Abstract
Serotonin (5HT) receptor signaling and 5HT-related agents, such as the anorexogen fenfluramine (Fen), have been associated with heart valve disease. We investigated the hypothesis that Fen may disrupt mitral valve interstitial cell (MVIC) homeostasis through its effects on mitogenesis and extracellular matrix biosynthesis. Normal and myxomatous mitral valves, both human and canine, were harvested, and primary MVIC cultures were established. 5HT caused increased phosphorylation of extracellular signal-related kinase in MVIC; Fen alone did not. However, Fen combined with 5HT increased the level of MVIC extracellular signal-related kinase, when compared with 5HT alone. In addition, MVIC mitogenesis per 3H-thymidine (3HTdR) demonstrated a 5HT dose-dependent increase, with no effect of Fen alone. In contrast, Fen combined with 5HT inhibited the MVIC 3HTdR response when compared with 5HT alone. Furthermore, fluoxetine, a 5HT transporter inhibitor, while having no effect alone, suppressed Fen-5HT 3HTdR inhibition when administered with Fen plus 5HT. Finally, MVIC incorporations of 3H-proline and 3H-glucosamine, measures of extracellular matrix collagen and glycosaminoglycan respectively, were increased with 5HT alone; however, Fen did not affect MVIC glycosaminoglycan or collagen either alone or in combination with 5HT. Taken together, the ratios of 3H-proline or 3H-glycosaminoglycan to 3HTdR in MVIC, normalized to 5HT alone, demonstrated a significant imbalance of extracellular matrix production versus proliferation in MVIC cultures with Fen plus 5HT exposure. This imbalance may explain in part the pathophysiology of Fen-related mitral valve disease.
Serotonin (5HT) is a neurotransmitter that has been demonstrated to be associated with heart valve disease in both clinical settings1,2,3,4,5,6,7,8 and in experimental animals.9,10,11,12 5HT-associated heart valve disease, affecting primarily the right-sided heart valves, was first noted with carcinoid tumors,6 which are chromaffin cell malignancies that affect the small intestine and produce serotonin and other catecholamines. Dopamine agonist administration has also been shown to be associated in rare cases with heart valve disease affecting either the mitral or aortic valves.7,8 5HT administration to mice9 and rats10,11 results in progressive heart valve disease, and transgenic mice that have the 5HT transporter (5HTT) gene deleted, resulting in delayed processing of 5HT, also develop heart valve disease that affects predominantly the mitral and aortic valves.12 Interestingly, fenfluramine (Fen) has never been demonstrated to cause an experimental valvulopathy.
In the mid-1990s, heart valve disease was shown to be associated with the use of Fen as a diet drug.1,2,3,4,5 Fen has been reported to have 5HT receptor (5HTR) agonist activity in neuronal cells and 5HT-releasing activity from 5HTT.13 Fen-related heart valve disease was reported both with administration of Fen alone, or in combination with phentermine (Phen), a monamine oxidase inhibitor that was co-administered to sustain Fen’s effects.1,2,3,4,5 Fen was withdrawn from human use by the U.S. Food and Drug Administration in 1997.14 The pathogenesis of Fen-induced heart valve disease is still incompletely understood. However, since Fen affects 5HT mechanisms and the pathology of the Fen valve lesions in some, but not all of the published cases4,5 resembled the carcinoid syndrome valvulopathy,4,5 it has been strongly suggested that a 5HT mechanism may be involved.1,2,3,4,5 Prior studies15,16,17,18,19,20 explored the pathogenesis of Fen-associated heart valve disease, using a variety of model systems, and in general concluded that Fen was likely acting as a 5HTR agonist. Since cardiac valve anatomy, physiology, and pathophysiology are unique for each of the different cardiac valves, we sought to focus the present investigations on the mitral valve. Mitral valves were also the most frequently affected in the Fen cases reported in both of the largest human pathology series.4,5
Thus, the present study examined the mitral valve interstitial cell (MVIC) response to 5HT and Fen, to investigate why Fen may have caused mitral valve disease. Our working hypothesis is that Fen may disrupt MVIC homeostasis through its effects on mitogenesis and associated extracellular matrix (ECM) biosynthetic activity via mechanisms involving 5HTR signal transduction and off-target effects. We investigated this hypothesis with cell culture studies using both human and canine MVIC assessing the effects of 5HT and Fen on canine and human MVIC with endpoints assessing signal transduction, mitogenesis, and ECM biosynthesis.
Materials and Methods
Reagents
Chemicals, including pharmaceuticals, were obtained from Sigma (St. Louis, MO) unless otherwise stated. Cell culture disposables were obtained from Corning Life Sciences (Lowell, MA) unless indicated otherwise.
Mitral Valves
Normal and diseased canine mitral valves were obtained at elective euthanasia (University of Pennsylvania School of Veterinary Medicine). All diseased canine mitral valves were from animals confirmed to have myxomatous mitral valve disease by echocardiograms. 10 normal canine mitral valves were obtained from seven female and three male animals, age range 2 to 17 years. Similarly, nine myxomatous mitral valves were obtained from four male and five female animals, age range 9 to 19 years. Diseased human myxomatous mitral valves were obtained at cardiac surgery (under an Institutional Review Board-approved protocol; University of Pennsylvania School of Medicine); 16 female (age range 51 to 83 years) and 27 male (age range 23 to 82 years) were assessed. Five normal human mitral valves (age range 43 to 54 years, four male, one female) were obtained from explanted hearts at the time of cardiac transplantation, as exempted by the Institutional Review Board. Whenever possible, valvular samples were divided for both fixation in 10% formalin for histochemistry and for cell culture as described below. Additional normal human mitral valves, three male and three female (age range 64 to 76 years) were obtained from autopsy specimens (under an approved Institutional Review Board exemption; Department of Pathology, University of Pennsylvania School of Medicine) for fixation and histochemistry. Formalin fixed specimens were paraffin embedded and sectioned according to standard procedures. Movat’s pentachrome staining for assessment of the ECM was performed as previously described.21 Immunostaining for human α-smooth muscle actin and human vimentin were each performed using standard peroxidase methodology, following heated citrate buffer antigen recovery with diaminobenzidine as a final chromogen, using anti-α-smooth muscle actin (M0851; DAKO, Carpinteria, CA) and anti-vimentin (M7020, DAKO) respectively as primary antibodies. These same human specific antibodies were used for both canine and human derived samples.
