Abstract
This study sought to determine if microdermabrasion can selectively remove stratum corneum to increase skin permeability. Although, microdermabrasion has been used for cosmetic treatment of skin for decades, no study has assessed the detailed effects of microdermabrasion conditions on the degree of skin tissue removal. Therefore, we histologically characterized the skin of rhesus macaques and human volunteers after microdermabrasion at different conditions. Using mobile tip microdermabrasion, an increase in the number of treatment passes led to greater tissue removal ranging from minimal effects to extensive damage to deeper layers of the skin. Of note, these data showed for the first time that at moderate microdermabrasion conditions selective yet full-thickness removal of stratum corneum could be achieved with little damage to deeper skin tissues. In the stationary mode of microdermabrasion, selective stratum corneum removal was not observed, but micro-blisters could be seen. Similar tissue removal trends were observed in human volunteers. As proof of concept for drug delivery applications, a model fluorescent drug (fluorescein) was delivered through microdermabraded skin and antibodies were generated against vaccinia virus after its topical application in monkeys. In conclusion, microdermabrasion can selectively remove full-thickness stratum corneum with little damage to deeper tissues and thereby increase skin permeability.
Keywords: microdermabrasion, transdermal drug delivery, vaccination, vaccinia virus, skin histology, skin permeability, stratum corneum removal
1. Introduction
Microdermabrasion is an FDA approved process first introduced in 1985 and is a popular method used to treat scars, acne and other cosmetic-dermatologic conditions (Spencer, 2005). Recently there has been interest in using microdermabrasion to enable transdermal drug delivery. It is known that skin’s top-most layer, the stratum corneum is the main transport barrier to delivery of drugs and vaccines across the skin and that removal of stratum corneum dramatically increases skin permeability (Prausnitz and Langer, 2008). Accordingly, to enable transdermal drug delivery various approaches have been investigated for the selective removal of stratum corneum to increase drug transport without damaging living cells of the viable epidermis layer just beneath the stratum corneum or causing widespread damage that is difficult for the skin to repair rapidly. While tape stripping has been employed to remove stratum corneum in many laboratory studies (Godefroy et al., 2005, Guy and Hadgraft, 2002), thermal ablation and mechanical abrasion have been emphasized for transdermal drug delivery with clinical potential. Thermal ablation of the stratum corneum has been carried out using lasers (Fang et al., 2004a), radiofrequency energy (Sintov et al., 2003) and direct application of heat (Bramson et al., Arora et al., 2008) and mechanical abrasion of the stratum corneum has been carried out using abrasive-pads (Glenn et al., 2007), blunt-tipped microneedles (Mikszta et al., 2002) and microdermabrasion (Fujimoto et al., 2005, Lee et al., 2006).
Mechanistically, microdermabrasion involves impingement of sharp microparticles on the skin surface, which are then removed under vacuum into a waste container along with the abraded skin tissue. Using microdermabrasion, various researchers have shown increased permeability of freshly excised animal skin to very low molecular weight compounds (<300 Da) recording a 10 to 20 fold flux enhancement of estradiol (Fujimoto et al., 2005), vitamin C (Lee et al., 2003), and 5-aminolaevulinic acid (Fang et al., 2004a). In contrast to microdermabrasion, which uses vacuum to produce flow of microparticles, a related approach called microscission uses a positive pressure to accelerate and impinge microparticles on the skin. Using this approach, lidocaine delivery and blood glucose measurement were demonstrated in human volunteers (Herndon et al., 2004).
Although various studies have shown that microdermabrasion enhances transdermal flux, a significant challenge in the development of microdermabrasion for clinical applications is the limited understanding of the effects of microdermabrasion on skin. Of note, while the different studies have demonstrated flux enhancement, they have not demonstrated full thickness removal of the stratum corneum layer, which is critical for reproducible and controlled transdermal delivery of large molecular weight compounds and vaccines. Furthermore, a mechanistic understanding is lacking even for cosmetic applications of microdermabrasion for which the device is already approved by the FDA. This is largely because the microdermabrasion device was originally classified as a class-I device, which according to the FDA is a device that presents minimal potential for harm to the user and general controls are sufficient to ensure safety. Consequently the microdermabrasion device never underwent phase-III clinical trials or the associated detailed characterization (Spencer, 2005).
For transdermal drug delivery applications it is important to understand the effects of microdermabrasion on the skin layers such that the full thickness of stratum corneum can be removed in a controlled fashion with minimum collateral damage to the underlying viable cells of the epidermis. Therefore, in this study we carried out detailed histological examination of the skin after performing microdermabrasion in vivo on the skin of rhesus macaques and human volunteers. Multiple skin biopsies were obtained from the microdermabraded sites and histologically analyzed to assess the effects of microdermabrasion on the different skin layers.
2. Materials and Methods
2.1 Microdermabrasion apparatus
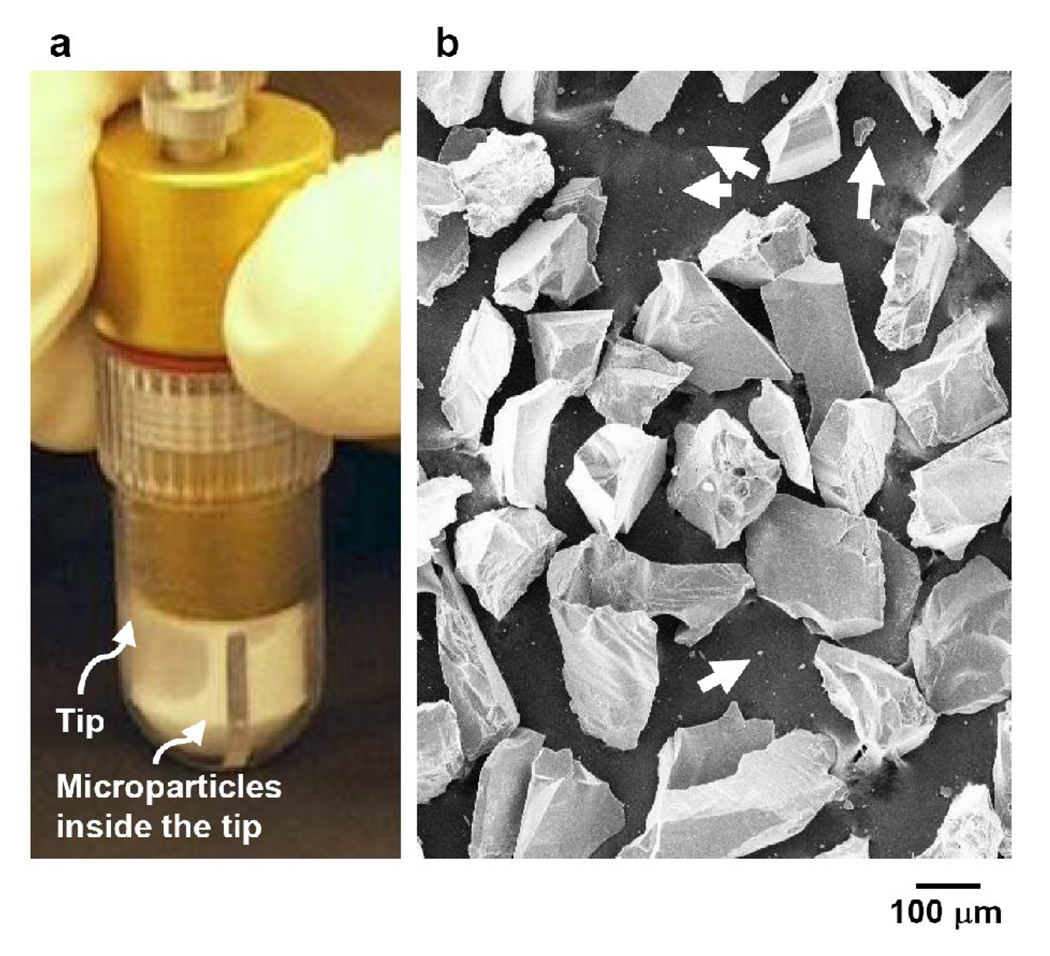
Microdermabrasion was performed using an FDA-approved device (MegaPeel® Gold Series, DermaMed International, Lenni, PA, USA) with disposable microdermabrasion tips having a skin-contacting hole with a 7 mm inner diameter (Fig 1a). The size and morphology of the aluminum oxide particles (DermaMed International) used for microdermabrasion were determined through scanning electron microscopy (LEO-1530, LEO electron microscopy, Cambridge, England). The particles were found to have highly irregular shapes with sharp edges (Fig 1b). The largest dimension of the majority of the particles was between 100 to 300 µm, although a small amount of particulate debris (about 1 µm) was also found.
