Abstract
Immune activation represents an adaptive reaction triggered by both noxious exogenous (microbes) and endogenous [high mobility group box-1 protein (HMGB1), S100 calcium binding proteins] inducers of inflammation. Cell stress or necrosis lead the release of HMGB1 and S100 proteins in the extracellular compartment where they act as damage-associated molecular pattern molecules (or alarmins) by engaging the receptor for advanced glycation end-products (RAGE). Although the biology of RAGE is dictated by the accumulation of damage-associated molecular pattern molecules at sites of tissue injury, the role of RAGE in mediating antenatal fetal injury remains unknown. First, we studied the relationships at birth between the intensity of human fetal inflammation and sRAGE (an endogenous RAGE antagonist), HMGB1, and S100β protein. We found significantly lower sRAGE in human fetuses that mounted robust inflammatory responses. HMGB1 levels correlated significantly with levels of interleukin-6 and S100β in fetal circulation. We then evaluated the levels and areas of tissue expression of RAGE, HMGB1, and S100β in specific organs of mouse fetuses on E16. Using an animal model of endotoxin-induced fetal damage and preterm birth, we determined that inflammation induces a significant change in expression of RAGE and HMGB1, but not S100β, at sites of tissue damage. Our findings indicate that RAGE and HMGB1 may be important mediators of cellular injury in fetuses delivered in the setting of inflammation-induced preterm birth.
Conventional wisdom holds that the primary causes of the high neonatal morbidity and mortality attendant preterm birth are complications of immature organ systems.1,2,3,4 However, a growing body of investigation suggests that the poor outcome observed in many preterm children is not entirely dependent on their gestational age at birth.2,5,6 After correcting for gestational age, several risk factors remain significantly associated with an increased risk of cerebral palsy, such as intra-amniotic infection, histological chorioamnionitis, prolonged rupture of the membranes, and hypoxemic fetal growth restriction.7,8,9 Therefore, particularities of the fetal innate immune response to infection appear to cause pathology unique to the premature fetus. This includes a heightened inflammatory and oxidative stress state that acts synergistically with microbial insult to induce cell damage and multisystem organ failure.7,10,11,12
The host’s response to microbial pathogens involves a series of carefully orchestrated mechanisms that include the newly described damage-associated molecular pattern molecules (DAMPs).13,14 DAMPs, also known as “alarmins,”15 are a pleiotropic group of intracellular proteins that include among others the high-mobility group box-1 (HMGB1 or amphoterin) and S100β proteins.13,16 When released into the extracellular compartment in excess as a result of cell activation or injury, DAMPs become “danger signals” that specifically activate the receptor of advanced glycation end-products (RAGE).14,17 RAGE is a transmembrane receptor,18 a member of the immunoglobulin superfamily, and functions as a chief receptor for products of nonenzymatic glycoxidation (advanced glycation end-products, AGEs), HMGB1, and S100β proteins.14 In adult humans and animals, RAGE has been shown to be expressed on the cellular surface of cortical neurons and numerous endothelial, smooth muscle, inflammatory, and vascular cells positioned in vital organs such as the brain, lung, heart, liver, and bowel.19,20,21,22 Binding of DAMPs to the RAGE extracellular domain results in sustained activation of nuclear factor (NF)-κB and recruitment of inflammatory cells (CD68- and Cd11c-positive mononuclear phagocyte), which in turn amplify the process of tissue damage.14 That RAGE and HMGB1 play a fundamental role in inflammation and oxidative stress-induced tissue injury is demonstrated by experiments in animal models where administration of quercetin (flavonoid with potent antioxidant properties and HMGB1 inhibitor)23 or soluble RAGE (sRAGE, an extracellular truncated form of RAGE that acts as a decoy receptor) or antibodies or peptides targeted against RAGE or HMGB1 attenuate the lethal effects of endotoxin, acetaminophen and ischemia-reperfusion.24,25,26,27,28,29,30
Recently, we demonstrated that the S100A12-RAGE axis is actively engaged in modulating the intensity of the human intra-amniotic inflammatory response to infection.31,32 We attributed a key role to the presence and activity of amniotic fluid (AF) sRAGE.31 In this study we sought to evaluate the role of RAGE, HMGB1, and S100β proteins as mediators of fetal organ injury in the context of infection and/or inflammation. Specifically, we have begun by assessing whether the intensity of the human maternal and fetal inflammation impacts on the fetal systemic levels of sRAGE (as marker of the RAGE system activation),33 HMGB1, or S100β levels at birth. Given that sRAGE acts as a decoy for RAGE we anticipated that in the setting of a robust fetal inflammatory response the circulatory levels of sRAGE are low. We thought that this may be related to inhibition or dysfunction of the mechanisms responsible for synthesis of this decoy receptor in the setting of overwhelming cytokemia. Alternatively, low levels of total sRAGE may be related to successful removal/detoxification of AGEs known to be generated in high amounts in the context of increased metabolic and oxidative stress, such as that associated with fetal prematurity and infection.34,35 To elucidate whether RAGE, HMGB1, and S100β are participants in the mechanisms underlying infection/inflammation induced fetal cell/organ damage, we turned to an animal model of preterm birth induced by maternal administration of endotoxin (lipopolysaccharide, LPS).35 First, we evaluated the level of RAGE, HMGB1, and S100β tissue expression and regional distribution in specific organs of mouse fetuses at developmental stage E16. Furthermore, we aimed to determine whether altered expression of RAGE, HMGB1, or S100β co-exists with tissue inflammation and cellular damage in vital organs such as fetal brain and liver.
Materials and Methods
Patients and Amniotic Fluid Collection
In a prospective study design, we enrolled 121 consecutive preterm singletons born to mothers who had a clinically indicated amniocentesis to rule out infection. All women were recruited at Yale New Haven Hospital (YNHH) following their admission to Labor and Birth or to the High Risk antepartum units. This study extended from May 2004 to October 2007. The Human Investigation Committee of Yale University approved the study protocol.
Amniocentesis was indicated independent of our research protocol. Amniotic fluid was collected by ultrasound-guided amniocentesis and each woman was followed prospectively until delivery. Eligible women had a gestational age at delivery ≥23.1 weeks, preterm labor contractions refractory to tocolysis, preterm premature rupture of membranes (PPROM), or advanced cervical dilatation (≥3 cm). Exclusion criteria were the presence of anhydramnios, human immunodeficiency or hepatitis viral infections, congenital anomalies, abnormal karyotype, or any known maternal medical conditions. Gestational age was determined based on last menstrual period confirmed by an ultrasound examination before 20 weeks.36 Preterm labor was defined as the presence of regular uterine contractions and documented cervical effacement and/or dilatation in patients <37 weeks of gestation. PPROM was confirmed by vaginal AF “pooling,” “nitrazine,” “ferning,” or an amniocentesis-dye positive test. Corticosteroids and antibiotics were recommended as clinically indicated.37 The neonatology resuscitation team was present at the time of delivery for all neonates.
Chemical and Microbiological Studies of the Amniotic Fluid
Following retrieval under sterile conditions, amniotic fluid was analyzed by the YNHH clinical and microbiological laboratories for glucose concentration, lactate dehydrogenase activity, white blood cell count, Gram stain, and standard culturing methods for aerobic and anaerobic bacteria, including Ureaplasma and Mycoplasma species. These results were available to the clinical team for management of the case. An amniotic fluid glucose cut-off of ≤15 mg/dL, an lactate dehydrogenase level ≥419 U/L, a positive Gram stain and/or culture result were considered suggestive of intra-amniotic infection. The results of the microbiological tests were available for case management and were reported as final 5 days after culturing. The remaining amniotic fluid was transported to the research laboratory, spun at 3000 × g at 4°C for 20 minutes, aliquoted into polypropylene cryotubes, and stored at −80°C until analysis.
Mass Spectrometry of the Amniotic Fluid
To confirm or exclude the presence of intra-amniotic inflammation, an amniotic fluid proteomic fingerprint (mass restricted [MR] score) was generated using surface enhanced laser desorbtion ionization-time of flight mass spectrometry. The method for generation of the MR score has been previously described.32 Briefly, the MR score is comprised of four proteomic biomarkers: defensin-2, defensin-1, S100A12 (calgranulin C), and S100A8 (calgranulin A). The MR score ranges from 0 to 4, depending on the presence or absence of each of the 4 protein biomarkers. A value of 1 was assigned if a biomarker peak was present and 0 if absent. Based on our previous results, we stratified the study population based on the “severity” of inflammation (MR 0: “no” inflammation; MR 1–2: “minimal” inflammation; MR 3–4: “severe” inflammation).38 Scorings of the amniotic fluid surface enhanced laser desorbtion ionization-time of flight tracings were performed by an investigator without knowledge of the maternal outcome, the results of the placental histological examination, or umbilical cord HMGB1, S100β protein, and interleukin (IL)-6 levels. The results of the MR score were not used for patient clinical management.
Cord Blood Specimens and Assessment of the Fetal Acid-Base Status at Birth
Within 10 minutes of delivery, cord blood samples (umbilical artery and vein) were collected in pre-heparinized 1-ml syringes, which were then capped and transported to the laboratory. The acid-base status was determined with the ABL 800 FLEX blood gas analyzer (Radiometer Medical A/S, Denmark). Umbilical cord blood was also obtained by aseptic puncture of the clamped umbilical vein. Following collection, the cord blood was immediately centrifuged at 1000 × g at 4°C for 15 minutes. Serum was aliquoted in sterile polypropylene tubes and stored at −80°C until IL-6, HMGB1, and S100β protein levels were examined.
Histological Evaluation of the Human Placenta
Placental tissues were available for all 121 neonates included in this analysis. H&E-stained sections of the amniochorionic membranes and umbilical cord were read by a perinatal pathologist unaware of the results of the MR score, fetal outcome, or umbilical cord blood analyses. Each section was examined systematically for the presence or absence of inflammation, and funisitis was diagnosed when neutrophils infiltrated the umbilical vessel walls or Wharton’s jelly. Three histological stages of chorioamnionitis were complemented by the histological grading system devised by Salafia et al, which includes four grades of inflammation of the amnion, chorion-decidua, and umbilical cord.39,40 Histopathological evidence of maternal and/or fetal immunoresponse was assessed as previously described by Ghidini and collaborators,41 and scored as either absent, mild (grades 1–2), or severe (grades 3–4). Severe inflammation in the amnion was considered a marker of activation of the maternal immune system in response to ascending infection, whereas severe inflammation of umbilical cord and/or chorionic vessels was considered to indicate a response of the fetal immune system.41
Evaluation for Early-Onset Neonatal Sepsis
Following delivery, all 121 neonates were admitted to the YNHH Newborn Special Care Unit were per institutional clinical guidelines; all had blood specimens and cultures obtained within 2 hours from the time of birth. Blood cultures were performed in the clinical microbiology laboratory for aerobic bacteria, anaerobic bacteria, and Mycoplasma species. Cerebrospinal and urine cultures were performed when clinically indicated. Antibiotic treatment was begun in all neonates after blood cultures were obtained. Early-onset neonatal sepsis (EONS) was defined as the presence of confirmed or suspected sepsis at ≤72 hours after birth. Confirmed sepsis represented the presence of a positive blood or any other body fluid microbial culture. A diagnosis of EONS was based on clinical symptoms corroborated with hematological laboratory results.42,43 Sepsis was suspected in the presence of ≥2 hematological criteria in the absence of a positive blood culture.44 EONS was dichotomized into present (when sepsis was either confirmed or suspected) or absent.38 Sepsis categorization and neonatal hematological indices were assessed by investigators who analyzed all of the data, unaware of the results of the umbilical cord analyte levels, proteomic profiling of the amniotic fluid, or histological evaluation of the placenta.