Cells and Cell Culture
Normal and diseased human and canine mitral valves were obtained as described above, and heart valve interstitial cells were characterized and cultured in M199 (Invitrogen, Carlsbad, CA)/10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA) as previously published.22 For most experimental protocols, cells were plated in M199/10% fetal bovine serum at 2 × 104 cells/cm2. Before exposure to pharmacological agents or to 5HT, quiescence was induced in all cultures by 48 hours exposure to M199/0.5% fetal bovine serum. This treatment was begun at plating for Western blots or proliferation (3H-thymidine, TdR, incorporation) studies (see below); induction of quiescence was delayed until confluency was reached for collagen synthesis (3H-proline incorporation) or glycosaminoglycan (GAG) synthesis (3H-GA incorporation) studies. All studies were performed in serum-free medium, and pharmacological agents were applied 30 minutes before any addition of 5HT. In experiments containing both fluoxetine (Flu) and Fen, cells were initially pretreated with Flu for 30 minutes, followed by addition of Fen for 30 minutes, before the addition of 5HT.
Western Blots
Five minutes after treatment with 5HT or other agents, cells were placed on ice, in the presence of both sodium orthovanadate and complete protease inhibitor (Boehringer-Mannheim, Mannheim, Germany) cocktail, scraped, concentrated, and lysed in a Triton-X114 buffer as previously described.22,23 Protein separation was performed by SDS-polyacrylamide gel electrophoresis under denaturing conditions, and blots were transferred to polyvinylidene difluoride membranes (Invitrogen, Carlsbad, CA). These were probed first with an antibody for human phosphorylated extracellular signal-related kinase (pERK1/2) (anti-phospo-p44/42 MAPK Cell Signaling Technologies, Danvers, MA), then stripped and re-probed with anti-ERK1/2 (ERK1/2; Santa Cruz Biotechnologies). Representative blots of at least triplicate studies are shown.
Radiometric Assays
Mitogenesis was assessed as follows: after treatment for 24 hours in 24-well plates, 1μCi/well 3HTdR (Perkin Elmer, Waltham, MA) was added for the final 6 hours of incubation. Cells were washed, treated with 6% trichloroacetic acid, and solubilized for scintillation counting. Collagen synthesis was estimated as follows: After treatment for 30 hours in 24-well plates, in the presence of 3μCi/well 3H-proline (Perkin Elmer), cells were washed, treated with 6% trichloroacetic acid, and solubilized for scintillation counting. For GAG synthesis, after treatment for 30 hours in 24-well plates, in the presence of 3μCi/well 3H-glucosamine HCl (American Radiolabeled Chemicals, St. Louis, MO), aliquots of conditioned medium were removed and 3H-labeled secreted GAG was precipitated with Alcian blue as previously described24 and the washed pellets solubilized for scintillation counting. Cells from the same cultures were washed, treated with 6% trichloroacetic acid, and solubilized for scintillation counting; these results closely paralleled those obtained from conditioned medium precipitates (data not shown). All radiometric experiments were run with either triplicate or quadruplicates of each treatment, and statistically analyzed individually as raw counts per minute; these data were subsequently compiled as averages within an experimental series. Each experimental series used two or more different cell lines. To compile averages within an experimental series, fold-changes compared with untreated controls within each experiment were used due to variation in raw baseline incorporation of all labels between cell lines.
Statistical Methods
Graphical results are presented as mean ± SEM Statistical analysis was performed using analysis of variance or non-parametric analysis of rank testing as needed, with appropriate subsequent post hoc analysis between groups.
Results
Human and Canine Mitral Valve Morphology and MVIC Heterogeneity
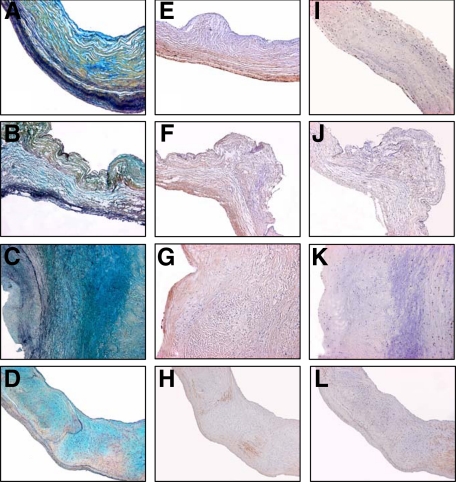
Movat’s staining of representative cross-sections of normal human and canine mitral valve specimens demonstrated comparable morphology in terms of the distribution of a number of ECM components including GAG, collagen, and elastic-laminae (Figure 1A and B). Furthermore, myxomatous mitral valves also demonstrated comparable pathological changes versus normal mitral valves in both human and canine specimens, including an increase in GAG and in a general a loss of organization of collagen and elastin components (Figure 1, C and D). Smooth muscle actin immunostaining of normal mitral valve tissue, including human and canine samples (Figure 1, E and F), demonstrated positive immunostaining of MVIC, chiefly localized near the ventricular surface of the leaflet cross-sections; in general the majority of MVIC were not immunopositive for smooth muscle actin. Myxomatous mitral valves, both human and canine, demonstrated occasional smooth muscle actin positive MVIC (Figure 1, G and H) with a loss of the ventricular orientation seen in normal mitral valves. Vimentin staining revealed sparse immunostaining for this cytoskeletal marker (Figure 1, I–L) that did not differ between human and canine leaflets regardless of disease status.
Figure 1.
Micrographs of canine and human mitral valves after Movat’s pentachrome (normal and myxomatous human, A and C; normal and myxomatous canine, B and D, respectively), α-smooth muscle actin (normal and myxomatous human, E and G; normal and myxomatous canine, F and H, respectively), and vimentin staining (normal and myxomatous human, I and K; normal and myxomatous canine J and L, respectively), demonstrating comparable organization and staining between human and canine normal, and human and canine myxomatous, valves. Normal valves demonstrate a well organized collagen structure (yellow) with interrelated glycosaminoglycans (GAG, stained blue) and elastin staining near the ventricular surface in A and B, whereas myxomatous (C and D) show increased GAG staining and disorganization of the collagen and elastin staining. Similarly, diseased valves from both species show disruption of the α-smooth muscle actin distribution (G and H) compared with normal valves (E and F). Vimentin expression is sparse in all examples, with no overt pattern differences. The magnification for all micrographs is ×50.
5HTR Signal Transduction in Human and Canine Mitral Valve Interstitial Cells
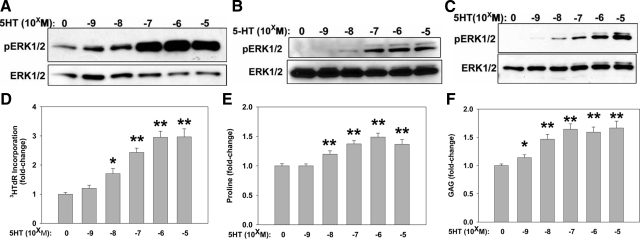
Since all of the 5HT receptors, except one, the type 3B receptor, which is an ion channel,25 use G-protein-coupled signal transduction mechanisms, we chose to monitor pERK1/2 per Western blot as a marker of 5HTR activity. 5HT increased pERK1/2 in a dose dependent manner in both human and canine myxomatous MVIC (Figure 2, A and B). In general, we found no discernable difference between normal and myxomatous MVIC in terms of the 5HT dose response (Figure 2C; normal canine MVIC). Canine MVIC were successfully cultured in 50% of cultures attempted (7 of 14), while only 17% of the human MVIC cultures proved useful for data acquisition (7 of 41), and these were only useable between passages 2 and 5 due to senescence with associated diminishing proliferation rates (data not shown). The canine MVIC, however, retained consistent growth characteristics between passages 2 and 10, and thus were used more extensively.
