Abstract
Acute liver failure (ALF) remains a disease with poor patient outcome. Improved prognosis is associated with spontaneous liver regeneration, which supports the relevance of exploring ‘regenerative’ therapies. Therefore, the role of the Wnt/β-catenin pathway in liver regeneration following ALF was investigated. ALF was induced in mice by acetaminophen overdose, which is also a leading cause of liver failure in patients. β-catenin distribution was also studied in liver sections from acetaminophen-induced ALF patients. A nonlethal dose of acetaminophen, which induces liver regeneration, led to stabilization and activation of β-catenin for 1 to 12 hours. These data were also verified by increased expression of the β-catenin surrogate target glutamine synthetase. β-Catenin activation occurred secondary to the inactivation of glycogen synthase kinase-3β and an increase in levels of casein kinase 2α, and led to increased cyclin-D1, another known β-catenin target. These observations were next substantiated in β-catenin conditional-null mice (β-catenin-null), which show dampened regeneration after acetaminophen injury following induction of CYP2e1/1a2 expression. In light of decreased acetaminophen injury in β-catenin-null mice despite CYP induction, equitoxic studies in control mice were performed. Significant differences in regeneration persisted following comparable injury in β-catenin-null and control animals. Retrospective analysis of liver samples from acetaminophen-overdose patients demonstrated a positive correlation between nuclear β-catenin, proliferation, and spontaneous liver regeneration. Thus, our studies demonstrate early activation of β-catenin signaling during acetaminophen-induced injury, which contributes to hepatic regeneration.
Acute liver failure (ALF) is an ominous liver disease that is on the rise in the Western world.1,2 The current treatments for ALF include an infusion of N-acetyl cysteine, a glutathione precursor, or orthotopic liver transplantation (OLT). While N-acetyl cysteine has been used with success in a subset of ALF patients secondary to acetaminophen (APAP) overdose, its effectiveness in non-APAP-associated ALF cases is being debated.3,4 OLT remains a mainstay treatment in severe cases and is associated with high cost, morbidity and mortality; and is limited by available donor livers. With over 50% of ALF cases being due to overdose of the commonly used over-the-counter anti-pyretic and analgesic APAP, efforts are under to way to investigate significance of regenerative therapies in ALF patients.5,6,7 Recently, studies have demonstrated that patients who have a timely increase in spontaneous liver regeneration following APAP overdose have improved prognosis.8,9 The role of liver regeneration in protection against APAP-induced ALF has also been demonstrated in experimental models.
Liver possesses a remarkable self-regenerative capability, as has been shown extensively in rodents after partial hepatectomy or by toxicant-induced injury with carbon tetrachloride, chloroform and thioacetamide.10,11,12,13,14 Regeneration is mediated by intricate signaling via growth factors such as hepatocyte growth factor and epidermal growth factor, and cytokines such as tumor necrosis factor-α. Recent studies have demonstrated a critical role of the Wnt/β-catenin pathway in hepatocyte proliferation during liver development, cancer, and regeneration.15 Rapid activation of β-catenin in rat liver was observed within 5 minutes of partial hepatectomy and knockdown of β-catenin with antisense treatment resulted decreased liver regeneration.16,17 In addition, lack of β-catenin in mice results in delayed liver regeneration due to inadequate G1 to S transition.18,19 These studies also identified CYP2E1 and CYP1A2 to be dramatically lower in β-catenin-null livers, thus precluding the use of these mice to test regeneration after APAP-injury. CYP2E1 and CYP1A2 are essential to metabolize APAP to induce injury and regeneration.20
The present study was designed to investigate changes in the Wnt/β-catenin pathway during liver regeneration in ALF induced by APAP. We identified β-catenin activation early during this event, which appears to be important in stimulating regeneration in this model. In addition, we showed high correlation between nuclear/cytoplasmic β-catenin and ongoing regeneration in hepatic biopsies from ALF patients. Our data suggest β-catenin activation as one of the mechanisms that may contribute to spontaneous regeneration following ALF in preclinical and clinical scenario.
Materials and Methods
Animals, Treatments, and Tissue Collection
Male CD-1 mice (20 to 25 g, n = 5 per time point) were injected intraperitoneally with 500 mg/kg APAP dissolved in warm 0.45% saline (pH 8). Mice were sacrificed at specific time points-1 hour, 3 hours, 6 hours, 12 hours, 24 hours, and 48 hours and the livers were removed and divided into three parts. One part was fixed in 10% buffered formalin and used for paraffin embedding and sectioning. Second portion of the liver was frozen in optimal cutting temperature compound for cryosections and third portion was snap frozen in liquid N2 for further analysis. Serum from animals was analyzed for alanine aminotransferase (ALT) as a marker of injury. All animals studies were performed in accordance with institutional animal use and care committee at the University of Pittsburgh, School of Medicine and the National Institutes of Health guidelines.
APAP-induced hepatic toxicity was also examined in β-catenin conditional null (β-cateninloxP/loxP, α-fetoprotein-albumin Cre+/−, or KO) and β-cateninloxP/loxP, α-fetoprotein-albumin Cre−/− or Con) mice that were generated as described elsewhere.21,22
For equitoxic studies, Con mice (n = 3/dose) were injected with IP APAP at 200 to 350 mg/kg dose and serum ALT levels estimated. A dose of 225 mg/kg induced modest liver injury that was reflected by ALT levels of around 1000 IU/L.
Patient Biopsies
Paraffin sections in the form of liver biopsies or resected livers from anonymized patients with APAP-induced ALF were obtained from Health Sciences Tissue Bank or Department of Pathology at the University of Pittsburgh Medical Center under an Institutional Review Board-exempt protocol. The patient summary and relevant information is provided in Table 1.
Table 1.
