Abstract
Skeletal distortions impose grave health disparities with potentially devastating consequences, including bone pain, immobility, and morbidity. Bone erosion is chiefly caused by hyperactive osteoclasts summoned to bone in response to circulating factors produced by tumor and inflammatory cells. Intense research in the past two decades has identified crucial elements and intricate circulatory systems that maintain and exacerbate inflammatory osteolysis. This progress led to better understanding of the mechanisms underlying this response and to developing advanced therapeutic interventions. Nevertheless, the multi-factorial causes of inflammatory osteolysis continue to impose a great challenge for these therapies. This article provides an overview of some of the prominent facets contributing to this process.
Introduction
Inflammation and tumors contribute to osteolysis causing bone pain and debilitating skeletal instability [1,2]. Skeletal integrity depends on bone homeostasis which is achieved by balanced function of bone cells. Bone formation by osteoblasts and bone resorption by osteoclasts are life long events delicately balanced in healthy individuals. This homeostasis is compromised under pathologic conditions such as metabolic and inflammatory diseases including osteoporosis, inflammatory osteolysis, and skeletal tumor metastases, wherein heightened osteoclast activity leads in most cases to increased bone loss. The consequences of overall bone weakening and localized focal bone erosions range from bone pain to bone fractures, hypercalcemia, and other mineral imbalances that erode skeletal stability. Conceptually, inflammatory and metastatic factors generally highjack bone cells and signaling cascades from their basally balanced state and coerce them into a continuously fueled hyperactive state to establish debilitating osteolysis.
Bone homeostasis and patho-physiology
Normal activity of osteoclasts and osteoblasts is essential for maintenance of bone homeostasis. Osteoclasts are the principal cells regulating bone resorption and remodeling, and absence of these cells ultimately leads to osteopetrosis [3]. Differentiation of osteoclasts depends primarily on two hematopoietic cytokines; M-CSF and receptor activator of NF-κB ligand (RANKL) [3]. These two cytokines are crucial for basal skeletal homeostasis. However, under certain pathological conditions, including inflammation and bone tumors the production of these factors is exacerbated resulting with increased osteoclastogenesis and subsequent bone destruction. A major breakthrough in regulation of osteoclastogenesis was achieved with the discovery of osteoprotegerin (OPG), a soluble protein of the TNF-receptor family [4]. OPG acts as a decoy receptor through binding to circulating RANKL and decreasing its bioavailability. Several studies have demonstrated that OPG is a potent inhibitor of bone loss thus regulating bone density and mass in mouse and man [1,5,6]. As expected, overexpression or targeted deletion of the OPG gene in animals led to osteopetrosis or bone loss, respectively. This secreted cytokine was also proven effective in blockade of metabolic, pathologic and tumor-induced bone loss. Subsequently, these functions led to identification of the OPG target protein, i.e. RANK ligand (RANKL) [7**].
RANKL/RANK signaling cascade is initiated by assembly of signal transduction complex at the cytoplasmic tail of RANK. Assembly begins with recruitment of signaling and adaptor molecules such as TNF receptor-associated factor-6 (TRAF6) [8]. Subsequently, several down stream tyrosine and serine/threonine kinases, including NIK, IKKs, c-src, Akt/PKB, and MEKK-1 are recruited to the complex and undergo activation [9]. The most notably activated pathways by RANK are NF-κB and mitogen-activated protein (MAP) kinase pathways [10*,11]. The functional relevance of these proteins to RANK-induced osteoclastogenesis has been established. In this respect, interfering with NF-κB activation [12,13], or deleting certain NF-κB subunits (combined deletion of p50 and p52) arrests osteoclastogenesis [14,15]. Likewise, dominant-negative forms of various MAP kinases and selective inhibitors of the MAP kinase pathways inhibited osteoclastogenesis or reduced osteoclast survival. A number of other genes such as PU.1, cfms (M-CSF receptor), c-fos, RANK, and NF-κB (p50, p52 subunits) have been shown to be critical for osteoclast differentiation and function. Other gene deletion studies implicated the protooncogene c-src, the proton ATPase, NFATc1, TRAP, and cathepsin-k genes at later stages of osteoclast activation and function [3,16].