Enzyme-Linked Immunosorbent Assays of Human Cord Blood IL-6, HMGB1, S100β, and sRAGE Proteins
IL-6 (Pierce-Endogen, Rockford, IL), sRAGE (R&D Systems, Minneapolis, MN), HMGB1 (IBL International, Hamburg, Germany), and S100β (BioVendor, Candler, NC) immunoassays were performed in duplicate according to manufacturers’ instructions by investigators unaware of the clinical presentation and umbilical cord blood sample origin. The minimal detectable concentration for IL-6, HMGB1, S100β, and sRAGE proteins was 1 pg/ml, 1 ng/ml, 20 pg/ml, or 4.12 pg/ml, respectively. The inter- and intra-assay coefficients of variation were <10% for all analytes. Several of the cord blood samples were previously used in studies aimed at exploring the relationships between fetal inflammation (IL-6) and the proteomic biomarkers of the MR score.7
Mouse Model of Inflammation-Induced Antenatal Fetal Injury and Preterm Birth
The animal model of preterm birth and fetal injury has previously been reported.35 The University of Maryland, Institutional Animal Review Board (Baltimore, MD) approved the animal experimentation. Briefly, C57BL/6 mice were purchased from Harlan Sprague Dawley (Madison, WI), maintained on a 12:12 hours light cycle and given free access to food and water. On E15.5 (E0 = sperm plug observed), ten pregnant mice were assigned to receive either one 10 μg of i.p. injection of LPS (from E. Coli 0111:B4, Calbiochem, La Jolla,) diluted in 0.2 ml endotoxin-free saline (LPS, n = 5) or saline alone (control: CRL, n = 5).
To evaluate the effect of maternal inflammation on fetal survivability, we sacrificed all pregnant animals by cervical dislocation at a specific time following LPS or saline injection. The uterus was quickly opened and the fetuses assessed for viability. A viable fetus was denoted by presence of a detectable motor response to mechanical stimulation. We found that 58% of fetuses were already dead when animals were sacrificed on E16 at 16 hours post-LPS administration.35 Viable fetuses were fixed in phosphate-buffered formalin and embedded in paraffin. Already dead fetuses were excluded to avoid nonspecific postmortem changes. We assessed the tissue distribution of HMGB1, RAGE, and S100β isoform in fetal organs (brain, thymus, lung, liver, bowel, skin, and cartilage) using five random fetuses (one from each LPS mouse) exposed to intrauterine inflammation and found alive at 16 hours post-LPS treatment. For comparison, we used five random fetuses (one from each CRL mouse) obtained at 16 hours after saline injection.
Immunohistochemistry and Image Processing
Five-μm sagittal serial paraffin sections were deparaffinized in xylene and rehydrated with graded ethanol to potassium-PBS solution, pH 7.2. Following antigen retrieval with citrate buffer, the sections were pretreated with 1% hydrogen peroxide for 15 minutes, followed by overnight incubation at 4°C with either rabbit anti-HMGB1 polyclonal (1:1000, Shino-Test, Japan), goat anti-RAGE polyclonal (5 μg/ml, R&D Systems), rabbit anti-S100β monoclonal (1:100, Abcam, clone EP1576Y), or mouse anti-ED1 monoclonal (1:100, Novus Biologicals, Littleton, CO) antibodies. The ED1 antigen is specific to infiltrating macrophages and is homologous to human CD68.45,46 Detection was performed with biotinylated donkey anti-goat, goat anti-rabbit, or goat anti-mouse secondary antibodies (1:600, Jackson ImmunoResearch, West Grove, PA) as appropriate. Detection was performed with horseradish peroxidase avidin-biotin complex (Elite ABC system, Vector Laboratories, Burlington VT) and Vector NovaRED was used as a substrate. Sections were counterstained with hematoxylin. For ED1 antigen, a mouse-on-mouse blocking reagent (Vector) was used before exposure to the first antibody. Erythroid lineage cells were identified in double immunoperoxidase staining using a biotinylated monoclonal antibody raised against TER-119, an antigen associated with cell-surface glycophorin A on developing erythroblasts and mature erythrocytes).47 Detection of TER-119 was accomplished with nickel-diaminobenzidine as the chromogen. In initial experiments optimizing concentration of each primary antibody, specificity of immunostaining was validated with negative sections where the primary antibody has been preabsorbed with fivefold excess recombinant antigen. Subsequently, negative control slides with omitted primary antibody were included with each run.
Sections were first screened for general anatomical landmarks, followed by a systematic examination of fetal organs for markers of tissue and cell damage (hemorrhage, edema, structural loosening and breakdown, nuclear morphological changes, changes in chromatin distribution, or karyorrhexis). Immunohistochemical staining was graded using the HSCORE (histo-score) system, according to the method as described by McCarty et al, which considers the intensity and percentage of cells staining at each intensity.48,49 Three randomly selected areas were imaged (×400 magnification) under a light microscope (Olympus IX71, Melville, NY) and digitized using an OLY-200 camera (Olympus) under the same light intensity settings. Cells in each field were scored by two independent investigators (C.S.B., I.A.B.) for staining intensity in the following categories: 0, no staining; 1+, weak; 2+, moderate; 3+, intense staining. HSCORE values (cellular or nuclear component) were calculated for each area using the formula Σ Pi (i + l), where i represents one of the four degrees of intensity staining and Pi is its corresponding percentage of cells, which fluctuates from 0% to 100%. The HSCORE is a numerical figure from 100 to 400. A lack of immunoreactivity results in an HSCORE value of 100, while 400 is the highest possible HSCORE (when 100% of cells are stained at a 3+ level).48 Tissue HSCOREs were derived by averaging values from the individual areas. The coefficient of variation for HSCORE was <7% for all tissues.
Statistical Analysis
Statistical analyses were performed with Sigma Stat, version 2.03 (SPSS Inc., Chicago, IL) and MedCalc (Broekstraat, Belgium) statistical software. Normality testing was performed using the Kolmogorov-Smirnov test. Data were compared with one way analysis of variance, followed by Student Newman Keuls tests (parametric), or Kruskal-Wallis on ranks, followed by Dunn’s tests (non-parametric), to adjust for multiple comparisons as appropriate. Although in tables, we present the absolute values, statistical analyses of data derived from immunoassays was performed after logarithmic transformation of data. Spearman or Pearson correlations were used to measure co-linearity between the selected independent variables, as well as other relevant relationships between dependent and independent variables. Comparisons between proportions were done with χ2 tests. Stepwise multivariable regression analysis was used to determine concurrent relationships between variables and to correct for possible influences of gestational age and birth weight. A P value of <0.05 was considered significant throughout the analysis.
Results
sRAGE and DAMP Levels in Human Fetuses at Birth
In Table 1 we present the demographic and clinical characteristics of the study population at the time of enrollment. Women with “severe” intra-amniotic inflammation (MR score 3–4) were more frequently of African American ethnicity, presented with symptoms of preterm birth at an earlier gestational age, and had shorter amniocentesis to delivery intervals, as compared with women with “no” (MR score 0) or “minimal” (MR score 1–2) amniotic fluid inflammation.
Table 1.
Demographic and Clinical Characteristics of Women at Enrollment
| Inflammatory status of the amniotic fluid
|
P value | |||
|---|---|---|---|---|
| MR 0 (absent) n = 24 | MR 1–2 (minimal) n = 38 | MR 3–4 (severe) n = 59 | ||
| Age, years* | 28 [16–39] | 29 [17–39] | 30 [17–40] | 0.634 |
| Nulliparity† | 10 (42) | 23 (76) | 24 (41) | 0.135 |
| Race† | <0.001 | |||
| Caucasian | 13 (54) | 26 (68) | 12 (20) | |
| African-American | 3 (12) | 8 (21) | 29 (49) | |
| Hispanic | 4 (17) | 4 (11) | 12 (20) | |
| Other | 4 (17) | 0 (0) | 6 (11) | |
| Gestational age, weeks* | 30.8 [28.1–33.5] | 29.8 [23.2–33.5] | 27.6 [23.4–32.4] | <0.001 |
| PPROM† | 19 (79) | 21 (55) | 37 (63) | 0.159 |
| Cervical dilatation* | 0 [0–6] | 1 [0–5] | 1 [0–5] | 0.251 |
| Uterine contractions† | 10 (42) | 15 (39) | 29 (49) | 0.612 |
| Steroid exposure during pregnancy† | 23 (96) | 34 (89) | 56 (95) | 0.497 |
| Prenatal antibiotic treatment† | 21 (87) | 32 (84) | 52 (88) | 0.850 |
| Amniocentesis to delivery interval, days* | 2.2 [0.05–19] | 2.5 [0.02–45] | 0.4 [0.02–7.4] | <0.001 |
Data presented as median with 5th and 95th percentiles in brackets as analyzed by Kruskal-Wallis analysis of variance.
Data presented as n (%) and analyzed by Chi square tests.
PPROM = preterm premature rupture of the membranes.
The clinical laboratory results for amniotic fluid and pathological examination of the placenta were more often consistent with presence of infection, inflammation, and/or histological chorioamnionitis and funisitis in women with MR scores 3–4 (Table 2). Neonatal outcome data, results of the acid-base status and the levels of the cord blood analytes at birth are presented in Table 3. Overall, neonates delivered in the setting of “severe” intra-amniotic inflammation were delivered at an earlier gestational age, and had lower birth weights and Apgar scores. Women with “severe” amniotic fluid inflammation delivered more frequently at <30 weeks of gestation compared with the other groups. These clinical findings support the biological relevance of the MR score for case classification based on severity of intra-amniotic inflammation. Six neonates had sepsis confirmed by blood culture results <72 hours after birth (Escherichia coli, n = 4; Streptococcus group B, n = 1; Staphylococcus aureus, n = 1). Neonates delivered by mothers with MR scores 3–4 more frequently had hematological indices suggestive of EONS.
Table 2.
Results of Amniotic Fluid Laboratory Analysis and Histological Examination of the Placenta
| Variable | Inflammatory status of the amniotic fluid
|
P value | ||
|---|---|---|---|---|
| MR 0 (absent) n = 24 | MR 1–2 (minimal) n = 38 | MR 3–4 (severe) n = 59 | ||
| Amniotic fluid analysis | ||||
| Glucose, mg/dL* | 27.5 [11–82] | 26.0 [11–55] | 5 [2–40] | <0.001 |
| Lactate dehydrogenase activity, U/L* | 123 [80–324] | 197 [82–373] | 644 [228–4560] | <0.001 |
| White blood cell count, cells/mm3* | 4 [0–10] | 6 [0–282] | 783 [123–5327] | <0.001 |
| Positive Gram stain† | 0 (0) | 3 (8) | 29 (49) | <0.001 |
| Positive cultures† | 0 (0) | 4 (11) | 41 (69) | <0.001 |
| Histological examination of the placenta | ||||
| Maternal inflammation† | <0.001 | |||
| Absent | 21 (88) | 28 (74) | 10 (17) | |
| Mild (grades 1–2) | 2 (8) | 7 (18) | 19 (32) | |
| Severe (grades 3–4) | 1 (4) | 3 (8) | 30 (51) | |
| Fetal inflammation† | <0.001 | |||
| Absent | 17 (71) | 18 (47) | 8 (14) | |
| Mild (grades 1–2) | 2 (8) | 8 (21) | 4 (7) | |
| Severe (grades 3–4) | 5 (21) | 12 (32) | 47 (80) | |
Data presented as median with 5th and 95th percentiles in brackets as analyzed by Kruskal-Wallis analysis of variance.
