Abstract
Anterograde and retrograde tracing techniques were used to characterize projections from the auditory cortex to the pedunculopontine and laterodorsal tegmental nuclei (PPT and LDT, respectively) in the midbrain tegmentum in guinea pigs. For anterograde tracing, tetramethylrhodamine dextran (FluoroRuby) was injected at several sites within auditory cortex. After sufficient time for transport, the brain was processed for immunohistochemistry with anti-choline acetyltransferase to reveal presumptive cholinergic cells. Anterogradely-labeled axons were observed ipsilaterally and, in smaller numbers, contralaterally, in both the pedunculopontine and laterodorsal tegmental nuclei. In all 4 nuclei, tracer-labeled boutons appeared to contact immunolabeled (i.e., cholinergic) cells. The contacts occurred on cell bodies and dendrites. The results were similar following injections that spread across multiple auditory cortical areas or injections that were within primary auditory cortex. In order to confirm the anterograde results, in a second series of experiments, retrograde tracers were deposited in the pedunculopontine tegmental nucleus. These injections labeled layer V pyramidal cells in the auditory cortex. The results suggest an excitatory projection from primary auditory cortex bilaterally to cholinergic cells in the midbrain tegmentum. Such a pathway could allow auditory cortex to activate brainstem cholinergic circuits, possibly including the cholinergic pathways associated with arousal and gating of acoustic stimuli.
Keywords: arousal, startle, corticofugal, pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus, acetylcholine
1. Introduction
Two midbrain cholinergic nuclei – the pedunculopontine tegmental nucleus (PPT) and the laterodorsal tegmental nucleus (LDT) – have been associated with the ascending arousal system (reticular activating system) [13, 19, 56, 61]. The cholinergic cells in PPT and LDT project to many brainstem nuclei. They are also the source of cholinergic projections to the thalamus. Indeed, it is their projection to the thalamus that has been studied in most detail and has been most closely related to arousal. The cholinergic cells show different levels of activity during different stages of the sleep-wake cycle, with cellular spiking activity higher during waking and during paradoxical sleep than during slow-wave sleep.
The PPT and LDT are in a position to have a major influence on auditory processing via projections to nuclei at numerous levels of the ascending auditory pathway. Targets of the cholinergic projections to the thalamus include the multiple subdivisions of the medial geniculate nucleus [15, 32, 52, 55, 59, 65]. Recent studies have also identified cholinergic projections from the PPT and LDT to the cochlear nucleus [36] and to the inferior colliculus [38].
Physiological recordings suggest that a large percentage of the cells in the PPT and LDT respond to acoustic stimuli [24, 43]. The exact route by which acoustic information reaches the midbrain cholinergic cells is unknown but may include direct projections from the superior colliculus and possibly from the inferior colliculus as well [11, 51, 66]. It is possible that other auditory structures project to the cholinergic nuclei, but the proximity of these nuclei to auditory pathways has made it difficult to identify other inputs. The lateral part of the PPT overlaps with the medial portion of the lateral lemniscus such that the cholinergic cells are intermingled with axons of this large auditory pathway. In addition, the dorsal nucleus of the lateral lemniscus is also nearby. This large auditory nucleus gives rise to a commissural pathway (the commissure of Probst) with fibers that directly traverse the caudal PPT and travel near the LDT. Injections of retrograde tracers into the LDT (or PPT) label cells in the DNLL but it has been difficult to conclude whether this label represents a direct projection or labeling of fibers of passage [51]. The issue is further complicated if one is interested in identifying auditory inputs that are related specifically to cholinergic cells because both the PPT and the LDT contain significant numbers of cells that use neurotransmitters other than acetylcholine (e.g., glutamate, GABA) [5, 18, 19, 26, 27, 29, 53, 58, 64].
The present study stems from an earlier series of experiments in which we made injections of anterograde tracer into the auditory cortex to examine projections to brainstem auditory nuclei in guinea pigs [6, 41, 48, 49]. Subsequent analysis has revealed labeled axons in the area of both the PPT and LDT (unpublished observations). This was surprising because, to the best of our knowledge, there are no reports of such projections. The present study examines these projections directly, and includes staining with a cholinergic marker to relate the cortical axons to the cholinergic brainstem cells. We also use retrograde tracers injected into the PPT to confirm the projections from auditory cortex.
2. Materials and methods
2.1 Surgery and perfusion
Experiments were performed on 8 adult pigmented guinea pigs (450−900 gm, either gender) obtained from Elm Hill Laboratories (Chelmsford, MA, USA). Appropriate measures were taken to minimize pain and suffering. Sterile instruments and aseptic technique were used for all surgical procedures. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Surgical methods have been described in detail previously (e.g., [6]). Briefly, the animal was anesthetized with isoflurane in oxygen (4% for induction; 1.5−2.5% for maintenance). Atropine (0.05 mg/kg, i.m.) was administered to reduce bronchial secretions. Body temperature was maintained with a feedback-controlled heating pad. Once anesthetized, the animal's scalp was prepared for surgery and the animal was mounted in a stereotaxic frame. The skin was incised and the margins were injected with a long-acting local anesthetic (0.25% bupivacaine with epinephrine 1:200,000; Hospira, Inc., Lake Forest, IL). The skin was retracted and a dental drill was used to open the skull at an appropriate location. For cortical injections (6 animals), FluoroRuby (FR, tetramethylrhodamine dextran, 10 000 MW, dissolved as 10% in saline, Invitrogen, Eugene, OR) was injected at 5−13 sites in temporal cortex. The injections were placed across an area spanning 2−3 mm caudal to Bregma in the rostrocaudal axis. In the mediolateral axis, the injections spread 2−3 mm ventrolateral to the pseudosylvian sulcus. The injections were thus aimed at primary auditory cortex (A1; as mapped and defined by Wallace et al. [62, 63]). Injections of 0.15 μl were made with a 10 μl Hamilton microsyringe angled 50° laterally in the transverse plane (approximately perpendicular to the cortex). Deposit sites were separated by about 0.5 mm and formed a grid within the dimensions described above. After completion of injections, Gelfoam (Harvard Apparatus, Holliston, MA, USA) was placed over the skull opening and the skin was sutured. Ketofen (ketoprofen 3 mg/kg, i.m.; Henry Schein, Melville, NY) was given to provide postoperative analgesia. The animal was returned to its cage and monitored until it was returned to the animal facility.