In sum, deficiency of osteoclasts leads to osteopetrosis, whereas excessive osteoclast activity under pathologic conditions leads to devastating bone loss diseases, such as osteoporosis, peri-articular osteolysis, inflammatory arthritis, periodontitis, tumor osteolysis and other forms of osteopenia.
Inflammatory osteolysis
Inflammatory responses are a module of the host defense immune response. The persistence of these responses is often associated with skeletal pathology ranging from localized focal bone erosion, peri-articular osteolysis in the vicinity of inflamed joints, to generalized osteopenia. The better known examples of such responses are inflammatory rheumatoid arthritis, seronegative spondyloarthropathies, and systemic lupus erythematosus. Other inflammatory bone pathologies include local and systemic responses to orthopedic implants and periodontitis. The common denominator of these inflammatory responses is bone loss owing to elevated osteoclast activity due to higher circulating levels of inflammatory mediators that promote and support osteoclast differentiation and activity. The inflamed synovium contains inflammatory cells that secrete a multitude of cytokines and growth factors including RANKL, TNF, IL-1, IL-6, PGE2, PTHrP, and IL-17. The hyperplastic synovium is infiltrated by synoviocytes, lymphocytes, plasma cells, activated macrophages, neutrophils and other cell types [17]. This microenvironment is superlative for recruitment and differentiation of osteoclasts that contribute to bone erosion.
Pathologically, inflammatory osteolysis is composed of two primary components, inflammatory factors and osteoclasts. Whereas excessive osteoclast activity relies heavily on the bioavailability of certain inflammatory factors, the osteolytic phase hinges solely on availability of osteoclasts [18].
Factors and modulators of the inflammatory osteolytic response
The RANKL/RANK signaling pathway occupies the center stage of the vast majority of osteolytic responses owing to its critical role in osteoclast differentiation and survival. Gene targeting studies have shown that RANKL and its receptor RANK are required for osteoclastogenesis and mice deficient of these genes are osteopetrotic and lack osteoclasts [7,19**]. Similarly, overexpression of OPG, the RANKL decoy receptor, or administration of OPG-Fc in mouse models of inflammatory osteolysis, eradicates osteoclasts and halts bone resorption. These animal models are resistant to focal and systemic bone loss in most common inflammatory osteolytic diseases despite existence of the inflammatory response.
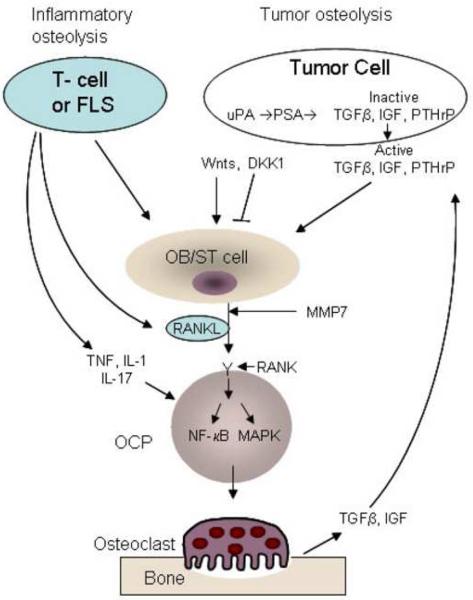
At its capacity as the sole osteoclast differentiation pathway, the RANKL/RANK signaling cascade is also a target for other modulators. These factors, such as TNF, IL-1, IL-17, bacterial endotoxins and other factors, impact osteoclastogenesis and bone resorption directly and indirectly (figure 1). The most notable cytokine that modulates both inflammatory and osteolytic arms is TNF which contributes dramatically to inflammatory osteolysis in rheumatoid arthritis, orthopedic osteolysis, and periodontitis [10,17]. The most described model is the human TNF transgenic mouse which develops polyarthritis associated with bone erosion solely dependent on osteoclast presence [20]. Related studies highlight the role of TNF as the inflammatory more so than an osteolytic effector. This notion is supported by studies in which inhibition of RANK signaling halted osteolysis whereas inflammation persisted. Mechanistically, TNF augments RANK/RANKL signaling tremendously when the latter has been initiated. It appears that TNF exacerbates osteoclastogenesis of RANKL-treated precursor cells using reciprocal mechanistic elements and that TNF osteolytic activity requires RANKL/RANK system. Further details regarding the RANKL/TNF molecular convergence point may identify the precise effector of osteolysis.