Clinical Features of APAP-Induced ALF Patients
| Number | Age | Sex* | β-Catenin score† | PCNA score‡ | OLT status |
|---|---|---|---|---|---|
| 1 | 50–59 | M | 1 | 0 | Yes |
| 2 | 50–59 | F | 0 | 0 | Yes |
| 3 | 40–49 | F | 0 | 1 | Yes |
| 4 | 30–39 | M | 0 | 0 | Yes |
| 5 | 20–29 | F | 1 | 1 | Yes |
| 6 | 40–49 | F | 1 | 1 | Yes |
| 7 | 20–29 | F | 0 | 0 | Yes |
| 8 | 30–39 | M | 8.6 | 4 | No |
| 9 | 40–49 | F | 4.7 | 1 | Yes |
| 10 | 40–49 | F | 6.4 | 1 | Yes |
| 11 | 50–59 | F | 20.6 | 4 | No |
| 12 | 30–39 | F | 1 | 0 | No |
| 13 | 30–39 | F | 16 | 4 | No |
| 14 | 20–29 | M | 3.5 | 2 | No |
| 15 | 40–49 | F | 12.5 | 4 | No |
| 16 | 20–29 | F | 1 | 1 | No |
| 17 | 50–59 | F | 7.2 | 3 | Yes |
| 18 | 20–29 | F | 11.8 | 4 | No |
| 19 | 20–29 | M | 7.6 | 3 | Yes |
| 20 | 40–49 | F | 1 | 2 | Yes |
| 21 | 20–29 | F | 1 | 2 | Yes |
M, male; F, female.
Number of cells with nuclear β-catenin staining in a high power field.
Arbitrary score: 1, <2%; 2, 2% to 10%; 3, 10% to 20%; 4, >20% PCNA-positive hepatocytes.
Protein Extraction, Microsome Preparation, and Western Blots
Whole cell lysates were prepared from pooled mice livers (n = 5) and human liver samples using radioimmunoprecipitation assay buffer (9.1mmol/L dibasic sodium phosphate, 1.7mmol/L monobasic sodium phosphate, 150mmol/L sodium chloride, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate [pH adjusted to 7.4]) containing fresh protease and phosphatase inhibitors (Sigma, St. Louis, MO) as described previously.19 Mouse liver microsomes were prepared from homogenized minced tissue in homogenization buffer (10 mmol/L sodium potassium phosphate, pH 7.4, 1.15% potassium chloride, and 10 mmol/L EDTA) as described previously.19 Protein concentration was determined by bicinchoninic acid protein assay. SDS-polyacrylamide gel electrophoresis analysis was performed using 50 μg of protein on 7.5% ready gels using the mini-PROTEIN 3-electrophoresis module (Biorad, Hercules, CA). Following 1 hour transfer at 100V (constant) to the Immobilon-PVDF membranes (Millipore, Bedford, MA), blots were incubated with primary and secondary antibodies with intermittent washes with the washing buffer. The primary antibodies used were against CYP2E1 (1:1000; Abcam, Cambridge, MA); active-β-catenin that recognizes hypophosphorylated β-catenin at serine-37 and threonine-41(1:100; Upstate Biotechnology); β-catenin, CYP1A2, glycogen synthase kinase-3β (GSK-3β), phospho-Ser9-GSK3β, c-met, E-cadherin, casein kinase 2α (CK2α) (1:200; Santa Cruz Biotechnology, Santa Cruz, CA); and anti-actin (1:1000; Millipore, Bedford, MA). Horseradish peroxidase-conjugated secondary antibodies (Chemicon, Temecula, CA) were used at 1:20,000. Blots were subjected to fresh Super-Signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and visualized by autoradiography. The autoradiographs were scanned and changes in protein expression assessed by changes in integrated optical density (IOD) that was obtained using Image J software.
Immunoprecipitation
Five hundred micrograms of whole cell lysate was pre-cleared by normal goat IgG and 20 μg A/G-agarose for 30 minutes at 4°C (Santa Cruz). The supernatant obtained after centrifugation (1000 × g) at 4°C was incubated with 20 μl of agarose-conjugated, goat-anti-β-catenin antibody (Santa Cruz) for 1 hour or overnight at 4°C. Pellets collected by centrifugation (1000 × g) were washed four times with radioimmunoprecipitation assay buffer at 4°C and resuspended in standard electrophoresis loading buffer. After boiling for 5 minutes, 30 μl of the samples were resolved on ready gels, transferred, and subjected to Western blot analysis as described above.
Real-Time PCR Analysis
Frozen livers (n = 5) were used to isolate mRNA using Qiagen RNeasy kit (Qiagen, San Diego, CA) according to the manufacturers protocol. Following DNase treatment mRNA was converted to cDNA using M-MuLV Reverse transcriptase enzyme (Invitrogen, Carlsbad, CA) in a reverse transcription (RT)-mastermix containing random primers, 5× RT buffer, dNTP mix, RiboLock, and M-MuLV Reverse transcriptase enzyme. β-Catenin message was estimated using the mouse TaqMan Gene Expression Assay (Assay ID-Mm00483033_m1, Applied Biosystems, Foster City, CA) in ABI PRISM 7000 machine according to manufacturers protocol. β-Actin was used as internal control. Changes in β-catenin mRNA were normalized to β-actin mRNA and data expressed as fold change compared with 0 hour.
Histology and Immunohistochemistry
Paraffin sections from mouse livers and patient biopsies were stained for H&E and observed under light microscope for assessment of necrosis. Paraffin liver sections were subjected to immunohistochemistry for β-Catenin (BD Biosciences, San Jose, CA), cyclin-D1, glutamine synthetase, and proliferating cell nuclear antigen (PCNA, Santa Cruz Biotechnology, Santa Cruz, CA) to determine expression and localization using the indirect immunoperoxidase technique. Briefly, 4-μm thick paraffin sections were passed through xylene, and graded alcohol, and rinsed in PBS. Endogenous peroxide was inactivated using 3% hydrogen peroxide (Sigma, St. Louis, MO). Slides were microwaved in citrate buffer for 20 minutes and blocked in blue blocker (Shandon Lipshaw, Pittsburgh, PA) followed by overnight incubation with anti-β-catenin antibody at 4°C (Santa Cruz). Sections were washed and incubated in secondary anti-mouse, horseradish peroxidase-conjugated antibody (Chemicon, Temecula, CA) for 1 hour at room temperature, then in ABC reagent (Vector Laboratories, Burlingame, CA) for 30 minutes and signal detected using AEC chromogen. Sections were counterstained with Harris hematoxylin (Sigma, St. Louis, MO) and passed through dehydration process followed by coverslipping and mounting in DPX (Fluka Labs, St. Louis, MO). For negative control, sections were incubated with secondary antibodies only.