After 7−14 days, the animals were given an overdose of anesthesia (inhalation of 5% isoflurane in oxygen until cessation of breathing and absence of withdrawal reflex). The animal was then perfused through the aorta with Tyrode's solution (similar to artificial cerebrospinal fluid) followed by approximately 300 ml of fixative (4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4) and then by a similar volume of fixative with 10% sucrose added. The brain was removed and stored overnight at 4°C in fixative with 25% sucrose.
A retrograde tracer was injected into the PPT in two animals. In one animal, 23 nl of FluoroGold (FG, FluoroChrome, Inc., Englewood, CO; 4% in water) was injected into the PPT through a micropipette (tip inside diameter = 30 μm) attached to a Nanoliter injector (World Precision Instruments, Sarasota, FL, USA). The micropipette was left in place for 5 minutes after injection and then withdrawn. In a second animal, 23 nl of red beads (RB; Lumafluor, Naples, FL, USA) was injected through a micropipette (tip inside diameter = 35 μm) attached to a Nanoliter injector, as described above. After a survival time of 5 days, the animals were perfused as described above.
2.2 Histology
On the day following perfusion, the brain was frozen and sectioned at 50 μm in the transverse plane on a sliding microtome. Sections were collected in 6 series; at least 3 series were stained with a cholinergic marker (described below). Some sections were stained with thionin for identification of brainstem nuclei and cortical layers. Cholinergic cells were identified with an antibody to choline acetyltransferase (ChAT) as described previously [37]. Briefly, sections were treated with a blocking solution (20% normal rabbit serum, 0.1 % Triton-X 100 in 0.01M phosphate buffer/0.9% saline) then with goat anti-ChAT (Chemicon, AB144P, diluted 1:50 to 1:100), biotinylated rabbit anti-goat IGg (BA-5000; diluted 1:100; Vector Laboratory) and visualized with green fluorescent streptavidin Alexa Fluor 488 (product # S11223, Invitrogen).
In 2 cases, synaptic boutons were stained by treating the sections with mouse anti-SV2 (Developmental Studies Hybridoma Bank, U Iowa; 1:500 dilution), biotinylated goat-anti-mouse antibody (BA-9200, Vector Laboratory) and streptavidin-Alexa Fluor 647 (S20992, Invitrogen). The sections were mounted on slides as described above.
2.3 Data analysis and photography
Sections were viewed on a Zeiss Axio Imager Z1 fluorescence microscope equipped with appropriate filters and an Apotome for optical sectioning. The borders of the PPT and LDT can be difficult to identify in standard Nissl stains; we used the distribution of ChAT-immunolabeled cells as a working definition for the limits of these nuclei.
The near-infrared of the Alexa Fluor 647 was visualized with a Zeiss AxioCam HRm monochrome digital camera and Zeiss AxioVision 4.6 software, which was used to pseudo-color the SV2 yellow. Photographs were taken with this camera and software or, in cases without infrared label, with a Zeiss AxioCam HRc color camera mounted on the same microscope. In some cases, the Apotome was used to collect a z-stack of images optically sectioned. These images are collected with the monochrome camera and pseudocolored with the AxioVision Apotome software. Photographs of injection sites were taken with an Optronics Magnafire camera mounted on a Zeiss Axioskop fluorescence microscope fitted with a 0.5x camera lens. Adobe Photoshop CS3 was used to adjust brightness and contrast, to overlay images taken with different fluorescence filters, and to add labels. In some cases, (Figs. 1, 4) images were adjusted with Photoshop as just described and then imported into Adobe Illustrator CS3 for additional labeling.
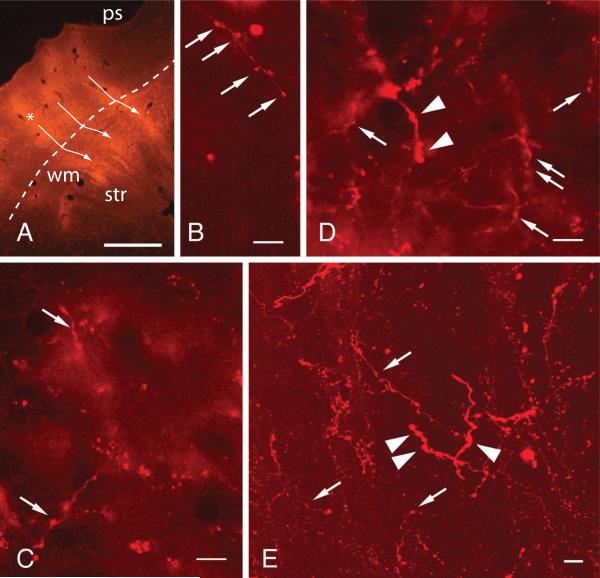
Figure 1.
Fluorescence photomicrographs showing a typical FluoroRuby (FR) injection into auditory cortex and representative labeled axons in the brainstem. A. Transverse section showing one of 12 FR deposit sites (*). The injections are in auditory cortex, just ventro-lateral to the pseudosylvian sulcus (ps). Bundles of labeled axons (white arrows) traverse the white matter (wm) and continue into the striatum (str) from the indicated injection site (*) as well as from two more dorsal sites (centered in nearby sections). Dashed line – border between cortex and white matter. Dorsal - up; medial - right. GP553. scale bar = 1 mm. B-E. FR-labeled axons in the laterodorsal tegmental nucleus (B, C) and the pedunculopontine tegmental nucleus (D, E) ipsilateral to the injected cortex. Thin axons exhibit many boutons (arrows) in the LDT and the PPT. Thicker axons with boutons (arrowheads) are visible in the PPT. The image in E is a z-stack (through-focal series) containing 24 optical sections. GP553 (C, D); GP556 (B, E). Scale bars = 10 μm. Adobe Photoshop CS3 was used to adjust brightness and contrast and Adobe Illustrator CS3 was used to add labels.