Figure 1. Inflammation and tumor-induced osteolysis.
Illustration of principal cells and factors mediating inflammatory and tumor osteolytic response. For details refer to text. FLS – fibroblast-like synovial cell, OB/ST – osteoblast/stromal cells, OCP – osteoclast precursors
Other prominent pro-inflammatory and pro-osteolytic factors include IL-17, IL-1 and IL-6. IL-17 is secreted by a distinct lymphocyte subset cells and induces expression of RANKL by osteoblasts and synovial fibroblasts [21]. It also increases the RANKL/OPG ratio by decreasing expression of OPG by stromal cells. Furthermore, it has been shown that IL-17 regulates IL-1, TNF, and IL-6 and plays an important role in inflammation and bone erosion in a mouse model of collagen-induced arthritis. Regulation of pro-inflammatory cytokines appears to be a major function of IL-17. For example, IL-17 directly regulates IL-1 and TNF evident by reduced IL-1 and TNF-induces inflammatory responses when IL-17 was inactivated. Overall, a cascade of events emanating from inflammatory cells lead to secretion of IL-17 which in turn upregulates expression of RANKL, TNF and IL-1, while down regulating expression of OPG and IL-1Ra, thus providing an intricate system supporting inflammation and subsequent osteolysis.
Cancer and Osteolysis
Mechanisms underlying bone tumor development
Breast and prostate cancer preferentially metastasize to bone. This event is not random and depends on tumor cell and host tissue properties. The initial step begins with detachment of individual tumor cells from the primary tumor. Aided by the tumor support system which includes tumorigenic, angiogenic and growth factors, these cells enter the circulation and invade target organs in a guided specific manner. Although tumorigenic cells metastasizing to skeletal compartments are commonly incapable of causing osteolysis on their own, this process involves consensual interactions between tumor cells and cellular components in the bone microenvironment causing osteolysis by osteoclasts and abnormal new bone formation by osteoblasts.
Cancer-induced osteolysis
The predominance of breast cancer metastases is osteolytic, whereas prostate cancer metastases are mostly osteoblastic. In most cases, ripple effects involving matrix-derived factors and soluble factors intensify these responses. These exaggerated responses are often referred to as the “vicious cycle” that maintains continuous momentum of tumor growth in bone which in turn exacerbates osteolysis. According to this scheme, tumor-secreted factors such as VEGF stimulate bone cells, which then degrade bone matrix and release cytokines and factors such as transforming growth factor-beta (TGFβ) and insulin-like growth factor (IGF) (figure 1). These factors act back on tumor cells and stimulate their metastatic potential. TGFβ released during the osteolytic response stimulates secretion of parathyroid hormone related protein (PTHrP) by tumor cells. This factor targets stromal cells and stimulates the secretion of RANKL, thus promoting osteoclast differentiation. Ensuing elevated osteoclast numbers results in increased bone resorption, leading to more TGFγ being released from bone and this cycle of events continues to maintain itself and aggrandize [22,23]. At the core of the osteolytic response stands the stromal factor RANKL, which naturally became a major target for therapeutic intervention.
Nevertheless, numerous other factors contribute to the mechanisms underlying cancer bone metastasis. In this regard, it has been shown that prostate cancer cells express adhesion molecules including αvβ3 which may facilitate homing to bone tissue enriched with integrin-specific binding matrices. On the other hand, a recent study by Nadiminty et al., suggest that prostate-specific antigen (PSA), a serine protease, directly contributes to bone metastasis through cleavage and activation of prominent bone related proteins such as TGFβ and PTHrP [24**]. Interestingly, gene microarrays conducted in the same study identified PSA regulation of RANKL, RUNX2, and Wnt signaling pathways which are considered the pillars of bone metabolism. Specifically, this study suggests that PSA may cause high bone resorption by increasing circulating levels of the osteoclastogenic factor RANKL and reducing levels of the antagonist osteoprotegerin. Furthermore, it is proposed that PSA upregulates the osteoblast differentiation factor Runx2 in osteoblasts/stromal cells and tumor cells , a finding consistent with the observation that levels of β-catenin, the primary mediator of Wnt signaling, were also increased.