Figure 1.
Microdermabrasion tip and particles. A microdermabrasion tip with aluminum oxide microparticles flowing inside the tip (a). Scanning electron microscopy image of aluminum oxide microparticles with a small population of very small particles (pointed to by arrows) (b).
To facilitate microdermabrasion of the same skin area during repeated passes, rectangular polyethylene medical foam tape (TM9716, MACtac, Stow, OH, USA) stencils (55 × 30 mm) with rectangular slits (40 × 10 mm) were attached to the skin of rhesus macaques and human volunteers. The slits were precisely cut using a computer-controlled CO2 laser (LS500XL. New Hermes, Duluth, GA, USA). For the mobile tip mode, the tips were moved manually back and forth within the slit of the stencil. The slit width of 10 mm closely accommodated the outer diameter of the microdermabrasion tip and thereby facilitated repeated motion over the same path on the skin. For the stationary tip mode, the microdermabrasion tip was placed directly on the skin surface without the use of a stencil.
2.2 In vivo rhesus macaque microdermabrasion
Microdermabrasion was performed on the backs of rhesus macaque monkeys (Yerkes National Primate Research Center, Emory University, Atlanta, GA) after trimming the hair and cleaning the skin with ethanol swabs. This protocol was approved by the Emory University IACUC.
For the mobile mode of microdermabrasion, two vacuum pressure settings of 25 kPa and 50 kPa were tested; the 25 kPa setting was operated at 100, 200 and 300 passes and the 50 kPa setting was operated at 10, 30, 50, 80 and 100 passes. To perform microdermabrasion in triplicate, three monkeys were assigned for each pressure level group. One pass is defined as the movement of the microdermabrasion tip from one end to the other on the treated skin site exposed through the stencil slit. The hand-piece was manually moved at a speed of approximately 40 mm/s and the flow rate of microparticles was kept at 70% of the maximum flow rate of the instrument. The vacuum pressure of 50 kPa was selected because it is considered a medium microdermabrasion setting according to manufacturer specifications, and in preliminary experiments on porcine cadaver skin this pressure resulted in removal of full-thickness of stratum corneum (data not shown). The other pressure of 25 kPa was selected because we wanted to identify the effect at a lower suction pressure. A non-microdermabraded, but trimmed and ethanol-cleaned section of skin from the back of the monkeys was used as a negative control.
The stationary mode of microdermabrasion was performed by keeping the hand-piece stationary on the skin and exposing the skin to a vacuum pressure of 30 kPa or 50 kPa for 3 s or 6 s each. The conditions were tested in triplicate by performing microdermabrasion at each condition on three monkeys.
To facilitate direct comparison between microdermabrasion in the mobile mode and the stationary mode, the “effective” microdermabrasion exposure time in the mobile mode was calculated by dividing the tip diameter (7 mm) by the speed of tip movement (40 mm/s) and then multiplying by the number of passes.
A 6 mm skin biopsy was obtained from the center of each microdermabraded and control skin site (mobile mode = center of stencil; stationary mode = center of treated skin), mounted in OCT (optimal cutting temperature) fluid (Tissue-Tek®, Fisher Scientific, Pittsburg, PA, USA), and frozen in liquid nitrogen for histological analysis.
2.3 Microdermabrasion of human volunteers
Sixteen healthy human volunteers, consisting of 6 males and 10 females aged 19 to 40 years old, were recruited for the study from the general population of Atlanta, GA, USA. ‘Healthy volunteers’ excludes persons with impaired immunity, chronic dermatological disorders, diabetes, liver disease, those in need of immunosuppressive agents or with tattoos on the inner forearm. Volunteers provided their written informed consent prior to microdermabrasion. The protocols followed the declaration of Helsinki and were approved by the IRBs at Emory University and the Georgia Institute of Technology.
A group of six volunteers was exposed to the mobile mode of microdermabrasion at 40 kPa vacuum pressure with seven passes of the hand-piece at a speed of approximately 13 mm/s (i.e., three times slower than that used in monkeys). Two groups of five volunteers each were exposed to the stationary mode of microdermabrasion at a vacuum pressure of 30 kPa and 45 kPa, respectively, for 3 s each. The flow rate of microparticles was always kept at 70% of the maximum flow rate of the instrument.
For each subject, microdermabrasion was performed on the volar forearm. One forearm was randomly selected for microdermabrasion and the other forearm acted as the negative control. The mobile and the stationary modes of microdermabrasion and the subsequent biopsy collection were performed in a manner similar to that of the monkeys, with the exception that the size of the skin biopsy was 1 mm.
2.4 Histology and microscopy
Skin biopsies embedded in OCT were cut into 10-µm thick sections using a cryostat (Cryo-Star HM 560 MV, Microm, Waldorf, Germany) and mounted onto glass slides (Fisher Scientific). All the skin sections except those from sites exposed to sodium fluorescein were stained with hematoxylin and eosin (H&E). Skin sections were then examined either for removal of skin layers or for delivery of sodium fluorescein using brightfield or fluorescence microscopy, respectively (Eclipse 600, Nikon, Tokyo, Japan), with a CCD camera (Retiga 1300, QImaging, Surrey, BC, Canada).
2.5 Histological assessment of skin layers
Quantitative analysis was carried out on biopsies taken from monkeys exposed to mobile-mode of microdermabrasion. Thirty consecutive H&E-stained skin sections were independently scored by two investigators to determine percentages of stratum corneum or viable epidermis layers from which the full-thickness of the respective tissues had been removed. Stratum corneum and viable epidermis of each skin section was categorized into the intervals 0–25% (score ‘0’), 26–50% (score ‘1’), 51–75% (score ‘2’) or 76–100% (score ‘3’) removal, representing the fractional percentage of a skin layer from which the tissue had been removed to its full depth. For example, a skin section in which the stratum corneum layer showed full-thickness stratum corneum removal on slightly more than half of its length but showed no full-thickness removal of the viable epidermis, was scored ‘2’ for stratum corneum removal and ‘0’ for epidermal removal.
