Abstract
An increasing number of breast cancer patients are diagnosed with small, localized, early-stage tumors. These patients are typically thought to have a good prognosis for long-term disease-free survival, but epidemiological studies indicate that up to 30% may have a recurrence within 3 to 5 years of diagnosis. Identifying patients with a high risk of recurrence and/or progression is important because they could be more aggressively treated at diagnosis to improve their chances for disease-free survival. Recent evidence suggests that elevated levels of the matrix metalloproteinase inhibitor, tissue inhibitor of metalloproteinase (TIMP)-4, are associated with malignant progression of ductal carcinoma in situ, a precancerous lesion. To examine the association of TIMP-4 with survival outcomes, we conducted a retrospective immunohistochemical analysis of 314 cases from patients with early-stage disease, defined as tumors smaller than 2 cm and no spread to lymph nodes (tumor-node-metastasis staging: T1N0MX). We found that tumors with elevated levels of TIMP-4 were correlated with a reduced probability of long-term disease-free survival, especially in patients with estrogen receptor-negative tumors. Our findings prompt further evaluation of TIMP-4 as a simple prognostic marker that may help identify patients with early-stage breast cancer who could benefit from more aggressive treatment at diagnosis.
Tissue inhibitor of metalloproteinase (TIMP)-4 is one of four members of the family of TIMPs, which modify the breakdown of extracellular matrix by the matrix metalloproteinases. Studies of the TIMPs in mammalian organisms have revealed distinctions in structure, biochemical properties, and tissue-specific expression patterns, suggesting unique roles in normal physiology.1,2,3 Because of their key roles in cell motility and tissue organization, the interactions between TIMPs and matrix metalloproteinases have been widely studied in cancer research. Until recently, TIMPs were recognized primarily only as matrix metalloproteinase inhibitors. However, recent work has made it increasingly clear that TIMPs have non-matrix metalloproteinase-associated functions in cancer,4 including roles in growth promotion,5,6,7 apoptosis,8,9,10 and angiogenesis.10,11 In the mammary gland, the importance of TIMPs has been demonstrated during normal physiological processes such as glandular development and involution during pregnancy.12 Although TIMPs have been studied in breast cancer, TIMP-4 has received limited attention and there is conflicting information about its significance. In one study, human breast cancer cells engineered to ectopically express TIMP-4 displayed a reduction in growth and metastasis after implantation in mice.13 In contrast, another study showed that a gene therapy strategy to express TIMP-4 promoted mammary tumor formation.14 Notably, in human breast cancer, TIMP-4 has been associated with the transition of ductal carcinoma in situ into invasive, infiltrating ductal carcinoma (IDC).15 With an increasing number of patients being diagnosed with small early-stage tumors (<20 mm in diameter) and no signs of spread to lymph nodes, the majority of patients are predicted to have a favorable prognosis for long-term disease-free survival according to traditional tumor-node-metastases (TNM) staging. Nevertheless, epidemiological studies indicate that 20 to 30% of these patients will have a recurrence of their breast cancer within 3 to 5 years of diagnosis.16,17 Markers that could identify this subgroup of patients who are at higher risk of relapse and/or malignant progression would be useful to stratify them for more aggressive treatment that might improve their chances for long-term disease-free survival. In this study, we tested the hypothesis that TIMP-4 expression correlates inversely with disease-free survival for patients with early-stage disease.