Figure 2.
MVIC culture data showing dose-dependent responsiveness to 5HT for ERK phosphorylation (pERK1/2) by Western blots (A–C), proliferation by tritiated thymidine (3HTdR) incorporation (D), collagen biosynthesis by tritiated proline incorporation (E), and glycosaminoglycan (GAG) biosynthesis by tritiated glucosamine incorporation (F). 5HT concentrations from 10−9 to 10−5M/L (indicated as −5 to −9) were studied. A–C: Representative Western blot results showing pERK1/2 with loading controls of total ERK1/2, from mitral valve interstitial cell (MVIC) cultures of human and canine mitral valve specimens.5HT concentrations from 10−9 to 10−5 M/L (indicated as −5 to −9) were studied. A: Human myxomatous MVIC results showing a 5HT dose-dependent increase in pERK1/2. B: Canine myxomatous MVIC data demonstrating a 5HT dose-dependent increase in pERK1/2. C: Normal canine MVIC results showing a comparable 5HT dose response for pERK1/2 to A and B. D: Canine MVIC showing 3H-thymidine (3H] TdR) incorporation results as an index of proliferation. E: Canine MVIC 3H-proline incorporation as an index of collagen biosynthesis. F: Canine MVIC 3H-glycosamine incorporation as an index of GAG biosynthesis. Data in D–F are shown as fold changes relative to controls without serotonin, and showed a significant dose-dependent increase in response to the addition of 5-HT alone. *P < 0.05, **P < 0.001 vs. 0 control.
5HT Has a Mitogenic Effect on MVIC, and Increases ECM Biosynthesis
Using 3HTdR as an endpoint, it was observed that 5HT administration to MVIC resulted in a dose-dependent increase in 3HTdR incorporation (Figure 2). In representative experiments for both human (data not shown) and canine (Figure 2D) cell lines, MVIC demonstrated overall a nearly three fold 5HT-mediated increase in 3HTdR incorporation. Increased collagen synthesis as monitored by 3H-proline uptake in vitro was noted with addition of 5HT to canine MVIC cultures (Figure 2E). Similarly, GAG biosynthesis was assessed with 3H-glucosamine incorporation in canine MVIC cultures (Figure 2F). 5HT addition alone resulted in increased 3H-glucosamine (Figure 2F); in the absence of any other additions, 5HT-mediated increases in these two ECM components were more modest (1.5-fold; Figure 2, E and F) than the increase observed in proliferation (Figure 2D).
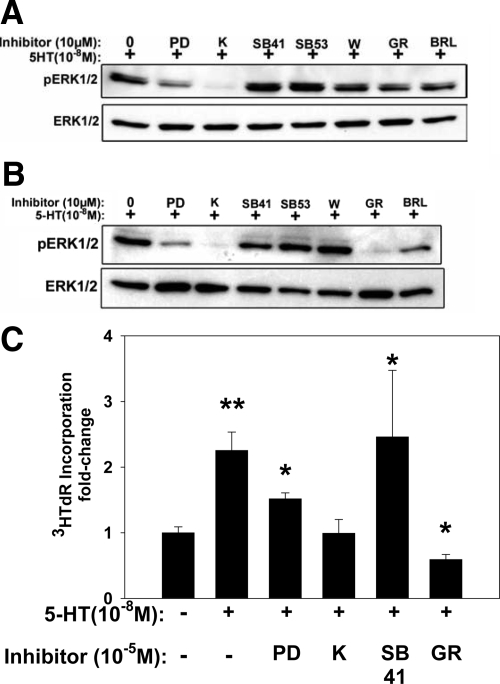
5HT Signals in MVIC through Specific 5HTR Subtypes
The 5HTR responsiveness of canine and human MVIC was studied with Western blots for pERK1/2 with commercially available pharmaceutical agents using either 5HTR-specific antagonists or a pERK1/2 inhibitor, PD98059. PD98059 resulted in decreased pERK1/2 in both human (Figure 3A) and canine MVIC (Figure 3B). Ketanserin, a 5HTR-2A antagonist, inhibited pERK1/2 in both human and canine MVIC, and was the only 5HTR antagonist studied that inhibited human MVIC pERK1/2. Canine MVIC also demonstrated diminished pERK1/2 with GR55562, a 5HTR-1B antagonist. Other agents studied, SB204741 (a 5HTR-2B antagonist), SB206553 (a 5HTR-2B/2C antagonist), and BRL15572 (a 5HTR-1D antagonist), had no effect on inhibiting pERK1/2 in either human or canine MVIC. Thus, these results, based on the use of pharmaceutical antagonists of 5HTR, indicate that in the MVIC studied, human MVIC appeared to have active 5HTR-2A and canine MVIC had both type 5HTR-2A and 1B receptors.
Figure 3.
The effects of inhibitors of 5HT receptors (5HTR) and a pERK1/2 inhibitor on MVIC cultures; data shown are pERK1/2 Westerns (A and B) and 3HTdR incorporation (C). All cultures were assessed with the addition of 10−8M/L 5HT. A: Human myxomatous MVIC results demonstrating that only K (see abbreviations below) inhibited pERK1/2. B: Canine myxomatous MVIC data showing that both K and GR inhibit 5HT induced pERK1/2. C: The 5HTR inhibitors K and GR inhibited the 5HT stimulation of 3[H]-Tdr, as did the pERK1/2 inhibitor, PD. *P < 0.05 vs. 0; **P < 0.001 vs. 0. Abbreviations - pERK1/2 inhibitor = PD98059 (PD); Receptor antagonists: 5HTR2A = Ketanserin (K); 5HTR2B = SB204741 (SB41); 5HTR2B & 5HTR2C = SB206553 (SB53); 5HTR1A = WAY100635 (W); 5HTR1B = GR55562 (GR); 5HTR1D = BRL15572 (BRL).