The resulting data were presented in two ways. First, the average score was calculated for each condition to provide a single number that characterized the degree of tissue removal for each layer. To provide additional information about variability, the distribution of scores for each condition was also reported as a histogram. Qualitative analysis was carried out for the remaining biopsies taken from monkeys exposed to stationary-mode microdermabration and all biopsies taken from humans. In this case, 3 to 5 consecutive sections each were visually assessed for damage and characterized qualitatively.
2.6 Topical delivery to microdermabraded skin
To assess the ability to deliver drugs through microdermabraded skin, 200 µl of an aqueous solution containing 10 % (w/v) sodium fluorescein (parenteral injection-grade, Akron, Buffalo Grove, IL, USA) was topically applied for 2 h on skin microdermabraded at 25 kPa for 100, 200 and 300 passes, and at 50 kPa for 50 passes (n=3 monkeys). To hold the solution in contact with the skin, a square channel 10 mm × 10 mm wide and 3.2 mm high was formed by stacking two identical pieces of stencils cut from medical foam tape using laser as described in section 2.1. After removing the larger stencil used during microdermabrasion, this construct was then attached to the skin exposing just the middle 10 mm × 10 mm region of the 40 mm × 10 mm microdermabraded area. The channel was then filled with the solution and a piece of tape was used to cover the channel to minimize evaporation. Topical application of the fluorescein solution to a non-microdermabraded skin site was used as a negative control. An 8-mm diameter skin biopsy was collected from each skin site to assess delivery into the skin by fluorescence microscopy of histological sections.
In a separate study, we assessed the delivery of MVA by measuring the immune response from topical application of this model vaccine (Tatsis et al., 2007) onto microdermabraded skin. The skin of monkeys (n=3) was exposed to 65 µl of phosphate-buffered saline containing 6 × 10 PFUs of MVA virus after microdermabrasion at 25 kPa and 10–15 passes as a primary immunization. A booster dose was applied 11 weeks later after microdermabrasion at 50 kPa and 50 passes at a different site on the back of the monkeys. Blood samples were collected 1 day before the primary immunization, 6 weeks after primary immunization and 1 week after the booster dose.
The blood samples were analyzed in triplicate using ELISA to determine the end point titers of the MVA-specific IgG antibodies (Harrop et al., 2004). Briefly, 96 well plates (Maxisorp, Nunc) were coated overnight at 4°C with MVA (0.5 × 106 pfu/well) in 0.05 M carbonate buffer pH 9.6, washed thrice with PBST (PBS with 0.05% Tween-20) and blocked with 3% bovine serum albumin in PBS for 1 h at 37°C. After washing with PBST, hundred-fold prediluted pre-immune and post-immune sera for each monkey were added to the wells and two-fold serially diluted. After 1 h incubation at 37°C and washing with PBST, 1:4000 dilution of horseradish peroxidase-labeled goat anti-rhesus IgG antibodies were added to the wells and incubated for 1 h at 37°C. After washing, color was developed with tetramethyl benzidine substrate (BD Biosciences, San Jose, CA). The reaction was stopped by adding 2 N H2SO4 and read at 450 nm (SpectraMax Plus384 Microplate Spectrophotometer, Molecular Devices). Each sample was run in triplicate. End point titer was defined as the dilution for which post-immunization mean absorbance value was greater than at least two times the absorbance of the pre-immunized blood samples for the respective monkey.
3. Results
3.1 Mobile microdermabrasion in monkeys
3.1.1 Effect of number of passes (qualitative measure)