Materials and Methods
Breast Tumor Specimens
Two collections of archival formalin-fixed and paraffin-embedded breast cancer specimens were used in this retrospective study. The use of de-identified archival material was approved by the institutional review boards at Lankenau Hospital and Basel University Hospital. All personal identifiers were removed from the clinical and histopathological information that was stored in the pathology database before transfer to the research laboratory. The collection used as a pilot group for hypothesis testing was obtained from the archives of the Department of Pathology of Bryn Mawr Hospital (Bryn Mawr, PA). Specimens were collected from 183 consecutive, consenting breast cancer patients who had undergone breast cancer resection during 1990 to 1996 at Bryn Mawr Hospital. For the pilot study to establish whether TIMP-4 is associated with stage or survival phenotype, we used the 67 cases of infiltrating ductal carcinoma smaller than 20 mm in diameter, as determined by the pathologist, that were node-negative (T1N0MX). The histological data of the pilot cohort, which had been used previously for studies of other biomarkers,18 is shown in Table 1. The collection used as an experimental group was obtained from a large collection of cases arrayed in a tissue microarray (TMA) made available from TriStar, Inc. (Bethesda, MD). The arrays consisted of one core from each of 2518 tissue blocks obtained from distinct patients, including control normal tissues from various organs. The total number of cored breast cancer specimens on the TMA was 2197 cases and of these 460 cores were from tumors smaller than 20 mm (T1), with 314 cases of the T1 tumors also being node-negative (T1N0MX). The staining results of these 314 cores evaluated by the pathologist were used in analysis of T1N0 IDC. Estrogen receptor (ER) status was determined by immunohistochemical analysis and scored according to Allred et al,19 with all tumors showing at least weak staining in at least 10% of tumor cells being regarded as positive for ER expression. Analysis of estrogen receptor status demonstrated that 156 of the 314 T1N0 IDC cores also lacked expression of the estrogen receptor (ie, were ER-negative). Histological subtype, pathological stage, tumor diameter, nodal status, and histological grade according to Elston and Ellis (BRE) were provided with the TMA. The histological description for the breast cancer specimens is summarized in Table 2.
Table 1.
Characteristics of Exploratory Pilot (Bryn Mawr) Specimens
| No. of patients | Value | % | Comments | |
|---|---|---|---|---|
| Continuous variables | ||||
| Age at diagnosis, years | ||||
| Median | 64.3 | |||
| Range | 26–89 | |||
| No. of patients | 178 | |||
| Follow-up time (months) | ||||
| Median | 72 | |||
| Range | 6–132 | |||
| No. of patients | 183 | |||
| Tumor diameter (mm) | ||||
| Median | 20 | |||
| Range | 1–100 | |||
| No. of patients | 168 | |||
| Discrete variables | ||||
| Lymph node status | ||||
| 0 | 70 | 53.8 | ||
| 1 | 30 | 23.1 | ||
| 2 | 30 | 23.1 | ||
| Unknown | 26 | |||
| Tumor grade | ||||
| I | 23 | 14.9 | ||
| II | 93 | 60.4 | ||
| III | 38 | 24.7 | ||
| Unknown | 2 | |||
| ER status | ||||
| Positive | 96 | 62.3 | ||
| Negative | 58 | 37.7 | ||
| Unknown | 2 | |||
| Histological type | ||||
| IDC | 156 | |||
| Other | 27 | |||
| IDC tumor size | ||||
| ≤20 mm | 88 | |||
| ≥20 mm | 57 | |||
| Unknown | 11 | |||
| Node status in IDC ≤20 mm (T1) | ||||
| N0 | 67 | Used in pilot study | ||
| N1–N3 | 19 | |||
| Unknown | 2 |
Table 2.
Characteristics of Experimental Test (TMA) Specimens
| No. of patients | Values | % | Comments | |
|---|---|---|---|---|
| Continuous variables | ||||
| Age at diagnosis (years) | ||||
| Median | 63 | |||
| Range | 26–101 | |||
| No. of patients | 1884 | |||
| Follow-up time (months) | ||||
| Median | 62 | |||
| Range | 1–176 | |||
| No. of patients | 2221 | |||
| Tumor diameter (mm) | ||||
| Median | 24 | |||
| Range | 2–140 | |||
| No. of patients | 2173 | |||
| Discrete variables | ||||
| Lymph node status | ||||
| 0 | 503 | 46.1 | ||
| 1 | 511 | 46.9 | ||
| 2 | 76 | 7.0 | ||
| Unknown | 204 | |||
| Tumor grade | ||||
| I | 276 | 23.2 | ||
| II | 449 | 37.7 | ||
| III | 466 | 39.1 | ||
| Unknown | 103 | |||
| TIMP-4 status | ||||
| 0 | 635 | 49.1 | ||
| I | 437 | 33.8 | ||
| II | 197 | 15.2 | ||
| III | 25 | 1.9 | ||
| ER status | ||||
| Positive | 972 | 77.3 | ||
| Negative | 285 | 22.7 | ||
| Unknown | 37 | |||
| Histological type | ||||
| IDC | 1294 | Analyzable | ||
| Other | 489 | |||
| IDC tumor size | ||||
| ≤20 mm | 460 | |||
| ≥20 mm | 834 | |||
| Node status in IDC ≤20 mm (T1) | Used in study of all T1N0 | |||
| N0 | 314 | |||
| N1–N3 | 146 | |||
| ER status among T1N0 | ||||
| ER-positive | 158 | |||
| ER-negative | 156 | Used in study of ER-negative T1N0 |
Antibodies
The primary antibody used was a rabbit polyclonal anti-human TIMP-4 antibody (Chemicon International, Temecula, CA) selected for its ability to stain formalin-fixed, paraffin-embedded specimens. To ensure the use of the same batch of antibody throughout the work, a large number of vials were purchased, and the antibody solution was pooled. An aliquot of the pooled TIMP-4 antibody was used for staining of the TMAs (shipped on ice to the laboratory of G.S.).