Data presented as n (%) and analyzed by Chi square tests.
Table 3.
Neonatal Outcome at Birth and Results of Cord Blood Analysis
| Variable | Inflammatory status of the amniotic fluid
|
P value | ||
|---|---|---|---|---|
| MR 0 (absent) n = 24 | MR 1–2 (minimal) n = 38 | MR 3–4 (severe) n = 59 | ||
| Neonatal clinical outcome characteristics | ||||
| Gestational age at delivery, weeks* | 32.0 [28.1–34.2] | 31.3 [24.0–33.8] | 27.6 [24.1–32.6] | <0.001 |
| Delivery <30 weeks† | 4 (17) | 12 (38) | 42 (71) | <0.001 |
| Birthweight, grams* | 1802 [1145–2592] | 1655 [676–2304] | 1127 [598–2127] | <0.001 |
| Birth by Cesarean delivery† | 5 (21) | 18 (47) | 27 (46) | 0.074 |
| 1 minute Apgar score* | 8 [4–9] | 7 [1–9] | 6 [1–9] | 0.018 |
| 5 minute Apgar score* | 9 [8–9] | 9 [4–9] | 8 [3–9] | 0.019 |
| Male gender† | 16 (66) | 21 (55) | 26 (79) | 0.156 |
| Cord blood gas analysis | ||||
| pH arterial* | 7.34 [7.30–7.36] | 7.30 [7.15–7.41] | 7.32 [7.16–7.48] | 0.131 |
| Base deficit–arterial, mmols/L* | 2.8 [9.3–0.61] | 5.05 [11.2–1.25] | 4.4 [13.9–0.55] | 0.245 |
| pH venous* | 7.38 [7.16–7.41] | 7.34 [7.21–7.43] | 7.37 [7.27–7.47] | 0.330 |
| Base deficit–venous, mmols/L* | 3.8 [11.7–1.7] | 3.65 [11.1–0.52] | 3.75 [6.29–0.09] | 0.550 |
| Early-onset neonatal sepsis categorization | ||||
| Confirmed sepsis† | 0 (0) | 2 (5) | 4 (7) | 0.433 |
| Early-onset neonatal sepsis† | 3 (12) | 4 (11) | 24 (41) | 0.001 |
| Cord blood analytes | ||||
| IL-6, pg/ml* | 5.8 [4–134] | 7.1 [4–31,186] | 23.6 [6–1624] | <0.001 |
| sRAGE, ng/ml* | 2.2 [1.08–3.7] | 2.2 [0.61–4.9] | 1.5 [0.26–4.09] | 0.025 |
| HMGB1, ng/ml* | 21.4 [5–264] | 15.9 [5–318] | 21.2 [7–184] | 0.232 |
| S100β, pg/ml* | 332 [157–3396] | 340 [84–4017] | 304 [107–1329] | 0.664 |
Data presented as median with 5th and 95th percentiles in brackets as analyzed by Kruskal-Wallis analysis of variance.
Data presented as n (%) and analyzed by Chi square tests.
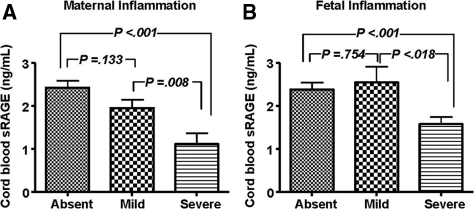
As expected, fetuses delivered by women with severe intra-amniotic inflammation (MR 3–4) had significantly higher cord blood IL-6. In contrast, this subgroup had significantly lower fetal umbilical levels of sRAGE (Table 3). The HMGB1 and S100β levels remained unaffected by intra-amniotic inflammation as assessed by the MR score. To identify the potential impact of other factors, in a multivariate analysis with sRAGE as the dependent variable and maternal and fetal variables (maternal age, parity, race, tocolytics, antibiotics, antenatal steroids, histological chorioamnionitis or funisitis, gestational age at birth, birth weight, cesarean delivery, MR score, fetal acid-base status, EONS, cord blood IL-6, and amniocentesis-to-delivery interval) as independent variables, we determined that a diagnosis of severe histological chorioamnionitis was the strongest predictor of a decreased cord blood sRAGE (R = −0.517, P < 0.001), independently of gestational age at birth or birth weight. Presence of severe histological inflammation in either the maternal (one way analysis of variance, P < 0.001, Figure 1A) or fetal (P < 0.001, Figure 1B) compartment was characterized by significantly lower cord blood sRAGE concentrations.
Figure 1.
Relationship between histological evidence of maternal (A) or fetal (B) inflammation and umbilical cord blood levels of soluble RAGE (sRAGE). sRAGE was measured using an immunoassay (R&D Systems) that detects a heterogeneous population of soluble RAGE proteins that encompass both alternative splice variants and cleavage forms of RAGE. Maternal inflammation was assessed by the extent of inflammatory infiltration of the amnion as absent (n = 59), mild (n = 29), or severe (n = 33), as described in Materials and Methods. The extent of inflammatory infiltrate in umbilical cord and/or chorionic vessels was used to establish the level of fetal inflammation as either absent (n = 43), mild (n = 14), or severe (n = 64). One-way analysis of variance followed by posthoc Student-Newman-Keuls tests was used for statistical analysis. Error bars indicate SE.
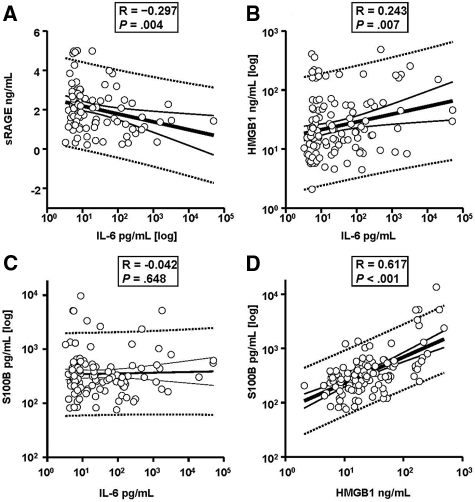
Overall, there was a significant inverse correlation between cord blood IL-6 and sRAGE levels (P = 0.003, Figure 2A). Conversely, a significant direct correlation was identified between cord blood IL-6 and cord blood HMGB1 (P = 0.007, Figure 2B), but not S100β (R = −0.042, P = 0.648, Figure 2C). However, levels of S100β correlated strongly with those of HMGB1 (P < 0.001, Figure 2D) and this correlation was significantly stronger than that of HMGB1 with IL-6 (Z statistic = −4.7, P < 0.001). In multivariate regression analysis, cord blood HMGB1 levels were the strongest predictor of an elevated cord S100β, independently of gestational age at delivery, birth weight, histological chorioamnionitis, PPROM, race, fetal acid-base status, or cord blood IL-6 (r = 0.744, F-ratio 113.1, P < 0.001).
Figure 2.
Relationships between severity of fetal inflammation at birth as depicted by cord blood interleukin-6 concentration and levels of sRAGE (A), HMGB1 (B), and S100β (C). Cord blood serum was collected at birth from 121 premature newborns who were admitted to the Newborn Intensive Special Care Unit (NBSCU). HMGB1 and S100β are damage associated molecular pattern molecules (DAMPs) and recognized ligands of the RAGE receptor. sRAGE represents a heterogeneous population of soluble RAGE proteins that have the ability to bind RAGE ligands thus preventing RAGE engagement. sRAGE values are normally distributed and thus presented in a non-logarithmic format. Data for IL-6 (x-axes of A, B, and C), HMGB1 (y axis of B and x axis of D), and S100β (Y-axes of C and D, respectively) are non-normally distributed and are presented in logarithmic format. The thick black lines represent the respective linear regression lines. The 95% confidence intervals are shown by the respective dotted lines. For each graph the Spearman correlation coefficient R and the level of statistical significance is shown.
In Normal Mouse Fetal Organs HMGB1, RAGE, and S100β Are Expressed in a Select Tissue Distribution Pattern
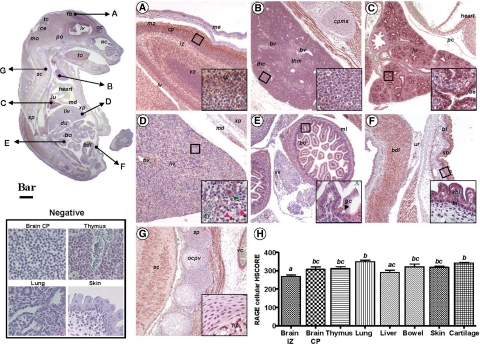
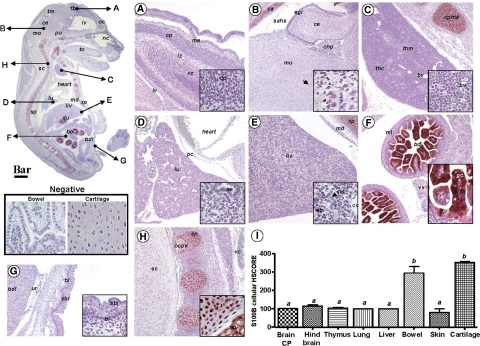
We first set out to provide a systematic mapping of normal mouse fetuses at E16 by immunohistochemistry of tissue levels and localization of HMGB1, RAGE, and S100β in vital organs. The anti-RAGE antibody demonstrated an intense and uniform pattern of RAGE expression throughout the fetal body (Figure 3). We determined that all zones of the brain cortical areas were intensely RAGE-positive (Figure 3A). This is consistent with the observations of Hori and collaborators,50 and the important physiological role of RAGE in neurite outgrowth during development. Neurons in the cortical plate, external germinal layer of the cerebellum and Purkinje neurons were RAGE-positive, with the immunohistochemical signal predominantly localized to plasma membranes and cellular processes. A conspicuous membrane RAGE expression was also observed in epithelial cells of the choroid plexus. Outside the central nervous system, RAGE appeared to be expressed in numerous tissues and organs throughout the fetal body. The thymus had prominent RAGE staining in both cortical and medullar areas (Figure 3B). The lung parenchyma also stood out as intensely RAGE-positive (Figure 3C), with cells of the airway epithelium appearing to exhibit the strongest RAGE expression (Figure 3C, inset) and in a predominant membrane location. In the fetal liver, RAGE immunostaining appeared less prominent than in the developing lung and was primarily localized to erythroblastic and myeloblastic progenitors (Figure 3D) scattered throughout the liver, consistent with the active role of fetal liver in extramedullary hematopoiesis at this developmental stage. The most conspicuous hepatic RAGE expression was detected in either a population of small cells with basophilic nuclei and scant cytoplasm (Figure 3D, inset, red arrowhead) likely nucleated red blood cells as judged by their TER119 positive status (see below) or in a population of large cells with abundant cytoplasm likely representing macrophage precursors (Figure 3D, inset, green arrowhead) as judged by their ED1-positive status. Hepatic endothelial cells were also RAGE-positive. In contrast, RAGE appeared to be expressed less in hepatocytes and mature small erythroblasts. The epithelial surface of the intestinal villi, intestinal glands, and muscular layer of the fetal bowel stained RAGE-positive (Figure 3E). Both basal and suprabasal layers of the fetal skin epidermis displayed intense RAGE immunoreactivity whereas the underlying connective tissue of the dermis was devoid of staining (Figure 3F, inset). Chondrocytes in the primordial cartilages of the vertebral bodies, and of the nucleus pulposus of the intervertebral disks had intense RAGE staining (Figure 3G, inset). Of note, muscle tissues throughout the embryo including that of the tongue, intestine, heart, urinary bladder, and media of blood vessels displayed conspicuous RAGE immunoreactivity. The HSCORE for RAGE demonstrated significant differences among fetal organs (Figure 3H, one way analysis of variance, P < 0.001) with the lung appearing to harbor the strongest RAGE signal.