To test the hypothesis that microdermabrasion can selectively remove full-thickness stratum corneum with little damage to deeper tissues, we histologically characterized the skin of rhesus macaques after exposure to microdermabrasion at 50 kPa vacuum pressure as a function of the number of passes across the skin. The microdermabrasion tip and particles used for microdermabrasion are shown in Fig. 1.
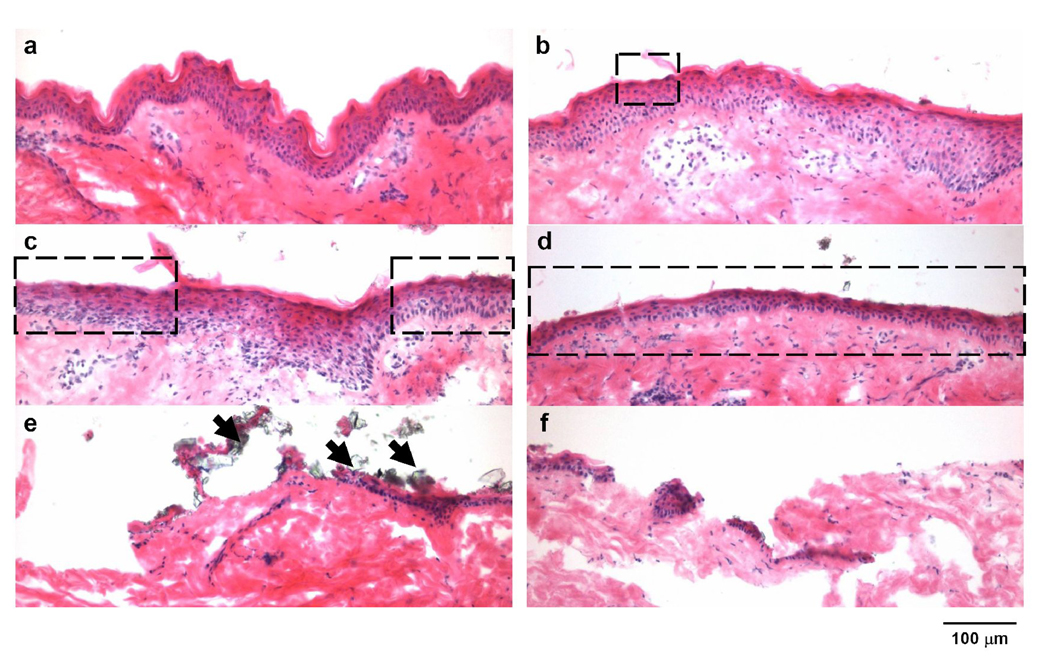
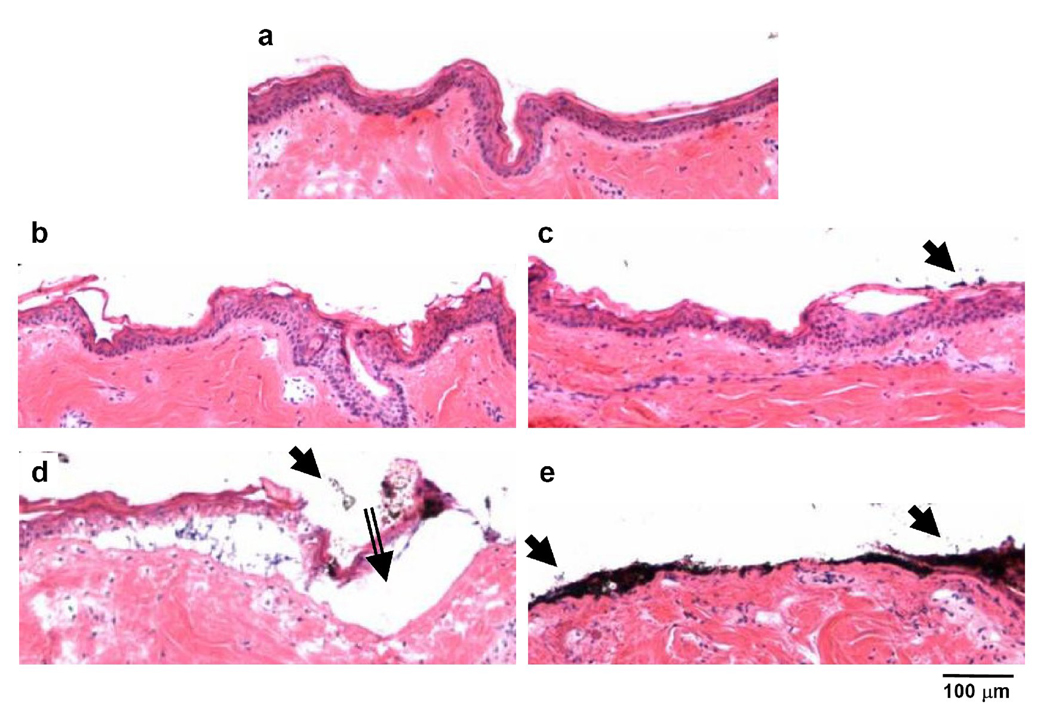
Histological analysis showed a broad range of removal effects on the stratum corneum and the viable epidermal layers of the skin, as compared to the untreated skin (Fig. 2a). In general, more skin was removed with increasing number of passes. At 10 passes, the stratum corneum and viable epidermis resembled the negative control, exhibiting full-thickness stratum corneum removal only at occasional focal regions (Fig. 2b). At 30 and 50 passes, larger areas of the skin were seen to have full-thickness removal of stratum corneum accompanied by little or no visible damage to the viable epidermis (Fig. 2c and 2d). At 80 and 100 passes there was extensive removal of full-thickness of stratum corneum accompanied by extensive damage to the viable epidermis and some damage to the dermis (Fig. 2e and 2f). In support of our hypothesis, selective removal of full-thickness of stratum corneum with little damage to deeper tissues was observed at 30 to 50 passes.
Figure 2.
Effect of the number of passes on removal of skin layers in monkeys after mobile microdermabrasion. Brightfield images of H&E stained skin sections from biopsies obtained from an untreated control site (a), and sites exposed to mobile-mode microdermabrasion with 50 kPa vacuum pressure for 10 passes (b), 30 passes (c), 50 passes (d), 80 passes (e) and 100 passes (f). Dotted rectangles indicate areas of selective yet full-thickness stratum corneum removal, and arrows point to residual aluminum oxide particles.
Aluminum oxide particles with diameters less than 5 µm were sometimes observed sticking to the skin after microdermabrasion (indicated by arrows in Fig. 2e). Note that most microdermabrasion particles are on the order of 100 µm in size and that these small adherent particles are from the particulate debris (Fig. 1b). Adherent particles were almost always seen after 80 and 100 passes (> 90% of skin sections), but only occasionally present after 10, 30 and 50 passes (< 5% of skin sections). This result may be explained by the observation that body fluid including blood was often observed on the skin surface after 80 and 100 passes, whereas fluid was generally not seen after 10, 30 and 50 passes, although mild to strong erythema was common. We hypothesize that the small particles adhered to the wet skin created after 80 and 100 passes due to surface tension forces.
3.1.2 Effect of number of passes (quantitative measure)
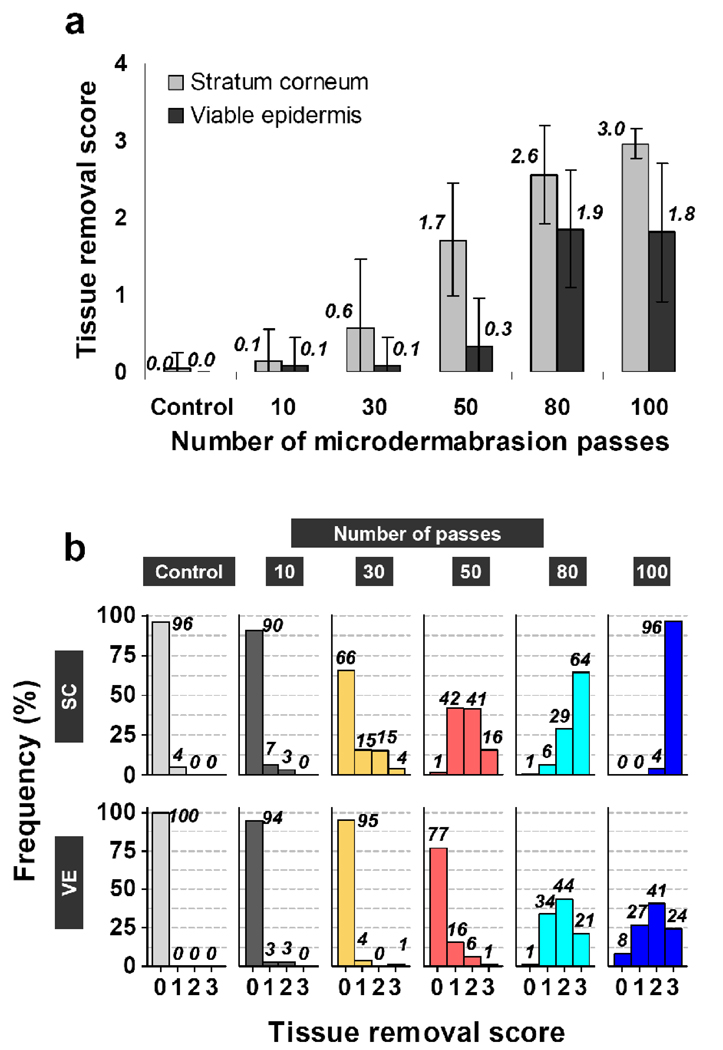
To quantify the effects of microdermabrasion on skin tissue removal, the extent of full-thickness removal of stratum corneum and of viable epidermis was visually scored on a scale of 0 to 3 (see Materials and Methods section). Based on these scores, the mean tissue removal scores were computed as a function of the number of microdermabrasion passes (Fig. 3a). The mean tissue removal scores represent the average fraction of a given skin section from which the full thickness of stratum corneum or viable epidermis was removed. The distribution of these scores for each microdermabrasion condition (collectively for all monkeys for that condition) is also reported in Fig. 3b.
Figure 3.
Tissue removal scores of skin layers. Quantitative representation of removal scores of stratum corneum and viable epidermis for an untreated control site and sites exposed to different numbers of passes of mobile-mode microdermabrasion at 50 kPa vacuum pressure, displayed as the mean values (a) and the frequency distribution for a total of 180 scores for each site (b). Numbers above the bars represent means (a) and percent frequency (b). Error bars correspond to standard deviations. SC=stratum corneum, VE=viable epidermis.
