Abstract
Background: No standard treatment options are available for patients with advanced, recurrent or metastatic vulvar carcinoma not amenable for locoregional treatment.
Patients and methods: In this phase II study, patients with advanced vulvar cancer received paclitaxel (Taxol) every 3 weeks for up to 10 cycles. Primary objective was response rate. Secondary objectives were response duration and toxicity. Response evaluation was assessed by World Health Organisation criteria, toxicity according to Common Toxicity Criteria.
Results: Thirty-one women from 10 institutions were included, with a median age of 64 (range 47–84), of which 29 were assessable for response. On study patients received a median of four cycles (range 1–10). Safety: Grade 3 and 4 neutropenia was seen in eight patients (8/29 = 27.6%), which in one patient resulted in neutropenic fever and treatment-related death. Further treatment-related grade 3/4 toxicity includes fatigue in three patients (10.3%) and neuropathy in one patient (3.4%). Efficacy: Overall response was 13.8% (n = 4; two complete responses + two partial responses). With a median follow-up of 24 months, median PFS was 2.6 months (95%confidence interval 2.04–4.21).
Conclusion: Paclitaxel shows moderate activity for local control in advanced vulvar cancer.
Keywords: advanced, cancer, paclitaxel, vulvar
introduction
Carcinoma of the vulva represents ∼4% to 5% of gynaecological malignancies [1]. The annual incidence is 2 per 100 000 women. In 70% of the cases, histology indicates squamous cell cancer while basal cell cancer, melanoma and adenocarcinoma allow for the remaining 30%. Vulvar cancer is a disease of older women as indicated by the median age of 74 years [2].
About 30% of patients present at a clinically late stage of the disease [2].
The management of early disease is based on surgery, performing local radical excision and—in case the sentinel node is positive—proceeding to groin dissection. For more advanced disease, surgery combined with radiotherapy or chemoradiation is the most widely used approach.
Even with multimodality treatment, 40%–50% of patients with advanced disease will ultimately represent with recurrence and 40% will die from their disease [3, 4].
Currently, there is no standard treatment for recurrent disease. Only in the case of local recurrence is curative surgery, radiation or chemoradiation possible in ∼50% of the patients [5, 6]. Unfortunately, in the patient group initially presenting with late-stage disease and a high risk of death of recurrence, 70% of recurrences either present with a regional (groin/pelvis) or distant component of recurrence [3]. This precludes radiotherapy as effective treatment in this setting. As of today, there is no effective therapy for the group of patients with a regional and/or distant recurrence, reflected in a 5-year survival of <10% [3, 4]. Although chemotherapy has shown activity in the neoadjuvant setting with response in up to 60% of chemo-naive patients [7–10], both single-agent and multiagent chemotherapy have shown minimal activity in (frequently heavily pretreated) recurrent vulvar cancer [11–14].
Single-agent cisplatin, piperazinedione, mitoxantrone or bleomycin did not result in any objective response or response duration in recurrent vulvar cancer [12, 13]. There is even less experience with multiagent chemotherapy in this setting. In recurrent squamous cell carcinoma of the vulva, we face a scarcity of data and disappointing results with chemotherapy [10]. Therapeutic approaches with better response rate and longer response duration are desperately needed. Paclitaxel is a cytotoxic agent that stabilizes the tubulin polymer bundles and therefore interfering with microtubular assembly and cell replication. It has broad activity against a number of solid tumours, including squamous cell carcinomas such as head and neck and cervical cancers [15–17]. Thus, a phase II trial in advanced or recurrent vulvar cancer was undertaken. The three-weekly single-agent schedule was chosen because it seemed feasible in this older age group.
patients and methods
The study was conducted by the European Organisation for Research and Treatment of Cancer (EORTC) Gynecological Cancer Group. The protocol was reviewed and approved by the EORTC Protocol Review Committee (study number 55985) and the institutional review board of each participating center. Written informed consent was obtained from all patients before registration.