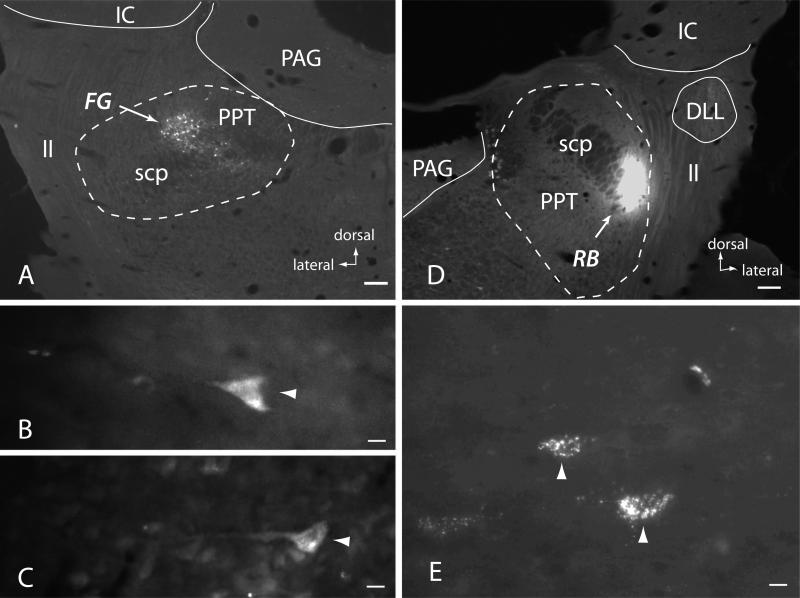
Figure 4.
Fluorescence photomicrographs showing injection of retrograde tracers into the PPT and resulting labeled cells in cerebral cortex. A. Injection site of FluoroGold (FG) into the left pedunculopontine tegmental nucleus (PPT). The PPT does not have distinct cytoarchitectonic borders; the dotted line indicates the extent of the PPT as indicated by the distribution of immunolabeled (cholinergic) cells (which were visualized with a different filter). Scale bar = 200 μm. GP575. Lateral is left; dorsal is up. B, C. FG-labeled pyramidal cells (arrowheads) in layer V of ipsilateral auditory cortex. Scale bars = 10 μm. Lateral (cortical surface) is to the left; dorsal is up. D. Injection site of red beads (RB) into the right PPT. Scale bar = 200 μm. GP578. E. RB-labeled layer V cortical cells (arrowheads) in ipsilateral auditory cortex in GP578. Scale bar = 10 μm. Lateral (cortical surface) is to the right; dorsal is up. Adobe Photoshop CS3 was used to adjust brightness and contrast and Adobe Illustrator was used to add labels. Abbreviations: DLL – dorsal nucleus of the lateral lemniscus; IC – inferior colliculus; ll – lateral lemniscus; PAG – periaqueductal gray; PPT – pedunculopontine tegmental nucleus; scp – superior cerebellar peduncle.
Cells that were immunopositive for ChAT were presumed to be cholinergic. Axons labeled with FluoroRuby were presumed to originate from cortical cells in the injected area. Swellings, or boutons, along the labeled axons or at the ends of branches were presumed to be sites of synapses. Potential contacts between labeled boutons and immunolabeled profiles were identified under careful examination with a 63x oil-immersion objective (NA = 1.4). Boutons in close apposition to an immunolabeled cell body or dendrite were considered potential synapses. In the cases with retrograde tracers, labeled cells were presumed to send an axon to or through the injection site.
3. Results
3.1 Anterograde transport from auditory cortex
Figure 1A shows a typical injection of FR into the left temporal cortex. Transverse sections showed multiple deposits of tracer centered on the middle cortical layers. The deposits did not appear to extend into the white matter directly, although it is difficult to be certain because of a large, bright bundle of labeled axons that extended directly from each deposit site into the white matter (e.g., white arrows in Fig. 1A). In the thalamus, retrogradely labeled cells were concentrated in the medial geniculate body, with the majority in the ventral division, consistent with an injection in A1 [42]. Labeled axons could be traced through the internal capsule and into the brainstem to many sites (e.g., inferior colliculus, superior olivary complex, cochlear nucleus) as described in previous studies of guinea pigs [6, 17, 48]. The remainder of this section focuses on labeled axons in the PPT and LDT.
The caudal end of the PPT lies medial to the dorsal nucleus of the lateral lemniscus. The cholinergic cells of the PPT spread rostro-ventrally toward the substantia nigra; we observed a few labeled cortical axons among the more rostral PPT cells, but the vast majority of the cortical boutons within the PPT were located in the caudal part of the nucleus (around the superior cerebellar peduncle). Figure 1B-D show examples of FR-labeled axons and boutons in the PPT and LDT ipsilateral to the cortical injections. The majority of labeled axons were thin, with small boutons located along the axon and, less often, at the ends of short branches. Thicker axons were visible occasionally; these had correspondingly larger boutons along their length (Fig. 1D, E). Thin axons were also labeled, in fewer numbers, in the contralateral PPT and LDT.