Abnormal cancer-induced bone formation
Tumor-induced sclerotic new bone formation by osteoblasts is uniquely mediated by prostate cancer. In general, patients with metastatic bone formation suffer from reduced or poor bone quality, frequent fractures, and bone pain. As of yet, the precise mechanism underlying this metastatic lesion is not fully understood. However, it has been reported that cancer cells produce a number of factors, such as platelet-derived growth factor (PDGF), IGFs, FGFs, VEGF, PTHrP, Wnt, PSA, urokinase-type plasminogen activator (uPA), endothelin-1 (ET-1) and BMPs. In this regard, it has been shown that prostate cancer cell-produced Wnts promote osteoblast and tumor cell proliferation, function, and life span. Wnts are secreted cysteine-rich glycoproteins essential for embryonic bone development and support bone formation in the developed skeleton. According to recent findings, Wnts exhibit an autocrine tumor proliferative and anti-apoptotic effects as well as paracrine induction of mesenchymal and osteoblastic activity. Intriguingly, it appears that prostate cancer cells maintain very sophisticated machinery capable of regulating the activity of tumor-secreted Wnts. In this regard, it has been shown that prostate cancer cells express the soluble Wnt inhibitor dickkopf-1 (DKK1) and the antagonist soluble frizzled related receptors (sFrp). It is proposed that DKK-1 production occurs at early stages in the development of skeletal metastases, which leads to inhibition of the osteogenic effects of Wnts, thus promoting metastatic osteolysis (reviewed in [25]). Decreasing DKK1 expression level as metastases progress permits Wnt's osteoblastic activity to resume. This uncontrolled activity ultimately results with osteosclerosis at the metastatic site. Therefore, DKK1 is considered a molecular switch of prostate cancer cells between osteolysis and osteosclerosis. According to this model, prostate cancer-produced factors such as the protease PSA cause cleavage and release of TGFβ, IGFs, and PTHrP. These factors, especially PTHrP, target osteoblasts and stromal cells, and lead to production of RANKL which then triggers the osteolytic phase. This osteolytic phase is further controlled by the Wnt inhibitor DKK1 which blunts osteoblast stimulation and activity. On the other hand, transition from the osteolytic to the osteoblastic/osteosclerotic phase occurs following expression of endothelin-1, BMPs and Wnts coincident with decreased levels of DKK1. A different study by Lynch and colleagues has identified upregulation of MMP7, Cathepsin-k and apolipoprotein-D at the tumor-bone interface. The study demonstrates that MMP7-deficient mice exhibit reduced prostate cancer-induced osteolysis due to diminished processing of membrane bound RANKL to a soluble form [26**].
NF-κB at the crossroads of inflammation, cancer, and osteolysis
The transcription factor NF-κB family is central to osteoclastogenesis and inflammation. Activation of NF-κB dimers is regulated by the inhibitory proteins of which IκBα has been widely investigated [10,27*]. Various stimuli trigger a kinase complex which phosphorylates IκBα leading to its degradation thus allowing nuclear translocation of the transcription factor. The predominant kinase complex found in most cells contains two catalytic subunits, IKK1 (also known as IKKα) and IKK2 (IKKβ), and a regulatory subunit, IKKγ (also known as NEMO) [28*-31]. IKK1 and IKK2 catalyze p100 processing and phosphorylate serines 32 and 36 of IκBα, respectively. The N-terminal alpha-helical region of NEMO associates with a hexapeptide sequence within the distal carboxyl terminus of IKK2 and IKK1 termed NEMO-binding domain (NBD) [32, 33].
The requirement of IKK1 and IKK2 to normal bone homeostasis has been established [34,35]. Mice devoid of IKK1 or IKK2 exhibit both in vitro and in vivo defects in osteoclastogenesis [35]. Further, IKK2-null mouse is refractory to inflammatory arthritis, whereas IKK1 has been implicated as an anti-inflammatory kinase [36]. Collectively, the available information provides evidence that the IKK complex is the gatekeeper for NF-κB-transcriptional regulation which facilitates inflammatory responses and osteolysis.