This analysis showed that the extent of full-thickness stratum corneum removal and the extent of full-thickness viable epidermis removal both increased with increasing number of passes, as measured by the mean tissue removal score (ANOVA, p < 0.05, Fig. 3a) and the frequency of ‘non-zero’ tissue removal scores (Fig. 3b). Stratum corneum was mostly intact until 30 microdermabrasion passes, at which point 34% of the skin sections had substantial removal of stratum corneum (with a mean tissue removal score of 0.6 ± 0.9), which was accompanied by just 5% of the skin sections exhibiting substantial removal of viable epidermis (with a mean tissue removal score of 0.1 ± 0.4). At 50 passes, 99% of the skin sections had substantial stratum corneum removal (with a mean tissue removal score of 1.7 ± 0.7), which was accompanied by 23% of the skin sections exhibiting substantial removal of viable epidermis (with a mean tissue removal score of 0.3 ± 0.6). At 80 and 100 passes, 99 – 100% of skin sections had substantial stratum corneum removal (with mean tissue removal scores of 2.6 ± 0.6 and 3.0 ± 0.2, respectively), which was accompanied by 92 – 99% of skin sections exhibiting substantial removal of viable epidermis (with means tissue removal scores of 1.9 ± 0.8 and 1.8 ± 0.9, respectively).
It is important to note that the variability in tissue removal scores represents the collective variability in the fractional removal of stratum corneum and viable epidermis for each animal group corresponding to a microdermabrasion condition. It does not represent inter-animal variability within the group. The inter-animal variability was not statistically significant for tissue removal scores of stratum corneum and the viable epidermis except for 30 passes and 50 passes, for which one animal showed a higher removal stratum corneum removal score as compared to the two other animals within the group (p<0.05).
Collectively these results are consistent with the qualitative analysis above and show that significant stratum corneum removal can be achieved with almost no damage to viable epidermis at 30 passes and that widespread stratum corneum removal can be achieved with low levels of damage to viable epidermis at 50 passes.
3.1.3 Effect of vacuum pressure (quantitative measure)
To determine the effect of microdermabrasion vacuum pressure on skin tissue removal, microdermabrasion was also performed at 25 kPa. Because vacuum pressure is the driving force for the flow of microparticles, a decrease in the vacuum pressure from 50 to 25 kPa is expected to decrease the microparticle kinetic energy and flow rate. In an attempt to compensate for this, more passes were used at this lower pressure, which yielded full-thickness stratum corneum tissue removal scores of 2.9 ± 0.4, 3.0 ± 0.1 and 2.3 ± 1.1 and viable epidermis tissue removal scores of 2.9 ± 0.4, 3.0 ± 0.1 and 2.3 ± 1.1at 100, 200 and 300 passes, respectively (histology images not shown). Blood was generally observed following microdermabrasion, and residual particles were also seen on the histology sections. These results suggest that we overcompensated for the expected reduction in efficacy at the lower pressure, because all three conditions led to extensive removal of full-thicknesses of both the stratum corneum and the viable epidermis. Indeed, the tissue removal scores at 25 kPa and 100 passes were similar to those at 50 kPa and 100 passes, which suggests that this two-fold change of pressure had less effect than, for example, a two-fold change of the number of passes.
3.2 Stationary microdermabrasion in monkeys
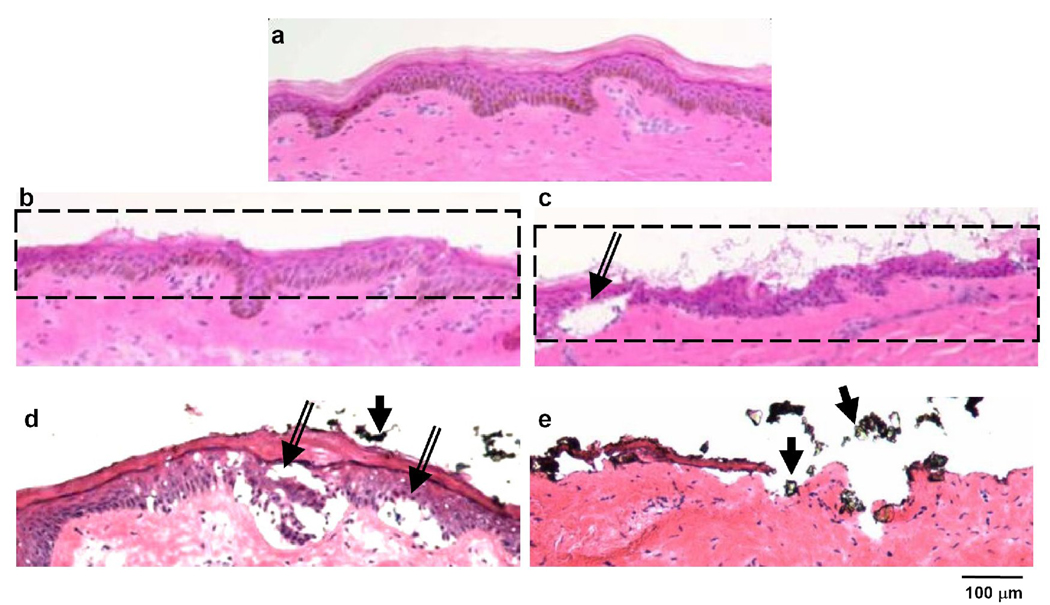
The stationary mode of microdermabrasion is simpler to perform than the mobile mode, because the microdermabrasion tip just needs to be held against the skin for a fixed period of time. We therefore characterized the histological effects on skin tissue removal from stationary microdermabrasion to assess its ability to selectively remove full-thickness stratum corneum. Similar to the untreated skin (Fig. 4a), the stratum corneum and the viable epidermis were both generally intact following exposure at 30 kPa for 3s (Fig. 4b) and 6s (Fig. 4c). However, using a higher vacuum pressure of 50 kPa with an exposure time of 3 s led to microscopic blisters (separation of viable epidermis from the dermis, indicated by the double-line arrow in Fig. 4d) accompanied by partial removal of stratum corneum (Fig. 4d), while a 6 s exposure time led to extensive removal of both stratum corneum and viable epidermis (Fig. 4e).
Figure 4.
Effect of exposure time and vacuum pressure on removal of skin layers in monkeys after stationary-mode microdermabrasion. Brightfield images of H&E stained skin sections from biopsies obtained from an untreated control site (a), and sites exposed to stationary-mode microdermabrasion with 30 kPa vacuum pressure and exposure time of 3 s (b) or 6s (c), and 50 kPa vacuum pressure and exposure time of 3 s (d) or 6s (e). Double-lined arrows point to blisters and single-lined arrows point to residual aluminum oxide particles.
For most drug delivery scenarios, blister formation is an unnecessary injury that should be avoided. However, for vaccine delivery applications, blisters may enhance the immune response by virtue of a wound healing response by recruiting inflammatory molecules and cells to the microblister site (Kuhns et al., 1992).
Residual particles were observed on some histology sections (indicated by single-line arrows in Fig. 4c, 4d and 4e). Overall, selective full-thickness removal of the stratum corneum was not observed, but was instead mostly accompanied by extensive damage to the viable epidermis.
