA, Jin J, Harper JW, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol. 2007;81(18):9737–9747. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102(32):11492–11497. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. The EMBO Journal. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin PJ, Kaur P, Smith PP, Perez-Reyes N, Blanton RA, McDougall JK. Progression of human papillomavirus type 18-immortalized human keratinocytes to a malignant phenotype. Proceedings of the National Academy of Sciences USA. 1991;88:571–574. doi: 10.1073/pnas.88.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SG, Lee D, Kim J, Seo T, Choe J. Human papillomavirus type 16 E7 binds to E2F1 and activates E2F1-driven transcription in a retinoblastoma protein-independent manner. J Biol Chem. 2002;277:2923–2930. doi: 10.1074/jbc.M109113200. [DOI] [PubMed] [Google Scholar]
- Jablonska S, Fabjanska L, Formas I. On the viral etiology of epidermodysplasia verruciformis. Dermatologica. 1966;132(5):369–385. doi: 10.1159/000254437. [DOI] [PubMed] [Google Scholar]
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- Jeon S, Allen-Hoffmann BL, Lambert PF. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J Virol. 1995;69:2989–2997. doi: 10.1128/jvi.69.5.2989-2997.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: Implications for cervical carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing M, Bohl J, Brimer N, Kinter M, Vande Pol SB. Degradation of tyrosine phosphatase PTPN3 (PTPH1) by association with oncogenic human papillomavirus E6 proteins. J Virol. 2007;81(5):2231–2239. doi: 10.1128/JVI.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Alani RM, Münger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes & Development. 1997a;11:2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Münger K. Analysis of the p53- mediated G1 growth arrest pathway in cells expressing the human papillomavirus type 16 E7 oncoprotein. Journal of Virology. 1997;71:2905–2912. doi: 10.1128/jvi.71.4.2905-2912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Thompson DA, Münger K. Destabilization of the RB tumor suppressor and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology. 1997b;239:97–107. doi: 10.1006/viro.1997.8851. [DOI] [PubMed] [Google Scholar]
- Kadaja M, Sumerina A, Verst T, Ojarand M, Ustav E, Ustav M. Genomic instability of the host cell induced by the human papillomavirus replication machinery. Embo J. 2007;26(8):2180–2191. doi: 10.1038/sj.emboj.7601665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katich SC, Zerfass-Thome K, Hoffmann I. Regulation of the Cdc25A gene by the human papillomavirus Type 16 E7 oncogene. Oncogene. 2001;20:543–550. doi: 10.1038/sj.onc.1204130. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen RA, Egelkrout EM, Vliet-Gregg P, Gewin LC, Gafken PR, Galloway DA. NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells. J Virol. 2007;81(8):3786–3796. doi: 10.1128/JVI.02007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RD, D’Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005;19(24):2925–2940. doi: 10.1101/gad.1370505. [DOI] [PubMed] [Google Scholar]
- Kessis TD, Connolly DC, Hedrick L, Cho KR. Expression of HPV16 E6 or E7 increases integration of foreign DNA. Oncogene. 1996;13(2):427–431. [PubMed] [Google Scholar]
- Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci U S A. 1997;94(21):11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102(5A):3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, Shah KV, Gillison ML. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189(4):686–698. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- Kutler DI, Wreesmann VB, Goberdhan A, Ben-Porat L, Satagopan J, Ngai I, Huvos AG, Giampietro P, Levran O, Pujara K, Diotti R, Carlson D, Huryn LA, Auerbach AD, Singh B. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2003;95(22):1718–1721. doi: 10.1093/jnci/djg091. [DOI] [PubMed] [Google Scholar]
- Lee C, Laimins LA. Role of the PDZ domain-binding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. J Virol. 2004;78(22):12366–12377. doi: 10.1128/JVI.78.22.12366-12377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Glaunsinger B, Mantovani F, Banks L, Javier RT. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol. 2000;74(20):9680–9693. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci U S A. 1997;94(13):6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Baleja JD. Structure and function of the papillomavirus E6 protein and its interacting proteins. Front Biosci. 2008;13:121–134. doi: 10.2741/2664. [DOI] [PubMed] [Google Scholar]