To be eligible, patients had to meet the following criteria: 18 years of age or older; histological proven squamous cell carcinoma of the vulva; measurable [according to World Health Organisation (WHO) criteria [18]] or evaluable disease not amenable to radiotherapy or surgery as first-line treatment or measurable metastatic disease outside previously irradiated areas or local recurrences within a previously treated area or local lesions showing progression while on treatment; no prior chemotherapy unless given as concurrent with radiotherapy; WHO performance less than two or two and adequate haematological, renal, cardiac and hepatic function.
treatment plan
The study was designed as an open-label, single-arm, multicenter phase II trial to determine the therapeutic effect of paclitaxel (Taxol, Bristol-Meyers Squibb, Waterloo, Belgium) in patients with recurrent, metastatic or locally advanced vulvar cancer not amenable to surgery or radiotherapy. Patients received paclitaxel 175 mg/m2 i.v. in a three-weekly regimen. Patients received the standard premedication for hypersensitivity reactions: dexamethasone, diphenhydramine and ranitidine for each administration and antiemetics according to the local institutional standard.
Toxicity was evaluated according to the National Cancer Institute's Common Toxicity Criteria for adverse events version 1.0 (Bethesda 1988, [19]).
No dose escalation was allowed. The paclitaxel dose was reduced for haematological and non-haematological effects: decrease by one dose level to 150 mg/ m2 in case of absolute neutrophil count < 500 lasting for 7 days, any episode of febrile neutropenia, grade 3 thrombocytopenia, stomatitis or myalgia. Decrease by two levels to 135 mg/ m2 was done in case of grade 4 thrombocytopenia.
Patients underwent evaluation of measurable disease every third cycle of chemotherapy. Response was assessed according to WHO criteria: a 'set of target lesions' was chosen before the first treatment administration and measured in their two perpendicular dimensions. Surface area was calculated by multiplying the two diameters. Lesions were eligible as target lesions when they had at least a diameter of 2.5 cm. A complete response (CR) was defined as complete disappearance of all known disease, determined by two observations not less than 4 weeks apart. A partial response (PR) was defined as a 50% or more decrease in the sum of tumour areas of all target lesions, determined by two observations not less than 4 weeks apart and no appearance of new lesions or progression of any existing lesion. In case a 25% or more increase was assessed or a new lesion appeared, progressive disease was defined. Treatment was continued until documented disease progression, unacceptable toxicity, patient refusal or in the case of PR or CR until a maximum of 10 cycles was reached.
statistical considerations
The Simon one-sample two-stage minimax design was applied [20]. A sample size of 29 assessable patients was required in order to discriminate between a response rate of 5% and 25% with a type I error = 10% and a type II error = 5%. Four or more responses needed to be observed for the treatment to be considered successful.
Patients and characteristics were summarised using appropriate descriptive statistics. Survival time was calculated from the date of registration until death from any cause, and surviving patients were censored at the time of last follow-up. Duration of response was calculated from the date of treatment start to the date of documented progression. If a new treatment was started before progression, the duration of response was censored on the day of start of new treatment. The Kaplan–Meier method was used to estimate survival and corresponding confidence intervals (CIs) [21]. These analyses were carried out using SAS version 8.2 (SAS Institute,. Cary, NC). All eligible patients are included in the efficacy analysis while safety data are presented on all patients who started protocol treatment regardless of eligibility.
results
patients' characteristics
From February 2001 to December 2004, 31 patients were registered in the study by 11 institutions. One patient included in the study did not meet the eligibility criteria because of a creatinine clearance <60 ml/min and another patient because of no suitable target lesions. Both patients were excluded from efficacy analyses. Therefore, 29 eligible patients were followed with a median follow-up time of 28 months. Age varied between 47 and 84 years with a median of 64. Ten patients had a WHO performance status (PS) of zero, eleven patients a PS score of one and eight patients of two. At entry, four patients had locally advanced disease and 22 had locoregional recurrences, of which eight were with distant metastases. Three patients had distant metastases only. On study patients received a median of four cycles (range 1–10).
Baseline characteristics including site of recurrence and prior therapy are given in Table 1. A total of 42 lesions were assessed as target lesions. Twelve lesions were located in a prior irradiation field and eight lesions were previously irradiated. Twenty-one lesions neither were nor in the previous irradiated field or previous irradiated. In one lesion, the radiotherapy status was unknown.