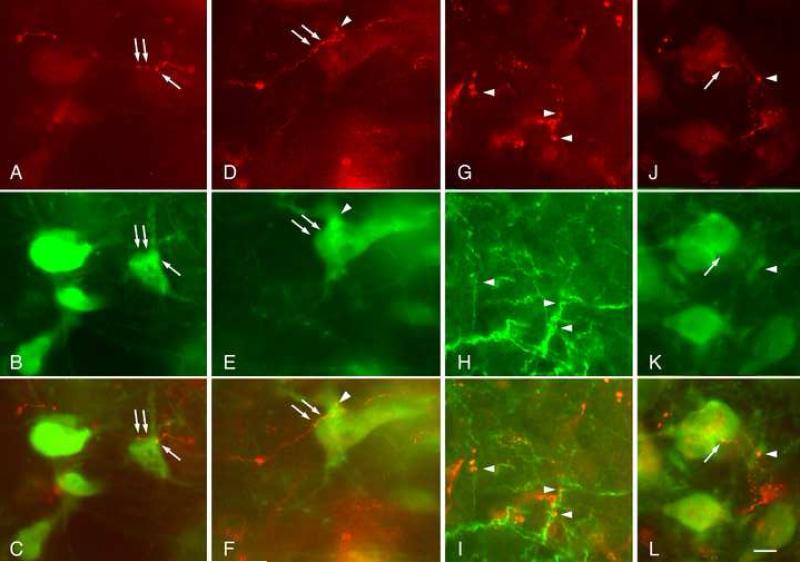
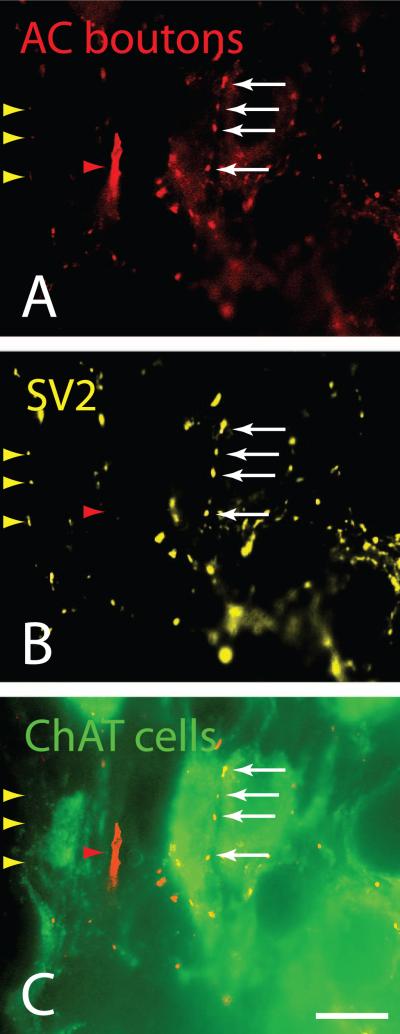
The PPT and LDT contain non-cholinergic cells intermingled with cholinergic cells (as discussed in the Introduction). In order to determine whether the cortical projections contact the cholinergic cells, we combined anterograde tracing with immunohistochemistry. Figure 2 shows FR-labeled cortical axons in close apposition to ChAT-immunoreactive cell bodies and dendrites in the PPT and LDT. Putative contacts were observed most frequently in the ipsilateral PPT and, much less frequently (about one fourth as many contacts), in the ipsilateral LDT. Contacts were less numerous in the contralateral cholinergic nuclei, but were observed in both the PPT and the LDT (Fig. 2JL). Each case yielded many examples of close appositions, suggesting that the cortical axons form synapses with cholinergic cells. This conclusion was supported by staining with the anti-SV2 antibody. This antibody stains a protein associated specifically with synaptic vesicles, and is considered a specific marker of synaptic sites [28]. Fig. 3 shows FR-labeled cortical boutons that are also stained with the SV2 marker; the boutons are in close contact with ChAT-positive profiles. Unfortunately, the penetration of the SV2 antibody was limited to tissue near the section surfaces, so it was not meaningful to quantify the percentage of FR-labeled boutons that were SV2-positive.
Figure 2.
Fluorescence photomicrographs showing FluoroRuby-labeled (FR) cortical axons in contact with presumptive cholinergic cells. Each column shows an area viewed for FR axons (red), choline acetyltransferase (ChAT)-immunopositive cells (green) and an overlay of the images to illustrate the putative contacts. Labeled boutons appeared to contact cell bodies (arrows) as well as dendrites (arrowheads). Contacts are shown in the left (ipsilateral) pedunculopontine tegmental nucleus (A-C) and laterodorsal tegmental nucleus (D-I) and also in the right (contralateral) laterodorsal tegmental nucleus (J-L). Scale bar = 10 μm. Adobe Photoshop CS3 was used to adjust brightness and contrast and to add labels.
Figure 3.
Fluorescence photomicrographs showing FluoroRuby-labeled cortical axons (red, panel A) with boutons (white arrows) that are immunoreactive for the synaptic marker SV2 (yellow; panel B). The boutons are also in apparent contact with presumptive cholinergic cells (green; panel C). Some boutons contained SV2 staining but not FR label (yellow arrowheads). A thick axon was labeled with FluoroRuby (red arrowhead) but was not immunoreactive for SV2. Left pedunculopontine nucleus, ipsilateral to the FluoroRuby injection in auditory cortex. GP 557. Scale bar = 10 μm. Adobe Photoshop CS3 was used to adjust brightness and contrast and to add labels. Abbreviations: AC – auditory cortex; ChAT cells – choline acetyltransferase-immunoreactive cells.
While there were many examples of apparent contacts between labeled axons and ChAT-immunopositive cells, there were many additional boutons not associated with immunolabeled profiles. It is possible, of course, that the immunohistochemistry failed to label some cholinergic profiles (e.g., distal dendrites?), but given the large number of boutons, many found near immunolabeled cell bodies or dendrites, it is likely that the cortical axons contact non-cholinergic cells. Such contacts are likely to occur in both PPT and LDT ipsilateral and contralateral to the cortical origins of the projection.