All slides were viewed under an Axioskop 40 (Zeiss) upright research microscope and digital images were obtained by Nikon Coolpix camera. Collages were prepared using the Adobe Photoshop 5.0 software.
Scoring for β-Catenin and PCNA Immunohistochemistry and Statistical Analysis
The results of liver injury in mice were expressed as + SEM. Significant differences between the groups were evaluated by Student’s t-test. Slides (blinded for transplantation status) stained for β-catenin and PCNA were observed under a light microscope at high power (×40) and number of hepatocytes with nuclear β-catenin and PCNA staining were counted in five different fields to calculate averages (areas with frank necrosis were excluded). Higher magnification (×63) photomicrographs were obtained for better depiction of nuclear β-catenin. For correlation analysis, the averages for β-catenin and PCNA-positive cells for each sample were assessed for correlation coefficient (r).
Results
Liver Regeneration after Sublethal Dose of APAP
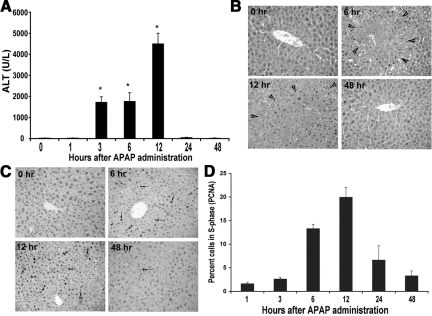
To study regeneration after toxicant induced acute liver injury, male CD-1 mice were injected with a nonlethal dose of APAP (500 mg/kg, IP) as described in Materials and Methods. Serum ALT and histopathology indicated increased liver injury at 3 hours after APAP treatment, which sustained through 6 hours and 12 hours, and abolished at 24 hours (Figure 1A). The histological findings in the liver were reminiscent of centrilobular necrosis at 3 to 12 hours (shown at 6 hours and 12 hours), with restoration of normal liver architecture observed after 24 hours (shown 48 hours) (Figure 1B). As shown previously, this recovery from liver injury was due to spontaneous liver regeneration estimated by PCNA analysis whereby cells in S-phase were evident at 6 hours, peaked at 12 hours, and declined at 24 hours after APAP treatment (Figure 1, C and D).12 Although extensive liver injury was observed following APAP administration, no mortality was noted at this dose.
Figure 1.
Increase in liver injury and subsequent regeneration following APAP. A: Changes in serum ALT following APAP administration (500 mg/kg) to male CD-1 mice. Significant differences between groups were observed by Student’s t-test (P < 0.01). B: Representative H&E-stained liver sections showing massive necrosis (areas between arrowheads) induced by APAP at 6 and 12 hours. Normal histology is regained by 48 hours following (×200). C: Representative PCNA immunohistochemistry on liver sections at 0, 6, 12, and 48 hours following APAP administration showing increased cell proliferation at 6 to 12 hours, indicated by cells in S-phase (arrows). D: Bar graph identifies percentage of cells in S-phase by PCNA immunohistochemistry over 0–48 hours after APAP administration. One thousand cells were counted at 400X magnification to calculate percentage.
β-Catenin Activation during APAP-Induced ALF
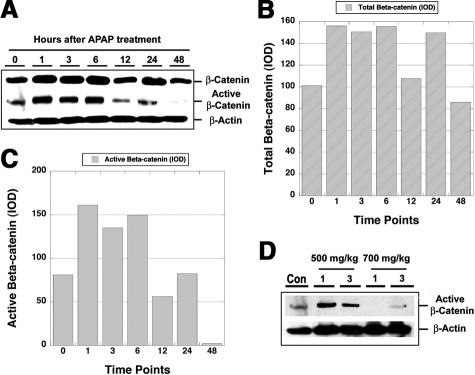
We estimated the levels of β-catenin protein (total and activated) in the livers of APAP-treated mice at various time points. Western blot analysis identified a dramatic increase in total β-catenin protein between 1 to 6 hours after APAP administration, followed by another increase at 24 hours (Figure 2A). The increases in total β-catenin at the corresponding time points were around 1.5-fold over the control liver when assessed by absolute IOD (Figure 2B). A concomitant increase in active-β-catenin that represents hypophosphorylated β-catenin at serine-37 and threonine-41 was evident at 1 to 6 hours only (Figure 2A). IOD assessment by Image J analysis revealed around twofold increase in active-β-catenin at 1 to 6 hours (Figure 2C). Thus, there is an early increase in total and active β-catenin stabilization during APAP-induced regeneration.
Figure 2.
An increase in total and active β-catenin during APAP-induced injury and regeneration. A: Western blot (WB) for total- and active-β-catenin in liver cell lysates following sublethal dose (500 mg/kg) of APAP. B: Analysis of absolute IOD by densitometry shows a 1.5 fold increase in total β-catenin at 1 to 6 hours and at 24 hours after APAP-injury. C: Analysis of absolute IOD shows around twofold increase in active β-catenin at 1 to 6 hours after APAP-injury. D: WB for active-β-catenin at 1 and 3 hours following 500 or 700 mg/kg dose of APAP. Con indicates control mice liver lysate without any treatment.
β-Catenin Activation Is Related to Liver Regeneration and Not Liver Injury
The relatively early increase in total and active β-catenin stabilization following APAP administration led us to investigate whether β-catenin activation itself played a role in mediating liver injury, as opposed to being a component of the immediate early response, to perhaps detect injury and promote liver regeneration. To answer this question, we performed a dose response study, where CD-1 mice were injected with nonlethal (500 mg/kg) or lethal (700 mg/kg) doses of APAP. Liver cell lysates obtained 1 hour or 3 hours after administration of lethal dose of APAP revealed a lack of any increase in active-β-catenin (Figure 2D). This is in contrast to the increase in active-β-catenin in the sub lethal group (Figure 2D).