Table 1.
Patients' characteristics (n = 29)
| Age | Median, 64 (range 47–84) |
| World Health Organisation performance | |
| 0 | 10 (34%) |
| 1 | 11 (38%) |
| 2 | 8 (28%) |
| Time since first histological diagnosis (months) | 18.3 (range 0.4–135.3) |
| Site target lesions | |
| Primary only | 10 (35 %) |
| Lymph nodes only | 6 (21 %) |
| Primary and lymph | 5 (17 %) |
| Skin only | 4 (14 %) |
| Other soft tissue only | 2 (7 %) |
| Skin and other soft | 1 (3 %) |
| Lung only | 1 (3 %) |
| Number of target lesions | |
| 1 | 17 (58%) |
| 2 | 11 (38%) |
| 3 | 1 (3%) |
| Total | 42 |
| Prior surgery | |
| No | 5 (17%) |
| Curative | 21 (72%) |
| Palliative | 2 (7%) |
| Prior radiotherapy | |
| No | 9 (31%) |
| Yes | 20 (69%) |
| Prior chemotherapy | |
| No | 24 (83%) |
| Prior chemoradiotherapy | 5 (17%) |
toxicity
One non-eligible patient did not receive any treatment and was therefore not included in the toxicity evaluation. The remaining 30 patients received a median of four cycles (range 1–10) of chemotherapy with a median dose intensity of 99% (range 78%–101%). Four patients discontinued treatment for toxicity. Three patients did so for neurotoxicity, mainly sensory neuropathy. One patient (3.3%) experienced a toxic death due to a grade 4 neutropenia starting at day 6 after the first administration of chemotherapy. The neutropenia was still ongoing when on day 22 the patient was hospitalized for fever with septic shock, and she died the same day. All toxic effects are given in Table 2. Most grade 3 and 4 toxic effects were assessed as mainly related to the chemotherapy. But skin toxicity and oedema were related to locoregional recurrence. One patient developed fatal pulmonary embolism while her disease was rapidly progressive.
Table 2.
Toxicity
| All grades, n (%) | Grade 3/4, n (%) | |
| Neutropenia | 14 (47.6%) | 8 (27.6%) |
| Neutropenic fever | 1 (3.3%) | 1 (3.3%) |
| Fatigue | 22 (73.3) | 3 (10%) |
| Hemoglobin | 29 (96.6%) | 2 (6.7%) |
| Skin toxicity | 7 (23.3%) | 2 (6.7%) |
| Oedema | 11 (36.7%) | 2 (6.7%) |
| Cardiovascular (arrhytmia) | 5 (16.6%) | 1 (3.3%) |
| Thrombosis/embolism | 2 (6.7%) | 1 (3.3%) |
| Vomiting | 9 (30%) | 1 (3.3%) |
| Other gastrointestinal | 14 (46.6%) | 1 (3.3%) |
| Hemorrhage | 3 (9.9%) | 1 (3.3%) |
| Neuropathy (sensory) | 13 (43.3%) | 1 (3.3%) |
| Arthralgia/myalgia | 10 (33.3) | 1 (3.3%) |
| Alopecia | 26 (87%) | 0 |
| Platelets | 1 (3.3%) | 0 |
response assessment and duration
In two patients out of 29 eligible patients, response could not be assessed as stipulated by the protocol. One patient died due to early progression after the second cycle of chemotherapy before the formal evaluation had taken place and another patient experienced fatal toxicity (septic shock) after the first cycle of chemotherapy.
Two patients had a CR and two other a PR, resulting in an overall response rate of 14%. Median duration of response was 18 months (range 6–23 months). Three patients had a stable disease (SD; neither a complete nor a partial response, nor a progression has been demonstrated, at least 9 weeks after treatment start), lasting at least 4 months, resulting in an overall clinical benefit of 24%.