- Longworth MS, Laimins LA. The binding of histone deacetylases and the integrity of zinc finger-like motifs of the E7 protein are essential for the life cycle of human papillomavirus type 31. J Virol. 2004;78(7):3533–3541. doi: 10.1128/JVI.78.7.3533-3541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ocejo O, Viloria-Petit A, Bequet-Romero M, Mukhopadhyay D, Rak J, Kerbel RS. Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19(40):4611–4620. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432(7015):307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Luscher-Firzlaff JM, Westendorf JM, Zwicker J, Burkhardt H, Henriksson M, Muller R, Pirollet F, Luscher B. Interaction of the fork head domain transcription factor MPP2 with the human papilloma virus 16 E7 protein: enhancement of transformation and transactivation. Oncogene. 1999;18:5620–5630. doi: 10.1038/sj.onc.1202967. [DOI] [PubMed] [Google Scholar]
- Lyons TE, Salih M, Tuana BS. Activating E2Fs mediate transcriptional regulation of human E2F6 repressor. Am J Physiol Cell Physiol. 2006;290(1):C189–C199. doi: 10.1152/ajpcell.00630.2004. [DOI] [PubMed] [Google Scholar]
- Majewski S, Jablonska S, Orth G. Epidermodysplasia verruciformis Immunological and nonimmunological surveillance mechanisms: role in tumor progression. Clin Dermatol. 1997;15:321–334. doi: 10.1016/s0738-081x(96)00169-1. [DOI] [PubMed] [Google Scholar]
- Mannhardt B, Weinzimer SA, Wagner M, Fiedler M, Cohen P, Jansen-Durr P, Zwerschke W. Human papillomavirus type 16 E7 oncoprotein binds and inactivates growth-inhibitory insulin-like growth factor binding protein 3. Mol Cell Biol. 2000;20:6483–6495. doi: 10.1128/mcb.20.17.6483-6495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik A, Runkorg K, Jaanson N, Ustav M, Ustav E. Induction of the bovine papillomavirus origin “onion skin”-type DNA replication at high E1 protein concentrations in vivo. J Virol. 2002;76(11):5835–5845. doi: 10.1128/JVI.76.11.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimi P, Pim D, Storey A, Banks L. HPV-16 E7 and adenovirus E1a complex formation with TATA box binding protein is enhanced by casein kinase II phosphorylation. Oncogene. 1996;12(11):2325–2330. [PubMed] [Google Scholar]
- Maufort JP, Williams SM, Pitot HC, Lambert PF. Human papillomavirus 16 E5 oncogene contributes to two stages of skin carcinogenesis. Cancer Res. 2007;67(13):6106–6112. doi: 10.1158/0008-5472.CAN-07-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek S, Zwerschke W, Jansen-Durr P, Eigenbrodt E. Effects of the human papilloma virus HPV-16 E7 oncoprotein on glycolysis and glutaminolysis: role of pyruvate kinase type M2 and the glycolytic-enzyme complex. Biochem J. 2001;356(Pt 1):247–256. doi: 10.1042/0264-6021:3560247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarelli JM, Atkins GB, Geisberg JV, Ricciardi RP. The viral oncoproteins Ad5 E1A, HPV16 E7 and SV40 TAg bind a common region of the TBP-associated factor-110. Oncogene. 1995;11:1859–1864. [PubMed] [Google Scholar]
- McCance DJ, Kopan R, Fuchs E, Laimins LA. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proceedings of the National Academy of Sciences USA. 1988;85:7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KM, Zhang J, Holst CR, Kozakiewicz BK, Singla V, Tlsty TD. p16(INK4a) prevents centrosome dysfunction and genomic instability in primary cells. PLoS Biol. 2006;4(3):e51. doi: 10.1371/journal.pbio.0040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Huh KW, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with E2F6. J Virol. 2008;82(17):8695–8705. doi: 10.1128/JVI.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray HR, McCance DJ. Human Papillomavirus Type 16 E6 Activates TERT Gene Transcription through Induction of c-Myc and Release of USF-Mediated Repression. J Virol. 2003;77(18):9852–9861. doi: 10.1128/JVI.77.18.9852-9861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10(23):3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- Mitelman F, Johansson B, Mertens F. Mitelman database of chromosome aberrations in cancer. 2007 http://cgap.nci.nih.gov/Chromosomes/Mitelman.
- Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger K. The role of human papillomaviruses in human cancers. Front Biosci. 2002;7:d641–d649. doi: 10.2741/a800. [DOI] [PubMed] [Google Scholar]
- Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. Journal of Virology. 1989a;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. The EMBO Journal. 1989b;8:4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151(12):1158–1171. doi: 10.1093/oxfordjournals.aje.a010166. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin- protein ligase. Mol Cell Biol. 2000;20(21):8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, Eichwald C, Nibert ML, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin. J Virol. 2007;81(24):13533–13543. doi: 10.1128/JVI.01669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, McLaughlin-Drubin ME, Munger K. Delocalization of the microtubule motor Dynein from mitotic spindles by the human papillomavirus E7 oncoprotein is not sufficient for induction of multipolar mitoses. Cancer Res. 2008;68(21):8715–8722. doi: 10.1158/0008-5472.CAN-08-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, Munger K. Direct association of the HPV16 E7 oncoprotein with cyclin A/CDK2 and cyclin E/CDK2 complexes. Virology. 2008;380(1):21–25. doi: 10.1016/j.virol.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CL, Munger K. Human papillomavirus E7 protein deregulates mitosis via an association with nuclear mitotic apparatus protein 1. J Virol. 2009;83(4):1700–1707. doi: 10.1128/JVI.01971-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Westbrook TF, McCance DJ. Human papillomavirus type 16 E7 maintains elevated levels of the cdc25A tyrosine phosphatase during deregulation of cell cycle arrest. J Virol. 2002;76(2):619–632. doi: 10.1128/JVI.76.2.619-632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6’s induction of epithelial hyperplasia in vivo. J Virol. 2003;77(12):6957–6964. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G, Jablonska S, Favre M, Croissant O, Jarzabek-Chorzelska M, Rzesa G. Characterization of two types of human papillomaviruses in lesions of epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1978;75:1537–1541. doi: 10.1073/pnas.75.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Kim EJ, Kwon HJ, Hwang ES, Namkoong SE, Um SJ. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000;275(10):6764–6769. doi: 10.1074/jbc.275.10.6764. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl3):S3/11–S3/25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Pass F, Reissig M, Shah KV, Eisinger M, Orth G. Identification of an immunologically distinct papillomavirus from lesions of epidermodysplasia verruciformis. J Natl Cancer Inst. 1977;59(4):1107–1112. doi: 10.1093/jnci/59.4.1107. [DOI] [PubMed] [Google Scholar]
- Patel D, Huang SM, Baglia LA, McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co- activation by CBP and p300. Embo J. 1999;18:5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SN. Enzymatic studies of glycogen metabolism in nonmalignant and malignant biopsies from the human uterine cervix. Acta Obstet Gynecol Scand. 1975;54:443–448. doi: 10.3109/00016347509157107. [DOI] [PubMed] [Google Scholar]
- Pei XF, Meck JM, Greenhalgh D, Schlegel R. Cotransfection of HPV-18 and v-fos DNA induces tumorigenicity of primary human keratinocytes. Virology. 1993;196(2):855–860. doi: 10.1006/viro.1993.1546. [DOI] [PubMed] [Google Scholar]
- Perea SE, Massimi P, Banks L. Human papillomavirus type 16 E7 impairs the activation of the interferon regulatory factor-1. Int J Mol Med. 2000;5(6):661–666. doi: 10.3892/ijmm.5.6.661. [DOI] [PubMed] [Google Scholar]
- Pett MR, Alazawi WO, Roberts I, Dowen S, Smith DI, Stanley MA, Coleman N. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 2004;64(4):1359–1368. doi: 10.1158/0008-5472.can-03-3214. [DOI] [PubMed] [Google Scholar]