Interestingly, ketanserin, a 5HTR-2A receptor inhibitor, significantly reduced 3HTdR in canine MVIC (Figure 3C) as did GR55562, a type 1B inhibitor. Both ketanserin and GR55562 inhibited pERK1/2 in canine MVIC (see Figure 3B), thus indicating that in canine MVIC 5HT receptor types 1B and 2A may be involved in receptor signaling related to 5HT-induced mitogenesis. PD98059, an inhibitor of pERK1/2, also reduced 3HTdR incorporation indicating the critical importance of signal transduction in the MVIC 5HT mitogenic response.
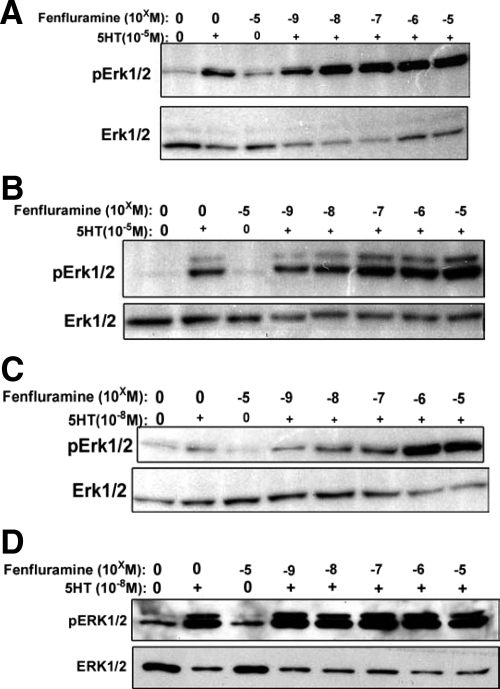
Fen Alone Has No Effect on MVIC pERK1/2, but Together with 5HT, Increases pERK1/2
Monitored using Western blots, even at the highest dosage used (10−5 M/L), Fen had minimal to no stimulatory effect on pERK1/2 in either human (Figure 4, A and C) or canine (Figure 4, B and D) MVIC. However, when either 10−5 M/L (Figure 4, A and B) or 10−8 M/L 5HT (Figure 4, C and D) was added to MVIC cultures together with Fen, a dose-dependent enhancement of the 5HT effect was observed in both human (Figure 4, A and C) and canine (Figure 4, B and D) MVIC cultures. Furthermore, the fact that Fen increases the 5HT effect suggests that Fen’s previously established 5HTT 5HT-releasing effects may be operative in MVIC and result in increased pERK1/2.
Figure 4.
The effects of fenfluramine (Fen) on 5HT stimulation of pERK1/2 in MVIC cultures; data shown are representative Western blots. Doses of Fen were 10−9 to 10−5 M/L, indicated as −5 to −9. A: Human myxomatous MVIC results demonstrating no effect of Fen alone, but when combined with 10−5M/L 5HT a dose-dependent increase in pERK1/2 was produced. B: Canine myxomatous MVIC results also showing a comparable effect to A, with Fen only increasing pERK1/2 when combined with 10−5M/L 5HT. C: Human myxomatous MVIC data using a lower dose of 5HT (10−8M/L) with comparable results to those seen in A, demonstrating that Fen only with 5HT demonstrates an increase in pERK1/2. D: Canine myxomatous MVIC, show that Fen alone has no effect, and combined with 10−8M/L 5HT gives results comparable with those in C.
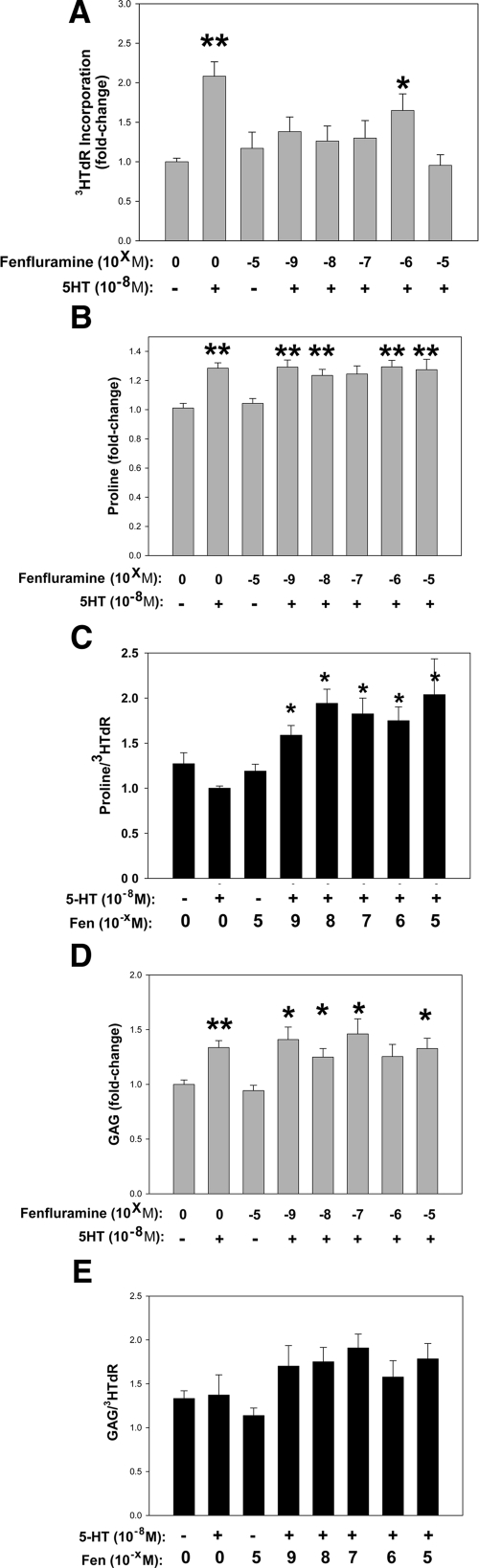
Fen Inhibits the 5HT-Associated Increase in MVIC 3HTdR, but Has No Effect on 5HT-Stimulated ECM Biosynthesis
In these experiments, an optimized dose of 5HT was used as a benchmark for mitogenesis per 3HTdR assays (per Figure 2D). In an escalating dosage study of Fen in both human (data not shown) and canine (Figure 5A) mitogenesis, MVIC did not show increased 3HTdR with increasing Fen, but instead showed that Fen significantly inhibited the 5HT induced mitogenic response. Thus, these data for human and canine MVIC demonstrated overall that while MVIC are significantly responsive to 5HT, Fen either alone or with 5HT, does not result in increased 3HTdR incorporation. Instead Fen alone has no effect on mitogenesis, but inhibits 5HT stimulation of MVIC 3H-Tdr incorporation.
Figure 5.