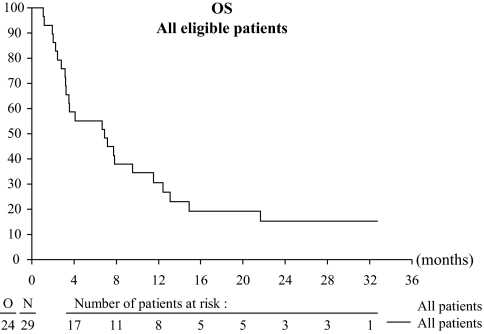
The median progression-free survival was 2.6 months (95% CI = 2–4.2 months). The progression-free survival rate at 1 year was 10.3% (95% CI = 2.6% to 24.3%). The median overall survival was 6.9 months (95% CI = 3.52–12.42 months) (Table 3). The 1-year overall survival rate was 31% (95% CI = 15.2% to 47.7%) (Figure 1). Five patients still alive at the time of analysis were censored at the last available date they were known to be alive.
Table 3.
Response to treatment and response duration (n = 29)
| Clinical response (WHO criteria) | No. of patients | % | 95% CI |
| CR | 2 | 6.9 | 0.8–22.8 |
| PR | 2 | 6.9 | 0.8–22.8 |
| SD | 3 | 10.3 | 2.2–27.4 |
| Progressive disease | 20 | 69 | 49.2–84.7 |
| Not assessable | 2 | 6.9 | 0.8–22.8 |
| Overall response (CR + PR) | 4 | 13.8 | 5.5–30.6 |
| Overall clinical benefit (CR + PR + SD) | 7 | 24.1 | 10.3–43.5 |
| Response duration | |||
| Median progression-free survival | 2.6 months | 2.0–4.2 months | |
| Progression-free survival at 1 year | 10.3% | 2.6%–24.3% | |
| Median overall survival | 6.8 months | 3.5–12.4 months | |
| Overall survival at 1 year | 30.6% | 15.2%–47.7% |
WHO, World Health Organisation; CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease.
Figure 1.
Overall survival (OS)
response in relation to radiotherapy status
In Table 4, the response per target lesion and prior radiation status are given. Half of the lesions (n = 21) did not receive radiotherapy at all. In three of these lesions (14%), a response was observed. Twelve lesions occurring in previous irradiated field did not show any response. On the other hand, in eight lesions that were irradiated and thereafter had progressed, three objective responses were seen (37%). It is very well possible that the site of lesions interferes as a confounding variable. The site of the lesion can be related both to its radiation status and its response outcome. Unfortunately, there were not enough data available to reliably test for such a site interaction. Therefore, we split the patients in three main groups: primary tumour lesions, lymph node lesions and other lesions. In Table 5, the results of this analysis are given. For lesions of primary tumour origin, previous radiotherapy appears to correlate with higher response rates (3/15 = 20% versus 0/5 = 0%). For lymph node lesions, the relation is reversed: irradiated lesions showed lower response rates (3/9 = 33.3% versus 0/3 = 0%).
Table 4.
Response per prior radiation status
| Previous radiotherapy | Total number of target lesions | Number of target lesions with response (CR + PR) |
| No RT at all | 21 | 3 (14.3 %) |
| Lesions within irradiated field | 12 | 0 |
| Previous irradiated lesions | 8 | 3 (37.5 %) |
RT, radiotherapy; CR, complete response; PR, partial response.
Table 5.
Response per site of lesions and prior radiation status
| RT status |
||
| No RT at all | Within irradiation field or previous irradiated | |
| n (% RR) | n (% RR) | |
| Primary tumour lesions (n = 18) | 5 | 13 |
| Response (CR + PR) | 0 (0%) | 3 (23%) |
| Lymph nodes lesions (n = 13)a | 9 | 3 |
| Response (CR + PR) | 3 (33%) | 0 (0%) |
| Other lesions (lung, skin and other soft tissue, n = 11) | 7 | 4 |
| Response (CR + PR) | 0 (0%) | 0 (0%) |
One lesion with unknown radiotherapy status.