- Pfister H, Nurnberger F, Gissmann L, zur Hausen H. Characterization of a human papillomavirus from epidermodysplasia verruciformis lesions of a patient from Upper-volta. Int J Cancer. 1981;27(5):645–650. doi: 10.1002/ijc.2910270511. [DOI] [PubMed] [Google Scholar]
- Phelps WC, Münger K, Yee CL, Barnes JA, Howley PM. Structure-function analysis of the human papillomavirus E7 oncoprotein. Journal of Virology. 1992;66:2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol JA, Stein RW, Moran E, Yaciuk P, Schlegel R, Lyons RM, Pittelkow MR, Münger K, Howley PM, Moses HL. TGFb1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990;61:777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Rueda LA, Bouadjar B, Montoya LS, Orth G, Favre M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32(4):579–581. doi: 10.1038/ng1044. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Taieb A, Rueda LA, Montoya LS, Bouadjar B, Favre M, Orth G. Evidence for a nonallelic heterogeneity of epidermodysplasia verruciformis with two susceptibility loci mapped to chromosome regions 2p21-p24 and 17q25. J Invest Dermatol. 2000;114:1148–1153. doi: 10.1046/j.1523-1747.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proceedings of the National Academy of Sciences USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB Journal. 2000;14:2185–2197. doi: 10.1096/fj.00-0029com. [DOI] [PubMed] [Google Scholar]
- Rhea WG, Jr, Bourgeois BM, Sewell DR. Condyloma acuminata: a fatal disease? Am Surg. 1998;64(11):1082–1087. [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2004 based on November 2006. 2007 SEER data submission http://seer.cancer.gov/csr/1975_2004/
- Rihet S, Lorenzato M, Clavel C. Oncogenic human papillomaviruses and ploidy in cervical lesions. J Clin Pathol. 1996;49(11):892–896. doi: 10.1136/jcp.49.11.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63(16):4862–4871. [PubMed] [Google Scholar]
- Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12(13):2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang B-C, Barbosa MS. Single amino acid substitutions in “low-risk” human papillomavirus (HPV) type 6 E7 protein enhance features characteristic of the “high risk” HPV E7 oncoproteins. Proceedings of the National Academy of Sciences USA. 1992;89:8063–8067. doi: 10.1073/pnas.89.17.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer FR, Moser B, Spoden GA, Jansen-Durr P, Zwerschke W. Human papillomavirus type 16 E7 oncoprotein inhibits apoptosis mediated by nuclear insulin-like growth factor-binding protein-3 by enhancing its ubiquitin/proteasome-dependent degradation. Carcinogenesis. 2007;28(12):2511–2520. doi: 10.1093/carcin/bgm199. [DOI] [PubMed] [Google Scholar]
- Schaeffer AJ, Nguyen M, Liem A, Lee D, Montagna C, Lambert PF, Ried T, Difilippantonio MJ. E6 and E7 oncoproteins induce distinct patterns of chromosomal aneuploidy in skin tumors from transgenic mice. Cancer Res. 2004;64(2):538–546. doi: 10.1158/0008-5472.can-03-0124. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP Complex Functions as a Ubiquitin-Protein Ligase in the Ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Scheurer ME, Tortolero-Luna G, Adler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15(5):727–746. doi: 10.1111/j.1525-1438.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences incervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Shamanin V, Glover M, Rausch C, Proby C, Leigh IM, zur Hausen H, de Villiers EM. Specific types of human papillomavirus found in benign proliferations and carcinomas of the skin in immunosuppressed patients. Cancer Res. 1994;54:4610–4613. [PubMed] [Google Scholar]
- Skyldberg B, Fujioka K, Hellstrom AC, Sylven L, Moberger B, Auer G. Human papillomavirus infection, centrosome aberration, and genetic stability in cervical lesions. Mod Pathol. 2001;14(4):279–284. doi: 10.1038/modpathol.3880303. [DOI] [PubMed] [Google Scholar]