The mobile and the stationary modes at 50 kPa can be compared by calculating an equivalent exposure time for the mobile-mode procedures (see Materials and Methods). Stationary-mode microdermabrasion for 6 s can be compared to mobile-mode microdermabrasion at 30 passes and 50 passes, which have effective exposure times of 5.3 s and 8.8 s, respectively. While both of these mobile-mode conditions yielded selective stratum corneum removal with little damage to deeper tissues, the stationary-mode procedure caused extensive epidermal tissue damage. These different responses may be explained by the possibly stronger effects of continuous exposure for 6 s compared to intermittent exposure for a similar amount of time, but broken up into 30 or 50 segments.
3.3 Mobile and stationary microdermabrasion in humans
To determine the applicability of results observed in rhesus macaques to humans, we carried out an additional study of the mobile and stationary modes of microdermabrasion on the skin of human volunteers. As compared to control skin (Fig. 5a), selective removal of stratum corneum was observed using a mobile tip at 40 kPa (Fig. 5b and 5c) and blisters were also sometimes seen (Fig. 5c). Because the IRB-approved protocol limited the maximum number of tip passes to seven and the vacuum pressure to 40 kPa, we used a slower speed of tip movement to increase the exposure time of microdermabrasion, which may explain the occurrence of microscopic blisters. However, this result validated the ability to selectively remove the stratum corneum in humans similar to that observed in monkeys. Inter-subject variability in full-thickness stratum corneum removal was high; full-thickness removal of stratum corneum was observed in two of the six volunteers.
Figure 5.
Mobile- and stationary-mode microdermabrasion in humans. Brightfield images of H&E stained human skin sections from biopsies obtained from an untreated control site (a), sites exposed to mobile-mode microdermabrasion with 40 kPa vacuum pressure and 7 passes (b and c), and sites exposed to stationary-mode microdermabrasion with an exposure time of 3 s and a vacuum pressure of 30 kPa (d) and 45 kPa (e). Dotted rectangles indicate areas of selective yet full-thickness removal of stratum corneum, double-lined arrows point to blisters and single-lined arrows point to residual aluminum oxide particles.
Stationary microdermabrasion performed for 3 s at 30 kPa produced microscopic blisters generally beneath intact stratum corneum (indicated by double-lined arrows in Fig. 5d) and at 45 kPa caused extensive removal of both stratum corneum and viable epidermis (Fig. 5e). Similar to the observation in monkeys, the stationary mode was unable to produce selective removal of the stratum corneum. Residual particles were also seen on the skin surface (indicated by single-lined arrows in Fig 5d and 5e).
Despite the occurrence of microscopic blisters, the procedure was well tolerated by the volunteers and no visible signs of damage were observed at the treated sites by study personnel. Mild erythema was often present, but no bleeding was seen.
3.4 Drug and vaccine delivery to monkeys
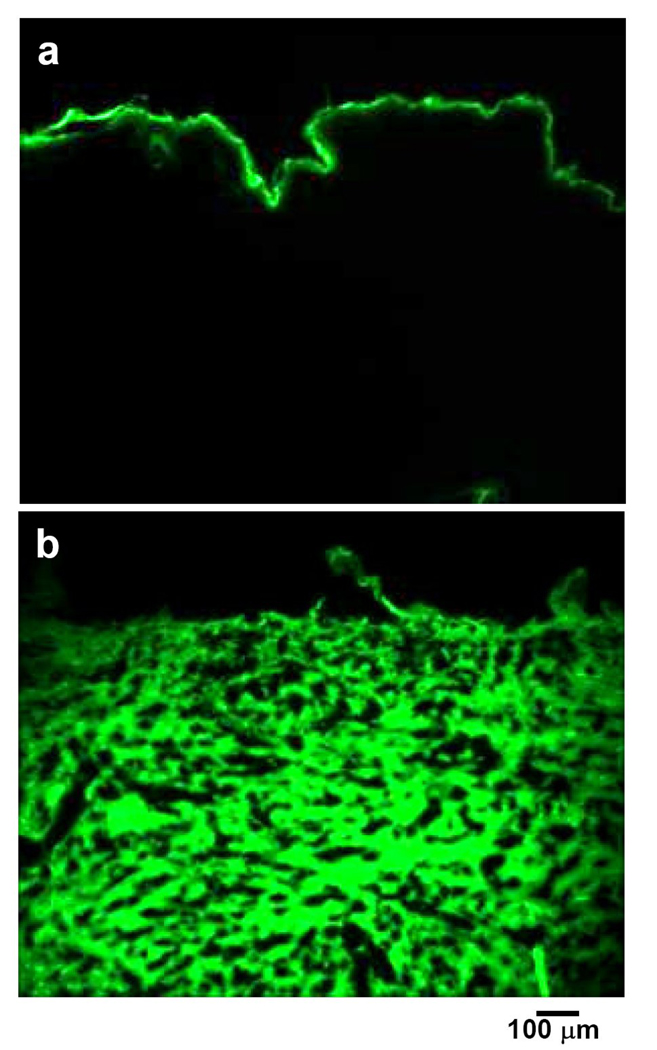
To validate the expectation that full-thickness stratum corneum removal can increase skin permeability, we measured the extent of topical delivery through microdermabraded skin. Topical application of sodium fluorescein, a model fluorescent hydrophilic compound, on non-microdermabraded skin resulted in essentially no detectable delivery across the stratum corneum (Fig. 6a). However, application to microdermabraded skin enhanced transdermal flux and delivered fluorescein deep into the dermis (Fig. 6b), consistent with increased skin permeability.
Figure 6.
In vivo delivery through microdermabraded skin. Fluorescence images of skin sections obtained from sites topically exposed to sodium fluorescein solution for 2 h without microdermabrasion treatment (a) and after mobile-mode microdermabrasion at 50 kPa and 50 passes (b).
Topical application of MVA, which is a live attenuated virus that serves as a model vaccine, generated virus-specific antibodies measured in the blood after primary immunization (antibody titer: 530 ± 230, p<0.05, Student’s t-test) and a still greater response after the booster dose (antibody titer: 5300 ± 1800, p<0.05, Student’s t-test). This result demonstrates increased skin permeability to viral particles through microdermabraded skin, which produced an antibody response and suggests a strategy for epidermal vaccination.
4. Discussion
4.1 Histological changes in skin layers
Despite widespread use of microdermabrasion in clinical practice, the microscopic changes in skin that are caused by this procedure have not been studied in detail before. The few studies that have done histologic evaluation of skin after microdermabrasion have reported thinning of stratum corneum at the specific conditions tested (Tan et al., 2001, Shim et al., 2001). The present study showed for the first time that microdermabrasion can cause a range of skin tissue removal effects, including selective removal of full-thickness stratum corneum at some conditions and removal of the viable epidermis or formation of micro-blisters at other conditions.