The effects of fenfluramine (Fen) on 5HT stimulation of proliferation (A), collagen biosynthesis (B), and GAG biosynthesis (D) in MVIC cultures; data shown are 3H incorporations as shown respectively in Figures 2D, E, and F above. Doses of Fen were 10−9 to 10−5 M/L, indicated as −5 to −9. Data in A, B, and D are shown as fold changes relative to controls without serotonin. C and D are ratios as described below. A: Fen reduces 5HT-mediated 3HTdR incorporation. B: Fen fails to inhibit 5HT-mediated 3H-proline incorporation C: The ratio of proline incorporation to 3HTdR incorporation shown as fold changes relative to serotonin, showing a net disruption of MVIC ECM biosynthesis by Fen, as collagen biosynthesis significantly exceeds the cellular proliferative index. D: Fen does not inhibit 5HT-mediated GAG biosynthesis. E: The ratio of GAG biosynthesis to 3HTdR incorporation, shown as fold changes relative to serotonin, showing a lesser trend in disruption of MVIC ECM biosynthesis. *P < 0.05, **P < 0.001 vs. control.
Increased collagen synthesis as monitored by 3H-proline uptake in vitro was noted with addition of 5HT to canine MVIC cultures (Figures 2E and 5B). However, Fen had no effect on 3H-proline incorporation in canine MVIC cultures, either alone or in combination with 5HT (Figure 5B). Similarly, GAG biosynthesis was assessed with 3H-glucosamine incorporation in canine MVIC cultures (Figures 2F and 5D). 5HT addition alone resulted in increased 3H-glucosamine, and the addition of Fen either alone or together with 5HT had no effect on 3H-glucosamine (Figure 5D). Ratios of both 3H-proline and 3H-GAG to 3HTdR (Figure 5, C and E, respectively) in MVIC were calculated and normalized to 5HT alone. These ratios demonstrated a significant imbalance of ECM production versus proliferation in MVIC cultures with Fen plus 5HT exposure as normalized to the 5HT response. Thus, these studies of two ECM markers, collagen and GAG, indicate that under the cell culture conditions studied, while 5HT stimulates proliferation, and collagen and GAG production, Fen does not significantly affect these ECM markers, while inhibiting 5HT stimulated mitogenesis, and thus a relative excess of ECM production versus proliferation may result from Fen exposure.
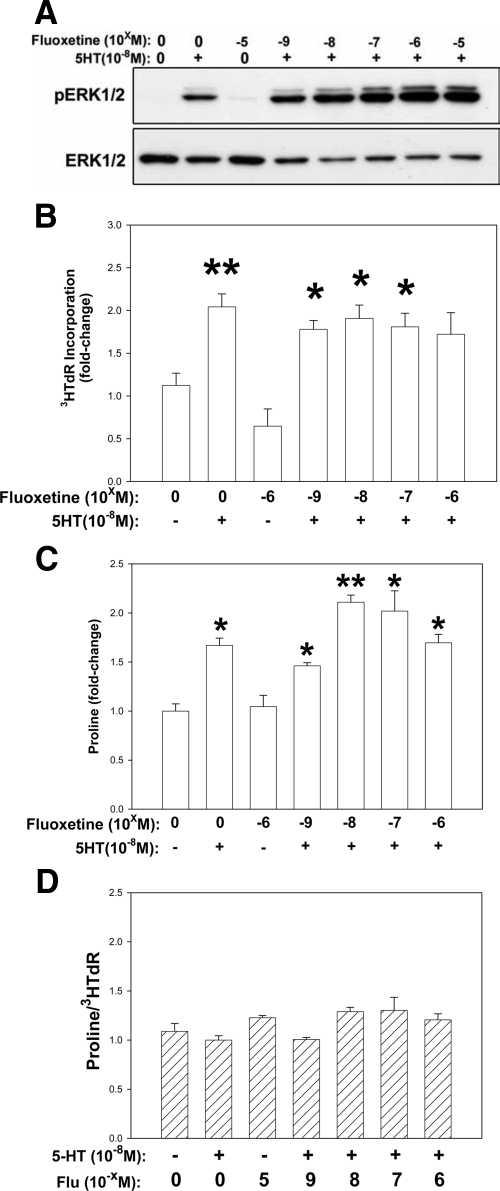
Fluoxetine Increases 5HT-Associated pERK1/2 in MVIC, but Has No Effect on ECM Biosynthesis or 3H-Tdr
Parallel studies to those described above were repeated with the substitution of the specific 5HTT inhibitor Flu for Fen. Similar results were obtained with Flu using Western blots to monitor pERK1/2 (Figure 6A), and radiometrics to monitor proline incorporation (Figure 6C) and glucosamine incorporation (data not shown), with the exception that doses of Flu higher than 10−6 M/L were cytotoxic (data not shown). Flu together with 5HT increased pERK1/2 signaling (Figure 6A), and had no effect on 5HT-mediated ECM biosynthesis (Figure 6C). However, unlike Fen, Flu had no effect on 5HT-mediated MVIC 3HTdR (Figure 6B). Ratios of both 3H-proline and 3H-GAG to 3HTdR (Figure 6D and data not shown) in MVIC were calculated and normalized to 5HT alone. These ratios demonstrated no net imbalance of ECM production versus proliferation in MVIC cultures with Flu plus 5HT exposure. Thus, these studies suggest that the disruption of MVIC homeostasis caused by Fen is not caused directly by increased 5HTR signaling, but rather an off-target effect.
Figure 6.
The effects of Fluoxetine (Flu) on 5HT-mediated ERK1/2 phosphorylation (A), proliferation (B), and collagen biosynthesis (C) in MVIC cultures; data shown are representative Western blot (A), and 3H incorporations as shown respectively in Figures 4D, 5A, and 5B above, with the substitution of Flu for Fen in experimental protocols. Doses of Flu were 10−9 to 10−5 M/L, indicated as −5 to −9. Data in B and C are shown as fold changes relative to controls without serotonin. Data in D are ratios as described below. A: Representative Western blot showing that like Fen in Figure 4 (above) only Flu plus 5HT demonstrates an increase in pERK1/2. B: 3H-TdR incorporation shows that unlike Fen (Figure 5A), Flu has no effect on 5HT-mediated 3HTdR incorporation. C: 3H-proline incorporation shows that Flu, like Fen, also does not inhibit 5HT-mediated 3H- proline incorporation or GAG biosynthesis (GAG data not shown). D: When ratios of 3H-proline incorporation to 3HTdR incorporations (B) are calculated, no disruption of MVIC ECM biosynthesis by Flu is seen. Data in D are shown as fold changes relative to serotonin. *P < 0.05, **P < 0.001 vs. control.