- Smith PP, Bryant EM, Kaur P, McDougall JK. Cytogenetic analysis of eight human papillomavirus immortalized human keratinocyte cell lines. Int J Cancer. 1989;44(6):1124–1131. doi: 10.1002/ijc.2910440631. [DOI] [PubMed] [Google Scholar]
- Smith PP, Friedman CL, Bryant EM, McDougall JK. Viral integration and fragile sites in human papillomavirus-immortalized human keratinocyte cell lines. Genes Chromosomes Cancer. 1992;5(2):150–157. doi: 10.1002/gcc.2870050209. [DOI] [PubMed] [Google Scholar]
- Southern SA, Evans MF, Herrington CS. Basal cell tetrasomy in low-grade cervical squamous intraepithelial lesions infected with high-risk human papillomaviruses. Cancer Res. 1997;57(19):4210–4213. [PubMed] [Google Scholar]
- Spardy N, Duensing A, Charles D, Haines N, Nakahara T, Lambert PF, Duensing S. The human papillomavirus type 16 E7 oncoprotein activates the Fanconi Anemia (FA) pathway and causes accelerated chromosomal instability in FA cells. J Virol. 2007;81 doi: 10.1128/JVI.01121-07. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitkovsky D, Hehner SP, Hofmann TG, Moller A, Schmitz ML. The human papillomavirus oncoprotein E7 attenuates NF-kappa B activation by targeting the Ikappa B kinase complex. J Biol Chem. 2002;277(28):25576–25582. doi: 10.1074/jbc.M201884200. [DOI] [PubMed] [Google Scholar]
- Stearns T. Centrosome Duplication A Centriolar Pas de Deux. Cell. 2001;105(4):417–420. doi: 10.1016/s0092-8674(01)00366-x. [DOI] [PubMed] [Google Scholar]
- Steinbeck RG. Proliferation and DNA aneuploidy in mild dysplasia imply early steps of cervical carcinogenesis. Acta Oncol. 1997;36:3–12. doi: 10.3109/02841869709100723. [DOI] [PubMed] [Google Scholar]
- Stoppler H, Malerczyk C, Block K, Aigner A, Czubayko F. The human papillomavirus (HPV) 16 E6 oncoprotein leads to an increase in gene expression of the angiogenic switch molecule FGF-BP in non-immortalized human keratinocytes. Oncogene. 2001;20(50):7430–7436. doi: 10.1038/sj.onc.1204957. [DOI] [PubMed] [Google Scholar]
- Stoppler H, Stoppler MC, Johnson E, Simbulan-Rosenthal CM, Smulson ME, Iyer S, Rosenthal DS, Schlegel R. The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene. 1998;17:1207–1214. doi: 10.1038/sj.onc.1202053. [DOI] [PubMed] [Google Scholar]
- Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5(1):45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- Storrs CH, Silverstein SJ. PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6. J Virol. 2007;81(8):4080–4090. doi: 10.1128/JVI.02545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F, Laimins LA. Human papillomavirus life cycle: active and latent phases. Semin Cancer Biol. 1999;9:379–386. doi: 10.1006/scbi.1999.0141. [DOI] [PubMed] [Google Scholar]
- Sur M, Cooper K. The role of the human papilloma virus in esophageal cancer. Pathology. 1998;30(4):348–354. doi: 10.1080/00313029800169616. [DOI] [PubMed] [Google Scholar]
- Talbert-Slagle K, DiMaio D. The bovine papillomavirus E5 protein and the PDGF beta receptor: it takes two to tango. Virology. 2009;384(2):345–351. doi: 10.1016/j.virol.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki T, Mulder LC, Iwamatsu A, Lee MJ, Davydov IV, Varshavsky A, Muesing M, Kwon YT. A family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons. Mol Cell Biol. 2005;25(16):7120–7136. doi: 10.1128/MCB.25.16.7120-7136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasca RA, Clarke RW. Recurrent respiratory papillomatosis. Arch Dis Child. 2006;91(8):689–691. doi: 10.1136/adc.2005.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber B, Dobner T. Adenovirus early E4 genes in viral oncogenesis. Oncogene. 2001;20(54):7847–7854. doi: 10.1038/sj.onc.1204914. [DOI] [PubMed] [Google Scholar]
- Thierry F, Yaniv M. The BPV-1 E2 trans-acting protein can be either an activator or repressor of the HPV-18 regulatory region. The EMBO Journal. 1987;6:3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21(33):5088–5096. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Zacny V, Belinsky GS, Clason M, Jones DL, Schlegel R, Münger K. The HPV E7 oncoprotein E7 inhibits tumor necrosis factor a-mediated apoptosis in normal human fibroblasts. Oncogene. 2001;20:3629–3640. doi: 10.1038/sj.onc.1204483. [DOI] [PubMed] [Google Scholar]
