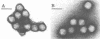Abstract
Virus particles morphologically resembling adenovirus were found in fecal specimens from infants and were examined for cultivability with standard cell culture techniques and for characteristics of human adenoviruses. Specimens from 13 of 15 infants could not be cultivated in cell cultures. The two adenoviruses that were cultivated, types 1 and 31, reacted in the expected manner in all tests. Counterimmunoelectrophoresis with group-specific anti-hexon serum confirmed that the observed particles in the 15 specimens were human adenoviruses. The buoyant density in sucrose of five of the noncultivable adenoviruses in original stool suspensions averaged 1.335 g/cm3 and that of the two cultivable ones averaged 1.332 g/cm3; both groups had typical adenovirus morphology by electron microscopy. Treatment of the specimens and of a variety of tissue culture cells with proteolytic and other enzymes did not improve cultivability. Examination of partially purified virus by immunoelectron microscopy did not reveal evidence of immunoglobulin A, G, or M coating on the particles, an indication that coproantibody inhibition was not the cause of noncultivability. Fluorescent-antibody studies with an antihexon conjugate and counterimmunoelectrophoresis studies of serially passaged noncultivable viruses indicated that the viruses are infecting cells but are not undergoing effective replication. Antisera to three of the noncultivable viruses demonstrated homologous reactions in counterimmunoelectrophoresis with the respective immunizing antigens but showed only low levels of hemagglutination-inhibiting and neutralizing activity to a few of the known human adenoviruses. We concluded that the noncultivable viruses in these infant diarrhea cases were indeed human adenoviruses, were not defective particles, were not bound to coproantibody, were infectious but incapable of effective relication in conventional cell cultures, were serologically related to types 11, 17, 32, and 33, and should be considered a new, distinct subgroup.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDUCCI D., TYRRELL D. A., ZAIMAN T. E. Acute infections of the respiratory tract and the adenoviruses. Lancet. 1956 Dec 29;271(6957):1326–1330. doi: 10.1016/s0140-6736(56)91484-2. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe R. B., Mufson M. A. Identification by immunofluorescence of adenoviral antigen in exfoliated bladder epithelial cells from patients with acute hemorrhagic cystitis. Proc Soc Exp Biol Med. 1974 Jul;146(3):754–758. doi: 10.3181/00379727-146-38187. [DOI] [PubMed] [Google Scholar]
- Birch C. J., Lewis F. A., Kennett M. L., Homola M., Pritchard H., Gust I. D. A study of the prevalence of rotavirus infection in children with gastroenteritis admitted to an infectious diseases hospital. J Med Virol. 1977;1(1):69–77. doi: 10.1002/jmv.1890010109. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973 Dec 8;2(7841):1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- DASCOMB H. E., HILLEMAN M. R. Clinical and laboratory studies in patients with respiratory disease caused by adenoviruses (RI-APC-ARD agents). Am J Med. 1956 Aug;21(2):161–174. doi: 10.1016/0002-9343(56)90049-3. [DOI] [PubMed] [Google Scholar]
- Dowdle W. R., Lambriex M., Hierholzer J. C. Production and evaluation of a purified adenovirus group-specific (hexon) antigen for use in the diagnostic complement fixation test. Appl Microbiol. 1971 Apr;21(4):718–722. doi: 10.1128/am.21.4.718-722.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T. H., Bryden A. S., Davies H. Letter: Virus particles in gastroenteritis. Lancet. 1973 Dec 29;2(7844):1497–1497. doi: 10.1016/s0140-6736(73)92760-8. [DOI] [PubMed] [Google Scholar]
- Flewett T. H. Diagnosis of enteritis virus. Proc R Soc Med. 1976 Sep;69(9):693–696. [PMC free article] [PubMed] [Google Scholar]