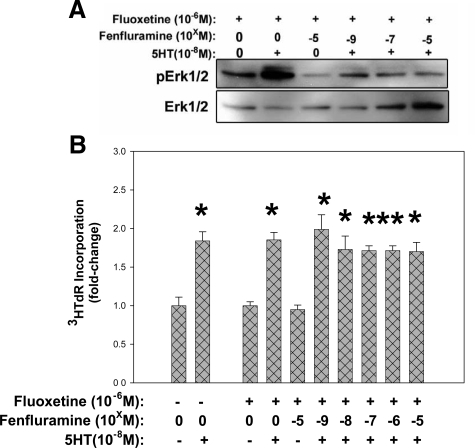
Flu-Mediated 5HTT Inhibition Prevents Fen Effects on 5HT-Stimulated pERK1/2 Signaling and Mitogenesis
Pretreatment of canine MVIC with Flu before Fen exposure and subsequent 5HT treatment prevents increased pERK1/2 signaling (Figure 7A) and decreased 3HTdR incorporation (Figure 7B) that occurs with Fen combined with 5HT (Figures 4 and 5A respectively). These results strongly suggest that Fen processing by 5HTT is required for its disruptive effect on 5HT stimulated mitogenesis and pERK1/2. Surprisingly, combining Fen with Flu also inhibits the pERK1/2 enhancement seen with 5HT plus either Flu or Fen (Figure 7A versus Figure 6A), suggesting the occurrence additional off-target effects of Fen and Flu unrelated to 5HTR signal transduction.
Figure 7.
Pre-exposure of MVIC cultures to Flu changes the effect of Fen on 5HT-mediated ERK1/2 phosphorylation and 3HTdR incorporation. A: Western blot of canine MVIC showing that the combination of Flu with Fen reduces pERK1/2 when combined with 10−8M/L 5HT. B: 3HTdR incorporation study with canine MVIC shows that after pretreatment with Flu, Fen fails to reduce 5HT-mediated 3HTdR incorporation, Data in (B) are shown as fold changes relative to controls without serotonin; *P < 0.05, **P < 0.001 vs. control.
Discussion
Fen-related valve disease remains an enigma that is likely related to other 5HT-associated valvulopathies. The results of the present studies provide some novel insights concerning the effects of Fen on MVIC that reveal an important perspective on the pathophysiology of Fen-related mitral valve disease. Overall Fen alone had no effect on any of the endpoints of interest, which included pERK1/2, 3HTdR, and ECM biosynthesis. However, Fen combined with 5HT had an overall disruptive effect versus 5HT alone, increasing pERK1/2, while diminishing 3H TdR, but with no effect on the ECM markers. 5HT’s role in the physiological function of MVIC is as yet unknown. The fact that MVIC have 5HT receptors and respond to 5HT per the endpoints studied in this paper strongly suggests that 5HT receptor signaling is likely an integral part of MVIC physiology. Thus, the present results demonstrating Fen causing a disruption of the 5HTR response is likely indicative of the pathophysiologic mechanisms related to Fen mitral valvulopathy.
Fen has been the subject of only one prior study concerning its effects on heart valve cells in culture.17 However, the effects of Fen on the central nervous system and neuronal-related cell cultures provide some insights concerning the present results. Nevertheless, the role of 5HT as a neurotransmitter in the nervous system is unique, and thus observations concerning neuronal results with Fen and Flu must also be viewed with a critical perspective when extended to other cell types. For example, Fen can deplete 5HT centrally in the nervous system.26,27 However, neuronal cells have a unique 5HT storage and reuptake capacity26,27,28,29 that is not present in fibroblasts and myofibroblasts, such as heart valve interstitial cells. In addition, studies of chronic Fen administration to rats demonstrate increased 5HT plasma levels,30 presumably due to the central depletion just mentioned, with lowering of blood levels of 5HT, likely due to impaired 5HT uptake by platelets, which normally store 5HT.31,32 However, human studies of Fen-treated patients demonstrated lowering of plasma 5HT levels.33 Furthermore, it is unknown to what extent plasma or blood 5HT levels may affect tissue levels of 5HT in the mitral valve interstitium, and only microdialysis studies, such as those performed in 5HT central nervous system experiments33 could help elucidate this. The present experiments focused on the rapid MVIC pERK1/2 response to 5HT, which was increased in combination with Fen. Our experiments used established pharmaceutical inhibitors of 5HTRs to elucidate the 5HTR-specific responsiveness of MVIC to 5HT. These compounds were all developed for human 5HTRs, and are not entirely 5HTR specific.34 Furthermore, 5HTR inhibitors for all of the receptors are not available. In addition, we used these compounds in primary cell cultures under serum-starved conditions. Thus, broad conclusions about the receptor profile of MVIC based on the present results are not possible. Despite this, our studies demonstrated strong 5HT responsiveness with dose-dependence of the pERK1/2 response in human and canine MVIC, with significant enhancement of 5HT effects due to Fen, which was ineffective alone. In contrast, Fen has been demonstrated to have specific 5HTR agonist effects in neuronal studies.26
Fen-Phen heart valve pathology has been reported in a limited number of papers. Only two of these reports4,5 included human pathology results on sizable clinical series of both affected mitral and aortic valves.4,5 In these papers4,5 it was demonstrated that in general both Fen-Fen and Fen alone were associated with heart valve disease, and that left-sided valves tended to be affected. However, the microscopic pathology was not entirely comparable with the carcinoid valvulopathy,4 and in particular the pathology of mitral valve disease in Fen-treated patients demonstrated fibrous plaques predominantly on the ventricular surface of the leaflets, with associated inflammatory infiltrates, which are atypical in carcinoid valve disease. In addition studies of Fen-valves with Ki-67 immunostaining as a proliferation marker4 demonstrated only occasional to absent staining, thus indicating that proliferative events were not prominent in Fen valve disease, despite its relatively rapid onset.4,5
Only a single prior publication17 reported Fen effects on human heart valve interstitial cells in culture. The results in this paper17 demonstrated that Fen alone caused an increase in pERK1/2, 3HTdR, and ECM biosynthesis, in contrast to the results of the present study. However, this previous investigation17 used mixed cardiac valve cell cultures, combining cells derived from pooling all four anatomical types of cardiac valves from hearts obtained at transplant, thus introducing the unknown impact of the heterogeneous nature of mixtures of these cell populations, thereby limiting the interpretation of the results.17 Furthermore, 5HT combined with Fen was not studied in this previous publication.17 The only other study16 to investigate Fen-mechanisms that used human cardiac valves, although not in culture, reported reverse transcription-PCR studies of both human and porcine cardiac valves, mitral and aortic, assessing RNA for the presence of 5HTR2 subtypes A, B, and C.
In addition, Fen has been shown to have 5HTR agonist effects in neural cells,26 and interferes with 5HTT, acting as a so-called 5HT-releasing agent.13,26 Fen has also been associated clinically with primary pulmonary hypertension,35 but paradoxically has been demonstrated to be effective for treating pulmonary hypertension in experimental animals.36,37 Thus, prior studies of Fen’s potential adverse effects on non-neuronal tissues are limited, and the previous conclusions of others that Fen-valvulopathy mechanisms were due to 5HTR agonist activity15,16,17 may not be applicable for MVIC, based on the results of the present studies.