RT, radiotherapy; RR, responserate; CR, complete response; PR, partial response.
discussion
The treatment with chemotherapy of recurrent vulvar cancer after primary surgery and radiotherapy is neither well defined nor extensively studied. Due to the low incidence of vulvar cancer, it is difficult to perform randomized trials in this relatively old age patient group. Cisplatin, bleomycin, methotrexate, 5-fluorouracil and mitomycin C have shown considerable activity in a preoperative setting with response percentages of 60% in chemo-naive patients [7, 8, 22]. Data about these drugs are scarce in recurrent disease not amenable to surgery or radiotherapy.
The current study is the first study on the use of paclitaxel in recurrent squamous cell cancer of the vulva showing a moderate response rate of 14%. Paclitaxel is a drug with antitumour activity in many types of cancer including cervical cancer [23, 24]. Monotherapy with 175 mg/m2 has shown to be a well-tolerated regimen, with bone marrow suppression, alopecia, nausea and vomiting and peripheral neuropathy being the most important side-effects. Hypersensitivity reactions with hypotension and bradycardia can be managed with premedications like dexamethasone and ranitidine.
Because paclitaxel had not yet been explored in vulvar cancer, it was chosen as experimental drug in the present study. Thirty-one patients were able to receive a median of four cycles with a very acceptable dose intensity of 99% (range 78% to 101%).
The treatment could be given on an outpatient basis and was well tolerated with neutropenia as most important drug-related toxicity. However, one patient experienced neutropenic fever in the very first days after the initial administration. This patient died during the hospitalization while the neutropenia was ongoing. She was a 78-year-old woman with no comorbidity or comedication. Beside age, there was no explanation for the quick onset and delayed recovery of the neutropenia. Polymorphisms in the enzyme system responsible for paclitaxel metabolism are described in the literature and one can speculate that such a genetic variability might have been responsible for the severe toxicity in this patient [25]. Other well-known paclitaxel-related toxic effects, such as fatigue, alopecia, myalgia and sensory neuropathy, were seen but did not exceed grade 2. Other toxic effects could be related to the local disease status, like edema, local infections and skin toxicity.
An objective response rate of 14% was achieved, while in 24% clinical benefit (CR, PR and SD) was shown. Two women had a CR, and two had a PR. Especially the PR rate is low in regard to other studies; however, only 4 of 29 women had primary locally advanced disease in contrast to 13 of 25 in the study of Wagenaar [7, 10].
A GOG trial studied monotherapy of low-dose cisplatin every 3 weeks in 24 patients. No objective response was observed. Of the 22 patients who were assessable for response, no objective regression of disease was observed. Ten had SD, while the remaining 12 had increasing disease. Thirteen other patients who were deemed ineligible for cisplatin therapy received piperzinedione. In this group also, no objective response was assessed [12]. An EORTC phase II investigated three-weekly combination therapy with bleomycin, methotrexate and CCNU in irresectable or recurrent vulvar cancer in 25 patients. Twelve patients with recurrent disease received prior surgery only (n = 9) or a combination of surgery and irradiation (n = 3). In the latter group, no responses were observed, while in the surgery-only group six PRs were seen. Another eight responses (two CRs) were observed in the group of patients with irresectable disease who did not receive any other treatment yet [7]. Although the response rate was encouraging, the toxicity related to methrotrexate was not acceptable and a modified regimen was studied in a subsequent EORTC study. Twenty-five patients with locally advanced or locoregional recurrences entered this phase II study. The response rate of 56% was again striking but with major haematological side-effects and mild signs of bleomycin-related pulmonary toxicity. Two patients died of toxicity. Again all patients showing responses only had surgery as previous treatment. The only patient who was assessable for response after surgery and radiotherapy showed progressive disease [10].
Prior radiation of target lesions seemed to have impact on the response rate of lesions, with a response difference between lesions in previous irradiated field and lesions that were irradiated before. This is hard to interpret because changes in vasculature and therewith changes in effect of chemotherapy are expected to be the same in both groups. Furthermore, there was a strong suggestion of site interaction. For lesions of primary tumour origin, previous radiotherapy appears to correlate with higher response rates while for lymph node lesions, the relation is reversed: irradiated lesions showed lower response rates. The available sample is, however, too small to formally confirm this interaction.