The conventional paradigm suggests that chronic inflammation aids the development of cancer and that inflammatory factors amplify the action of osteoclast inducers. These actions are frequently mediated by the action of NF-κB and constitute the core for tumor and inflammatory osteolysis. In support of this paradigm, inhibitors of the NF-κB pathway or genetic deletion of members of this family abolished both responses. These approaches clarified that whereas activation of IKK1 by RANKL/RANK pathway inhibits metastatic repression leading to enhanced metastasis, IKK2 appears to coordinate the inflammatory and osteolytic components. This is supported by studies showing that catalytically inactive IKK1 (IKK1AA) and IKK2 (IKK2AA) fail to support metastasis and inflammatory osteolysis, respectively (reviewed in [37]). Furthermore, in breast cancer cells, it was found that NF-κB promotes osteolytic metastasis by inducing osteoclastogenesis in a GM-CSF-dependent mechanism [38**]
The role of NEMO in osteoclasts has been described indirectly using NEMO-binding domain inhibitor [33,39]. Importantly, short cell-permeable peptide spanning the IKK2 NBD disrupts the association of NEMO with IKK2, blocks NF-κB activation, and ameliorates responses in animal models of inflammation [32,40]. These observations position the IKK2 NBD as a viable target for the design of anti-inflammatory drugs. Furthermore, given the central role of NF-κB (the down stream target of the IKK complex) in osteoclastogenesis and inflammatory bone loss diseases (reviewed in [10,41]), NBD is regarded as an attractive target to be tested in this subset of diseases. In addition, sporadic mutations in the nemo gene where manifested by bone abnormalities [42,43] suggesting that this gene plays a key role in bone homeostasis. NEMO was described as the hub for inflammatory diseases [44]. In this regard, it has been suggested that Lysine 63-linked poly-ubiquitination events of NEMO, situate it as a scaffold and signal integrator molecule [45]. Mutations specifically targeting the relevant lysine residues responsible for poly-ubiquitination of NEMO identified the role of NEMO as modulator of inflammatory disorders. Using this approach, Ni and colleagues have shown that Lys392 modulates TLR signaling and inflammation in vivo [46]. Another study demonstrated the role of NEMO Lysine 285 as crucial in the pathogenesis of Crohn's disease, an autoimmune inflammatory disorder of the gastrointestinal tract [47].
Canonical NF-κB activation is mediated by IKK2, the role of which in inflammation, osteoclastogenesis and cancer osteolysis has been widely described (reviewed in [37,48]). IKK2 is essential for NF-κB activation by pro-osteoclastogenic and pro-inflammatory factors. In this regard, targeted deletion of IKK2 in the myeloid compartment abrogates osteoclastogenesis in vitro and in vivo [35,49]. Similarly, inhibition of IKK2 activation by introduction of NEMO-binding domain (NBD) peptide abolished osteoclastogenesis, inflammatory osteolysis, and inflammatory arthritis in various models of inflammation [33,39,50]. These studies established that IKK2 is the link between inflammation and osteolysis. Specifically, IKK2 protects macrophages and osteoclasts from apoptosis and mediates pro-inflammatory cytokine actions, such as in the case of TNF. Deletion of IKK2 renders immune cells, macrophages and osteoclast precursors susceptible for TNF-induced apoptosis through a gain of function in the JNK (c-Jun N-terminal Kinase) pathway. This is evident by the findings that inhibition of TNF or JNK restores the osteoclastogenic and inflammatory potential of these cells [35,49].
The alternative NF-κB activation pathway is dominated by NIK (NF-κB inducing kinase) activation of IKK1 which in turn induces processing of p100/NF-κB2 subunit to generate p52. P52 then binds to RelB, the dimer translocates to the nucleus and activates target genes. In vitro studies have shown that cells devoid of NIK or IKK1 fail to differentiate into osteoclasts, whereas in vivo inactivation of these kinases did not affect the overall skeleton [34,35,51]. While the precise role of IKK1 in baseline osteoclastogenesis remains unclear, an important role for this kinase in regulating and restricting inflammation has been described. In this regard, Lawrence and colleagues have demonstrated that IKK1 accelerates the turnover of RelA and cRel subunits and their removal from pro-inflammatory gene promoters, resulting with suppression of NF-κB activity. The authors concluded that inactivation of IKK1 in mice increased inflammation [52]. Further, in a recent study, Liu and co-workers demonstrated that PIAS1 (protein inhibitor of activated STAT1), which is an E3 ligase, limits inflammatory responses through selective inhibition of NF-κB and STAT1 binding to relevant gene promoters [36]. IKK1 also promotes metastasis. In this regard, a recent study by Luo and colleagues [53**] suggests that IKK1 is positioned at the interface of inflammation and metastasis in prostate cancer. According to this study, the metastatic repressor maspin (mammary serine protease inhibitor) is transiently repressed by IKK1. It was proposed that RANKL-expressing prostate tumor cells activate IKK1 which in turn inhibits maspin gene transcription in the nucleus an action that promotes metastasis. The requirement of RANKL/RANK signaling for this phenomenon may partially explain the preferential prostate cancer cell metastasis to bone which is RANKL/RANK-rich microenvironment.