Previous research by our group focused on 5HT’s effects on sheep aortic valve interstitial cells, which occurred via G-protein coupled receptors,22,23 and were comparable with the pERK1/2 results of the present studies. These earlier investigations22,23 also demonstrated that 5HT signaling in sheep aortic valve interstitial cells resulted in increased pERK1/2, and showed comparable changes to the present MVIC studies with increased collagen and GAG biosynthesis in response to 5HT administration. Fen was not investigated in these prior experiments. However, selective inhibitors of 5HTR subtypes used in our previous sheep aortic valve interstitial cell studies22,23 demonstrated a predominance of the type 2A receptor.
While it is clear that increased 5HTR signaling is associated with heart valve disease, the pathogenesis of valvulopathies due to agents that affect 5HT mechanisms needs to be completely elucidated. The present study provides novel insights concerning 5HT-related heart valve disease, and also reveals that prior conclusions concerning Fen may have been incomplete in their scope. Furthermore, 5HTT inhibitors, especially the selective 5HT reuptake inhibitors, such as fluoxetine, are widely used for treating depression, and their role over time concerning their potential effects on heart valve disease, either de novo or pre-existing, has been investigated to a very limited extent in a single cross-sectional study.38 In addition, a 5HTT polymorphism in the promoter region of human 5HTT has a Mendelian distribution in the general population.39,40 This 47 bp deletion has been associated with diminished 5HTT function. Thus, a patient on a 5HTT inhibitor who is also homozygous for the short form of the 5HTT polymorphism could be more susceptible to hypothetical valvular adverse effects of 5HTT inhibition due to Flu or related agents. Thus, further long term studies of the potential importance of 5HTT pharmacogenetics related to 5HT mechanisms and heart valve disease are warranted.
The present study has several limitations that very likely do not impact on the conclusions, but nevertheless will be addressed. We compared MVIC results for canine and human mitral valves, both normal and myxomatous. While others have noted comparisons between human and canine myxomatous mitral valve disease,41,42 we performed a limited series of microscopy studies documenting both morphology and pathology comparisons. It was beyond the scope of the present study to perform a comprehensive comparative pathology study validating canine myxomatous mitral valve disease as a model of the human disorder. Nevertheless, although human MVIC were difficult to grow in primary cultures (compared with canine MVIC), we had no examples where the data involving either canine or human MVIC, regardless of disease status, disagreed concerning 5HT and Fen results. Furthermore, while MVIC from either normal or myxomatous mitral valves responded comparably in our studies, it could be argued that myxomatous MVIC should not be studied since these results from myxomatous MVIC may not be of general interest. However, many of the reported Fen-Phen cases4,5 were from patients with underlying myxomatous mitral valve disease, and thus MVIC studies from myxomatous valves are likely of translational relevance.
It is also acknowledged that the MVIC studied in our investigations represent a heterogeneous population of cells, and this is clear from the smooth muscle actin and vimentin immunostaining results (Figure 1). It should also be noted that our observations are consistent with those of a comprehensive study concerning the activation status of MVIC in normal and myxomatous mitral valves.43 However, in Fen valvulopathy, although it could be that only a phenotypic subset of MVIC is affected by this agent, this seems unlikely. Furthermore, the selectivity of Fen for specific MVIC phenotypes is unknown. Therefore, the fact that our cell culture results with Fen plus 5HT, in a heterogeneous MVIC culture, demonstrate a strong, uniform response to Fen/5HT is comparable, as a model system, with MVIC exposure to Fen in vivo, and thus may address the mechanisms of interest for this paper. Future studies that subclone MVIC phenotypes and assess the effects of Fen and 5HT on activation may be helpful to refine the understanding of the pathophysiology of Fen-related heart valve disease. Endothelial related research concerning Fen-associated valve disease has not been performed by others and was beyond the scope of the present studies. Nevertheless, the pathology studies of the Fen-Phen valvulopathy demonstrate inflammatory infiltrates,4,5 which differs from the pathology noted with the carcinoid valvulopathy, and suggests that endothelial activation may also be present as part of Fen-associated valvular pathophysiology. This is also an important subject for future research directions.
Conclusions
Fen in combination with 5HT increases pERK1/2 in MVIC with an associated disruption in MVIC related physiological activities including a significantly reduced mitogenic response that is 5HTT dependent, occurring at the same time as an unabated 5HT-induced stimulation of ECM production. Overall, these findings represent novel insights that relate to the pathogenesis of Fen-associated mitral heart valve disease.
Acknowledgments
We gratefully acknowledge the assistance of Drs. John Rush and Bruce Keene in obtaining canine mitral valve specimens for this study.
Footnotes
Address reprint requests to Jeanne M. Connolly, The Children’s Hospital of Philadelphia, Abramson Research Center, Suite 702, 3615 Civic Center Boulevard, Philadelphia, PA 19104-4318. E-mail: connolly@email.chop.edu.
Supported by a grant from the NHLBI, HL74731, The Kibel Foundation, and The William J. Rashkind Endowment of The Children’s Hospital of Philadelphia.