Sublethal Doses of APAP Induce Expression of β-Catenin-Responsive Genes
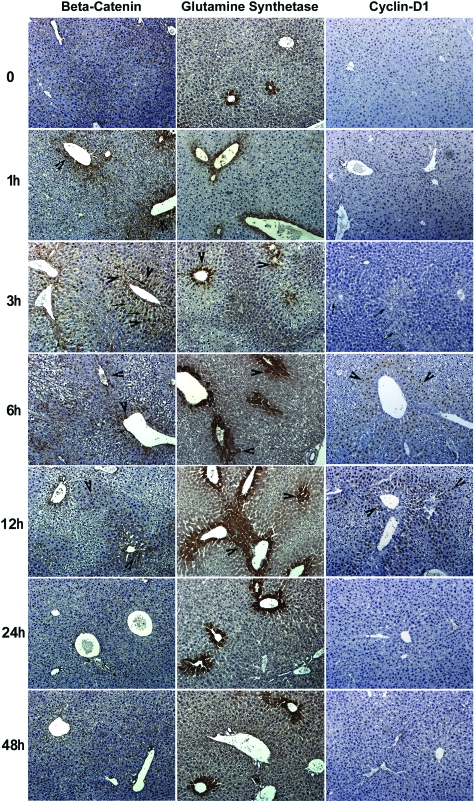
To further elucidate the effect of β-catenin activation in APAP-induced liver regeneration, we examined sections from livers at various times after administration of sublethal doses of APAP for distribution of β-catenin and its known targets-glutamine synthetase (GS) and cyclin-D119,23 (Figure 3). In a normal liver, β-catenin was observed at the hepatocyte membrane. At this time point, GS was localized to the cells around the central vein and most hepatocytes were negative for cyclin-D1. At 1 hour after APAP, we began to observe accentuation of β-catenin staining in centrizonal areas (arrowhead) that indicated stabilization of the protein in hepatocytes. No change in GS staining was evident at this time. At 3 hours, when several hepatocytes in the centrizonal underwent oncotic cell death (arrow), a subset hepatocytes in the same zone displayed a dramatic increase in nuclear and cytoplasmic localization (arrowhead) of β-catenin. There were also several cells in the centrizonal area, which at this time exhibited GS staining, but mostly in a normal pattern despite ongoing cell death. No cyclin-D1-positive cells were noted at 1 hour or 3 hours after APAP treatment. At 6 hours, β-catenin was predominantly cytoplasmic in many cell layers around the central veins (arrowhead) encompassing centrizonal and some midzonal areas of the hepatic lobule. At this time there was an associated widening of GS staining as evident by multiple layers of GS-positive cells around the central vein. Cyclin-D1-positive hepatocytes were observed for the first time at 6 hours and these cells are located in centrizonal area of the liver lobule. At 12 hours, pericentral accentuation of β-catenin expression was still apparent, although was less intense than the preceding time points, while a continued increase in GS and further increase in cyclin-D1-positive hepatocytes was evident at this stage. At 24 hours and 48 hours, predominant areas within the hepatic lobule showed normal cellular and zonal distribution of β-catenin, GS, and cyclin-D1 that is comparable with the pre-injury state.
Figure 3.
Immunohistochemical changes in β-Catenin, glutamine synthetase (GS), and cyclin-D1 in murine liver at various time points after sublethal doses of APAP. Increased centrizonal β-catenin is observed as early as 1 hour, with clear cytoplasmic and limited nuclear localization at 3, 6, and 12 hours. Increase GS levels are observed at 3, 6, and 12 hours, whereas increased cyclin-D1 is chiefly observed at 6 to 12 hours. All three proteins were predominantly observed in normal configuration at 24 and 48 hours. Positively staining cells are highlighted with arrowheads and oncotic cell death is indicated by arrows.
Mechanism of β-Catenin Stabilization and Activation after APAP Injury
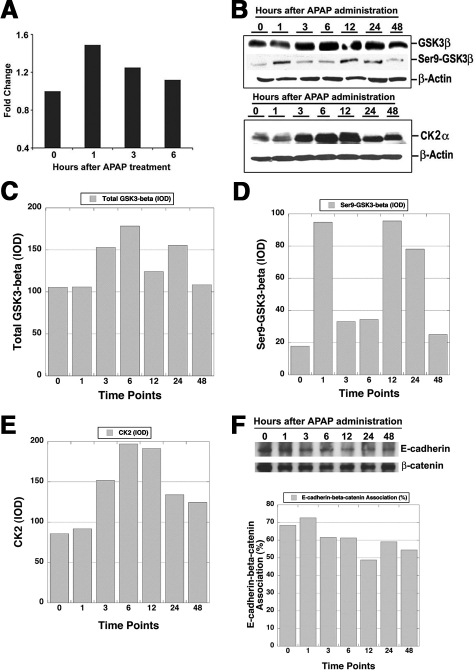
Since β-catenin stabilization and activation was apparent between 1 and 12 hours, we next investigated the molecular basis responsible for these changes. We examined the expression of β-catenin gene (CTNNB1) by real time PCR. Analysis of pooled livers overall showed only minor differences in β-catenin mRNA expression with highest expression being evident at 1 hour after APAP treatment (Figure 4A).
Figure 4.
Multifactorial basis of β-catenin activation after APAP-induced ALF and subsequent liver regeneration. A: Real-time PCR for β-catenin gene expression (fold-change) following APAP treatment from 0 to 6 hours. B: WB for total GSK3β and inactive GSK3β (phosho-Ser9) (upper panel) and CK2α (lower panel) in liver lysates following APAP treatment over 0 to 48 hours. C: Densitometry shows a minor increase in total GSK3β protein levels at 3, 6, and 24 hours after APAP treatment. D: Densitometry reveals around fivefold increase in inactive-GSK3β at 1, 12, and 24 hours after APAP treatment. E: Densitometry reveals around twofold increase in total CK2α levels at 3 to 12 hours after APAP treatment. F: Coprecipitation studies show relatively unchanged β-catenin-E-cadherin association in whole liver lysates after APAP treatment over 0 to 48 hours (upper panel). Densitometry revealed only minor changes in stoichiometry of β-catenin-E-cadherin association during APAP-induced regeneration.