Figure 3.
Photomicrograph of fetal mouse RAGE immunoreactivity in normal pregnancy. Parasagittal section of a representative mouse fetus at E16 stained immunohistochemically with a polyclonal antibody raised against the extracellular domain of RAGE (Scale bar = 1 mm). The arrows depict topographically fetal organs or tissues photographed at higher magnification (Scale bar = 150 μm) in the panels labeled as follows: A, forebrain; B, thymus; C, lung; D, liver (the red and green arrowheads point to the small and large RAGE expressing hepatic cells described in Results section respectively); E, bowel; F, skin; G, vertebral cartilage tissue. The areas delineated by the squares are further shown as higher magnification captions (Bar = 33 μm) in the right corner inset of each panel. H: distribution of RAGE staining intensity among fetal organs and tissues in n = 5 different fetuses. Error bars indicate SE one-way analysis of variance followed by posthoc Student-Newman-Keuls tests was used for statistical analysis. Bars with at least one common letter are not different at a value of P > 0.05. Representative fields of brain cortical plate (CP), thymus, lung and skin from slides processed identically but omitting the primary antibody are shown in the panel labeled Negative (Scale bar = 33 μm). Abbreviations: ae – airway epithelium; bdl – anterior wall of the urinary bladder; bl – basal layer of epidermis; bo – bowel; bv – blood vessel; ce – cerebellum; cp – cortical plate; cpms – cartilage primordium of manubrium sterni; du – duodenum; eb – erythroblastic islands; fb – forebrain; gc – goblet cells; iz – intermediate zone; liv – liver; lv – lateral ventricle; md – muscle diaphragm; me – meninges; ml – muscular layer; mo – medulla oblongata; nc – nasal cavity; np – nucleus pulposus in the central part of a lumbar intervertebral disk; oc – olfactory cortex; ocpv– ossification within a cartilage primordium of a vertebra; pc – pericardium; po – pons; sbl – suprabasal layer of epidermis; sc – spinal cord; sp – spine; thc – thymus cortical zone; thm – thymus medullar zone; tm – tectum mesencephali; to – tongue; ur – base of urachus; vc – vena cava; vz – ventricular zone; xp – xyphoid process.
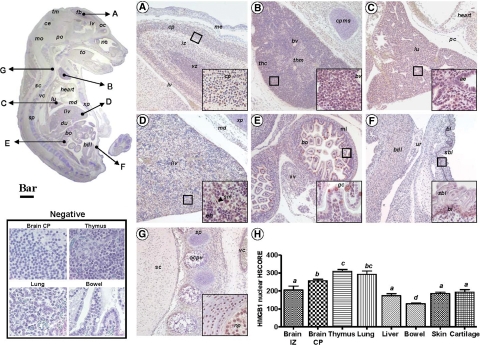
The anti-HMGB1 antibody allowed us to conclude that at E16, HMGB1 is ubiquitously present in nuclei of tissues and organs throughout the fetal body (Figure 4). Specifically, we determined that brain areas with densely packed nuclei (Figure 4A) such as the cortical plate, ventricular zone, or external germinal layer of the cerebellum, are rich in HMGB1 positive nuclei. Some nuclei stained more intensely than others, giving the whole area a patchy appearance. As expected, fetal thymus had prominent HMGB1 nuclear staining especially in the cortical area (Figure 4B). The lung parenchyma also had a patchy distribution of HMGB1 positive nuclei with most of them belonging to endothelial cells and to the airway epithelium of segmental and terminal bronchi (Figure 4C, inset). In the liver, hepatocyte nuclei stained more strongly for HMGB1 than nuclei in erythroblastic islands (Figure 4D). Importantly, neither hepatic erythroblasts nor hepatocytes displayed extranuclear HMGB1 staining in any of the CRL fetuses, which is consistent with the intranuclear predominance of HMGB1 in non-inflammatory cells.16 Conversely, nuclear and extranuclear HMGB1 was seen in both sinusoid and erythroblastic island-associated macrophages. Nuclear HMGB1 was distinctly identified in the fetal bowel (Figure 4E). Interestingly, “goblet” cells (mucus secreting) of the small and large intestine appeared intensely HMGB1-positive. We observed that the pattern of HMGB1 expression in the intestinal goblet cells was primarily extranuclear. In contrast with RAGE, HMGB1 was present only in the basal layer of the fetal skin (Figure 4F) in both nuclear and extranuclear location. Chondrocytes in the primordial vertebral body cartilages (Figure 4G) and in the nucleus pulposus had intense HMGB1 staining restrained to the nucleus. In addition, unossified primordial chondrocytes of the ribs, sternum, and other fetal bones also displayed HMGB1 positive nuclei. Next, we were interested in comparing the histological expression levels of HMGB1 in organs of the mouse fetus. Given that in normal fetuses most tissues displayed a predominant nuclear pattern of HMGB1 staining, we restricted our analysis to the nuclear component of the HSCORE, which revealed that the brain cortical plate, thymus, and lung display the most prominent levels of HMGB1 staining (Figure 4 H, P < 0.001). However, in contrast to RAGE, HMGB1 seemed to be expressed less uniformly throughout the embryonic body.
Figure 4.
Photomicrograph of fetal mouse HMGB1 immunoreactivity in normal pregnancy. Parasagittal section of a representative mouse fetus at E16 stained immunohistochemically with a polyclonal antibody against HMGB1 (Scale bar = 1 mm). The arrows depict topographically fetal organs or tissues photographed at higher magnification (Scale bar = 150 μm) in the panels labeled as follows: A, forebrain; B, thymus; C, lung; D, liver; E, bowel; F, skin; G, vertebral cartilage tissue. The areas delineated by the squares are further shown as higher magnification captions (Scale bar = 33 μm) in the right corner inset of each panel. H: distribution of RAGE staining intensity among fetal organs and tissues in n = 5 different fetuses. Error bars indicate SE one-way analysis of variance followed by posthoc Student-Newman-Keuls tests was used for statistical analysis. Bars with at least one common letter are not different at a value of P > 0.05. Representative fields of brain cortical plate (CP), thymus, lung, and bowel from slides processed identically but omitting the primary antibody are shown in the panel labeled Negative (Scale bar = 33 μm). Abbreviations: ae – airway epithelium; bdl – anterior wall of the urinary bladder; bl – basal layer of epidermis; bo – bowel; bv – blood vessel; ce – cerebellum; cp – cortical plate; cpms – cartilage primordium of manubrium sterni; du – duodenum; eb – erythroblastic islands; fb – forebrain; gc – goblet cells; iz – intermediate zone; liv – liver; lv – lateral ventricle; md – muscle diaphragm; me – meninges; ml - muscular layer; mo – medulla oblongata; nc – nasal cavity; np – nucleus pulposus in the central part of a lumbar intervertebral disk; oc – olfactory cortex; ocpv – ossification within a cartilage primordium of a vertebra; pc – pericardium; po – pons; sbl – suprabasal layer of epidermis; sc – spinal cord; sm – sinusoid macrophage; sp – spine; thc – thymus cortical zone; thm – thymus medullar zone; tm – tectum mesencephali; to – tongue; ur – base of urachus; vc – vena cava; vz – ventricular zone; xp – xyphoid process.
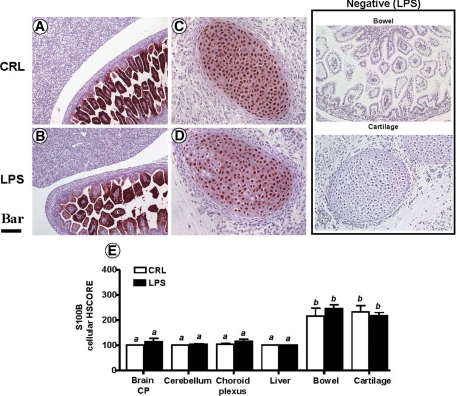
At E16, S100β was found to be even less expressed in the organs of the fetus, as compared with both RAGE and HMGB1 (Figure 5, A–I). Within the nervous system, S100β was not identified in the cortical plate, intermediate or ventricular zone of the forebrain (Figure 5A). However, S100β was selectively present in cells scattered within the pons and medulla oblongata regions of the hindbrain (Figure 5B) and anterior horns of the spinal cord (not shown). Overall, the most prominent S100β staining was concentrated in the villous epithelium of the small bowel (Figure 5F) and chondrocytes of the unossified cartilages including those of the developing calvaria (Figure 5B), sternum (Figure 5C), vertebra, and nucleus pulposus of the intervertebral disks (Figure 5H). The HSCORE analysis demonstrated that the fetal bowel and vertebral cartilage expressed the highest levels of staining for S100β (Figure 5I, P < 0.001).
Figure 5.
Photomicrograph of fetal mouse S100β immunoreactivity in normal pregnancy. Parasagittal section of a representative mouse fetus at E16 stained immunohistochemically with a monoclonal antibody against S100 β (Scale bar = 1 mm). The arrows depict topographically fetal organs or tissues photographed at higher magnification (Scale bar = 150 μm) in the panels labeled as follows: A, forebrain; B, hindbrain; C, thymus; D, lung; E, liver; F, bowel; G, skin; H, vertebral cartilage tissue. The areas delimited by the squares are further shown as higher magnification captions (Scale bar = 33 μm) in the right corner inset of each panel. I: distribution of S100 β immunostaining intensity among fetal organs and tissues in n = 5 different fetuses. Error bars indicate SE; one-way analysis of variance followed by posthoc Student-Newman-Keuls tests was used for statistical analysis. Bars with at least one common letter are not different at a value of P > 0.05. Representative high magnification fields of fetal bowel and cartilage from slides processed identically but omitting the primary antibody are shown in the panel labeled Negative (Scale bar = 33 μm). Abbreviations: ae – airway epithelium; bdl – anterior wall of the urinary bladder; bl – basal layer of epidermis; bo – bowel; bv – blood vessel; ca – developing calvaria; ce – cerebellum; chp – choroid plexus; cp – cortical plate; cpms – cartilage primordium of manubrium sterni; du – duodenum; eb – erythroblastic islands; fb – forebrain; gc – goblet cells; iz – intermediate zone; liv – liver; lv – lateral ventricle; md – muscle diaphragm; me – meninges; ml – muscular layer; mo – medulla oblongata; nc – nasal cavity; np – nucleus pulposus in the central part of a lumbar intervertebral disk; ob – olfactory bulb; ocpv – ossification within a cartilage primordium of a vertebra; pc – pericardium; po – pons; sahs – subarachnoidal space; sbl – suprabasal layer of epidermis; sc – spinal cord; sm – sinusoid macrophage; sp – spine; thc – thymus cortical zone; thm – thymus medullar zone; tm – tectum mesencephali; to – tongue; ur – base of urachus; vc – vena cava; vz – ventricular zone; xp – xyphoid process.