Conclusions
A comprehensive survey of all osteolytic diseases incriminates activation of the RANK/ NF-κB pathway as central for the vast majority of osteolytic responses and underscores osteoclast activity as indispensable for inflammatory and tumor-induced osteolysis. It is natural, therefore, that therapeutic approaches are targeting the RANKL/RANK/NF-κB pathway to prevent osteoclast formation as well as targeting mature osteoclasts to block bone resorption. Disarming inflammatory and metastatic responses are also effective approaches to alleviate osteolysis and decrease tumor burden. These are achieved by targeting tumor-secreted and pro-inflammatory factors that fuel the vicious cycle of inflammatory osteolysis. An overview of available therapeutic tools points to the need for a combination therapy targeting various facets of the inflammatory-osteolytic response which then can be fine-tuned to fit individual needs.
Acknowledgments
The author is supported by grants R01-AR054326, R01-AR49192 from the National Institute of Health (NIH) and grants #8510, #8570 from the Shriners Hospital for Children. The author would like to thank Adam Abu-Amer for summarizing information for this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunological reviews. 2005;208:228–251. doi: 10.1111/j.0105-2896.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 2.Hagemann T, Balkwill F, Lawrence T. Inflammation and Cancer: A Double-Edged Sword. Cancer Cell. 2007;12:300–301. doi: 10.1016/j.ccr.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? The American journal of pathology. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 5.Schett G, Redlich K, Hayer S, Zwerina J, Bolon B, Dunstan C, Gortz B, Schulz A, Bergmeister H, Kollias G, et al. Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum. 2003;48:2042–2051. doi: 10.1002/art.11150. [DOI] [PubMed] [Google Scholar]
- 6.Ritchlin CT, Schwarz EM, O'Keefe RJ, Looney RJ. RANK, RANKL and OPG in inflammatory arthritis and periprosthetic osteolysis. Journal of Musculoskeletal Neuronal Interactions. 2004;4:276–284. [PubMed] [Google Scholar]
- 7**.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira dSA, Van G, Itie A, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. First report describing the discovery of OPGL (RANKL) [DOI] [PubMed] [Google Scholar]
- 8.Darnay BG, Besse A, Poblenz AT, Lamothe B, Jacoby JJ. TRAFs in RANK signaling. Advances in Experimental Medicine & Biology. 2007;597:152–159. doi: 10.1007/978-0-387-70630-6_12. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Amer Y. Advances in osteoclast differentiation and function. Current drug targets. Immune, endocrine and metabolic disorders. 2005;5:347–355. doi: 10.2174/1568008054863808. [DOI] [PubMed] [Google Scholar]
- 10*.Abu-Amer Y, Darwech I, Otero J. Role of the NF-kappaB axis in immune modulation of osteoclasts and bone loss. Autoimmunity. 2008;41:204–211. doi: 10.1080/08916930701694543. Comprehensive review on the role of NF-kB in inflammatory osteolysis. [DOI] [PubMed] [Google Scholar]
- 11.Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Clohisy J, Hirayama T, Frazier E, Han S, Abu-Amer Y. NF-kB signaing blockade abolishes implant particle-induced osteoclastogenesis. J.Orthop.Res. 2004;22:13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 13.Clohisy J, Roy B, Biondo C, Frazier E, Willis D, Teitelbaum S, Abu-Amer Y. Direct Inhibition of NF-kB Blocks Bone Erosion Associated with Inflammatory Arthritis. The Journal of Immunology. 2003;171:5547–5553. doi: 10.4049/jimmunol.171.10.5547. [DOI] [PubMed] [Google Scholar]
- 14.Franzoso G, Carlson L, Poljak L, Shores E, Brown K, Leonardi A, Tran T, Boyce B, Siebenlist U. Requirment for NF-kB in osteoclast and B-cell development. Genes & Development. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attar RM, Caamano J, Carraso D, Iotsova V, Ishikawa H, Ryseck R-P, Weih F, Bravo R. Genetic approaches to study Rel/NF-kB/IkB function in mice. Semin Cancer Biol. 1997;8:93–101. doi: 10.1006/scbi.1997.0060. [DOI] [PubMed] [Google Scholar]