3.2 Retrograde transport from the PPT
In order to confirm auditory cortex as a source of projections to the cholinergic nuclei, we injected retrograde tracers into the PPT. Fig. 4A shows the center of a small injection of FluoroGold (FG) into the left PPT. The injection is centered in caudal PPT just dorsal to the superior cerebellar peduncle. Layer V pyramidal cells were labeled in the temporal cortex (Fig. 4B, C). The locations of these cells, about half way between the rhinal and pseudosylvian sulci, are consistent with location in primary auditory cortex [42, 62, 63]. The labeled cells were located in layer V and most had a prominent apical dendrite, establishing their identity as pyramidal cells. Additional labeled cells were present in medial prefrontal cortex (the area previously described as the major source of cortical input to the PPT [51]; data not shown). Similar results were produced by a small injection of red beads (RB) into the right PPT in a different animal. The injection is centered just lateral to the superior cerebellar peduncle and medial to the lateral lemniscus (Fig. 4D, E). RB-labeled cells were present in layer V of auditory cortex as well as medial prefrontal cortex.
4. Discussion
The results provide evidence for a direct projection from primary auditory cortex to cholinergic cells in the PPT and LDT of guinea pigs. This appears to be the first report of a projection from any part of auditory cortex to the PPT or LDT, and the first anatomical evidence to associate cortical inputs directly with the cholinergic cells.
4.1 Immunohistochemistry
The ChAT immunohistochemistry is based on an antibody used with fixation and processing procedures validated previously in guinea pigs [37]. The results provide strong evidence that the ChAT-immunopositive profiles belong to cells that use acetylcholine as a neurotransmitter. The SV2 antibody has been used in numerous species, and has been used previously to identify synaptic sites in guinea pigs (e.g., [28]). The presence of SV2 immunoreactivity in some of the cortical boutons labeled with the anterograde tracer strongly supports the conclusion that these boutons form synapses.
The results suggest a substantial projection from auditory cortex to multiple cell types in the PPT and LDT. A portion of this projection appears to terminate directly on cholinergic cells. Thus it is likely that projections from each side of auditory cortex can activate cholinergic circuits on both sides of the brainstem. In addition, a substantial number of labeled boutons were observed among the immunolabeled cells but not in obvious contact with those cells. Some of the boutons may contact cholinergic cells that were not immunolabeled because of technical limitations (e.g., incomplete penetration of the antibody). It is also possible that some cells contacted by the cortical axons are in fact non-cholinergic cells. The PPT and LDT contain distinct cell populations that use acetylcholine, GABA, glutamate or a host of other putative neurotransmitters (see discussion in [4, 33, 64]). Cells in any of these groups could be among the noncholinergic targets of the cortical axons.
4.2 Comparison with previous studies
Several studies have used retrograde tracers to identify inputs to the PPT and LDT (e.g., [7, 51, 54]). The only areas of cerebral cortex so identified are in frontal and limbic areas. Retrogradely labeled cells were identified in rats in medial prefrontal, orbital and cingulate cortex after injections in PPT or LDT [51]. Labeled cells were found in parietal, temporal or perirhinal cortex only in two cases in which the injection encroached on the lateral lemniscus; the authors concluded that these cells may not project to the PPT. Motor and premotor cortex have been identified as projecting to the PPT in cats and monkeys [10, 25, 31, 35]. Of all the cortical projections, the most prominent in terms of the number of cells projecting to the PPT or LDT appears to be from the prefrontal cortex. The present experiments labeled more cells in prefrontal cortex than in temporal cortex. The cells in temporal cortex were clearly within the boundaries of auditory cortex; in addition, their location in layer V is consistent with a projection to the brainstem. It is possible that some of these cells have axons that traversed the injection site rather than terminating in the PPT. This issue is clarified by the anterograde tracers, which labeled many axons with boutons in both the PPT and the LDT. It will be important in subsequent studies to confirm with electron microscopy that the cortical axons form synapses with PPT and LDT cells.
It is unclear why a projection from auditory cortex to the PPT and LDT has not been described consistently in past studies in which anterograde tracers have been injected into auditory cortex. One study provided evidence for a projection from Te2 (a “belt” area of auditory cortex in rats) to the PPT [60]. That study used wheat germ agglutinin-horseradish peroxidase as an anterograde tracer. While the PPT was not explicitly described as a target, plots of the data show labeled terminals surrounding the lateral part of the caudal superior cerebellar peduncle, i.e., in the area of PPT ([60], Fig. 6, section j). The results suggest that a projection from auditory cortex to the PPT is not limited to guinea pigs, and may involve multiple areas of auditory cortex. While it remains possible that there are species differences, it seems more likely that there are technical differences. We have found FluoroRuby, as used here, to be a particularly sensitive tracer. We have obtained similar results with fluorescein dextran (unpublished observations). It seems likely that this sensitivity explains, at least in part, the large number of labeled axons observed in the present study.
4.3 Functional significance
The PPT and LDT have been implicated in arousal and cortical activation [9, 20], locomotor control [12], sensory gating and sensorimotor integration [8, 22], sleep-wake cycles [19, 34], and autonomic functions [2, 46, 47]. These functions are accomplished by projections that extend from the PPT and LDT as far as the frontal cortex and the spinal cord. Relating distinct functions to specific circuits and neurotransmitters will require not only that the inputs to the nuclei be identified but that the neurochemical specificity of their targets be identified. The present study suggests that auditory cortex projects to both cholinergic and non-cholinergic cells in the PPT and the LDT.
The retrograde tracing experiments identified layer V pyramidal cells as the source of auditory cortical projections to the PPT and LDT. It is likely that this pathway uses glutamate or a similar neurotransmitter and excites its targets. As described previously, cholinergic cells of the PPT and LDT are associated with arousal (as part of the ascending arousal, or reticular activating, system). Auditory cortical projections could potentially activate these cholinergic cells and trigger a broad arousal and activation of a wide region of cortex. Of course, a sufficiently loud sound can be arousing, and it is not assumed that auditory cortex necessarily would be involved in such arousal. Reese et al. [43, 44, 45] suggested that the PPT receives numerous auditory inputs, possibly including projections from the dorsal nucleus of the lateral lemniscus and other cells whose axons travel in the lateral lemniscus. Additional auditory inputs may arrive via the superior colliculus. One or more of these inputs presumably underlie the short-latency responses of PPT cells to acoustic stimulation [43, 45]. PPT cells also have longer latency responses that could reflect descending inputs from higher centers. LDT cells apparently lack short latency responses but do show long-latency responses (65 to several hundred milliseconds in rats; [24]). The present study suggests that auditory cortex provides inputs that could underlie long-latency responses in both the LDT and PPT.