Immunohistochemical Staining
Tissue sections from the pilot group were deparaffinized and rehydrated essentially as described previously.18 Steam-based antigen retrieval was performed, and endogenous peroxidase activity was blocked by incubating slides in 0.3% H2O2 in water before blocking with the ABC Elite kit (Vector Laboratories, Burlingame, CA). A rabbit polyclonal anti-human TIMP-4 antibody was added (10 μg/ml), and the mixture was incubated overnight in a moist chamber at +4°C. After extensive washes, the secondary biotin-conjugated antibody was added before addition of the avidin-peroxidase complex (ABC Elite kit). The diluted chromogen 3,3′-diaminobenzidine was added for 5 minutes after which specimens were thoroughly rinsed and counterstained with hematoxylin for 8 seconds. Coverslips were mounted with PermaMount (Vector Laboratories) on dry slides. The TMA slides from the experimental group were deparaffinized and rehydrated in a descending ethanol series followed by a wash in standard PBS. Pretreatments included epitope retrieval by autoclaving 5 minutes in citrate buffer (pH 6.0) followed by quenching of endogenous peroxidase activity by incubating slides in 0.3% H2O2/methanol. After blocking in normal horse serum (S-2000, Vector Laboratories), the primary TIMP-4 antibody was added (2.2 μg/ml) in PBS for 2 hours at 21°C in a moist chamber. After extensive washes, the horseradish peroxidase-conjugated secondary antibody was added (K4003 EnVision, anti-rabbit, Dako, Carpenteria, CA). The chromogen 3,3′-diaminobenzidine was added for 6 minutes followed by washes and counterstaining for 20 seconds with hematoxylin (Harris hematoxylin HTX 31000, Medite GmbH, Burgdorf, Germany). After drying in an ascending ethanol series and xylene, TMA slides were covered and examined.
As controls for specific staining in the pilot study, we used formalin-fixed and paraffin-embedded cell pellets of MDA-MB-231, a TIMP-4-expressing human breast carcinoma cell line (American Type Culture Collection, Manassas, VA), and MDA-MB-435, a TIMP-4-nonexpressing human breast carcinoma cell line (American Type Culture Collection) for quality control. All cases were also stained with a purified rabbit IgG immunoglobulin isotype standard serum as a specificity control. All controls were run in parallel with each case.
On the TMA, tissue cores from 18 organ types were present. These included colon, lung, heart, endometrium, pancreas, skin, and spleen.
Before the TMA was stained, smaller test arrays were used to establish staining conditions. In these tests, regular antibody, pre-immune serum, and pre-absorbed antibody were used to ensure that specific staining could be detected.
Statistical Analysis
TMA staining data were analyzed with GraphPad (version 5.0, GraphPad Software Inc., San Diego, CA) and SAS (version 9.2, SAS Institute, Cary, NC). Early-stage IDC samples were analyzed for staining correlations to overall survival by constructing Kaplan-Meier curves,20,21 and differences between curves were evaluated by a log-rank test. Multivariate and univariate hazard ratios were calculated by applying a Cox proportional hazards regression model (SAS phreg) on the results from the TMA. Because of incomplete or missing information on relapse, adjuvant and hormone therapy, progesterone receptor, and Her-2/neu status, these parameters were not included in the analysis.