This study involved a detailed histologic evaluation over a broad range of microdermabrasion parameters using the skin of monkeys and humans in vivo. In monkeys, 10 passes of microdermabrasion caused thinning of stratum corneum that rarely led to full-thickness stratum corneum removal. This observation is consistent with other reports in humans under conditions representative of conventional cosmetic microdermabrasion (Tan et al., 2001, Karimipour et al., 2005), which typically involves just a few passes (Spencer, 2005), although variations in vacuum pressure, microdermabrasion devices and tip designs, speed of tip movement and device operators complicate direct comparisons. We have also looked at the effect of larger numbers of passes and have shown that selective full-thickness removal of stratum corneum can be achieved at both 30 and 50 passes at the conditions used in this study. We expect that selective stratum corneum removal could similarly be achieved using other microdermabrasion devices at other optimized conditions.
It is also worth noting that full thickness removal of stratum corneum over just a fraction of the surface area should have a huge effect on skin permeability. Thus, it is not necessary to remove all of the stratum corneum from the microdermabraded area but removal of full thickness stratum corneum from at least some regions of the skin should enable delivery of large molecules through the skin. In contrast, while removal of some viable epidermis is not desirable, some damage is likely to be acceptable. Thus, data from this study suggests that at 30 – 50 passes, significant regions of full-thickness stratum corneum can be removed with little damage to viable epidermis. However, tight control over the area of the skin from which full thickness stratum corneum has been removed is critical for dosage reproducibility. Further studies will be needed to modify and optimize devices and procedures to reduce variability.
Micro-blisters were sometimes observed after microdermabrasion, especially after stationary microdermabrasion. These micro-blisters may represent an early stage of suction blister formation, which is a well-established technique used to harvest epidermis for transplantation or to study inflammatory reactions and wound healing in skin (Gupta and Kumar, 2000). Typically, about 40 kPa vacuum pressure is applied for 1 – 3 h to create suction blisters measuring 10 −30 mm in diameter to harvest the epidermis for grafting. It is, however, worth noting that blisters from microdermabrasion are microscopically small and of uncertain clinical significance; and their occurrence can be eliminated by optimizing the mobile mode of microdermabrasion.
4.2 Implications for transdermal drug delivery
Microdermabrasion has only recently been investigated for transdermal drug delivery. Previous studies using microdermabrasion have demonstrated transdermal flux enhancement only for very low molecular weight compounds (<300 Da) under conditions that were not shown to remove full-thickness stratum corneum. To deliver larger molecules, biopharmaceuticals and vaccines, removal of full-thickness stratum corneum is probably required. Even small regions (e.g., microns) of full-thickness stratum corneum removal should have a large impact on skin permeability to macromolecules, which are of nanometer dimensions. Such micron-sized “leaks” in the skin barrier have been created, for example, using microneedles or heat and shown to increase skin permeability by orders of magnitude (Bramson et al., McAllister et al., 2003, Fang et al., 2004b).
In this study, we have shown full-thickness removal of stratum corneum from areas on the skin surface measuring less than 100 µm up to approximately 1000 µm in width, depending on microdermabrasion conditions. As a proof-of-concept that microdermabrasion makes skin permeable, we have dramatically increased transdermal delivery of a model drug, fluorescein, and demonstrated an antibody response generated by a model live-virus vaccine. To our knowledge, this is the first demonstration of delivery of a molecule larger than 300 Da in size and the first demonstration of delivery of a viral vaccine to generate an immune response by microdermabrasion. These results were enabled by using large numbers of microdermabrasion passes to remove full-thickness stratum corneum, which differs from previous work that has involved much fewer passes that appeared to only thin the stratum corneum.
Overall, these results suggest that microdermabrasion under suitable conditions may be used as a skin pre-treatment followed by subsequent application of a transdermal patch to deliver drugs and vaccines. Because this process exposes the viable epidermis, gene delivery to epidermal keratinocytes may also be possible through direct application of cell transfection agents.
Because microdermabrasion can be used to cause full thickness removal of stratum corneum, appropriate precautions are required to prevent infection. However, the risk of infection is expected to be small, as long as clean materials are used. Even when compromised, the skin avoids infection after minor injuries, such as scratches, scrapes and nicks, and after more extensive cosmetic treatments such as dermabrasion, chemical peels, laser resurfacing and other cosmetic procedures. While full thickness stratum corneum takes approximately fourteen days to fully regenerate (Jansen et al., 1974), even partial regeneration of stratum corneum in a few days will significantly restore skin barrier function (Elias, 2005).
In a related process called microscission, microparticles were impinged on the skin using a high pressure carrier gas to create 50–200 µm deep microconduits in the skin (Herndon et al., 2004). Microscissioning is similar to the stationary mode of microdermabrasion, but differs in the use of high-pressure gas rather than vacuum suction to propel the microparticles. Lidocaine delivery and glucose measurements from blood extracted through the microconduits were demonstrated. Because these microconduits were at least 50 µm deep, microscissioning may produce more deep-tissue trauma than microdermabrasion with selective full-thickness stratum corneum removal (i.e., 10–15 µm deep). The microscissioning procedure was enhanced through the additional use of protective masks with micron-sized openings applied to the skin surface to control the diameter of the microconduits. This use of masks may also be applicable as an adjunct to microdermabrasion in order to better control and localize the microdermabrasion effects to smaller skin areas for better cosmetic appearance and decreased tissue trauma.
Current microdermabrasion machines are designed for manual cosmetic applications in a clinical environment. For transdermal drug delivery, the machines will need to be adapted to achieve tightly controlled microdermabrasion with low variability in selective removal of stratum corneum. Notably, the motion of the microdermabrasion tip on the skin may need automation to control its speed, frequency, movement path and force of application on the skin. In addition, fine control over the vacuum pressure and abrasive-particle flow rate may be required to reduce tissue-removal variability. Finally, home use of microdermabrasion will benefit from scaling down machine size to a hand-held device, possibly built using the tools of microfabrication. Home-based microdermabrasion might enable controlled release transdermal patches for delivery of current injectable drugs, such as insulin, human growth hormone, and erythropoietin.
4.3 Implications for cosmetic use
Microdermabrasion is presently approved as a class I device by the FDA (Spencer, 2005). As a result, phase III clinical trials demonstrating the safety and efficacy of microdermabrasion have not been done. Based on this study in monkeys and human volunteers, practitioners may want to avoid large numbers of passes in the mobile mode, which can cause full-thickness removal of stratum corneum and, possibly, deeper tissues. Small numbers of passes just removes partial-thickness stratum corneum, which has been documented by previous researchers as well (Karimipour et al., 2005, Spencer, 2005).
The stationary mode of microdermabrasion may also need to be avoided, because of its tendency to form micro-blisters. Furthermore, topical creams and ointments for cosmetic or other purposes should be used with caution after microdermabrasion, because the stratum corneum thinning may enhance absorption of the topical formulations, leading to increased and possibly adverse effects. The comments in this section are intended to alert clinicians using microdermabrasion to possible concerns, but should not be taken as formal recommendations or clinical guidelines.
5. Conclusion
Motivated by the goal to selectively remove full-thickness stratum corneum to increase skin permeability and the need to microscopically characterize the effects of microdermabrasion on skin, we performed mobile and stationary microdermabrasion on the skin of rhesus macaques and human volunteers in vivo. This produced a detailed characterization of the effects of microdermabrasion on skin over a broad range of microdermabrasion conditions.