The ability of an acoustic stimulus to rouse a sleeping individual depends in part on its salience [1, 39]. Determination of salience, in some cases requiring semantic analysis of the stimulus (e.g., recognizing a stimulus as one's own name) is likely to be accomplished in the forebrain, probably involving the auditory cortex as well as the amygdala and prefrontal cortex. The connections revealed in the present study, particularly the cortical inputs to the cholinergic cells, could potentially contribute to arousal that is itself gated by higher level analysis.
Descending auditory inputs to PPT or LDT could underlie the habituation that is characteristic of the responses of many PPT cells to acoustic stimuli [44]. Constant activation of arousal circuitry by ongoing acoustic stimuli would seem to be of little value; habituation would presumably allow the system to operate at a basal level that could then be adjusted by a stimulus that is particularly arousing, either because it is especially loud and thus threatening, or because it has particular behavioral salience. The mechanisms by which PPT cells habituate is not known, although it has been reported that habituation is reduced in a decerebrate preparation [44, 45]. How would cortical projections from layer V cells – presumably glutamatergic – underlie habituation of the cholinergic cells? One possibility is that the cholinergic cells are relatively insensitive to ascending acoustic inputs, but respond to those inputs when maintained at a relatively depolarized level by input from the auditory cortex. Another possibility is that some of the cortical projections contact GABAergic cells in the PPT or LDT (among the non-cholinergic cells in the present study), that could in turn inhibit the cholinergic cells. In this scenario, repetitive activation of the cortical cells would lead to activation of PPT/LDT GABAergic cells that would inhibit their cholinergic neighbors via local collaterals and thus suppress responses of the cholinergic cells to the repetitive stimuli. The present study provides evidence for cortical inputs to both cholinergic and non-cholinergic cells, though whether the latter include GABAergic cells has yet to be tested.
Koyama et al. [24] suggested that cholinergic cells in the LDT may be concerned with attention to a sensory stimulus and that a subset of the cholinergic cells may be “...concerned with producing the attentive state only upon novel, unfamiliar stimulation.” (page 1029). Recent studies have highlighted cells in the inferior colliculus that are selectively responsive to novel stimuli [30, 40]. These cells respond to a wide range of stimuli but show rapid habituation; i.e., they stop responding to a stimulus that is repeated. Cholinergic cells of the PPT and LDT show habituation [24, 43, 45] and project to the inferior colliculus [38]. We hypothesize that cholinergic projections could contribute to the ability of collicular cells to respond to novel stimuli, and that this response characteristic may be particularly related to arousal and attention. Preliminary findings suggest that cortical projections to the PPT and LDT contact cholinergic cells that project to the inferior colliculus [50]. Whether or not this is true, the present results suggest that the projections from auditory cortex to the brainstem tegmental cholinergic nuclei should be included in future models of top-down influences on acoustically-driven arousal.
The PPT and LDT have also been associated with pre-pulse inhibition of the acoustic startle response [11, 21, 23, 67]. The acoustic startle response is a brief muscular contraction in response to an intense sound. Pre-pulse inhibition is a phenomenon by which a less intense (sub-threshold) sound, presented just before the intense sound, can inhibit the startle response to the second sound. Destruction of the brainstem cholinergic cells has little effect on the startle response but reduces pre-pulse inhibition. The phenomenon is believed to rely on projections from the cholinergic cells to cells of the caudal pontine reticular nucleus [3, 16]. In fact, the projections from the cholinergic nuclei have been viewed as gating the flow of information in the ascending sensory pathways [57]. Graham [14] suggested that pre-pulse inhibition could “protect” neural circuits from the disruptions associated with startle, allowing pre-attentive processing to continue and facilitating orienting or approach behaviors. Fendt et al. [11] extended this model and reviewed circuitry associated with pre-pulse inhibition. Their model circuit includes “modulatory forebrain influences” on PPT and LDT; these would clearly include projections from amygdala and prefrontal cortex and, based on the current data, projections from auditory cortex. Future study of the effects of cortical inputs on pre-pulse inhibition may provide insights into the role of these cortical inputs not only on sensory processing but also on some of the motor functions for which the PPT and LDT are well known.
Acknowledgements
This work supported by NIH NIDCD DC04391 and DC08463. Thanks to Colleen Sowick and Megan Storey-Workley for expert technical assistance. Thanks to Dr. Kyle Nakamoto for comments on an earlier draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no competing financial interests.