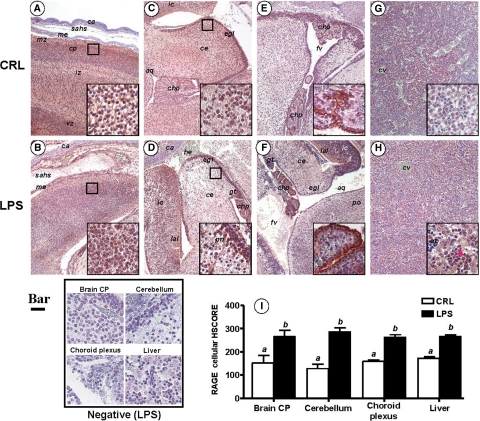
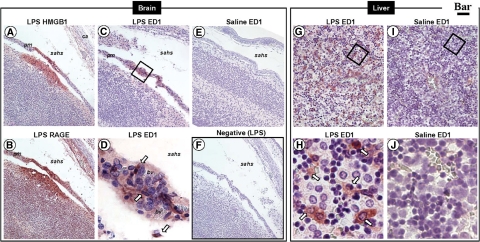
LPS-Induced Preterm Birth Is Associated with Elevated RAGE Expression in Fetal Brain and Liver
In view of the proposed role of RAGE-HMGB1 interaction in both physiological and pathological processes leading to tissue damage, we next turned to investigate expression of RAGE in LPS-induced inflammation and preterm birth. We found a specific and organ selective increase in RAGE immunostaining in both the brain and the liver of fetuses exposed to inflammation in utero, as compared with CRLs (Figure 6). In the brain, there was significant increase in RAGE expression in the cortical plate (Figure 6, A and B), external germinal layer of the cerebellum, Purkinje neurons (Figure 6, C and D, see inset), and choroid plexus (Figure 6, E and F). In the liver, in conjunction with the increase in RAGE-positive cells and immunostaining intensity, we also observed a change in the subcellular localization of RAGE, with LPS markedly increasing the cytoplasmic level of RAGE signal in hepatocytes, which were otherwise observed to express low RAGE levels in the absence of inflammation. Furthermore, compared with CRL, LPS also increased the population of small cells with a basophilic nucleus and intense membrane RAGE staining (Figure 6, G and H, see inset). Aggregates of these cells were observed in higher numbers in perivascular hepatic parenchyma, as well as in intravascular spaces, which was indicative of their blood-derived origin or function. In Figure 6I we present the results of the HSCORE analysis for RAGE cellular immunostaining, which demonstrated a significant up-regulation of RAGE in fetuses exposed to antenatal inflammation.
Figure 6.
Fetal RAGE immunoreactivity in a mouse model of inflammation-induced fetal injury and preterm birth. Pregnant C57Bl/6 mice were injected intraperitoneally with 10 μg lipopolysaccaride (LPS, n = 5) on day 15 of pregnancy. This regimen is known to trigger preterm birth of stillborn fetuses within an average of 18 hours after LPS injection. Control mice (CRL) received the same volume of saline i.p. To demonstrate that fetal damage preceding stillbirth involves altered RAGE expression in vital fetal organs such as brain (A–F) and liver (G–H), all animals were euthanized 16 hours after injection. Fetuses found alive were fixed in phosphate buffered formalin and processed for immunohistochemistry using a polyclonal antibody against the extracellular domain of RAGE. Representative photomicrographs of the fetal forebrain (A, CRL; B, LPS), cerebellum (C, CRL; D, LPS), choroid plexus (E, CRL; F, LPS) and liver (G, CRL; H, LPS) are shown (Scale bar = 100 μm). The areas delimited by the squares are further shown as higher magnification captions (Scale bar = 10 μm) in the right corner inset of each panel. The red arrowhead in (H) points to a cluster of small RAGE expressing hepatic cells described in Results section. I: Distribution of RAGE cellular staining intensity in LPS (black bars, n = 5) and CRL (open bars, n = 5) fetuses. Error bars indicate SE one-way analysis of variance followed by posthoc Student-Newman-Keuls tests was used for statistical analysis. Bars with different letters are statistically different at a value of P < 0.05. Representative high magnification fields from LPS slides processed identically but omitting the primary antibody are shown in the panel labeled Negative (Scale bar = 10 μm). Abbreviations: aq – aqueductus; ca – developing calvaria; ce – cerebellum; chp – choroid plexus within the fourth ventricle; cp – cortical plate; eb – erythroblastic islands; egl – external germinal layer of the cerebellum; fv – fourth ventricle; gt – germinal trigone; he –hemorrhage; ic – inferior colliculus mesencephali; iz – intermediate zone; lal – lateral lemniscus; me – meninges; sahs – subarachnoid space; ss – sagittal superior dural venous sinus; vc – vena cava; vz – ventricular zone.
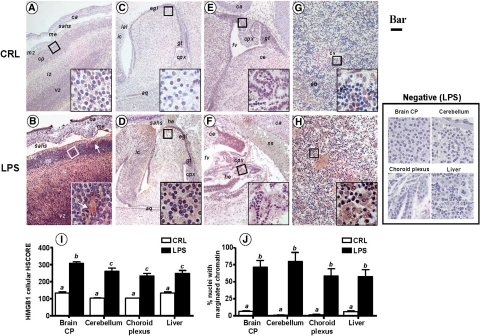
LPS-Induced Preterm Birth Is Associated with Elevated Extranuclear HMGB1 in Fetal Brain and Liver
Similar to RAGE, and compared with fetuses of CRL animals at the same embryonic stage, we noted a selective and marked up-regulation of HMGB1 in fetal brain and liver, but not in other organs such as thymus, cartilage, lung, or bowel. Immunohistochemical analysis of various brain regions demonstrated a significant elevation in HMGB1 staining intensity in specific brain areas. In the forebrain cortex, areas rich in nuclei such as the cortical plate (Figure 7, A and B) were specifically affected compared with the intermediate and ventricular zones. Of importance was the shift in cellular distribution from the primarily intranuclear pattern of staining in CRL fetuses to an intracytoplasmic and even intercellular location of HMGB1 in the brain of inflammation-exposed fetuses (Figure 7, A and B, insets). Brains of inflammation-exposed fetuses had widespread areas of neuropil cavitation suggestive of severe tissue damage (Figure 7B, white arrows). We next focused our attention on regions of the mouse hindbrain such as the cerebellum (Figure 7, C and D) and choroid plexus of the fourth ventricle (Figure 7, E and F), which is an important source of cerebrospinal fluid. We noted a significant up-regulation of HMGB1 in the external germinal layer of the cerebellum and a diffuse pattern of HMGB1 up-regulation in the inferior mesencephalic colliculus (Figures 7D). A significant change in HMGB1 was noted in the simple cubic epithelium of the choroid plexus (Figure 7F, inset) accompanied by diffusion of HMGB1 within the loose connective tissue rich in blood capillaries. An increased number of free red blood cells in the subarachnoidal space (Figure 7D), as well as in the fourth ventricle (Figure 7F), was indicative of subarachnoidal and interventricular hemorrhage, both consistently identified in the brains of fetuses whose mothers received LPS. The fetal liver was also markedly affected (Figure 7, G and H). Whereas in CRL fetuses HMGB1 staining of hepatocytes was confined to the nucleus (Figure 7G), the staining in LPS fetuses was primarily localized to hepatocyte cytoplasm and paracellular spaces, as shown in Figure 7H (inset). The overall increase in HMGB1 immunoreactivity in brain and liver sections of fetuses with antenatal exposure to maternal inflammation was confirmed by our semiquantitative HSCORE analysis as shown in Figure 7I (P < 0.001). Concurrently with the increase and extranuclear redistribution of HMGB1, we observed a significant increase in the number of nuclei with an irregular shape (karyorrhexis), chromatin disorganization, and loss of hematoxylin affinity (karyolysis). These histopathological changes appeared to be most prominent in brain and hepatocyte nuclei (Figure 7J, P < 0.001) and were consistent with those described in oxidative stress-induced cellular injury.51
Figure 7.
Fetal HMGB1 immunoreactivity in a mouse model of inflammation-induced fetal injury and preterm birth. Pregnant C57Bl/6 mice were injected intraperitoneally with 10 μg lipopolysaccaride (LPS, n = 5) on day 15 of pregnancy. This regimen is known to trigger preterm birth of stillborn fetuses within an average of 18 hours after LPS injection. Control mice (CRL) received the same volume of saline i.p. To demonstrate that fetal damage preceding stillbirth involves altered RAGE expression in vital fetal organs such as brain (A–F) and liver (G–H), all animals were euthanized 16 hours after injection and live fetuses processed for immunohistochemistry using a polyclonal antibody against HMGB1. Representative photomicrographs of the fetal forebrain (A, CRL; B, LPS), cerebellum (C, CRL; D, LPS), choroid plexus (E, CRL; F, LPS), and liver (G, CRL; H, LPS) are shown (Bar = 100 μm). The areas delimited by the squares are further shown as higher magnification captions (Bar = 10 μm) in the right corner inset of each panel. The white arrows in (B) point to cavitation areas in the fetal cortical plate with loss of nuclei, which are also intensely HMGB1 positive. I: Distribution of HMGB1 staining intensity in LPS (black bars, n = 5) and CRL (open bars, n = 5) fetuses. J: Antenatal fetal cellular damage estimated by the % of cells with vesicular aspect (marginated chromatin and loss of hematoxylin affinity). Error bars indicate SE. One-way analysis of variance followed by posthoc Student-Newman-Keuls tests was used for statistical analysis. Bars with different letters are statistically different at a value of P < 0.05. Abbreviations: aq – aqueductus; ca – developing calvaria; ce – cerebellum; chp – choroid plexus within the fourth ventricle; cp – cortical plate; eb – erythroblastic islands; egl – external germinal layer of the cerebellum; fv – fourth ventricle; gt – germinal trigone; he –hemorrhage; ic – inferior colliculus mesencephali; iz – intermediate zone; lal – lateral lemniscus; me – meninges; sahs – subarachnoid space; ss – sagittal superior dural venous sinus; vc – vena cava; vz – ventricular zone. Representative high magnification fields from LPS slides processed identically but omitting the primary antibody are shown in the panel labeled Negative (Scale bar = 10 μm).