In the canonical Wnt/β-catenin pathway, GSK-3β is the main regulator of β-catenin, which phosphorylates β-catenin at specific serine and threonine residues for eventual proteasomal degradation.24 We examined the levels of total and Ser9-phospho-GSK-3β (inactive form) by Western blot analysis in the liver cell lysates after APAP administration. While the total GSK3β levels appeared not to fluctuate dramatically, levels of Ser9-phospho-GSK-3β showed some cyclical changes in its protein levels (Figure 4B). Absolute IOD assessment showed around 30% increase in total GSK3β levels at 3, 6, and 24 hours only (Figure 4C). However, the levels of inactive GSK3β were increased around fourfold at 1, 12, and 24 hours above the levels at baseline (Figure 4D). This indicated significant temporal inactivity of GSK3β, which might be contributing toward overall β-catenin stabilization and activation.
CK2α is known to positively stimulate β-catenin signaling.25,26 CK2α levels also showed noteworthy changes especially at 3 to 12 hours after APAP-injury (Figure 4B). IOD obtained after densitometric analysis revealed at least twofold increase in total CK2α protein over baseline at these time points, suggesting yet another complementary mechanism of β-catenin activation (Figure 4E).
Finally, β-catenin is known to complex with E-cadherin at the hepatocyte membrane.27 Next, we performed co-precipitation studies to investigate changes in these complexes following APAP-induced acute liver injury and regeneration. E-cadherin-β-catenin association remained relatively unaffected over the course of the entire injury and regeneration after APAP administration as shown by co-precipitation studies (Figure 4F). In addition, densitometry verified any changes in the stoichiometry of association between the two protein during APAP-induced liver regeneration.
Response of β-Catenin Conditional Null Mice to APAP Administration Following CYP2E1 Induction
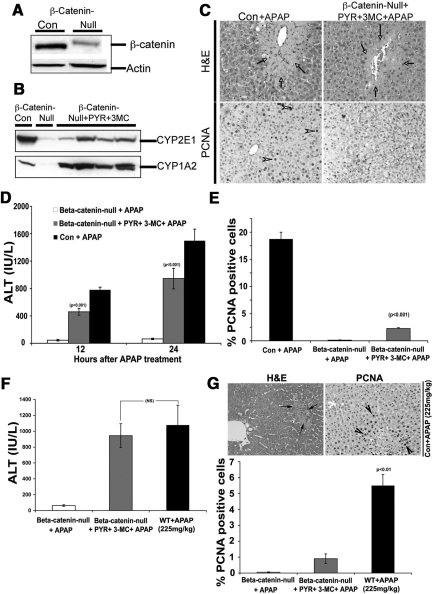
To further confirm the relevance of β-catenin activation in liver regeneration after APAP-injury, we decided to examine the β-catenin conditional null mice (β-cateninloxp/loxp;αFP-Alb-Cre+/−, or KO) and wild-type (β-cateninloxp/loxp;αFP-Alb- Cre−/− or Con) littermates that are available in C57BL/6 background in our laboratory.21,22 The C57BL/6 strain of mice displays prominent injury and proliferation in response to sublethal doses of acetaminophen at 24 hours as opposed to CD-1 mice at 6 to 12 hours.28 While the KO model would have been ideal to study role of β-catenin in APAP-induced hepatic regeneration, a major limitation was that the KO livers lack CYP2E1 and CYP1A2 (Figure 5A and B), the two main P450 enzymes involved in metabolism of APAP to its reactive metabolite N-acetyl benzoquinoneimine, which is ultimately responsible for liver injury.19,29 In fact KO mice have been shown to be resistant to APAP-injury due to absence of N-acetyl benzoquinoneimine formation.29 We attempted the use of KO and Con mice to compare and contrast APAP-injury and regeneration following administration of CYP1A2 inducer-3-methylcholanthrene and a CYP2E1 inducer-pyrazole, which greatly induced CYP1A2 and only modestly induced CYP2E1 (Figure 5B). When these mice were exposed to APAP, following CYP induction, they do demonstrate hepatic injury, both biochemically and histologically, albeit, at lower propensity than the controls (Figure 5, C and D). When the livers were examined for PCNA immunohistochemistry at 24 hours, <3% of the KO hepatocytes were observed to be PCNA-positive in KO despite P450 induction, as compared with around 20% in Con and none in KO in absence of P450 induction (Figure 5, C and E). This observation of disproportionately lower hepatocyte proliferation following liver injury, which was only modest as compared with the controls, does support an overall positive role of β-catenin in regulating regeneration following APAP-administration.
Figure 5.
β-Catenin-conditional-null mice (β-cateninloxP/loxP;Cre+/−) exhibit disproportionately lower liver regeneration following APAP-induced hepatic injury after CYP2E1 and CYP1A2 induction. A: WB for β-catenin in liver lysates from β-catenin-null and Con (β-cateninloxP/loxP; Cre−/−) mice. B: WB for CYP1A2 and CYP2E1 before and after 3-methylcholanthrene and pyrazole treatment in β-catenin-null mice. C: H&E staining (top) shows massive injury after APAP administration in controls (left) and only modest injury in KO despite CYP induction (right). While several PCNA+ve hepatocytes are observed in Con (bottom left), only occasional hepatocyte is detected in S-phase in KO after pyrazole, 3-MC, and APAP at 24 hours. Arrows indicate necrotic cells in top and arrowheads indicate PCNA-positive cells in bottom panel. D: Changes in serum ALT in Con+APAP, KO+APAP and KO+pyrazole + 3-MC+APAP mice at 24 hours post-APAP, identifies no injury in KO+APAP and significantly higher injury in KO-+pyrazole + 3-MC+APAP and highest injury in Con+APAP. E: Numbers of hepatocytes were counted in S-phase in the three groups at 24 hours. The bar graph indicates around ninefold lower number of hepatocytes in S-phase in KO+pyrazole + 3-MC+APAP group, as compared with Con+APAP group. F: APAP at a dose of 225 mg/kg induced hepatic injury at 24 hours in control mice that was comparable with KO at the same time. G: Histological evidence of injury in control mice (arrows) is observed in H&E (upper left panel), along with the presence of increased PCNA+ve hepatocytes (arrowheads) in the centrilobular area shown by PCNA immunohistochemistry (upper right panel). Around 6% to 7% of hepatocytes per high power field were PCNA-positive in control mice at 24 hours after the equitoxic dose, which were significantly greater than those observed in the KO mice at the same time after CYP-induction and APAP administration (P < 0.01) (lower panel).