Literature cited
- 1.Bastuji H, Perrin F, Garcia-Larrea L. Semantic analysis of auditory input during sleep: studies with event related potentials. Int J Psychophysiol. 2002;46:243–255. doi: 10.1016/s0167-8760(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 2.Bellingham MC, Ireland MF. Contribution of cholinergic systems to state-dependent modulation of respiratory control. Respir Physiol Neurobiol. 2002;131:135–144. doi: 10.1016/s1569-9048(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 3.Bosch D, Schmid S. Cholinergic mechanism underlying prepulse inhibition of the startle response in rats. Neuroscience. 2008;155:326–335. doi: 10.1016/j.neuroscience.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Boucetta S, Jones BE. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane-anesthetized rats. J Neurosci. 2009;29:4664–4674. doi: 10.1523/JNEUROSCI.5502-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements JR, Grant S. Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci Lett. 1990;120:70–73. doi: 10.1016/0304-3940(90)90170-e. [DOI] [PubMed] [Google Scholar]
- 6.Coomes DL, Schofield BR. Projections from the auditory cortex to the superior olivary complex in guinea pigs. Eur J Neurosci. 2004;19:2188–2200. doi: 10.1111/j.0953-816X.2004.03317.x. [DOI] [PubMed] [Google Scholar]
- 7.Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- 8.Diederich K, Koch M. Role of the pedunculopontine tegmental nucleus in sensorimotor gating and reward-related behavior in rats. Psychopharmacology (Berl) 2005;179:402–408. doi: 10.1007/s00213-004-2052-y. [DOI] [PubMed] [Google Scholar]
- 9.Dringenberg HC, Sparling JS, Frazer J, Murdoch J. Generalized cortex activation by the auditory midbrain: mediation by acetylcholine and subcortical relays. Exp Brain Res. 2006;174:114–123. doi: 10.1007/s00221-006-0427-5. [DOI] [PubMed] [Google Scholar]
- 10.Edley SM, Graybiel AM. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol. 1983;217:187–215. doi: 10.1002/cne.902170207. [DOI] [PubMed] [Google Scholar]
- 11.Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 1987;411:1–12. doi: 10.1016/0006-8993(87)90675-5. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Rill E. The pedunculopontine nucleus. Prog Neurobiol. 1991;36:363–389. doi: 10.1016/0301-0082(91)90016-t. [DOI] [PubMed] [Google Scholar]
- 14.Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 15.Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. The Journal of Comparative Neurology. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- 16.Homma Y, Skinner RD, Garcia-Rill E. Effects of pedunculopontine nucleus (PPN) stimulation on caudal pontine reticular formation (PnC) neurons in vitro. J Neurophysiol. 2002;87:3033–3047. doi: 10.1152/jn.2002.87.6.3033. [DOI] [PubMed] [Google Scholar]
- 17.Jacomme A-V, Nodal FR, Bajo VM, Manunta Y, Edeline J-M, Babalian A, Rouiller EM. The projection from auditory cortex to cochlear nucleus in guinea pigs: an in vivo anatomical and in vitro electrophysiological study. Experimental Brain Research. 2003;153:467–476. doi: 10.1007/s00221-003-1606-2. [DOI] [PubMed] [Google Scholar]
- 18.Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 19.Jones BE. The organization of central cholinergic systems and their functional importance in sleep-waking states. Prog Brain Res. 1993;98:61–71. doi: 10.1016/s0079-6123(08)62381-x. [DOI] [PubMed] [Google Scholar]
- 20.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 21.Jones CK, Shannon HE. Lesions of the laterodorsal tegmental nucleus disrupt prepulse inhibition of the acoustic startle reflex. Pharmacol Biochem Behav. 2004;78:229–237. doi: 10.1016/j.pbb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Isa T. Sensory-motor gating and cognitive control by the brainstem cholinergic system. Neural Networks. 2002;15:731–741. doi: 10.1016/s0893-6080(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 23.Koch M, Kungel M, Herbert H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Experimental Brain Research. 1993;97:71–82. doi: 10.1007/BF00228818. [DOI] [PubMed] [Google Scholar]
- 24.Koyama Y, Jodo E, Kayama Y. Sensory responsiveness of “broad-spike” neurons in the laterodorsal tegmental nucleus, locus coeruleus and dorsal raphe of awake rats: implications for cholinergic and monoaminergic neuron-specific responses. Neuroscience. 1994;63:1021–1031. doi: 10.1016/0306-4522(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 25.Kuypers HG, Lawrence DG. Cortical projections to the red nucleus and the brain stem in the Rhesus monkey. Brain Res. 1967;4:151–188. doi: 10.1016/0006-8993(67)90004-2. [DOI] [PubMed] [Google Scholar]
- 26.Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J Comp Neurol. 1994;344:232–241. doi: 10.1002/cne.903440205. [DOI] [PubMed] [Google Scholar]
- 27.Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J Comp Neurol. 1994;344:190–209. doi: 10.1002/cne.903440203. [DOI] [PubMed] [Google Scholar]
- 28.Layton MG, Robertson D, Everett AW, Mulders WH, Yates GK. Cellular localization of voltage-gated calcium channels and synaptic vesicle-associated proteins in the guinea pig cochlea. J Mol Neurosci. 2005;27:225–244. doi: 10.1385/JMN:27:2:225. [DOI] [PubMed] [Google Scholar]
- 29.Leonard CS, Kerman I, Blaha G, Taveras E, Taylor B. Interdigitation of nitric oxide synthase-, tyrosine hydroxylase-, and serotonin-containing neurons in and around the laterodorsal and pedunculopontine tegmental nuclei of the guinea pig. The Journal of Comparative Neurology. 1995;362:411–432. doi: 10.1002/cne.903620309. [DOI] [PubMed] [Google Scholar]
- 30.Malmierca MS, Cristaudo S, Perez-Gonzalez D, Covey E. Stimulus-specific adaptation in the inferior colliculus of the anesthetized rat. J Neurosci. 2009;29:5483–5493. doi: 10.1523/JNEUROSCI.4153-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumura M, Nambu A, Yamaji Y, Watanabe K, Imai H, Inase M, Tokuno H, Takada M. Organization of somatic motor inputs from the frontal lobe to the pedunculopontine tegmental nucleus in the macaque monkey. Neuroscience. 2000;98:97–110. doi: 10.1016/s0306-4522(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 32.McCormick DA, Prince DA. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. Journal of Physiology. 1987;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mena-Segovia J, Ross HM, Magill PJ, Bolam JP. The pedunculopontine nucleus: Towards a functional integration with the basal ganglia. In: Bolam JP, Ingham CA, Magill PJ, editors. The Basal Ganglia VIII. Springer Science and Business Media; New York: 2005. pp. 533–544. [Google Scholar]