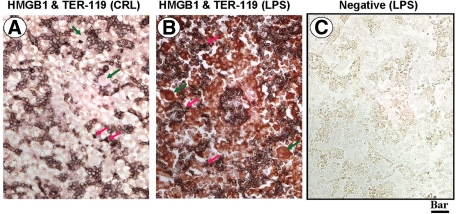
We further determined that the small cells with scant cytoplasm were TER-119 positive indicative of their erythroid lineage (Figure 8A, red arrows). In livers of CRL fetuses these cells were confined to erythroblastic islands and showed little HMGB1 expression. An increase in the number of TER119-positive cells were seen in fetal livers of the LPS treated animals (Figure 8, B and C).
Figure 8.
Topographical co-localization of HMGB1 in fetal liver relative to TER119+ cells of erythroid lineage. Paraffin sections from CRL (A) and LPS-treated animals (B and C) were stained first with a biotinylated mouse anti-TER119 antibody followed by detection with nickel-diaminobenzidine (black chromogen) followed by rabbit anti-HMGB1, which was detected using Vector-Red chromogen). C is from a negative slide with both primary antibodies omitted. The sections were not counterstained. Scale bar = 25 μm. The red arrows point to the small hepatic cells with scant cytoplasm, which are also TER119 positive indicating erythroid lineage. The large TER119 negative cells with abundant cytoplasm are marked with green arrows and show HMGB1 positive staining confined to the nucleus in CRL animals. LPS treatment results in induction of extranuclear HMGB1 signal in the TER119 negative cells (B).
LPS-Induced Preterm Birth Is Not Associated with Changes in Fetal S100β Expression
Next, we examined whether at this developmental stage, inflammation affects the levels of S100β expression in fetal organs that physiologically express either low (brain and liver) or high (small bowel and cartilage) levels of S100β.52 Using a monoclonal antibody selectively reactive with the S100β subunit, we showed by immunohistochemistry that the strong cellular positivity within intestinal cells and chondrocytes remained unaffected by the inflammatory status (Figure 9, A–D) of the fetus. There was no significant difference between LPS and CRL fetuses in terms of the number of S100β-positive cells scattered in different structures of the central nervous system. Similarly, the structures within fetal liver remained largely devoid of S100β staining. HSCORE analysis revealed that inflammation did not alter the normal pattern of fetal S100β expression at E16 (Figure 9E).
Figure 9.
Fetal S100β immunoreactivity in a mouse model of inflammation-induced fetal injury and preterm birth. Pregnant C57Bl/6 mice were injected intraperitoneally with 10 μg lipopolysaccaride (LPS, n = 5) on day 15 of pregnancy. This regimen is known to trigger preterm birth of stillborn fetuses within an average of 18 hours after LPS injection. Control mice (CRL) received the same volume of saline i.p. Maternal inflammation did not alter S100β immunoreactivity. Representative photomicrographs of the two tissues with high basal S100β expression, fetal small bowel (A, CRL; B, LPS; Scale bar = 77 μm) and vertebral developing cartilage (C, CRL; D, LPS; Scale bar = 50 μm) are shown. Panel I: Distribution of S100β staining intensity in LPS (black bars, n = 5) and CRL (open bars, n = 5) fetuses. Error bars indicate SE. One-way analysis of variance followed by posthoc Student-Newman-Keuls tests was used for statistical analysis. Bars with different letters are statistically different at a value of P < 0.05. Representative fields from LPS slides processed identically but omitting the primary antibody are shown in the panel labeled Negative (Scale bar = 77 μm).
HMGB1 and RAGE Are Coexpressed in Sites of Brain and Liver Tissue Injury
The developmental expression of RAGE and HMGB1 in the nervous system of mouse embryos at E15 was previously reported by Chou and colleagues.53 However, detailed studies on expression and subcellular localization of RAGE and HMGB1 in the setting of fetal inflammation and preterm birth were not available. We determined that at E16, RAGE, and HMGB1 are co-expressed at sites with morphologically altered brain tissue architecture and subarachnoid hemorrhage (Figure 10, A and B). To confirm the inflammatory nature of the observed fetal brain lesions, we investigated the involvement of infiltrating macrophages identified on the basis of ED1 antigen expression.45 Figure 10, C and E demonstrates that the affected area characterized by prominent extranuclear HMGB1 and RAGE staining co-exist with multiple cavitation lesions and is abundantly populated by ED1+ cells. The vascular pial membrane overlying the lesion could serve as the originating site of diapedesing monocytes (Figure 10D), a phenomenon not observed in CRL fetuses (Figure 10F). A rich co-expression of RAGE, extranuclear HMGB1 and ED1+ inflammatory infiltrate was also observed in livers of fetuses exposed to inflammation in utero (Figure 10, G and H) but not in fetuses of CRL mice (Figure 10, I and J).
Figure 10.
Topographical co-localization of altered RAGE and HMGB1 expression with ED1+ inflammatory infiltrates in fetuses exposed to LPS-induced maternal inflammation. Serial sections of fetal mouse brain (A–F) and liver (G–J) were stained with either HMGB1, RAGE, or the mouse monoclonal ED1 antibody, which recognizes an antigen expressed on infiltrating mouse macrophages, which is equivalent to human CD68. Panels D, H, and J are higher magnifications of the squared areas in panels C, G, and I, respectively. The arrows point to ED1+ macrophages in vascular rich pia mater (D) or liver (F) of a fetus from an endotoxin treated mother. Scale bars: 50 μm (A, B, C, E, and F); 10 μm (D); 25 μm (G and I); 8 μm (H and J). Abbreviations: ca – developing calvaria; bv – blood vessel; pm – pia mater; sahs – subarachnoidal space.
Discussion
In 1993 Leviton was the first to advance the concept of the fetal inflammatory syndrome,54,55 based on the observation that recognition of microorganisms and activation of the fetal immune system was followed by an outpouring of acute pro-inflammatory cytokines. Therefore, fetal cytokinemia is traditionally viewed as a fetal inflammatory response to infection, which represents an increased risk of perinatal injury that includes brain damage and multisystem organ failure.7,56,57,58 Yet not all fetuses exposed to acute inflammation or high levels of cytokines die or develop white matter injury or cerebral palsy.6,7,59 This suggests that the cellular mechanisms leading to fetal injury are multifactorial and cannot be ascribed to levels of a single acute cytokine or mutations in a specific gene.
Herein we address for the first time in the human fetus some of the complex interrelationships between RAGE and two well-recognized DAMP molecules, HMGB1, and S100β, respectively. It is well-established that the biology of RAGE is largely dictated by the expression and accumulation of its ligands including HMGB1 and S100 proteins, such as S100β and S100A12 (ENRAGE).29,60 Prompted by our prior proteomic discovery that amniotic fluid S100A12 is a late phase inducer of intra-amniotic inflammation, we had shown that the RAGE apparatus localized exclusively to amnion epithelial, decidual, and extravillous trophoblast cells, while the villous trophoblast was largely RAGE negative.31 Furthermore, we found an exponential increase in amniotic fluid sRAGE concentration with increasing gestational age. This finding may explain the higher incidence of infection-related preterm deliveries earlier in pregnancy.31 The current study takes the next step by extending our study of the rules of engagement for RAGE in the amniotic fluid, amnion and placenta, to the human fetus. We find a significant inverse relationship between the fetal systemic levels of sRAGE and severity of inflammation in the intra-amniotic, maternal, and fetal compartment. Our results suggest that the levels of sRAGE are significantly decreased in fetuses that mount an inflammatory reaction. Thereby, it is possible that sRAGE consumption allows excessive activation of multiple signal transduction pathways, such as the mitogen activated protein kinase family (p38, Erk1/2, jnk) or Rho GTPases (cdc42, rac), leading to tissue damage and multiple organ failure in fetuses with a robust inflammatory response to infection.61,62
A large number of spliced RAGE mRNA variants have been described for the human RAGE gene (AGER).63 The soluble form of RAGE (sRAGE) that potentially counteracts advanced glycation end-products and other RAGE ligands, such as HMGB1 and S100β, consists of several forms, including endogenous secretory RAGE (esRAGE; a C-terminally truncated splice variant of RAGE), as well as cleaved-type forms generated by shedding of the extracellular domain of RAGE. The stimulus for sRAGE shedding seems to involve HMGB1 binding to RAGE and the sheddase ADAM10.64 Given the complex transcriptional and post-transcriptional regulation of RAGE, future studies are needed to identify the relationships between inflammation and RAGE variants, including esRAGE in fetal tissues and fetal circulation. In this study we assessed the total sRAGE, which includes the sum of esRAGE and that of the proteolytically derived forms. Prior studies established a decrease in the systemic levels of total sRAGE in several clinical conditions with an inflammatory component such as coronary artery disease, diabetes, and rheumatoid arthritis.65,66,67 The current results expand this observation to the human fetus.
A significant number of human fetuses in this study were delivered shortly after a diagnosis of intra-amniotic infection and/or inflammation was established. Given that HMGB1 and S100β are chronic mediators of inflammation, our finding that their levels were not significantly elevated in the setting of “severe” intra-amniotic inflammation may imply that removal of the fetus from the hostile environment occurred before the onset of irreversible fetal damage. A bell-shaped response curve that reflects chronicity should be also considered in future studies. Moreover, we observed a significant direct correlation between the fetal circulatory levels of an acute (IL-6) versus a chronic (HMGB1) cytokine. This relationship, however, was not as strong as that of the two late phase cytokines (HMGB1 and S100β), implying that the two systems vary independently to a certain extent. It is known that among acute phase cytokines, IL-6 mediates the transition from the acute to chronic inflammation by promoting monocyte and macrophage chemoattraction and activation while concomitantly inhibiting neutrophil activity.68,69 However, owing to its ability to engage RAGE, HMGB1 is not the initiator of an acute inflammatory course, but rather the perpetuator of a chronic process leading to cellular dysfunction and tissue destruction.14,29 This premise has been extensively examined in humans and murine models of disorders with chronic components such as diabetes, inflammatory bowel and coronary artery disease and arthritis.70,71,72 Our findings may provide an explanation for the clinical observation that neonatal complications such as necrotizing enterocolitis, subependymal and intraventricular hemorrhage, or cerebral cavitation with subsequent development of porencephaly manifest clinically only days to weeks after birth.73 This suggests that the process leading to neonatal injury although initiated in utero, has an important chronic component that continues to evolves postnatally and may also explain why, at birth, levels of acute phase cytokines in either amniotic fluid or cord blood are not always predictive of neonatal outcome.6,8,59 By study design, we limited our analysis in preterm newborns to the timing of birth since a large number of neonates with postnatal complications would have been required to test the possible involvement of IL-6 in promoting fetal cellular damage and oxidative stress via release of HMGB1. We propose that following birth, the initial acute insult spirals into a self-perpetuated chronic inflammatory state leading to tissue damage. Further studies are needed to confirm this hypothesis. If true, in the postnatal period, sustained high levels of chronic mediators of inflammation such as HMGB1 may be better markers of outcome than IL-6.