Equitoxic Study in KO and Controls Reveal Significantly Lower Proliferation in KO Following APAP Injury
For meaningful comparison of the role of β-catenin in cell proliferation after APAP injury, we performed an equitoxic study in Con an KO mice. Based on the serum ALT levels of around 1000 IU/L achievable in KO mice after CYP-inductions and at 24 hours after APAP administration, a dose of 225 mg/kg in C57Bl/6 control mice was identified to be equitoxic as also stated in the methods (Figure 5F). Histology of livers from these animals revealed a modest centrizonal injury (Figure 5G). Around 6% to 7% of hepatocytes in the centrizonal area displayed PCNA-positive nuclei in these livers (Figure 5G). A direct comparison identified a significant difference (P < 0.01) in the numbers of PCNA-positive hepatocytes in KO and Con mice at 24 hours after APAP administration despite similar hepatic injury reflected by comparable serum ALT levels in both groups of mice (Figure 5G). Thus, absence of β-catenin leads to impaired hepatocyte proliferation and dampened regeneration after APAP injury.
β-Catenin Activation Correlates with Higher Spontaneous Liver Regeneration in Patients with APAP-Induced ALF
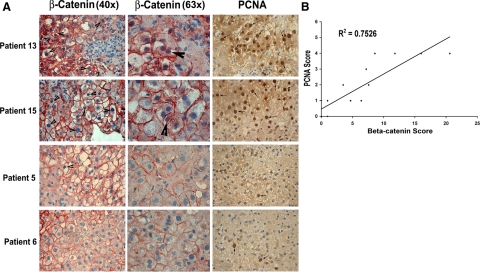
To investigate whether β-catenin activation could also be regulating regeneration in ALF patients, a retrospective analysis using sections from resected livers or biopsies was performed (Table 1). The investigation was aimed at identifying any correlation between β-catenin activation and ongoing cell proliferation (PCNA analysis) as a marker of spontaneous regeneration that eventually prevented OLT. The patient livers, which displayed spontaneous regeneration as indicated by high numbers of PCNA-positive hepatocytes alleviating the need for OLT, also exhibited higher number of hepatocytes with nuclear β-catenin (shown at lower and higher magnification) and vice versa as seen in representative photomicrographs (Figure 6A). When the numbers of hepatocytes with nuclear β-catenin (activation) were plotted against their individual PCNA scores, high correlation was observed between β-catenin activation and liver regeneration (R2 = 0.7526, Figure 6B). These observations also support a positive role of β-catenin in impelling regeneration following toxicant-induced liver failure.
Figure 6.
β-Catenin and PCNA in representative patients of APAP-induced ALF. A: Several hepatocytes showed nuclear β-catenin (arrowhead) shown at ×40 (left panel) and ×63 (middle panel) in the liver biopsies from ALF patients that exhibited spontaneous regeneration (Patients 13 and 15). A concomitant presence of PCNA+ve hepatocytes (arrows) was also observed (right panels, ×40). Only occasional hepatocytes show any nuclear β-catenin with most cells exhibiting membranous β-catenin only (×40 and ×63). Occasional PCNA+ve cell was observed in the same livers (×40) (Patients 5 and 6). B: A high correlation was observed between the nuclear β-catenin and cell proliferation (R2 = 0.7526) when the numbers of positive hepatocytes for nuclear β-catenin were plotted against the respective PCNA scores in ALF livers.
Discussion
Acute liver failure has multiple and diverse bases, ranging from acute to chronic insults to the liver. Acetaminophen overdose remains among the most common causes of ALF. Liver regeneration following surgery and toxic insult is unique to the organ and is responsible for replacing the lost mass for normal function.13 A number of studies have demonstrated that patients who exhibit timely stimulation of the timely compensatory liver regeneration following APAP overdose have a good prognosis.8,9,30 Such timely regeneration has been linked to survival following exposure to hepatotoxic drugs and chemicals such as APAP, CCl4, and chloroform.10,12,14,30 Here, we also demonstrate the importance of spontaneous regeneration in survival following APAP-induced ALF and identify the role of Wnt/β-catenin signaling in this process as well.15
Our observations indicate that β-catenin activation is an early event and is vital for regeneration after APAP-induced ALF. Interestingly, activation of β-catenin was observed even before biochemical or histological hallmarks of liver injury were evident. This observation, along with known regulation of various P450’s by β-catenin, leads to the question if β-catenin is involved in the initiation of liver injury after APAP overdose.29 However, β-catenin activation was suppressed following lethal doses of APAP that are coincidently also known to inhibit regeneration.12,14 Thus it appears that early activation of β-catenin especially in a subset of hepatocytes in pericentral area might be stimulating proliferation of these cells and eventually be the basis of observed regeneration. While the lack of β-catenin activation following lethal doses of APAP suggests no role of β-catenin in inducing injury through regulation of xenobiotic enzymes, it cannot be thoroughly ruled out due to the ongoing tissue damage, disarray, and necrosis, making it technically challenging to qualitatively and quantitatively assay proteins levels.