- 34.Mesulam MM. Cholinergic pathways and the ascending reticular activating system of the human brain. Ann N Y Acad Sci. 1995;757:169–179. doi: 10.1111/j.1749-6632.1995.tb17472.x. [DOI] [PubMed] [Google Scholar]
- 35.Monakow KH, Akert K, Kunzle H. Projections of precentral and premotor cortex to the red nucleus and other midbrain areas in Macaca fascicularis. Exp Brain Res. 1979;34:91–105. doi: 10.1007/BF00238343. [DOI] [PubMed] [Google Scholar]
- 36.Motts SD, Schofield BR. Olivary and extra-olivary sources of cholinergic input to the cochlear nucleus. Assoc. Res. Otolaryngol. Abs. 2005;27:242. [Google Scholar]
- 37.Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neuroscience. 2008;154:186–195. doi: 10.1016/j.neuroscience.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motts SD, Schofield BR. Sources of cholinergic input to the inferior colliculus. Neuroscience. 2009;160:103–114. doi: 10.1016/j.neuroscience.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oswald I, Taylor AM, Treisman M. Discriminative responses to stimulation during human sleep. Brain. 1960;83:440–453. doi: 10.1093/brain/83.3.440. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-González D, Malmierca MS, Covey E. Novelty detector neurons in the mammalian auditory midbrain. Eur J Neurosci. 2005;22:2879–2885. doi: 10.1111/j.1460-9568.2005.04472.x. [DOI] [PubMed] [Google Scholar]
- 41.Peterson DC, Schofield BR. Projections from auditory cortex contact ascending pathways that originate in the superior olive and inferior colliculus. Hear Res. 2007;232:67–77. doi: 10.1016/j.heares.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redies H, Brandner S, Creutzfeldt OD. Anatomy of the auditory thalamocortical system of the guinea pig. J Comp Neurol. 1989;282:489–511. doi: 10.1002/cne.902820403. [DOI] [PubMed] [Google Scholar]
- 43.Reese NB, Garcia-Rill E, Skinner RD. Auditory input to the pedunculopontine nucleus: II. Unit responses. Brain Res Bull. 1995;37:265–273. doi: 10.1016/0361-9230(95)00001-u. [DOI] [PubMed] [Google Scholar]
- 44.Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus--auditory input, arousal and pathophysiology. Prog Neurobiol. 1995;47:105–133. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- 45.Reese NB, Garcia-Rill E, Skinner RD. Auditory input to the pedunculopontine nucleus: I. Evoked potentials. Brain Res Bull. 1995;37:257–264. doi: 10.1016/0361-9230(95)00002-v. [DOI] [PubMed] [Google Scholar]
- 46.Ruggiero DA, Giuliano R, Anwar M, Stornetta R, Reis DJ. Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol. 1990;292:1–53. doi: 10.1002/cne.902920102. [DOI] [PubMed] [Google Scholar]
- 47.Saponjic J, Radulovacki M, Carley DW. Modulation of respiratory pattern and upper airway muscle activity by the pedunculopontine tegmentum: role of NMDA receptors. Sleep Breath. 2006;10:195–202. doi: 10.1007/s11325-006-0075-9. [DOI] [PubMed] [Google Scholar]
- 48.Schofield BR, Coomes DL. Auditory cortical projections to the cochlear nucleus in guinea pigs. Hear Res. 2005;199:89–102. doi: 10.1016/j.heares.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Schofield BR, Coomes DL. Projections from auditory cortex contact cells in the cochlear nucleus that project to the inferior colliculus. Hear Res. 2005;206:3–11. doi: 10.1016/j.heares.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Schofield BR. Cholinergic projections to midbrain cholinergic neurons that project to the inferior colliculus. Assoc. Res. Otolaryngol. Abs. 2009;32:130. [Google Scholar]
- 51.Semba K, Fibiger HC. Afferent connectiuons of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: A retro- and antero-grade transport and immunohistochemical study. The Journal of Comparative Neurology. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- 52.Shute CC, Lewis PR. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967;90:497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- 53.Spann BM, Grofova I. Cholinergic and non-cholinergic neurons in the rat pedunculopontine tegmental nucleus. Anat Embryol (Berl) 1992;186:215–227. doi: 10.1007/BF00174143. [DOI] [PubMed] [Google Scholar]
- 54.Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- 55.Steriade M, Paré D, Parent A, Smith Y. Projections of cholinergic and noncholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 56.Steriade M. Acetylcholine systems and rhythmic activities during the waking--sleep cycle. Prog Brain Res. 2004;145:179–196. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- 57.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 58.Takakusaki K, Shiroyama T, Yamamoto T, Kitai ST. Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J Comp Neurol. 1996;371:345–361. doi: 10.1002/(SICI)1096-9861(19960729)371:3<345::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 59.Tebecis AK. Cholinergic and non-cholinergic transmission in the medial geniculate nucleus of the cat. Journal of Physiology. 1972;226:153–172. doi: 10.1113/jphysiol.1972.sp009978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaudano E, Legg CR, Glickstein M. Afferent and Efferent Connections of Temporal Association Cortex in the Rat: A Horseradish Peroxidase Study. Eur J Neurosci. 1991;3:317–330. doi: 10.1111/j.1460-9568.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 61.Vincent SR. The ascending reticular activating system--from aminergic neurons to nitric oxide. J Chem Neuroanat. 2000;18:23–30. doi: 10.1016/s0891-0618(99)00048-4. [DOI] [PubMed] [Google Scholar]
- 62.Wallace MN, Rutkowski RG, Palmer AR. Identification and localisation of auditory areas in guinea pig cortex. Experimental Brain Research. 2000;132:445–456. doi: 10.1007/s002210000362. [DOI] [PubMed] [Google Scholar]
- 63.Wallace MN, Rutkowski RG, Palmer AR. Interconnections of auditory areas in the guinea pig neocortex. Exp Brain Res. 2002;143:106–119. doi: 10.1007/s00221-001-0973-9. [DOI] [PubMed] [Google Scholar]
- 64.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Research Bulletin. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- 66.Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Research Reviews. 1996;21:301–314. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- 67.Yeomans JS, Lee J, Yeomans MH, Steidl S, Li L. Midbrain pathways for prepulse inhibition and startle activation in rat. Neuroscience. 2006;142:921–929. doi: 10.1016/j.neuroscience.2006.06.025. [DOI] [PubMed] [Google Scholar]