References
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LEC. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med. 1998;339:719–724. doi: 10.1056/NEJM199809103391102. [DOI] [PubMed] [Google Scholar]
- Shively BK, Roldan CA, Gill EA, Najarian T, Loar SB. Prevalence and determinants of valvulopathy in patients treated with dexfenfluramine. Circulation. 1999;100:2161–2167. doi: 10.1161/01.cir.100.21.2161. [DOI] [PubMed] [Google Scholar]
- Volmar KE, Hutchins GM. Aortic and mitral fenfluramine-phentermine valvulopathy in 64 patients treated with anorectic agents. Arch Pathol Lab Med. 2001;125:1555–1561. doi: 10.5858/2001-125-1555-AAMFPV. [DOI] [PubMed] [Google Scholar]
- McDonald PC, Wilson JE, Gao M, McNeill S, Spinelli JJ, Williams OD, Harji S, Kenyon J, McManus BM. Quantitative analysis of human heart valves: does anorexigen exposure produce a distinctive morphological lesion? Cardiovasc Pathol. 2002;11:251–262. doi: 10.1016/s1054-8807(02)00110-2. [DOI] [PubMed] [Google Scholar]
- Moller JE, Connolly HM, Rubin J, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003;348:1005–1015. doi: 10.1056/NEJMoa021451. [DOI] [PubMed] [Google Scholar]
- Dewey RB, 2nd, Reimold SC, O'Suilleabhain PE. Cardiac valve regurgitation with pergolide compared with nonergot agonists in Parkinson disease. Arch Neurol. 2007;64:377–380. doi: 10.1001/archneur.64.3.377. [DOI] [PubMed] [Google Scholar]
- Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;356:39–46. doi: 10.1056/NEJMoa054830. [DOI] [PubMed] [Google Scholar]
- Musunuru S, Carpenter JE, Sippel RS, Kunnimalaiyaan M, Chen H. A mouse model of carcinoid syndrome and heart disease. J Surg Res. 2005;126:102–105. doi: 10.1016/j.jss.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Gustafsson BI, Tommeras K, Nordrum I, Loennechen JP, Brunsvik A, Solligard E, Fossmark R, Bakke I, Syversen U, Waldum H. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005;111:1517–1522. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- Hauso O, Gustafsson BI, Loennechen JP, Stunes AK, Nordrum I, Waldum HL. Long-term serotonin effects in the rat are prevented by terguride. Regul Pept. 2007;143:39–46. doi: 10.1016/j.regpep.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, Seif I, Benhaiem-Sigaux N, Kirsch M, Hamon M, Adnot S, Eddahibi S. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation. 2006;113:81–89. doi: 10.1161/CIRCULATIONAHA.105.554667. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Serotonin releasing agents. Neurochemical, therapeutic, and adverse effects. Pharmacol Biochem Behav. 2002;71:825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- Smith DA, Schmid EF. Drug withdrawals and the lessons within. Curr Opin Drug Discov Devel. 2006;9:38–46. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57:75–81. [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol. 2003;63:1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- Setola V, Dukat M, Glennon RA, Roth BL. Molecular determinants for the interaction of the valvulopathic anorexigen norfenfluramine with the 5-HT2B receptor. Mol Pharmacol. 2005;68:20–33. doi: 10.1124/mol.104.009266. [DOI] [PubMed] [Google Scholar]
- Setola V, Roth BL. Screening the receptorome reveals molecular targets responsible for drug-induced side effects: focus on ‘fen-phen’. Expert Opin Drug Metab Toxicol. 2005;1:377–387. doi: 10.1517/17425255.1.3.377. [DOI] [PubMed] [Google Scholar]
- Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- Rabkin E, Hoerstrup SP, Aikawa M, Mayer JE, Jr, Schoen FJ. Evolution of cell phenotype and extracellular matrix in tissue-engineered heart valves during in-vitro maturation and in-vivo remodeling. J Heart Valve Dis. 2002;11:308–314. [PubMed] [Google Scholar]
- Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, Levy RJ. Serotonin mechanisms in heart valve disease I: serotonin-induced up-regulation of transforming growth factor-beta1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol. 2002;161:2111–2121. doi: 10.1016/s0002-9440(10)64489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, Liang B. Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells. Am J Pathol. 2002;161:2209–2218. doi: 10.1016/S0002-9440(10)64497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry DE, Chopra RK, Ovenden J, Anastassiades TP. Differential use of Alcian blue and toluidine blue dyes for the quantification and isolation of anionic glycoconjugates from cell cultures: application to proteoglycans and a high-molecular-weight glycoprotein synthesized by articular chondrocytes. Anal Biochem. 2000;285:211–219. doi: 10.1006/abio.2000.4761. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Partilla JS, Rothman RB. Serotonin transporters, serotonin release, and the mechanism of fenfluramine neurotoxicity. Ann NY Acad Sci. 2000;914:172–186. doi: 10.1111/j.1749-6632.2000.tb05194.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Redmon JB, Raatz SK, Kwong CA, Swanson JE, Bantle JP. Chronic treatment with phentermine combined with fenfluramine lowers plasma serotonin. Am J Cardiol. 2000;85:913–915, A910. doi: 10.1016/s0002-9149(99)00896-6. [DOI] [PubMed] [Google Scholar]
- Tamir H, Gershon MD. Serotonin-storing secretory vesicles. Ann NY Acad Sci. 1990;600:53–66. doi: 10.1111/j.1749-6632.1990.tb16872.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. Neurotransmitter transporters: fruitful targets for CNS drug discovery. Mol Psychiatry. 2000;5:357–362. doi: 10.1038/sj.mp.4000728. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Baumann MH, Rothman RB. Chronic fenfluramine administration increases plasma serotonin (5-hydroxytryptamine) to nontoxic levels. J Pharmacol Exp Ther. 2008;324:791–797. doi: 10.1124/jpet.107.132654. [DOI] [PubMed] [Google Scholar]
- Humphreys CJ, Beidler D, Rudnick G. Substrate and inhibitor binding and translocation by the platelet plasma membrane serotonin transporter. Biochem Soc Trans. 1991;19:95–98. doi: 10.1042/bst0190095. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Humphreys CJ. Platelet serotonin transporter. Methods Enzymol. 1992;215:213–224. doi: 10.1016/0076-6879(92)15065-k. [DOI] [PubMed] [Google Scholar]
- Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- MacLean MR. Pulmonary hypertension, anorexigens and 5-HT: pharmacological synergism in action? Trends Pharmacol Sci. 1999;20:490–495. doi: 10.1016/s0165-6147(99)01389-9. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Mutlu A, Russell JC, Brindley DN, DeAlmeida J, Rabinovitch M. Dexfenfluramine protects against pulmonary hypertension in rats. J Appl Physiol. 2002;93:1770–1778. doi: 10.1152/japplphysiol.00500.2002. [DOI] [PubMed] [Google Scholar]
- Rochefort GY, Lemaire MC, Eder V, Hanton G, Hyvelin JM, Bonnet P, Antier D. Dexfenfluramine does not worsen but moderates progression of chronic hypoxia-induced pulmonary hypertension. Eur J Pharmacol. 2006;550:149–154. doi: 10.1016/j.ejphar.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Mast ST, Gersing KR, Anstrom KJ, Krishnan KR, Califf RM, Jollis JG. Association between selective serotonin-reuptake inhibitor therapy and heart valve regurgitation. Am J Cardiol. 2001;87:989–993. doi: 10.1016/s0002-9149(01)01435-7. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Stober G, Heils A, Lesch KP. Serotonin transporter gene polymorphism and affective disorder. Lancet. 1996;347:1340–1341. [PubMed] [Google Scholar]
- Pedersen HD, Haggstrom J. Mitral valve prolapse in the dog: a model of mitral valve prolapse in man. Cardiovasc Res. 2000;47:234–243. doi: 10.1016/s0008-6363(00)00113-9. [DOI] [PubMed] [Google Scholar]
- Disatian S, Ehrhart EJ, 3rd, Zimmerman S, Orton EC. Interstitial cells from dogs with naturally occurring myxomatous mitral valve disease undergo phenotype transformation. J Heart Valve Dis. 2008;17:402–411. [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]