We and others have demonstrated that β-catenin activation is crucial for liver regeneration and plays a key role in regulating expression of various cyclins (A, D, and E), which are critical for G1 to S phase transition of cell cycle.18,19 The data presented in the current study reveal for the first time that β-catenin plays an equally important role in liver regeneration following drug-induced liver injury. Within 1 hour of APAP-injury, pericentral stabilization of β-catenin was evident. Interestingly, at 3 hours, a subset of hepatocytes with enhanced nuclear and cyctoplasmic β-catenin persisted the ‘assault’ and these cells might eventually have been the basis of restoration of the hepatic lobules. This is supported by a dramatic increase in the centrizonal expression of β-catenin-responsive genes encoding cyclin-D1 and GS, which is evident at 6 to 12 hours. It should be noted that β-catenin stabilization is known to precede the expression of its target proteins, as also shown in our previous studies in liver regeneration.16 This study also reported a lag in normalization of the expression of β-catenin-target genes after β-catenin activation is no longer apparent. Specifically, cyclin-D1 increases after hepatectomy are noted at 24 to 72 hours after partial hepatectomy, whereas β-catenin nuclear translocation occurs within 5 minutes and is significantly diminished at 48 hours.17,31,32 Thus, our studies in CD-1 mice provide evidence for the first time that β-catenin activation may be playing a positive role in hepatic regeneration after APAP-injury by mediating proliferation through regulation of cyclin-D1 levels.
The stabilization and activation of β-catenin is primarily known to be a post-transcriptional event.33 β-Catenin stabilization and activation following APAP injury also appears to be post-translational and has a multifactorial basis. The most relevant observation is a noteworthy but transient inactivation of GSK3β. Phosphorylation of GSK3β at serine-9 has been shown to induce inactivity of GSK3β, which in turn leads to β-catenin stabilization.34,35 How is GSK-3β inactivated following APAP injury is unclear at the present time. One hypothesis is that oxidative stress resulting in 4-hydroxynonenal production, which has been previously demonstrated in APAP-induced liver injury, has also been shown to induce inactivation of GSK-3β in human neuroblastoma cells and in cardiomyocytes.36,37,38,39 The inactivation of GSK3β also appears to be complemented by an increase in total CK2α. The role of CK2α as a positive regulator of Wnt/β-catenin signaling is well accepted.25,26 Thus these factors and perhaps others lead to a regulated and temporal increase in β-catenin and in the expression of its targets, which in turn positively stimulate cell proliferation and eventually restore the liver after APAP injury. It should be emphasized that at any given time there is a balance between positive and negative regulators of Wnt/β-catenin signaling that determine the “on” or “off” status of the signaling pathway. In addition, many of the genetic targets of this pathway are known negative regulators of the Wnt/β-catenin signaling as well.40 All of the above factors allow for strict regulation of the pathway and in turn of the biological activity related to the pathway, which in the current case is cell proliferation. The fluctuations in levels of GSK3β and its phosphorylated state and in CK2α, which matches with the presence of active-β-catenin at certain, but not all time points iterates the many levels of regulation within the highly dynamic β-catenin signaling pathway. While we are aware of the possible roles of many other components of this pathway that were not investigated in the study, which might be impacting the state of signaling in the current scenario, we are certain of the observed activation of β-catenin at early stages of APAP-induced hepatic regeneration.
To conclusively address the role of β-catenin in mice liver regeneration following APAP-induced ALF, we used the β-catenin-null mice, generated by our laboratory.19 However, others and we have previously demonstrated that β-catenin-null mice lack expression of CYP2E1/1A2.19,29 Thus, β-catenin-null mice are resistant to APAP-induced hepatotoxicity due to lack of reactive metabolite formation.29 To overcome this issue, we successfully induced CYP2E1/1A2 using specific agents, albeit partially, which enabled injury in the β-catenin-conditional null mice. However these animals failed to exhibit cell proliferation, supporting a positive role of β-catenin in this process following APAP-induced ALF, as shown in other scenarios of hepatic regeneration.16,17,18,19 However, one has to take in to account the fact that liver injury is known to drive regeneration and hence the lower proliferation index in absence of β-catenin might in fact be due to lower hepatic injury despite modest induction of the relevant CYPs. To circumvent this issue we performed an equitoxic study, where dose of APAP was titrated in control mice to produce hepatic injury comparable with the KO following induction of the two CYPs. When compared, a significantly lower proliferation index persisted in KO despite comparable liver injury estimated by serum biochemistry. Thus, these studies highlight a positive role of β-catenin activation in regeneration after APAP-injury in preclinical setup.
Finally, to test whether Wnt/β-catenin β-catenin activation may be one of the factors responsible for spontaneous regeneration in patients of ALF secondary to APAP overdose, we investigated expression and activation of β-catenin in patients in two sets of patients. The first group included patients of APAP-induced ALF that did not require OLT due to evidence of spontaneous regeneration after conservative management and the second set of patients required OLT due to lack of spontaneous regeneration and declining status. Our data indicates that β-catenin activation (nuclear and cytoplasmic localization) correlates with increased spontaneous regeneration observed by PCNA immunohistochemistry, in patients with APAP-induced ALF (R2 = 0.76). While, we are aware of the heterogeneity in the patient population, timing of hepatic biopsies and multifactorial basis of management of APAP-induce ALF cases, based on our preclinical studies and in light of the positive correlation in patients, it is likely that the stimulation of β-catenin may be one of the mechanisms of spontaneous regeneration following APAP-injury. Furthermore, the correlation between liver regeneration and β-catenin nuclear localization in patient biopsies indicates that β-catenin immunohistochemistry may be used as a biomarker for liver regeneration in ALF patients. While the current analysis has been done on relatively low number of cases (n = 21), future studies with greater sample size including non-APAP-induced ALF cases, will be necessary to validate thesis observation. This is of particular interest since there is currently no sensitive marker for liver regeneration except α-fetoprotein.9
Thus, our observations highlight the role of liver regeneration in survival following ALF. Also we show the role of β-catenin activation for successful stimulation of liver regeneration. The rapid activation of β-catenin following APAP overdose may qualify β-catenin as a potential biomarker for liver regeneration following APAP-induced ALF. Examination of β-catenin activation, as a potential predictor of spontaneous liver regeneration, may be useful in excluding OLT candidates following ALF. Similarly, as new secreted proteins are being identified as targets of β-catenin pathway, these might have prognostic implications as indicators of spontaneous regeneration, thus avoiding high costs and complications of OLT. Finally, stimulating liver regeneration by mediators of β-catenin activation such as Wnt proteins or hepatocyte growth factor may have a therapeutic potential in ALF.