- Fox J. P., Brandt C. D., Wassermann F. E., Hall C. E., Spigland I., Kogon A., Elveback L. R. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969 Jan;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G., Norrby E., Wadell G. Isolation of incomplete adenovirus 16 particles containing viral and host cell DNA. Virology. 1977 Oct 15;82(2):449–461. doi: 10.1016/0042-6822(77)90018-6. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Atuk N. O., Gwaltney J. M., Jr New human adenovirus isolated from a renal transplant recipient: description and characterization of candiate adenovirus type 34. J Clin Microbiol. 1975 Apr;1(4):366–376. doi: 10.1128/jcm.1.4.366-376.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Barme M. Counterimmunoelectrophoresis with adenovirus type-specific anti-hemagglutinin sera as a rapid diagnostic method. J Immunol. 1974 Mar;112(3):987–995. [PubMed] [Google Scholar]
- Hierholzer J. C. Further subgrouping of the human adenoviruses by differential hemagglutination. J Infect Dis. 1973 Oct;128(4):541–550. doi: 10.1093/infdis/128.4.541. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Gamble W. C., Dowdle W. R. Reference equine antisera to 33 human adenovirus types: homologous and heterologous titers. J Clin Microbiol. 1975 Jan;1(1):65–74. doi: 10.1128/jcm.1.1.65-74.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONCAS J., MOISAN A., AVILANIS V. Incidence of adenovirus infection: a family study. Can Med Assoc J. 1962 Jul 14;87:52–58. [PMC free article] [PubMed] [Google Scholar]
- JONCAS J., PAVILANIS V. Diarrhoea and vomiting in infancy and childhood: viral studies. Can Med Assoc J. 1960 May 28;82:1108–1113. [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Dolin R., Thornhill T. S., Kalica A. R., Chanock R. M. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972 Nov;10(5):1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerla H. C., Hierholzer J. C. Physicochemical and serological characteristics of respiratory virus fluorescein-isothiocyanate conjugates for fluorescent-antibody diagnosis. J Clin Microbiol. 1975 May;1(5):451–461. doi: 10.1128/jcm.1.5.451-461.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. L., Palmer E. L., Middleton P. J. Ultrastructure of infantile gastroenteritis virus. Virology. 1975 Nov;68(1):146–153. doi: 10.1016/0042-6822(75)90156-7. [DOI] [PubMed] [Google Scholar]
- Mavromichalis J., Evans N., McNeish A. S., Bryden A. S., Davies H. A., Flewett T. H. Intestinal damage in rotavirus and adenovirus gastroenteritis assessed by d-xylose malabsorption. Arch Dis Child. 1977 Jul;52(7):589–591. doi: 10.1136/adc.52.7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P. J., Szymanski M. T., Petric M. Viruses associated with acute gastroenteritis in young children. Am J Dis Child. 1977 Jul;131(7):733–737. doi: 10.1001/archpedi.1977.02120200015004. [DOI] [PubMed] [Google Scholar]
- Moffet H. L., Shulenberger H. K., Burkholder E. R. Epidemiology and etiology of severe infantile diarrhea. J Pediatr. 1968 Jan;72(1):1–14. doi: 10.1016/s0022-3476(68)80394-4. [DOI] [PubMed] [Google Scholar]
- Prage L., Höglund S., Philipson L. Structural proteins of adenoviruses. 8. Characterization of incomplete particles of adenovirus type 3. Virology. 1972 Sep;49(3):745–757. doi: 10.1016/0042-6822(72)90531-4. [DOI] [PubMed] [Google Scholar]
- SPENDLOVE R. S., SCHAFFER F. L. ENZYMATIC ENHANCEMENT OF INFECTIVITY OF REOVIRUS. J Bacteriol. 1965 Mar;89:597–602. doi: 10.1128/jb.89.3.597-602.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H. S., Vastine D. W., Yamashiroya H., West C. E. Immunofluorescent detection of adenovirus antigen in epidemic keratoconjunctivitis. Invest Ophthalmol. 1976 Mar;15(3):199–207. [PubMed] [Google Scholar]
- Sohier R., Chardonnet Y., Prunieras M. Adenoviruses. Status of current knowledge. Prog Med Virol. 1965;7:253–325. [PubMed] [Google Scholar]
- Tjia S., Fanning E., Schick J., Doerfler W. Incomplete particles of adenovirus type 2. III. Viral and cellular DNA sequences in incomplete particles. Virology. 1977 Jan;76(1):365–379. doi: 10.1016/0042-6822(77)90309-9. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Gust I. D., Holmes I. H. Human rotavirus and its antibody: their coexistence in feces of infants. J Clin Microbiol. 1978 May;7(5):405–409. doi: 10.1128/jcm.7.5.405-409.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw A., Davies H., Parry J. Eelectron microscopy of fatal adenovirus gastroenteritis. Lancet. 1977 Feb 12;1(8007):361–361. doi: 10.1016/s0140-6736(77)91158-8. [DOI] [PubMed] [Google Scholar]
- Wigand R., Keller D. Relationship of human adenoviruses 12, 18, and 31 as determined by hemagglutination inhibition. J Med Virol. 1978;2(2):137–142. doi: 10.1002/jmv.1890020208. [DOI] [PubMed] [Google Scholar]