Innovative methods of neuro-imaging have identified that preterm birth perturbs the program of corticogenesis in the developing brain.74 The recognition that regions of the brain have diverse susceptibility to injury at various degrees of maturation has stimulated investigators to identify the cells within the fetal nervous system that are selectively vulnerable to antenatal insult and biomarker proteins predictive of brain damage.9,75,76 One such biomarker is S100β.77,78 We found that in human preterm fetuses, HMGB1 is a very strong predictor of systemic S100β levels, independently of gestational age, birth weight, or cord blood IL-6 levels. This finding is consistent with the intracellular location of these two DAMP molecules and absence of the classical NF-κB and AP1 response elements on the S100β promoter.79
Yet, correlation does not imply causation. Thus, experiments targeted at unraveling some of the complex interrelationships responsible for our end results in humans were required in an animal model of inflammation induced preterm birth, oxidative stress, and fetal damage.35 We first compared RAGE, HMGB1, and S100β immunostaining in the normal fetal mouse body. We determined that at E16, RAGE, HMGB1, and S100β are expressed in a variety of fetal vital organs, but out of the three inflammatory mediators, RAGE was most uniformly expressed throughout the body. Unlike RAGE, which localized primarily on the cellular surface, HMGB1 had a primarily intranuclear location. This observation is consistent with its role as a nonhistone DNA-binding protein that functions in nucleosome stabilization and activation of the transcriptional machinery.16 Using an antibody targeting the C-terminus of S100β, we found that this isoform is present in several structures of the brain with important roles in controlling autonomic (pons, medulla oblongata) or motor functions (cerebellum, anterior horn of the medulla). However, in comparison with both RAGE and HMGB1, S100β was much more sparsely expressed in the central nervous system at this stage of development. Interestingly, high levels of S100β expression were identified in the villous epithelium of the bowel and chondrocytes of unossified bones. This is consistent with other previous reports, which suggest that although S100β is considered a brain-specific DAMP with roles in neuro-inflammation and postinjury brain tissue repair, it can be also expressed in the kidney, bone, skin, and muscle.80 Together, our results suggest that the instigators of the mechanisms exaggerating the host response to infection leading to chronic tissue destruction are expressed in multiple fetal organs. Their potential for chronic tissue destruction when released in excess in the extracellular space, as noted for HMGB1 in mouse fetuses exposed to inflammation, may explain the large spectrum of complications associated with prematurity in the immediate postnatal period.29
Finally, as reflected in the differences in HSCOREs, exposure of the fetus to inflammation and tissue infiltration with ED1+ monocyte/macrophages was accompanied by a selective increase in staining intensity of RAGE- and HMGB1-positive brain, cerebellum, choroids plexus, and liver cells. Our anti-RAGE polyclonal antibody captured both RAGE and sRAGE. Interestingly, we observed an increase in the cytoplasmic level of RAGE staining in the brain and liver of fetuses exposed to inflammation in utero. This suggests that inflammation is either a transcription inducer for esRAGE or a stimulus for increased shedding of the extracellular domain of RAGE, or both. The choroid plexus is involved in the clearance and metabolism of sRAGE-DAMP complexes in the brain.81 Hence, an alternative explanation for the increased intracellular staining for RAGE is that both hepatocytes and cells of the choroid plexus sequester sRAGE from blood and cerebrospinal fluid, respectively. This may explain our finding that fetuses with a high level of inflammation have lower sRAGE levels. Further studies are needed to confirm this premise.
Under normal conditions, HMGB1 is mostly a nuclear protein.82 Yet, our data confirm that in the context of cellular stress and necrosis induced by inflammation, HMGB1 is released in the surrounding tissue of the fetal brain and liver, as demonstrated by marked differences in the cellular and nuclear HSCOREs. It is known that in response to cellular stress, S100β is released into the extracellular space where, similarly to HMGB1, it exhibits cytokine-like functions via RAGE-mediated NF-κB transactivation.83 However, in our experimental mouse model, we were unable to observe changes in expression or location of the S100β isoform in response to LPS. This may be explained by the low level of basal S100β expression observed in the mouse brain and liver at this stage of gestation.84,85 Previous studies have shown that expression of both RAGE and S100β is developmentally regulated with a marked increase in expression in the immediate postnatal period.53,81,85 Thus, our results cannot rule out an effect of S100β in mediating brain damage and/or repair following delivery.
RAGE, HMGB1 and the macrophage antigen ED1+ were co-expressed at sites with obvious tissue injury, such as brain cavitation and subarachnoidal hemorrhage. This provides evidence for the proposal that HMGB1 and components of the RAGE system play a key role in the pathophysiology of periventricular leukomalacia and intraventricular hemorrhage. Using the same animal model, we previously demonstrated that fetuses exposed to inflammation in utero have increased liver glutathione consumption, which is consistent with an increase state of antenatal fetal redox imbalance.35 The results of this study suggest that, along with oxidative stress and inflammation, HMGB1 and RAGE can act as mediators of hepatotoxicity. We further determined that nucleated cells of erythroid lineage (TER119+) are present in the liver of fetuses exposed to LPS in utero. This finding is consistent with our previous study in humans, which demonstrated that inflammation represents an important stimulus for nucleated red blood cell production or release in the absence of hypoxia.86
Thus, from our previously published data, coupled with the results presented here, we conclude that the process of fetal inflammation comprises a variety of acute and chronic pathological processes that collectively induce an imbalance in tissue homeostasis characterized by extracellular release of DAMPs and tissue damage. The current study puts forth the hypothesis that RAGE and HMGB1 are active participants in mediating the process of fetal and neonatal tissue injury that may lead to multiple organ dysfunction. There is substantial evidence that the process of fetal tissue destruction, in cases complicated by intra-amniotic inflammation, is initiated in utero. Given the ability of RAGE and HMGB1 to promote chronic, rather than acute cellular stress, our data highlight that the consequences of the fetal inflammatory response to infection may extend well beyond the antenatal period. The potential to refine therapeutic compounds to target DAMPs and RAGE to prevent fetal and neonatal damage is exciting and such studies should be pursued.
Acknowledgments
We are indebted to the nurses, fellows, and residents at Yale New Haven Hospital, Department of Obstetrics and Gynecology and Reproductive Sciences, and to all patients who participated in the study.
Footnotes
Address reprint requests to Dr. Catalin S. Buhimschi, Director, Perinatal Research, Yale University, Department of Obstetrics, Gynecology & Reprod. Sci., 333 Cedar Street, LLCI 804, New Haven, CT 06520. E-mail: catalin.buhimschi@yale.edu.
Supported by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD) Grant RO3 HD 50249 (C.S.B.) and the Yale WRHR Career Development Center (K12 HD 1027766) (C.S.B.); and NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grant RO1 HD 047321 (I.A.B.), and March of Dimes Basil O’Connor Award (I.A.B). The funding source had no involvement in study design, interpretation of data, writing of the report or decision to submit the paper for publication.
Contributions to authorship: C.S.B. and I.A.B. designed the study, collected, analyzed, and interpreted the human and animal data and drafted the manuscript. I.A.B., C.S.B., and C.P.W. designed, developed, and performed the animal experiments. V.B. and R.A.E. collected, analyzed, and interpreted the human neonatal data. M.A.B., A.T.D., E.A.O., and G.Z. conducted the enzyme-linked immunosorbent assays and collected part of the proteomics data. G.Z. also performed the immunohistochemistry experiments. C.S.B., A.T.D., and S.L. recruited patients, collected biological specimens prospectively, and reviewed the data analysis. J.A.M. collaborated with C.S.B. and I.A.B. in systematically examining, quantifying, and interpreting the animal immunohistochemistry data.
References
- Behrman RE, Butler AS, editors. Washington D.C.,: The National Academies Press,; Institute of Medicine Committee on Understanding Premature Birth and Assuring Health Outcomes Board on Health Sciences outcomes: Preterm Birth. Causes, Consequences, and Prevention. Institute of Medicine of the National Academies. 2007 [PubMed] [Google Scholar]
- Allen MC. Neurodevelopmental outcomes of preterm infants: Curr Opin Neurol. 2008;21:123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol. 2006;30:219–226. doi: 10.1053/j.semperi.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Robertson CM, Watt MJ, Yasui Y. Changes in the prevalence of cerebral palsy for children born very prematurely within a population-based program over 30 years. JAMA. 2007;297:2733–2740. doi: 10.1001/jama.297.24.2733. [DOI] [PubMed] [Google Scholar]
- Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. National Institute of Child Health and Human Development Neonatal Research Network. Intensive care for extreme prematurity–moving beyond gestational age. N Engl J Med. 2008;358:1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet LM, Smith GN. Cerebral palsy and chorioamnionitis: the inflammatory cytokine link. Obstet Gynecol Surv. 2001;56:433–436. doi: 10.1097/00006254-200107000-00023. [DOI] [PubMed] [Google Scholar]
- Buhimschi CS, Dulay AT, Abdel-Razeq S, Zhao G, Lee S, Hodgson EJ, Bhandari V, Buhimschi IA. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG. 2009;116:257–267. doi: 10.1111/j.1471-0528.2008.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, Leviton A, Gappa M, Dammann CE. Lung and brain damage in preterm newborns, and their association with gestational age, prematurity subgroup, infection/inflammation and long term outcome. BJOG. 2005;112 Suppl 1:4–9. doi: 10.1111/j.1471-0528.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, Laptook A, Walsh M, Oh W, Hale E. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24:635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- Eschenbach DA. Amniotic fluid infection and cerebral palsy. Focus on the fetus. JAMA. 1997;278:247–248. [PubMed] [Google Scholar]
- Haynes RL, Baud O, Li J, Kinney HC, Volpe JJ, Folkerth DR. Oxidative and nitrative injury in periventricular leukomalacia. Brain Pathol. 2005;15:225–233. doi: 10.1111/j.1750-3639.2005.tb00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tör M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs PAM: Ps and alarmins: all we need to know about danger. J Leukoc Biol. 2007;8:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Yang H, Tracey KJ. High mobility group box 1 (HMGB1). Crit Care Med. 2005;33:S472–S474. doi: 10.1097/01.ccm.0000187005.81616.a9. [DOI] [PubMed] [Google Scholar]
- Rauvala H, Rouhiainen A. RAGE as a receptor of HMGB1 (Amphoterin): roles in health and disease. Curr Mol Med. 2007;7:725–734. doi: 10.2174/156652407783220750. [DOI] [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Przysiecki C, Shaw A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712. [PMC free article] [PubMed] [Google Scholar]