Results
TIMP-4 Protein Is Present in Breast Cancer Specimens of All Histological Types
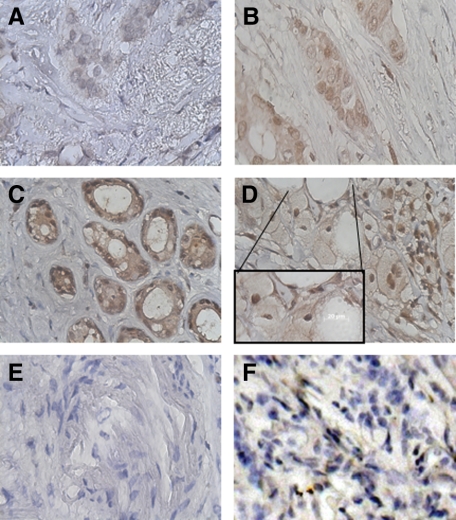
Immunohistochemical analysis of infiltrating ductal carcinoma demonstrated TIMP-4 staining associated with tumor epithelial cells. Staining results were obtained from 1783 of the total of 2197 (81%) tissue samples on the TMA. Reasons for failure among the nonstaining tissues included lack of tumor cells in the sample (N = 295; 13%) or missing sample spots on the TMA (N = 119; 6%). The relative levels of immunohistochemical expression of TIMP-4 on the TMA core specimens were evaluated by one pathologist, and the staining intensity was estimated by visual inspection in a four-step scale (score 0, 1, 2, and 3). Both nuclear and cytoplasmic patterns of staining were evident in the specimens (Figure 1, A–D). Using a proprietary threshold finder software tool (developed by G.S.), the following optimal scoring criteria were found. Cases for which no positive staining was observed were given the score 0, representing TIMP-4-negative tumors. Cases in which tumor cells had weak (light color) or ≤30% of tumor cells had a moderate staining intensity were given the score 1. The intermediate score 2 was used when >30% of tumor cells had moderate or <60% of tumor cells had strong staining intensity. Cases in which ≥60% of cells had a strong staining intensity (dark brown/black color) were assigned the score 3.
Figure 1.
Immunohistochemical detection of TIMP-4 in formalin-fixed, paraffin-embedded breast cancer tissues. Absence (A) or presence (B–D) of TIMP-4 stained infiltrating ductal carcinoma. B–D: Polyclonal antibody staining showed cytoplasmic and nuclear staining of three levels of intensity. E: Representative control staining with isotype rabbit IgG. F: Antibody pre-absorbed with recombinant human TIMP-4.
Cytoplasmic costaining was present in approximately 10% of breast cancers with nuclear positive staining and was recorded but scored separately. These patterns differed from those obtained with an isotype rabbit antibody or with primary antibody pre-absorbed with purified recombinant human TIMP-4 protein (Figure 1, E and F, respectively). Results from the TMA indicated that 50.9% of all IDC (N = 890) and 51.9% of the early-stage IDC (N = 343) stained positive for TIMP-4 protein. The results from the TMA also indicated that TIMP-4 expression is found in histological types of breast cancer other than IDC (Table 3).
Table 3.
TIMP-4 Expression in Various Histological Types of Breast Cancer
| Histological type | On array | Analyzable | TIMP-4 score 0 (%) | TIMP-4 score 1, 2, or 3 (%) |
|---|---|---|---|---|
| All | 2197 | 1783 | 52.3 | 47.7 |
| Ductal carcinoma | 1531 | 1294 | 49.1 | 50.9 |
| Lobular carcinoma | 311 | 192 | 67.7 | 32.3 |
| Mucinous carcinoma | 69 | 53 | 58.5 | 41.5 |
| Cribriform carcinoma | 64 | 55 | 58.2 | 41.8 |
| Medullary carcinoma | 57 | 49 | 40.8 | 59.2 |
| Tubular carcinoma | 56 | 49 | 52.3 | 47.7 |
| Papillary carcinoma | 30 | 26 | 65.4 | 34.6 |
| Other carcinoma | 79 | 70 | 62.9 | 37.1 |
Survival Analysis of Test Specimens
Because lymph node status is regarded as the single most critical prognostic factor for breast cancer, and there is a lack of simple immunohistochemical markers to predict prognosis of patients with node-negative breast cancer, we specifically analyzed the ability of TIMP-4 expression to predict prognosis of node-negative patients.22 Kaplan-Meier analyses of overall survival were performed on results from only IDC specimens on the TMA.
No other histological subtype had sufficient data information to perform such analysis. The results from all early-stage IDC (T1N0MX) (N = 314) on the TMA indicated that the presence of TIMP-4 (scores 1, 2, and 3) was associated with decreased survival expectancy relative to the presence of no TIMP-4 in the tumor material (score 0) (P = 0.0254 by log-rank analysis) (Figure 2A).
Figure 2.