- 16.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 17.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nature Reviews. Immunology. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 18.Schett G, Smolen JS. New insights in the mechanism of bone loss in arthritis. Current Pharmaceutical Design. 2005;11:3039–3049. doi: 10.2174/1381612054865046. [DOI] [PubMed] [Google Scholar]
- 19**.Wong B, esser D, Kim N, Arron J, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-src. Mol.Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. First report describing the RANK/TRANCE signal transduction pathway. [DOI] [PubMed] [Google Scholar]
- 20.Kollias G, Douni E, Kossiotis G, Kontoyiannis D. On the role of TNF and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol Rev. 1999;169:175–194. doi: 10.1111/j.1600-065x.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. Journal of Experimental Medicine. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato S, Futakuchi M, Ogawa K, Asamoto M, Nakao K, Asai K, Shirai T. Transforming growth factor beta derived from bone matrix promotes cell proliferation of prostate cancer and osteoclast activation-associated osteolysis in the bone microenvironment. Cancer Science. 2008;99:316–323. doi: 10.1111/j.1349-7006.2007.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cicek M, Oursler MJ. Breast cancer bone metastasis and current small therapeutics. Cancer & Metastasis Reviews. 2006;25:635–644. doi: 10.1007/s10555-006-9035-x. [DOI] [PubMed] [Google Scholar]
- 24**.Nadiminty N, Lou W, Lee SO, Mehraein-Ghomi F, Kirk JS, Conroy JM, Zhang H, Gao AC. Prostate-specific antigen modulates genes involved in bone remodeling and induces osteoblast differentiation of human osteosarcoma cell line SaOS-2. Clinical Cancer Research. 2006;12:1420–1430. doi: 10.1158/1078-0432.CCR-05-1849. [see comment][erratum appears in Clin Cancer Res. 2006 Jun 1;12(11 Pt 1):3552] This report documents the role of PSA in bone resorption and osteoblast differentiation. [DOI] [PubMed] [Google Scholar]
- 25.Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ, Shaughnessy JD., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. The study demonstrates that MMP7-deficient mice exhibit reduced prostate cancer-induced osteolysis due to diminished processing of membrane bound RANKL to a soluble form. [DOI] [PubMed] [Google Scholar]
- 27*.Karin M, Yamamoto Y, Wang M. The IKK NF-kB system: A treasure trove for drug development. Nat.Rev. 2004;3:17–26. doi: 10.1038/nrd1279. A comprehensive review focused on components of the NF-kB signaling pathways and identifies targets for therpies. [DOI] [PubMed] [Google Scholar]
- 28*.DiDonato J, Hayakawa M, Rothwarf D, Zandi E, Karin M. A cytokine-responsive I-κB kinase that activates the transcription factor Nf-kB. Nature. 1997;388:548–554. doi: 10.1038/41493. One of the first three simultaneously published reports that identified IKKs. [DOI] [PubMed] [Google Scholar]
- 29.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, et al. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science (New York, N.Y.) 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 30.Regnier C, Song H, Gao X, Goeddel D, Cao Z, Rothe M. Identification and characterization of an I-κB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 31.Stancovski I, Baltimore D. NF-kB activation:: The IkB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 32.May MJ, Marienfeld RB, Ghosh S. Characterization of the Ikappa B-kinase NEMO Binding Domain. Journal of Biological Chemistry. 2002;277:45992–46000. doi: 10.1074/jbc.M206494200. [DOI] [PubMed] [Google Scholar]
- 33.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. The Journal of biological chemistry. 2004;279:37219–37222. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 34.Chaisson ML, Branstetter DG, Derry JM, Armstrong AP, Tometsko ME, Takeda K, Akira S, Dougall WC. Osteoclast differentiation is impaired in the absence of IkB kinase-alpha. J Biol Chem. 2004;279:54841–54848. doi: 10.1074/jbc.M406392200. [DOI] [PubMed] [Google Scholar]