- Zen K, Chen CX, Chen YT, Wilton R, Liu Y. Receptor for advanced glycation endproducts mediates neutrophil migration across intestinal epithelium. J Immunol. 2007;178:2483–2490. doi: 10.4049/jimmunol.178.4.2483. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Müller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 1998;44:1147–1157. [PubMed] [Google Scholar]
- Butscheid M, Hauptvogel P, Fritz P, Klotz U, Alscher DM. Hepatic expression of galectin-3 and receptor for advanced glycation end products in patients with liver disease. J Clin Pathol. 2007;60:415–418. doi: 10.1136/jcp.2005.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Xiao W, Zhang H, Lotze MT, Wang H, Xiao X: Quercetin prevents lipopolysaccharide-induced HMGB1 release and proinflammatory function. Am J Respir Cell Mol Biol 2009. doi:10.1165/rcmb.2008.0119OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ochani M, Li J, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tasaka S, Shiraishi Y, Fukunaga K, Yamada W, Seki H, Ogawa Y, Miyamoto K, Nakano Y, Hasegawa N, Miyasho T, Maruyama I, Ishizaka A. Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am J Respir Crit Care Med. 2008;178:356–362. doi: 10.1164/rccm.200707-1069OC. [DOI] [PubMed] [Google Scholar]
- Lutterloh EC, Opal SM, Pittman DD, Keith JC, Jr, Tan XY, Clancy BM, Palmer H, Milarski K, Sun Y, Palardy JE, Parejo NA, Kessimian N. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit Care. 2007;11:R122. doi: 10.1186/cc6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekong U, Zeng S, Dun H, Feirt N, Guo J, Ippagunta N, Guarrera JV, Lu Y, Weinberg A, Qu W, Ramasamy R, Schmidt AM, Emond JC. Blockade of the receptor for advanced glycation end products attenuates acetaminophen-induced hepatotoxicity in mice. J Gastroenterol Hepatol. 2006;21:682–688. doi: 10.1111/j.1440-1746.2006.04225.x. [DOI] [PubMed] [Google Scholar]
- Zeng S, Feirt N, Goldstein M, Guarrera J, Ippagunta N, Ekong U, Dun H, Lu Y, Qu W, Schmidt AM, Emond JC. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology. 2004;39:422–432. doi: 10.1002/hep.20045. [DOI] [PubMed] [Google Scholar]
- Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002;54:1615–1625. doi: 10.1016/s0169-409x(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi IA, Zhao G, Pettker CM, Bahtiyar MO, Magloire LK, Thung S, Fairchild T, Buhimschi CS. The receptor for advanced glycation end products (RAGE) system in women with intra-amniotic infection and inflammation. Am J Obstet Gynecol. 2007;196:181.e1–181.e13. doi: 10.1016/j.ajog.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG. 2005;112:173–181. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- Bopp C, Hofer S, Weitz J, Bierhaus A, Nawroth PP, Martin E, Büchler MW, Weigand MA. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res. 2008;147:79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Azpurua H, Dulay AT, Buhimschi IA, Bahtiyar MO, Funai E, Abdel-Razeq SS, Luo G, Bhandari V, Copel JA, Buhimschi CS. Fetal artery impedance as assessed by Doppler ultrasound in pregnancies complicated by intra-amniotic inflammation and preterm birth. Am J Obstet Gynecol. 2009;200:203.e1–203.e11. doi: 10.1016/j.ajog.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003;188:203–208. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- Hadlock FP, Deter RL, Harrist RB, Park SK. Computer assisted analysis of fetal age in the third trimester using multiple fetal growth parameters. J Clin Ultrasound. 1983;11:313–316. doi: 10.1002/jcu.1870110605. [DOI] [PubMed] [Google Scholar]
- ACOG Committee on Practice Bulletins-Obstetrics ACOG Practice Bulletin No. 80: premature rupture of membranes. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007;109:1007–1019. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK, Pettker CM, Magloire L, Funai E, Norwitz ER, Paidas M, Copel JA, Weiner CP, Lockwood CJ, Buhimschi IA. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeye RL. Disorders of the placenta and decidua. Naeye RL, editor. St. Louis: Mosby,; Disorder of the Placenta, Fetus and NeonateDiagnosis and Clinical Significance. 1992:pp. 118–247. [Google Scholar]
- Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73:383–389. [PubMed] [Google Scholar]
- Ghidini A, Salafia CM, Kirn V, Doria V, Spong CY. Biophysical profile in predicting acute ascending infection in preterm rupture of membranes before 32 weeks. Obstet Gynecol. 2000;96:201–206. doi: 10.1016/s0029-7844(00)00908-x. [DOI] [PubMed] [Google Scholar]
- Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121:129–134. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- Rodwell RL, Taylor KM, Tudehope DI, Gray PH. Hematologic scoring system in early diagnosis of sepsis in neutropenic newborns. Pediatr Infect Dis J. 1993;12:372–376. doi: 10.1097/00006454-199305000-00004. [DOI] [PubMed] [Google Scholar]
- Smulian JC, Bhandari V, Campbell WA, Rodis JF, Vintzileos AM. Value of umbilical artery and vein levels of interleukin-6 and soluble intracellular adhesion molecule-1 as predictors of neonatal hematologic indices and suspected early sepsis. J Matern Fetal Med. 1997;6:254–259. doi: 10.1002/(SICI)1520-6661(199709/10)6:5<254::AID-MFM2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JG, Döpp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994;83:140–147. [PMC free article] [PubMed] [Google Scholar]
- Ramprasad MP, Fischer W, Witztum JL, Sambrano GR, Quehenberger O, Steinberg D. The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc Natl Acad Sci USA. 1995;92:9580–9584. doi: 10.1073/pnas.92.21.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- McCarthy KS, Miller LS, Cox EB, Konrath J, McCarthy KS., Sr Estrogen receptor analysis: correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- Zammit C, Barnard R, Gomm J, Coope R, Shousha S, Coombes C, Johnston C. Altered intracellular localization of fibroblast growth factor receptor 3 in human breast cancer. J Pathol. 2001;194:27–34. doi: 10.1002/path.846. [DOI] [PubMed] [Google Scholar]
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HM, Bergner AJ, Müller T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol. 2003;456:1–11. doi: 10.1002/cne.10448. [DOI] [PubMed] [Google Scholar]
- Chou DK, Zhang J, Smith FI, McCaffery P, Jungalwala FB. Developmental expression of receptor for advanced glycation end products (RAGE), amphoterin and sulfoglucuronyl (HNK-1) carbohydrate in mouse cerebellum and their role in neurite outgrowth and cell migration. J Neurochem. 2004;90:1389–1401. doi: 10.1111/j.1471-4159.2004.02609.x. [DOI] [PubMed] [Google Scholar]
- Leviton A. Preterm birth and cerebral palsy: is tumor necrosis factor the missing link? Dev Med Child Neurol. 1993;35:553–558. doi: 10.1111/j.1469-8749.1993.tb11688.x. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Montag AG, MacMillan W, Janeczek S, Pryde PG, Besinger RE, Gianopoulos JG, Roizen N. Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am J Obstet Gynecol. 2003;188:1438–1446. doi: 10.1067/mob.2003.380. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr. 2000;12:99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]
- Kantar M, Kültürsay N, Kütükçüler N, Akisü M, Cetingül N, Caglayan S. Plasma concentrations of granulocyte-macrophage colony-stimulating factor and interleukin-6 in septic and healthy preterms. Eur J Pediatr. 2000;159:156–167. doi: 10.1007/s004310050041. [DOI] [PubMed] [Google Scholar]
- Romagnoli C, Frezza S, Cingolani A, De Luca A, Puopolo M, De Carolis MP, Vento G, Antinori A, Tortorolo G. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr. 2001;160:345–350. doi: 10.1007/pl00008445. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Cliver SP, Biasini F, Peralta-Carcelen AM, Rector R, Alriksson-Schmidt AI, Faye-Petersen O, Carlo W, Goldenberg R, Hauth JC. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008;198:466.e1–466.e11. doi: 10.1016/j.ajog.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signaling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274:19919–19924. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22:1572–1580. doi: 10.1096/fj.07-9909com. [DOI] [PubMed] [Google Scholar]
- Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- Falcone C, Emanuele E, D'Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–1037. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- Devangelio E, Santilli F, Formoso G, Ferroni P, Bucciarelli L, Michetti N, Clissa C, Ciabattoni G, Consoli A, Davì G. Soluble RAGE in type 2 diabetes: association with oxidative stress. Free Radic Biol Med. 2007;43:511–518. doi: 10.1016/j.freeradbiomed.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Pullerits R, Bokarewa M, Dahlberg L, Tarkowski A. Decreased levels of soluble receptor for advanced glycation end products in patients with rheumatoid arthritis indicating deficient inflammatory control. Arthritis Res Ther. 2005;7:R817–R824. doi: 10.1186/ar1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Yen CF, Basar M, Kayisli UA, Martel M, Buhimschi I, Buhimschi C, Huang SJ, Krikun G, Schatz F. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008;172:1571–1579. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang H, Schmidt AM, Zhang C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2008;295:H491–H498. doi: 10.1152/ajpheart.00464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (ENRAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Katagiri Y, Kiire A, Momohara S, Kamatani N. Serum amyloid A activates nuclear factor-kappaB in rheumatoid synovial fibroblasts through binding to receptor of advanced glycation end-products. J Rheumatol. 2008;35:752–756. [PubMed] [Google Scholar]
- Bejar RF, Vaucher YE, Benirschke K, Berry CC. Postnatal white matter necrosis in preterm infants. J Perinatol. 1992;12:3–8. [PubMed] [Google Scholar]
- Ment LR, Vohr BR. Preterm birth and the developing brain. Lancet Neurol. 2008;7:378–379. doi: 10.1016/S1474-4422(08)70073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Fagel DM, Ganat Y, Maragnoli ME, Ment LR, Ohkubo Y, Schwartz ML, Silbereis J, Smith KM. Astroglial cells in development, regeneration, and repair. Neuroscientist. 2007;13:173–185. doi: 10.1177/1073858406298336. [DOI] [PubMed] [Google Scholar]
- Li Q, Michaud M, Stewart W, Schwartz M, Madri JA. Modeling the neurovascular niche: murine strain differences mimic the range of responses to chronic hypoxia in the premature newborn. J Neurosci Res. 2008;86:1227–1242. doi: 10.1002/jnr.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti F, Gazzolo D. S100B testing in pregnancy. Clin Chim Acta. 2003;335:1–7. doi: 10.1016/s0009-8981(03)00243-2. [DOI] [PubMed] [Google Scholar]
- Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim Biophys Acta. 2008;PMID:19110011. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Allore RJ, Friend WC, O'Hanlon D, Neilson KM, Baumal R, Dunn RJ, Marks A. Cloning and expression of the human S100 beta gene. J Biol Chem. 1990;265:15537–15543. [PubMed] [Google Scholar]
- Van Eldik LJ, Wainwright MS. The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restor Neurol Neurosci. 2003;21:97–108. [PubMed] [Google Scholar]
- Crossgrove JS, Li GJ, Zheng W. The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp Biol Med (Maywood) 2005;230:771–776. doi: 10.1177/153537020523001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Ponath G, Schettler C, Kaestner F, Voigt B, Wentker D, Arolt V, Rothermundt M. Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol. 2007;184:214–222. doi: 10.1016/j.jneuroim.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Stewart JA, Urban MI. The postnatal accumulation of S-100 protein in mouse central nervous system. Modulation of protein synthesis and degradation Dev Biol. 1972;29:372–384. doi: 10.1016/0012-1606(72)90078-4. [DOI] [PubMed] [Google Scholar]
- Hachem S, Laurenson AS, Hugnot JP, Legraverend C. Expression of S100B during embryonic development of the mouse cerebellum. BMC Dev Biol. 2007;7:17–31. doi: 10.1186/1471-213X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulay AT, Buhimschi IA, Zhao G, Luo G, Abdel-Razeq S, Cackovic M, Rosenberg VA, Pettker CM, Thung SF, Bahtiyar MO, Bhandari V, Buhimschi CS. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008;198:426.e1–426.e9. doi: 10.1016/j.ajog.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]