In monkeys, the degree of skin tissue removal increased with number of passes in the mobile mode. At 30 and 50 passes, selective full-thickness removal of stratum corneum was observed with little damage to the viable epidermis. At larger numbers of passes, viable epidermis was removed. Using the stationary mode, selective stratum corneum removal was not achieved. Typically, either the stratum corneum and epidermis were both intact, with possible formation of micro-blisters at the dermal-epidermal junction, or the stratum corneum and epidermis were both removed. Similar tissue removal trends were observed in human volunteers using both the mobile and stationary modes of microdermabrasion. Finally, delivery of sodium fluorescein and an enhanced antibody response to MVA virus were demonstrated.
We conclude that microdermabrasion can selectively remove full-thickness stratum corneum with little damage to deeper tissues and thereby increase skin permeability.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the assistance of Dr. Jack Orkin, Stephanie Ehnert, Christopher Souder and Lenox Franker of the veterinary and animal-care staff at the Yerkes National Primate Research Center for the care and preparation of monkeys for microdermabrasion; Judy Mathew, Susan Lalor and other staff at the Hope Clinic of Emory University for their assistance in human microdermabrasion; Dr. Sarah Wise at Emory University for her assistance in performing microdermabrasion on humans; Dr. Mark Allen for use of the CO2 laser in his lab; Dr. Young Bin Choy for preparing the scanning electron micrographs of the aluminum oxide particles; and Dr. Walter Orenstein and Dr. Mark Mulligan for their guidance in the human study at the Hope Clinic of Emory University. This work was supported in part by the National Institutes of Health (R01 EB006369, U01 AI074579) and Emory Center for AIDS Research (CFAR) (P30 AI050409), and took place at the Yerkes National Primate Research Center and the Hope Clinic at Emory University and the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008;364:227–236. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramson J, Dayball K, Evelegh C, Wan YH, Page D, Smith A. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10:251–260. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: an integrated view. J. Invest. Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Fang JY, Lee WR, Shen SC, Fang YP, Hu CH. Enhancement of topical 5-aminolaevulinic acid delivery by erbium:YAG laser and microdermabrasion: a comparison with iontophoresis and electroporation. Br. J. Dermatol. 2004a;151:132–140. doi: 10.1111/j.1365-2133.2004.06051.x. [DOI] [PubMed] [Google Scholar]
- Fang JY, Lee WR, Shen SC, Wang HY, Fang CL, Hu CH. Transdermal delivery of macromolecules by erbium:YAG laser. J. Controlled Release. 2004b;100:75–85. doi: 10.1016/j.jconrel.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Shirakami K, Tojo K. Effect of microdermabrasion on barrier capacity of stratum corneum. Chem. Pharm. Bull. 2005;53:1014–1016. doi: 10.1248/cpb.53.1014. [DOI] [PubMed] [Google Scholar]
- Glenn GM, Villar CP, Flyer DC, Bourgeois AL, McKenzie R, Lavker RM, Frech SA. Safety and immunogenicity of an enterotoxigenic Escherichia coli vaccine patch containing heat-labile toxin: use of skin pretreatment to disrupt the stratum corneum. Infect. Immun. 2007;75:2163–2170. doi: 10.1128/IAI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy S, Peyre M, Garcia N, Muller S, Sesardic D, Partidos CD. Effect of skin barrier disruption on immune responses to topically applied cross-reacting material, CRM(197), of diphtheria toxin. Infect. Immun. 2005;73:4803–4809. doi: 10.1128/IAI.73.8.4803-4809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kumar B. Suction blister induction time: 15 minutes or 150 minutes? Dermatol. Surg. 2000;26:754–756. doi: 10.1046/j.1524-4725.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Guy RH, Hadgraft J, editors. New York, USA: Marcel Dekker; 2008. Transdermal drug delivery. [Google Scholar]
- Harrop R, Ryan MG, Golding H, Redchenko I, Carroll MW. Monitoring of human immunological responses to vaccinia virus. In: Isaccs SN, editor. Methods in Molecular Biology -Vaccinia Virus and Poxvirology: Methods and Protocols. 1st ed. Clifton, New Jersey, USA: Humana Press; 2004. [DOI] [PubMed] [Google Scholar]
- Herndon TO, Gonzalez S, Gowrishankar TR, Anderson RR, Weaver JC. Transdermal microconduits by microscission for drug delivery and sample acquisition. BMC Med. 2004;2:12. doi: 10.1186/1741-7015-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen LH, Hojyo-Tomoko MT, Kligman AM. Improved fluorescence staining technique for estimating turnover of the human stratum corneum. Br. J. Dermatol. 1974;90:9–12. doi: 10.1111/j.1365-2133.1974.tb06356.x. [DOI] [PubMed] [Google Scholar]
- Karimipour DJ, Kang S, Johnson TM, Orringer JS, Hamilton T, Hammerberg C, Voorhees JJ, Fisher G. Microdermabrasion: a molecular analysis following a single treatment. J. Am. Acad. Dermatol. 2005;52:215–223. doi: 10.1016/j.jaad.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Kuhns DB, DeCarlo E, Hawk DM, Gallin JI. Dynamics of the cellular and humoral components of the inflammatory response elicited in skin blisters in humans. J. Clin. Invest. 1992;89:1734–1740. doi: 10.1172/JCI115775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W-R, Shen S-C, Wang K-H, Hu C-H, Fang J-Y. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J. Invest. Dermatol. 2003;121:1118–1125. doi: 10.1046/j.1523-1747.2003.12537.x. [DOI] [PubMed] [Google Scholar]
- Lee WR, Tsai RY, Fang CL, Liu CJ, Hu CH, Fang JY. Microdermabrasion as a novel tool to enhance drug delivery via the skin: an animal study. Dermatol. Surg. 2006;32:1013–1022. doi: 10.1111/j.1524-4725.2006.32224.x. [DOI] [PubMed] [Google Scholar]
- McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim EK, Barnette D, Hughes K, Greenway HT. Microdermabrasion: a clinical and histopathologic study. Dermatol. Surg. 2001;27:524–530. doi: 10.1046/j.1524-4725.2001.01001.x. [DOI] [PubMed] [Google Scholar]
- Sintov AC, Krymberk I, Daniel D, Hannan T, Sohn Z, Levin G. Radiofrequency-driven skin microchanneling as a new way for electrically assisted transdermal delivery of hydrophilic drugs. J. Controlled Release. 2003;89:311–320. doi: 10.1016/s0168-3659(03)00123-8. [DOI] [PubMed] [Google Scholar]
- Spencer JM. Microdermabrasion. Am. J. Clin. Dermatol. 2005;6:89–92. doi: 10.2165/00128071-200506020-00003. [DOI] [PubMed] [Google Scholar]
- Tan MH, Spencer JM, Pires LM, Ajmeri J, Skover G. The evaluation of aluminum oxide crystal microdermabrasion for photodamage. Dermatol. Surg. 2001;27:943–949. doi: 10.1046/j.1524-4725.2001.01120.x. [DOI] [PubMed] [Google Scholar]
- Tatsis N, Lin SW, Harris-McCoy K, Garber DA, Feinberg MB, Ertl HC. Multiple immunizations with adenovirus and MVA vectors improve CD8(+) T cell functionality and mucosal homing. Virology. 2007;367:156–167. doi: 10.1016/j.virol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]