In other squamous tumours, the combination of paclitaxel and cisplatin or carboplatin has showed considerable activity. In recurrent head and neck cancer, the combination resulted in an objective response of almost 30% [26]. In cervical cancer, response rates of 30%–50% are demonstrated, with superiority of combination therapy versus monotherapy [27, 28]. The combination of paclitaxel and carboplatin in vulvar cancer might be attractive and should be considered for earlier disease.
In summary, our study shows that paclitaxel can be given safely on an outpatient basis to patient with locally advanced or recurrent vulvar cancer, but the low response rate requires more studies with other cytotoxic or antiangiogenic agents or combinations.
funding
3U10 CA11488-31 through 5U10 CA011488-38 from the National Cancer Institute (Bethesda, MD); Fonds Cancer (FOCA) from Belgium.
Acknowledgments
We would like to thank the following investigators for supporting and contributing to this study: J. van der Velden (Amsterdam), I. Vergote (Leuven), N. Reed (Glasgow), C. Guerra (Coimbra), C. Scarabelli (Voghera), P. Witteveen (Utrecht), J. Vermorken (Antwerpen), P. Zola (Torino), N. Colombo (Milano) and N. Donadello (Varese). This study took place over a number of years and we would like to thank all of the data managers and study nurses who have supported this protocol. Presented at the Poster session IGCS 2005, Santa Monica. This study's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Velden van der J, Lindert van ACM, Gimbrere CHF, et al. Epidemiologic data on vulvar cancer: comparison of hospital with population-based data. Gynecol Oncol. 1996;62:379–383. doi: 10.1006/gyno.1996.0252. [DOI] [PubMed] [Google Scholar]
- 3.Moore DH, Thomas GM, Montana GS, et al. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the gynaecologic oncology group. Int J Radiat Oncol Biol Phys. 1998;42:79–85. doi: 10.1016/s0360-3016(98)00193-x. [DOI] [PubMed] [Google Scholar]
- 4.Lupi G, Raspaliesi F, Zucali R, et al. Combined preoperative chemoradiotherapy followed by radical surgery in locally advanced vulvar carcinoma. Cancer. 1996;77:1472–1478. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1472::AID-CNCR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Podratz KC, Symmonds RE, Taylor WF. Carcinoma of the vulva: analysis of treatment failures. Am J Obstet Gynecol. 1982;143:340–351. doi: 10.1016/0002-9378(82)90823-7. [DOI] [PubMed] [Google Scholar]
- 6.Ndubisi B, Kaminski PF, Olt G, et al. Staging and recurrence of disease in squamous cell carcinoma of the vulva. Gynecol Oncol. 1995;59:34–37. doi: 10.1006/gyno.1995.1264. [DOI] [PubMed] [Google Scholar]
- 7.Durrant KR, Mangioni C, Lacave AJ, et al. Bleomycin, methotrexate and CCNU in advanced inoperable squamous cell carcinoma of the vulva. A phase II study of the EORTC gynaecological cancer cooperative group (GCCG) Gynecol Oncol. 1990;37:359–362. doi: 10.1016/0090-8258(90)90367-t. [DOI] [PubMed] [Google Scholar]
- 8.Benedetti-Panici P, Greggi S, Scambia G, et al. Cisplatin (P), bleomycin(B), and methotrexate (M) preoperative chemotherapy in locally advanced vulvar carcinoma. Gynecol Oncol. 1992;50:49–53. doi: 10.1006/gyno.1993.1163. [DOI] [PubMed] [Google Scholar]
- 9.Geisler JP, Manahan KJ, Buller RE. Neoadjuvant chemotherapy in vulvar cancer: avoiding primary exenteration. Gynecol Oncol. 2006;100:53–57. doi: 10.1016/j.ygyno.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 10.Wagenaar HC, Colombo N, Vergote I, et al. Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable squamous-cell carcinoma of the vulva: an EORTC Gynaecological Cancer Cooperative Group Study. European Organization for Research and Treatment of Cancer. Gynecol Oncol. 2001;81:348. doi: 10.1006/gyno.2001.6180. [DOI] [PubMed] [Google Scholar]