- 35.Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu L-C, Cao Y, Schett G, Wagner EF, Karin M. IkB kinase-beta, but not IKK-alpha, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J. Exp. Med. 2005;201:1677–1687. doi: 10.1084/jem.20042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Yang Y, Chernishof V, Loo RRO, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, et al. Proinflammatory Stimuli Induce IKK[alpha]-Mediated Phosphorylation of PIAS1 to Restrict Inflammation and Immunity. Cell. 2007;129:903–914. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 37.Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Research. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 38**.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nature Medicine. 2007;13:62–69. doi: 10.1038/nm1519. [see comment] This report shows that NF-κB promotes osteolytic metastasis by inducing osteoclastogenesis in a GM-CSF-dependent mechanism. [DOI] [PubMed] [Google Scholar]
- 39.Jimi E, Aoki K, Saito H, D'Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nature medicine. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 40.May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective Inhibition of NF-kappa B Activation by a Peptide That Blocks the Interaction of NEMO with the Ikappa B Kinase Complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 41.Ruocco MG, Karin M. Control of osteoclast activity and bone loss by IKK subunits: new targets for therapy. Advances in Experimental Medicine & Biology. 2007;602:125–134. doi: 10.1007/978-0-387-72009-8_16. [DOI] [PubMed] [Google Scholar]
- 42.Courtois G, Smahi A, Israel A. NEMO/IKK-gamma: linking NF-kB to human disease. Trends in Molecular Medicine. 2001;7:427–430. doi: 10.1016/s1471-4914(01)02154-2. [DOI] [PubMed] [Google Scholar]
- 43.Ku CL, Dupuis-Girod S, Dittrich AM, Bustamante J, Santos OF, Schulze I, Bertrand Y, Couly G, Bodemer C, Bossuyt X, et al. NEMO mutations in 2 unrelated boys with severe infections and conical teeth. Pediatrics. 2005;115:e615–619. doi: 10.1542/peds.2004-1754. [DOI] [PubMed] [Google Scholar]
- 44.Burns KA, Martinon F. Inflammatory Diseases: Is Ubiquitinated NEMO at the Hub? Current Biology. 2004;14:R1040–R1042. doi: 10.1016/j.cub.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 45.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nature cell biology. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 46.Ni C-Y, Wu Z-H, Florence WC, Parekh VV, Arrate MP, Pierce S, Schweitzer B, Van Kaer L, Joyce S, Miyamoto S, et al. Cutting Edge: K63-Linked Polyubiquitination of NEMO Modulates TLR Signaling and Inflammation In Vivo. J Immunol. 2008;180:7107–7111. doi: 10.4049/jimmunol.180.11.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn's Disease Protein, NOD2, Requires RIP2 in Order to Induce Ubiquitinylation of a Novel Site on NEMO. Current Biology. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 48.Karin M. NF-kappaB and cancer: mechanisms and targets. Molecular Carcinogenesis. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 49.Otero JE, Dai S, Foglia D, Alhawagri M, Vacher J, Pasparakis M, Abu-Amer Y. Defective osteoclastogenesis by IKKbeta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)-induced JNK-dependent apoptosis and impaired differentiation. The Journal of biological chemistry. 2008;283:24546–24553. doi: 10.1074/jbc.M800434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata W, Maeda S, Hikiba Y, Yanai A, Ohmae T, Sakamoto K, Nakagawa H, Ogura K, Omata M. Cutting Edge: The I{kappa}B Kinase (IKK) Inhibitor, NEMO-Binding Domain Peptide, Blocks Inflammatory Injury in Murine Colitis. J Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- 51.Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL. The I-kappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198:771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKK-alpha- limits macrophage NF-kB activation and contributes to the resolution of inflammation. Nature. 2005;434:1138–1143. doi: 10.1038/nature03491. [DOI] [PubMed] [Google Scholar]
- 53**.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. This article describes the molecular mechanism of IKK regulation of the cancer repressor Maspin. [DOI] [PubMed] [Google Scholar]