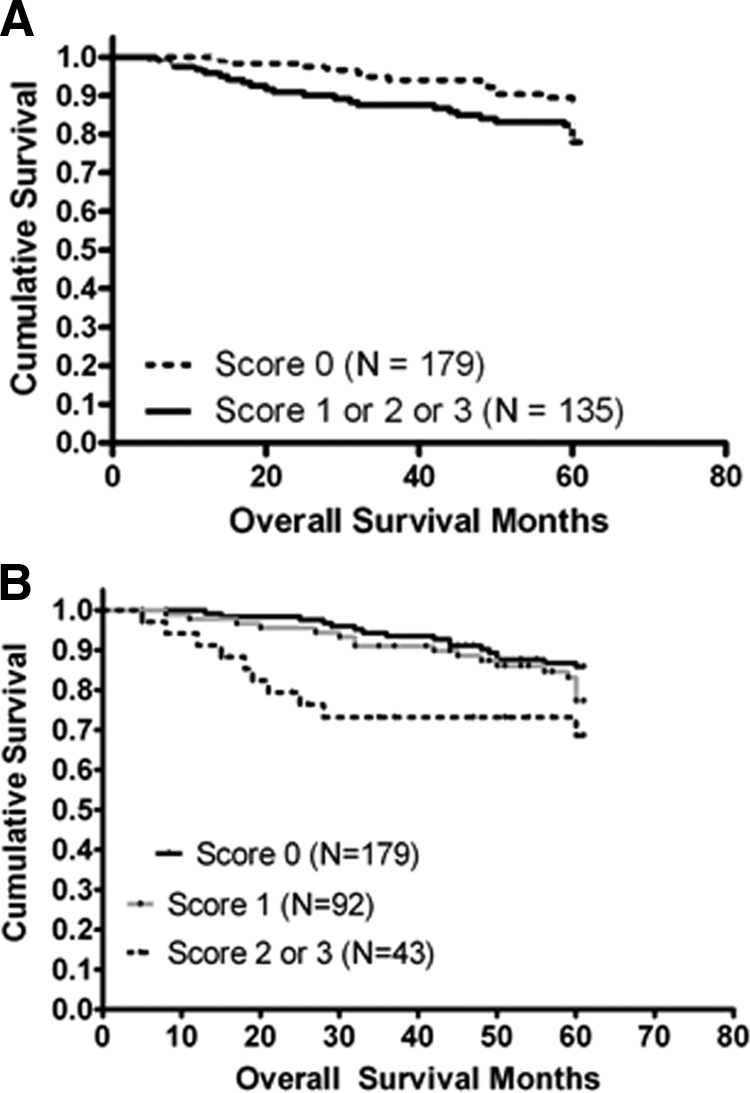
Kaplan-Meier survival graph for early-stage IDC. A: Overall survival analysis according to TIMP-4 status in patients from the TMA with infiltrating ductal carcinomas smaller than 20 mm in diameter without spread to local lymph nodes (N = 314) (log-rank P = 0.0254). B: Survival for the same patients group stratified according to undetectable (score 0) versus detectable levels of low (score 1) or intermediate/high (scores 2 and 3, respectively) of TIMP-4 in the tumor material.
To assess the ability of TIMP-4 to predict disease progression for early stage, node-negative IDC, breast cancer specimens were analyzed separately by univariate and multivariate analysis. By univariate analysis, the presence of TIMP-4 was associated with a significant risk of disease progression. Of the four markers examined (BRE, size, ER status, and TIMP-4), TIMP-4 was the only predictor with good prognostic capability (Table 4). Multivariate analysis using the Cox proportional hazards regression method,23,24 to take several prognostic factors into account (BRE, size, and TIMP-4 score), showed that the presence of TIMP-4 remained an independent prognostic factor for survival (hazard ratio, 1.423; P = 0.0308) (Table 5). At the 5% level, only TIMP-4 was statistically significant, with none of the other markers being statistically significant even at the 10% level (data not shown). In an initial analysis, ER status had the widest interval and an estimate of 1.175. With several specimens missing information on ER status, it was excluded from the final analysis to add confidence to the results for the other factors.
Table 4.