- 11.Trope C, Johnsson JE, Larsson G, Simonson E. Bleomycin alone or combined with mitomycin C in treatment of advanced or recurrent squamous cell carcinoma of the vulva. Cancer Treat Rep. 1980;64:639–642. [PubMed] [Google Scholar]
- 12.Thigpen JT, Blesing JA, Homesley HD, Lewis GD. Phase II trials of cisplatin and pierazinedione in advanced or recurrent squamous cell carcinoma of the vulva: a Gynecologic Oncology Group Study. Gynecol Oncol. 1986;23:358–363. doi: 10.1016/0090-8258(86)90138-1. [DOI] [PubMed] [Google Scholar]
- 13.Muss HB, Bundy BN, Christopherson WA. Mitoxantrone in the treatment of advanced vulvar and vaginal carcinoma. A Gynecologic Oncology Group study. Am J Clin Oncol. 1989;12:142–144. doi: 10.1097/00000421-198904000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Deppe G, Cohen CJ, Bruckner HW. Chemotherapy of squamous cell carcinoma of the vulva: a review. Gynecol Oncol. 1979;7:345–348. doi: 10.1016/0090-8258(79)90112-4. [DOI] [PubMed] [Google Scholar]
- 15.Schrijvers D, Vermorken JB. Taxanes in treatment of head and neck cancer. Curr Opin Oncol. 2005;17:218–224. doi: 10.1097/01.cco.0000158735.91723.0e. [DOI] [PubMed] [Google Scholar]
- 16.McGuire WP, Blessing JA, Moore D, et al. Paclitaxel has moderate activity in squamous cervix cancer: a Gynecological Oncology Group study. J Clin Oncol. 1996;14:792–795. doi: 10.1200/JCO.1996.14.3.792. [DOI] [PubMed] [Google Scholar]
- 17.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent or persistent squamous cell carcinoma of the cervix: a Gynecological Oncology Group study. J Clin Oncol. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 18.Miller AB, Hoogstraaten B, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.NCI-CTC Toxicity Grading (version 1.0). National Cancer Institute. Guidelines for the Reporting of Adverse Drugs Reactions. Bethesda, MD: Division of Cancer Treatment; 1988. [Google Scholar]
- 20.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1958;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Shimizi Y, Hasumi K, Masubuchi K. Effective chemotherapy consisting of bleomycin, vincristine, mitomycine C, and cisplatin (BOMP) for a patient with inoperable vulvar cancer. Gynecol Oncol. 1990:423–427. doi: 10.1016/0090-8258(90)90156-f. [DOI] [PubMed] [Google Scholar]
- 23.McGuire WP, Blessing JA, Moore D, et al. Paclitaxel has moderate activity in squamous cervix cancer: a Gynecological Oncology Group study. J Clin Oncol. 1996;14:792–795. doi: 10.1200/JCO.1996.14.3.792. [DOI] [PubMed] [Google Scholar]
- 24.Kadelka AP, Winn R, Edwards CL, et al. An update of a phase II study of paclitaxel in advanced or recurrent squamous cell cancer of the cervix. Anticancer Drugs. 1997;8:657–661. doi: 10.1097/00001813-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Spratlin J, Sawyer MB. Pharmacogenetics of paclitaxel metabolism. Crit Rev Oncol Hematol. 2007;61:222–229. doi: 10.1016/j.critrevonc.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Pivot X, Cals L, Cupissol D, et al. Phase II trial of paclitaxel-carboplatin combination in recurrent squamous cell carcinoma of the head and neck. Oncology. 2001;60:66–71. doi: 10.1159/000055299. [DOI] [PubMed] [Google Scholar]
- 27.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynaecological Oncology Group Study. J Clin Oncol. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 28.Moore KN, Herzog TJ, Lewin S, et al. A comparison of cisplatin/paclitaxel and carboplatin/paclitaxel in stage IVB, recurrent or persistent cervical caner. Gynecol Oncol. 2007;105:299–303. doi: 10.1016/j.ygyno.2006.12.031. [DOI] [PubMed] [Google Scholar]