Footnotes
Address reprint requests to Satdarshan P.S. Monga, M.D., Director-Division of Experimental Pathology (EP), Associate Professor of Pathology (EP) & Medicine (GI), University of Pittsburgh School of Medicine, 200 Lothrop Street S-421 BST, Pittsburgh, PA 15261. E-mail: mongass@upmc.edu.
Supported by NIH, R01DK62277 and R01CA124414 to S.P.S.M. and Rango’s Fund for Enhancement of Pathology Research. U.A. is the recipient of American Liver Foundation’s Postdoctoral Research Fellowship for 2006–2007.
References
- Khan SA, Shah N, Williams R, Jalan R. Acute liver failure: a review. Clin Liver Dis. 2006;10:239–258, vii-viii. doi: 10.1016/j.cld.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Lazerow SK, Abdi MS, Lewis JH. Drug-induced liver disease 2004. Curr Opin Gastroenterol. 2005;21:283–292. doi: 10.1097/01.mog.0000160043.10804.60. [DOI] [PubMed] [Google Scholar]
- Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- Horn KD, Wax P, Schneider SM, Martin TG, Nine JS, Moraca MA, Virji MA, Aronica PA, Rao KN. Biomarkers of liver regeneration allow early prediction of hepatic recovery after acute necrosis. Am J Clin Pathol. 1999;112:351–357. doi: 10.1093/ajcp/112.3.351. [DOI] [PubMed] [Google Scholar]
- Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 2005;41:26–31. doi: 10.1002/hep.20511. [DOI] [PubMed] [Google Scholar]
- Apte UM, Limaye PB, Ramaiah SK, Vaidya VS, Bucci TJ, Warbritton A, Mehendale HM. Upregulated promitogenic signaling via cytokines and growth factors: potential mechanism of robust liver tissue repair in calorie-restricted rats upon toxic challenge. Toxicol Sci. 2002;69:448–459. doi: 10.1093/toxsci/69.2.448. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 2005;33:41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K, Vaidya VS, Apte UM, Manautou JE, Ronis MJ, Bucci TJ, Mehendale HM. Type 1 diabetic mice are protected from acetaminophen hepatotoxicity. Toxicol Sci. 2003;73:220–234. doi: 10.1093/toxsci/kfg059. [DOI] [PubMed] [Google Scholar]
- Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi D, Micsenyi A, Bowen WC, Monga DK, Talavera JC, Monga SP. Morpholino oligonucleotide-triggered beta-catenin knockdown compromises normal liver regeneration. J Hepatol. 2005;43:132–141. doi: 10.1016/j.jhep.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
- Apte U, Zeng G, Muller P, Tan X, Micsenyi A, Cieply B, Dai C, Liu Y, Kaestner KH, Monga SP. Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology. 2006;44:992–1002. doi: 10.1002/hep.21317. [DOI] [PubMed] [Google Scholar]
- Apte U, Zeng G, Thompson M, Muller P, Micsenyi A, Cieply B, Kaestner KH, Monga SP. Beta-Catenin is critical for early postnatal liver growth. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1578–1585. doi: 10.1152/ajpgi.00359.2006. [DOI] [PubMed] [Google Scholar]
- Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, Kitajewski J, Kahn A, Perret C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Dominguez I, Mizuno J, Wu H, Imbrie GA, Symes K, Seldin DC. A role for CK2alpha/beta in Xenopus early embryonic development. Mol Cell Biochem. 2005;274:125–131. doi: 10.1007/s11010-005-3073-5. [DOI] [PubMed] [Google Scholar]
- Dominguez I, Mizuno J, Wu H, Song DH, Symes K, Seldin DC. Protein kinase CK2 is required for dorsal axis formation in Xenopus embryos. Dev Biol. 2004;274:110–124. doi: 10.1016/j.ydbio.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Gardner CR, Dambach DM, Durham SK, Brittingham JA, Laskin JD, Laskin DL. Role of tumor necrosis factor receptor 1 (p55) in hepatocyte proliferation during acetaminophen-induced toxicity in mice. Toxicol Appl Pharmacol. 2003;193:218–227. doi: 10.1016/j.taap.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- Rao KN, Virji MA, Moraca MA, Diven WF, Martin TG, Schneider SM. Role of serum markers for liver function and liver regeneration in the management of chloroform poisoning. J Anal Toxicol. 1993;17:99–102. doi: 10.1093/jat/17.2.99. [DOI] [PubMed] [Google Scholar]
- Monga SP, Tang Y, Candotti F, Rashid A, Wildner O, Mishra B, Iqbal S, Mishra L. Expansion of hepatic and hematopoietic stem cells utilizing mouse embryonic liver explants. Cell Transplant. 2001;10:81–89. [PubMed] [Google Scholar]
- Rickheim DG, Nelsen CJ, Fassett JT, Timchenko NA, Hansen LK, Albrecht JH. Differential regulation of cyclins D1 and D3 in hepatocyte proliferation. Hepatology. 2002;36:30–38. doi: 10.1053/jhep.2002.33996. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Blivet-Van Eggelpoel MJ, Beurel E, Boissan M, Delelo R, Cadoret A, Capeau J. Dysregulation of glycogen synthase kinase-3beta signaling in hepatocellular carcinoma cells. Hepatology. 2002;36:1528–1536. doi: 10.1053/jhep.2002.37192. [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Dozza B, Smith MA, Perry G, Tabaton M, Strocchi P. Regulation of glycogen synthase kinase-3beta by products of lipid peroxidation in human neuroblastoma cells. J Neurochem. 2004;89:1224–1232. doi: 10.1111/j.1471-4159.2004.02413.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Knight TR, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- Li HL, Wang AB, Huang Y, Liu DP, Wei C, Williams GM, Zhang CN, Liu G, Liu YQ, Hao DL, Hui RT, Lin M, Liang CC. Isorhapontigenin, a new resveratrol analog, attenuates cardiac hypertrophy via blocking signaling transduction pathways. Free Radic Biol Med. 2005;38:243–257. doi: 10.1016/j.freeradbiomed.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]