Univariate Analyses of Early-Stage Breast Cancer Survival to Prognostic Factors in Breast Cancer
| Parameter | Range or category | Hazard ratio (95% confidence interval) |
|---|---|---|
| BRE | 1–3 | 0.876 (0.726–1.057) |
| TIMP-4 score | Positive vs. negative | 1.389 (1.012–1.907) |
| Tumor size | 5–20 mm | 0.984 (0.943–1.029) |
| ER | Positive vs. negative | 1.104 (0.690–1.687) |
Statistical analyses were performed by the χ2 test (Wald method) in Cox proportional hazards regression analysis to evaluate the prognostic power of factors in a multivariate manner.
Table 5.
Multivariate Analyses of Early-Stage Breast Cancer Survival to Prognostic Factors in Breast Cancer
| Parameter | Range or category | Estimate | SE | χ2 statistic | P | Hazard ratio (95% confidence interval) |
|---|---|---|---|---|---|---|
| BRE grade | 1–3 | −0.13086 | 0.09909 | 1.7442 | 0.1866 | 0.877 (0.723–1.066) |
| TIMP-4 score | Positive vs. negative | 0.35253 | 0.16323 | 4.6643 | 0.0308 | 1.423 (1.032–1.959) |
| Tumor size | 5–20 mm | −0.01434 | 0.02324 | 0.3809 | 0.5286 | 0.985 (0.942–1.032) |
Statistical analyses were performed by the χ2 test (Wald method) in Cox proportional hazards regression analysis to evaluate the prognostic power of factors in a multivariate manner.
Stratification of the TIMP-4 level in subgroups of low (score 1) or medium/high (scores 2 and 3) staining intensities demonstrated not just a lower proportion of patients with 5-year disease-free survival within the medium/high subset, but also a greater likelihood of early onset of disease progression (P = 0.0052 by log-rank analysis) (Figure 2B). The age at time of death ranged from 34 to 84 years (median of 68 years) with 57% of all deaths occurring among patients younger than 70 years. However, the follow-up data provided with the TMA specimens did not discriminate between death due to breast cancer and death due to other causes. Thus, the Kaplan-Meier analysis was performed on patients for whom censoring information was available during the first 5 years of follow-up. When the results were considered together, they strongly suggested an unfavorable survival prognosis associated with TIMP-4 elevation in primary breast carcinoma.
TIMP-4 Expression Is Associated with a Particularly Unfavorable Survival Prognosis in ER-Negative Early-Stage Ductal Carcinoma
Estrogen receptor status has been used as a prognostic marker with receptor presence indicating favorable long-term disease-free survival. Patients with ER-negative tumors are generally regarded as more likely to have a relapse or progression of cancer within a shorter time. Still, some patients within this group will experience long-term survival without further complications. However, there is no known marker that can predict survival prognosis among patients with early stage (T1N0) ER-negative breast tumors. Our analysis clearly indicated that the presence of TIMP-4 protein was associated with a shorter disease-free survival time. Patients in the TIMP-4-positive group had early progression to more aggressive disease and were less likely to survive 5 years after diagnosis (Figure 3). Comparison of the overall survival for early-stage ER-negative tumors showed a strong correlation between the presence of TIMP-4 (score = 1, 2, or 3) and poor survival (P = 0.0011 by log-rank analysis), relative to the survival period for those without detectable levels of TIMP-4. Clinically, these findings suggest that ER-negative tumors are more sensitive to growth-promoting pathways involving TIMP-4 that are as yet undefined.
Figure 3.

Kaplan-Meier survival graph for estrogen receptor-negative early-stage IDC. Overall survival according to the presence (scores 1, 2, and 3) or absence (score 0) of TIMP-4 in tumors on the TMA for patients with estrogen receptor-negative early-stage IDC (N = 156) (log-rank P = 0.0011).
Discussion
This study demonstrates that breast cancer patients with early-stage infiltrating ductal carcinoma whose tumors express TIMP-4 have shorter disease-free periods compared with patients whose tumors have undetectable TIMP-4. Further, high levels of TIMP-4 were found predominantly in patients with a survival period of less than 3 years, indicating that TIMP-4 is a marker for aggressive disease and shorter survival expectancy.