- Toussaint-Smith E, Donner DB, Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene. 2004;23(17):2988–2995. doi: 10.1038/sj.onc.1207442. [DOI] [PubMed] [Google Scholar]
- Veldman T, Liu X, Yuan H, Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc Natl Acad Sci U S A. 2003;100(14):8211–8216. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa LL, Vieira K-B-L, Pei XF, Schlegel R. Differential effect of tumor necrosis factor on proliferation of primary human keratinocytes and cell lines containing human papillomavirus types 16 and 18. Molecular Carcinogenesis. 1992;6:5–9. doi: 10.1002/mc.2940060103. [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Warburg O. Ueber den Stoffwechsel der Tumoren. Berlin: Springer; 1936. [Google Scholar]
- Watson RA, Thomas M, Banks L, Roberts S. Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J Cell Sci. 2003;116(Pt 24):4925–4934. doi: 10.1242/jcs.00809. [DOI] [PubMed] [Google Scholar]
- Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- White AE, Livanos EM, Tlsty TD. Differential Disruption of Genomic Integrity and Cell Cycle Regulation in Normal Human Fibroblasts by the HPV Oncoproteins. Genes & Development. 1994;8:666–677. doi: 10.1101/gad.8.6.666. [DOI] [PubMed] [Google Scholar]
- Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E. Association between an oncogene and an antioncogene: the adenovirus E1a proteins bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Winkler B, Crum CP, Fujii T, Ferenczy A, Boon M, Braun L, Lancaster WD, Richart RM. Koilocytotic lesions of the cervix The relationship of mitotic abnormalities to the presence of papillomavirus antigens and nuclear DNA content. Cancer. 1984;53(5):1081–1087. doi: 10.1002/1097-0142(19840301)53:5<1081::aid-cncr2820530511>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Woodworth CD, Notario V, DiPaolo JA. Transforming growth factors beta 1 and 2 transcriptionally regulate human papillomavirus (HPV) type 16 early gene expression in HPV-immortalized human genital epithelial cells. Journal of Virology. 1990;64:4767–4775. doi: 10.1128/jvi.64.10.4767-4775.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerfass K, Schulze A, Spitkovsky D, Friedman V, Henglein B, Jansendurr P. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. Journal of General Virology. 1995;69:6389–6399. doi: 10.1128/jvi.69.10.6389-6399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz JW, Jansen-Durr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13:2323–2330. [PubMed] [Google Scholar]
- Zhou X, Munger K. Expression of the human papillomavirus type 16 E7 oncoprotein induces an autophagy-related process and sensitizes normal human keratinocytes to cell death in response to growth factor deprivation. Virology. 2009;385(1):192–197. doi: 10.1016/j.virol.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegert C, Wentzensen N, Vinokurova S, Kisseljov F, Einenkel J, Hoeckel M, von Knebel Doeberitz M. A comprehensive analysis of HPV integration loci in anogenital lesions combining transcript and genome-based amplification techniques. Oncogene. 2003;22(25):3977–3984. doi: 10.1038/sj.onc.1206629. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Degenkolbe R, Bernard HU, O’Connor MJ. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- Zwerschke W, Mannhardt B, Massimi P, Nauenburg S, Pim D, Nickel W, Banks L, Reuser AJ, Jansen-Durr P. Allosteric activation of acid alpha-glucosidase by the human papillomavirus E7 protein. J Biol Chem. 2000;275(13):9534–9541. doi: 10.1074/jbc.275.13.9534. [DOI] [PubMed] [Google Scholar]
- Zwerschke W, Mazurek S, Massimi P, Banks L, Eigenbrodt E, Jansen-Durr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1999;96:1291–1296. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]