A biological mechanism to explain the link between TIMP-4 level and poor survival prognosis in breast cancer patients remains to be determined. Even though the TIMPs are usually only regarded as regulators of matrix metalloproteinase activities, there is a growing body of evidence demonstrating that the TIMPs are multifunctional proteins that can affect cell growth, apoptosis, and angiogenesis.1,2,3 Expression of TIMP-4 might be an early sign of malignancy as it has been shown that TIMP-4 expression is associated with the progression of ductal carcinoma in situ to infiltrating ductal carcinoma.15 In addition, others have demonstrated that TIMP-4 expression is associated with poor survival prognosis among colon cancer patients.25 Still others have shown that TIMP-1 inhibits intrinsic apoptosis by inducing survival pathways involving focal adhesion kinase.26 For example, high levels of TIMP-1 are associated with poor survival outcome for breast cancer patients,27 and they are also predictive of response to chemotherapy.28 Based on our current observation, TIMP-4 in breast cancer tissue would be predicted to mediate a tumor progression event(s) contributing to reduced life expectancy.
It is becoming increasingly important to identify markers that can stratify patients with small but aggressive tumors so that appropriate treatment can commence at the earliest possible time point. In the past, patients with early-stage breast cancer were generally given a good prognosis for disease-free survival. However, it now clear that even tumors with low TNM scores can recur or progress within a few years of diagnosis. This occurrence is mostly observed among the ER-negative tumors, which frequently also lack the progesterone receptor (progesterone receptor-negative), making them unresponsive to estrogen and therapies that interfere with estrogen signaling. Among the most aggressive and difficult types of breast cancers to treat are “triple-negative” tumors, ie, those lacking ER, progesterone receptor, and amplified Her-2. For these patients, standard chemotherapy is the only modality of systemic therapy. However, triple-negative tumors are frequently associated with a high rate of local and systemic relapse even after chemotherapy.29 With no access to the Her-2 status of the TMA tumors, it is a possibility that at least some tumors identified as ER-negative are also triple-negative. With a substantial overlap between the triple-negative and the basal-like breast carcinomas,30 the results could be affected by these subcharacteristics. On-going prospective studies of TIMP-4 in early-stage breast cancer will provide clarification as to whether TIMP-4 is associated with ER status alone or whether it is associated with triple-negative/basal-like tumor types.
Another factor that could influence the observed association between TIMP-4 and survival is the therapies given after surgery. This question will also need to be addressed. However, because it is known that some but not all patients with ER-negative tumors will have a relapse within a few years after diagnosis,16,17 it is tempting, based on the survival outcome among those with TIMP-4-positive/ER-negative tumors, to suggest that TIMP-4 can identify these patients. On the basis of the results from this retrospective study, TIMP-4 might help satisfy the need for markers to identify the tumors that pose the most risk in this regard.
Acknowledgments
We thank Ms. Gwendolyn Gilliard for technical assistance and Ms. Kate Ciavarelli for editorial support. This study is dedicated to the memory of our friend and supporter who battled breast cancer, Karen R. Schneider (1964−2008).
Footnotes
Address reprint requests to G. C. Prendergast, Ph.D., Lankenau Institute for Medical Research, 100 Lancaster Ave., Wynnewood, PA, 19096 USA. E-mail: prendergast@limr.org.
Supported by the Sharpe-Strumia Foundation of the Bryn Mawr Hospital, the Pennsylvania Department of Health, Fight from the Fairway, Inc., the Martha W. Rogers Charitable Trust, and the Lankenau Hospital Foundation.
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University was involved in the peer review process or final disposition for this article.
References
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer. 2003;89:1817–1821. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993;59:329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- Koop S, Khokha R, Schmidt EE, MacDonald IC, Morris VL, Chambers AF, Groom AC. Overexpression of metalloproteinase inhibitor in B16F10 cells does not affect extravasation but reduces tumor growth. Cancer Res. 1994;54:4791–4797. [PubMed] [Google Scholar]
- Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993;157:351–358. doi: 10.1002/jcp.1041570219. [DOI] [PubMed] [Google Scholar]
- Bond M, Murphy G, Bennett MR, Amour A, Knauper V, Newby AC, Baker AH. Localization of the death domain of tissue inhibitor of metalloproteinase-3 to the N terminus: metalloproteinase inhibition is associated with proapoptotic activity. J Biol Chem. 2000;275:41358–41363. doi: 10.1074/jbc.M007929200. [DOI] [PubMed] [Google Scholar]
- Fata JE, Leco KJ, Moorehead RA, Martin DC, Khokha R. Timp-1 is important for epithelial proliferation and branching morphogenesis during mouse mammary development. Dev Biol. 1999;211:238–254. doi: 10.1006/dbio.1999.9313. [DOI] [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- Khokha R, Zimmer MJ, Graham CH, Lala PK, Waterhouse P. Suppression of invasion by inducible expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in B16–F10 melanoma cells. J Natl Cancer Inst. 1992;84:1017–1022. doi: 10.1093/jnci/84.13.1017. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liu YE, Greene J, Sheng S, Fuchs A, Rosen EM, Shi YE. Inhibition of tumor growth and metastasis of human breast cancer cells transfected with tissue inhibitor of metalloproteinase 4. Oncogene. 1997;14:2767–2774. doi: 10.1038/sj.onc.1201245. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wang M, Celiker MY, Liu YE, Sang QX, Goldberg ID, Shi YE. Stimulation of mammary tumorigenesis by systemic tissue inhibitor of matrix metalloproteinase 4 gene delivery. Cancer Res. 2001;61:2365–2370. [PubMed] [Google Scholar]
- Zhao YG, Xiao AZ, Park HI, Newcomer RG, Yan M, Man YG, Heffelfinger SC, Sang QX. Endometase/matrilysin-2 in human breast ductal carcinoma in situ and its inhibition by tissue inhibitors of metalloproteinases-2 and -4: a putative role in the initiation of breast cancer invasion. Cancer Res. 2004;64:590–598. doi: 10.1158/0008-5472.can-03-1932. [DOI] [PubMed] [Google Scholar]
- Hess KR, Pusztai L, Buzdar AU, Hortobagyi GN. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003;78:105–118. doi: 10.1023/a:1022166517963. [DOI] [PubMed] [Google Scholar]
- Kiba T, Inamoto T, Nishimura T, Ueno M, Yanagihara K, Teramukai S, Kato H, Toi M, Fukushima M. The reversal of recurrence hazard rate between ER positive and negative breast cancer patients with axillary lymph node dissection (pathological stage I-III) 3 years after surgery. BMC Cancer. 2008;8:323. doi: 10.1186/1471-2407-8-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta Soler A, Knudsen KA, Salazar H, Han AC, Keshgegian AA. P-cadherin expression in breast carcinoma indicates poor survival. Cancer. 1999;86:1263–1272. doi: 10.1002/(sici)1097-0142(19991001)86:7<1263::aid-cncr23>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- Chernick MR, Friis RH, editors. New York: John Wiley and Sons, Inc.,; Introductory Biostatistics for the Health SciencesModern Applications Including Bootstrap. 2003 [Google Scholar]
- Lachin JM. New York: John Wiley and Sons, Inc.,; Biostatistical MethodsThe Assessment of Relative Risk. 2000 [Google Scholar]
- Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6:375–392. doi: 10.1023/a:1014778713034. [DOI] [PubMed] [Google Scholar]
- Kalbfleisch JD, Prentice RL, editors. New York: John Wiley and Sons, Inc.,; The Statistical Analysis of Failure Time Data. 2002 [Google Scholar]
- Therneau TM, Grambsch PM. New York: Springer Verlag, Inc.,; Modeling Survival DataExtending the Cox Model. 2000 [Google Scholar]
- Hilska M, Roberts PJ, Collan YU, Laine VJ, Kossi J, Hirsimaki P, Rahkonen O, Laato M. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007;121:714–723. doi: 10.1002/ijc.22747. [DOI] [PubMed] [Google Scholar]
- Liu XW, Taube ME, Jung KK, Dong Z, Lee YJ, Roshy S, Sloane BF, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells from extrinsic cell death: a potential oncogenic activity of tissue inhibitor of metalloproteinase-1. Cancer Res. 2005;65:898–906. [PubMed] [Google Scholar]
- Schrohl AS, Holten-Andersen MN, Peters HA, Look MP, Meijer-van Gelder ME, Klijn JG, Brunner N, Foekens JA. Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res. 2004;10:2289–2298. doi: 10.1158/1078-0432.ccr-03-0360. [DOI] [PubMed] [Google Scholar]
- Schrohl AS, Meijer-van Gelder ME, Holten-Andersen MN, Christensen IJ, Look MP, Mouridsen HT, Brunner N, Foekens JA. Primary tumor levels of tissue inhibitor of metalloproteinases-1 are predictive of resistance to chemotherapy in patients with metastatic breast cancer. Clin Cancer Res. 2006;12:7054–7058. doi: 10.1158/1078-0432.CCR-06-0950. [DOI] [PubMed] [Google Scholar]
- Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]